Abstract
Background.
Management of glioblastoma is complicated by pseudoprogression, a radiological phenomenon mimicking progression. This retrospective cohort study investigated the incidence, prognostic implications, and most clinically appropriate definition of pseudoprogression.
Methods.
Consecutive glioblastoma patients treated at the Juravinski Hospital and Cancer Centre, Hamilton, Ontario between 2004 and 2012 with temozolomide chemoradiotherapy and with contrast-enhanced MRI at standard imaging intervals were included. At each imaging interval, patient responses as per the RECIST (Response Evaluation Criteria in Solid Tumors), MacDonald, and RANO (Response Assessment in Neuro-Oncology) criteria were reported. Based on each set of criteria, subjects were classified as having disease response, stable disease, pseudoprogression, or true progression. The primary outcome was overall survival.
Results.
The incidence of pseudoprogression among 130 glioblastoma patients treated with chemoradiotherapy was 15%, 19%, and 23% as defined by RANO, MacDonald, and RECIST criteria, respectively. Using the RANO definition, median survival for patients with pseudoprogression was 13.0 months compared with 12.5 months for patients with stable disease (hazard ratio [HR]=0.70; 95% confidence interval [CI], 0.35–1.42). Similarly, using the MacDonald definition, median survival for the pseudoprogression group was 11.8 months compared with 12.0 months for the stable disease group (HR=0.86; 95% CI, 0.47–1.58). Furthermore, disease response compared with stable disease was also similar using the RANO (HR=0.52; 95% CI, 0.20–1.35) and MacDonald (HR=0.51: 95% CI, 0.20–1.31) definitions.
Conclusions.
Of all conventional glioblastoma response criteria, the RANO criteria gave the lowest incidence of pseudoprogression. Regardless of criteria, patients with pseudoprogression did not have statistically significant difference in survival compared with patients with stable disease.
Keywords: Chemoradiotherapy, glioblastoma, magnetic resonance imaging, pseudoprogression.
Glioblastoma (GBM) is the most common primary central nervous system malignancy. In the United States, approximately 10000 cases are diagnosed every year.1 Even with significant advances in therapy, overall survival (OS) remains poor. With modern therapy – maximal surgical excision and adjuvant chemoradiotherapy (CRT) – 2-year OS is 26.5%.2 At the time of recurrence, salvage treatment may consist of further chemotherapy, radiation or surgery.3 As greater GBM tumor volume may be prognostic of lower OS,4 initiating salvage treatment at the earliest suspicion of disease recurrence is crucial.
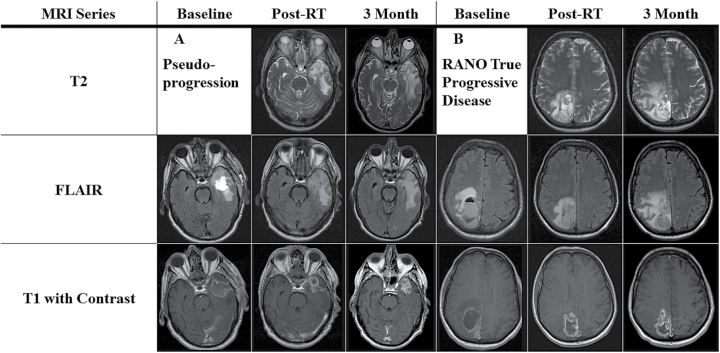
However, it can be difficult to determine the optimal timing of salvage therapy when patterns of disease response on radiographic imaging are confounded by CRT. The result is the phenomenon known as pseudoprogression, where the diffusion of contrast mimics radiographic progression (Fig. 1). Mechanistic hypotheses for pseudoprogression include radiated normal tissue and tumor facilitating contrast transit through a disrupted blood brain barrier, anti-neoplastic inflammatory responses, edema production, radiation necrosis, or radiation-induced enhancement.5,6 The proposed definition of pseudoprogression is when the post-CRT magnetic resonance imaging (MRI) shows in-field early progression, but is followed by disease stability or response on the subsequent scan.7 For the purpose of this study, the term “early progression” is used until pseudoprogression or true disease progression is established.8–10
Fig. 1.
T2, FLAIR, and gadolinium-enhanced images at baseline—immediately prior to CRT; post-treatment—at the completion of CRT; and at the second post-treatment scan. (A) Pseudoprogression example. The rim of the surgical cavity demonstrates progressive thickening on T1 with increased FLAIR and T2 signal. Subsequent stabilization at the second post-treatment scan was observed. (B) True progression example. T1 imaging demonstrates minimal change in nodularity of surgical cavity post-CRT. Progressive changes on T2 and FLAIR are consistent with progression as per RANO.
With early progression, families can wait with anxiety for the recommended 12 weeks to determine their response to treatment.7 This wait is justified as approximately 12% to 65% of these early progression patients will have pseudoprogression, not requiring salvage therapy.11–13 However, if the eventual diagnosis is disease progression, salvage therapy has been postponed. Simpler approaches to rule out pseudoprogression are limited. True progression unfortunately appears in a similar anatomical distribution14–17 and has a scarcity of specific radiological characteristics18,19 to distinguish itself from pseudoprogression.
A better understanding of pseudoprogression could facilitate more timely salvage. The Response Assessment in Neuro-Oncology (RANO) Criteria’s design was heavily influenced by pseudoprogression in order to better separate it from true disease progression. Following its introduction it became the gold standard for assessing progression.7 As it was published in 2010, relevant literature still holds results describing pseudoprogression within the older MacDonald20,21 or exclusively radiology-based criteria.12,22,23 Even modern radiological series frequently use the Response Evaluation Criteria in Solid Tumours (RECIST)24 or small variations, foregoing an aspect of clinical assessment (ie, changes in neurological status or corticosteroid dose).25–29 Other series will omit cases with the subcentimeter findings, defined as non-measurable disease by RANO, preventing application of their data to subtler GBM recurrences.7,9,26,27,30,31 Further complicating matters, the literature holds a myriad of nuances for defining pseudoprogression within these various criteria.8,9,21,32,33 This heterogeneity limits the ability of neuro-oncologists to compare and apply the evidence.
Confidence in clinical decision making would be improved with more applicable data that indicated which response criteria defined pseudoprogression in a fashion that reliably predicted for survival. This study used clinical features already gathered by neuro-oncology clinics to determine which commonly used response criteria provided the most clinically relevant definition of pseudoprogression.
Materials and Methods
Study Population
This study included consecutive patients with GBM treated at the Juravinski Cancer Centre, Ontario, Canada, between 2004 and 2012. The inclusion criteria were temozolomide-based CRT treatment, a GBM diagnosis confirmed by histology, an age of 18 or over, and adequate imaging were included in the study. Adequate imaging was defined as gadolinium-enhanced MRI digitally available to the authors: (1) after surgical resection and prior to CRT; (2) at 4 weeks post-CRT; and (3) at the second post-treatment scan, at least 12 weeks post-CRT. The study was approved by the Institutional Research Ethics Board.
Data Extraction
Data including age, treatment dates, date of death, extent of surgery, dexamethasone dose, Karnofsky Performance Status (KPS), and clinical changes were extracted from clinical charts. From each MRI report, the size of the dominant enhancing lesion, the radiologist’s impression of non-measurable disease, T2 and FLAIR findings, progression at other lesions, and the radiologists’ overall impression were also extracted. If the enhancing lesion was not measured, the authors (MJK, JNG) evaluated the scans as per RANO.7 Briefly, the largest or most reliably measurable enhancing lesion(s), as directed by the radiologist’s report, was assessed for its largest bidimensional measurements on the T1 series. New enhancing lesions were evaluated in relation to the patient’s 80% isodose line on his or her radiation plan, as per RANO.
The extracted data were used to evaluate a patient’s progression status at the time of each MRI, as evaluated by common response criteria—MacDonald,34 RANO,7 or RECIST24 criteria. Table 1 outlines these response criteria.
Table 1.
Response criteria evaluating glioblastoma progression
| Response Criteria | Extent of Response | |||
|---|---|---|---|---|
| Complete Response | Partial Response | Stable Disease | Progression | |
| Response Evaluation Criteria In Solid Tumours (RECIST)24 | No enhancing disease for 4 weeks | ≥50% decrease in enhancing lesion size and no new lesions | Does not qualify as any other Extent of Response | ≥25% increase in enhancing lesion size or any new lesion |
| MacDonald Criteria34 | No enhancing disease for 4 weeks, no corticosteroid, and neurologically stable or improved | ≥50% decrease in enhancing lesion size, no new lesions, and stable or improved clinically | Does not qualify as any other Extent of Response | ≥25% increase in enhancing lesion size, any new lesion, or clinical deterioration related to disease progression |
| Response Assessment in Neuro- Oncology Working Group (RANO)7 | No enhancing disease for 4 weeks*, stable or improved T2/ FLAIR lesions, no corticosteroid, and neurologically stable or improved | ≥50% decrease in enhancing lesion size, stable or improved T2/ FLAIR lesions, no new lesions, and stable or improved clinically | Does not qualify as any other Extent of Response | ≥25% increase in enhancing lesion size, increasing T2/FLAIR lesion size, any new lesion**, or clinical deterioration related to disease progression |
* Patients with non-measurable disease cannot be described as having a complete response
** New lesions outside of the radiation field are not considered to be secondary to pseudoprogression
Patients with disease progression on their post-CRT scan were considered to have early progression and were categorized into 4 response classifications based on their follow-up scan results and performance as follows:
True Progression: early progression then subsequent disease progression
Pseudoprogression: early progression then subsequent disease stability or response
Disease Response: a partial or complete response post-treatment without interval disease progression OR stable disease post-treatment with subsequent disease response
Stable Disease: describes the remainder of the patients.
These response classifications were generated for each patient for each of the MacDonald, RECIST, or RANO criteria individually. A computational algorithm was developed to perform this in a blinded fashion.
Statistical Analysis
The primary objective of the study was to quantify the incidence and prognostic impact of pseudoprogression. Incidence was defined as the proportion of patients with pseudoprogression. Confidence intervals (CI) for incidence were calculated using the Wilson Score method. Overall survival was estimated using Kaplan-Meier methods. Cox models were used to examine the relationship between progression status and overall survival. Stable disease was chosen a priori to be the reference group. In addition, sex, age, KPS, and post-surgery disease extent were selected a priori to be included in all models. To minimize bias, the start of the observation time for each patient was selected as the date of the follow-up scan.19,20,21
Results
Inclusion criteria were met by 159 patients. Patients were then excluded if their MRIs were unavailable on digital film (n = 17), treatment involved a vascular endothelium growth factor modulator (n = 8), treatment involved a surgical debulking between their MRI scans (n = 2), or a contrast agent other than gadolinium was used for imaging (n = 2). One of the 2 patients that underwent surgical debulking had pathological findings consistent with disease progression. The baseline demographics of the 130 patients included in the study are reported in Table 2. All patients with adequate KPS were offered adjuvant temozolomide. Survival analysis was only performed on the 95 patients (73.1%) who underwent all the required imaging.
Table 2.
Baseline characteristics of study population
| Characteristic | All Subjects (n = 130) |
|---|---|
| Age (years): mean (SD) | 56 (9) |
| KPS: mean (SD) | 80 (13) |
| Tumour size: n (%) | |
| No detectable disease | 14 (11) |
| Non-measurable disease | 44 (34) |
| Measurable disease* | 72 (55) |
| Gender: n (%) | |
| Male | 84 (64) |
| Female | 46 (36) |
* mean = 6.6 cm, standard deviation (SD) = 2.9 cm
Pseudoprogression incidence is reported in Table 3. RANO criteria had the lowest incidence of pseudoprogression at 15% (95% CI, 10%–22%). For MacDonald and RECIST criteria, the incidence of pseudoprogression was 19% (95% CI, 13%–27%) and 23% (95% CI, 17%–31%), respectively.
Table 3.
Incidence of pseudoprogression by each response criteria
| RANO | MacDonald | RECIST | |
|---|---|---|---|
| Cases / Subjects | 19 / 130 | 25 / 130 | 30 / 130 |
| Incidence | 0.15 | 0.19 | 0.23 |
| 95% Confidence Interval | 0.10–0.22 | 0.13–0.27 | 0.17–0.31 |
RANO, Response Assessment in Neuro-Oncology; RECIST, Response Evaluation Criteria in Solid Tumours
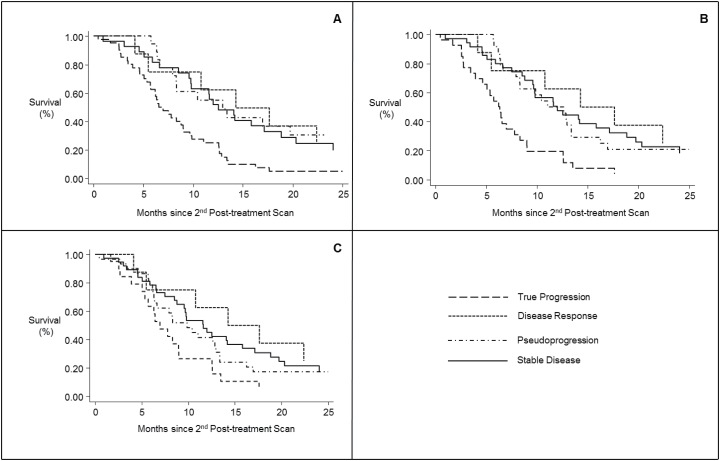
Median survival for the total cohort was 13.6 months when measured from the date of diagnosis and 9.8 months when measured from the second post-treatment scan. Fig. 2 illustrates OS for each of the response criteria’s possible outcomes. Median OS for each of these cohorts is reported in Table 4. None of the response criteria could statistically significantly differentiate survival between disease response, stable disease, and pseudoprogression (Table 5). RANO-defined pseudoprogression had the greatest signal for improved OS, relative to stable disease, with a hazard ratio (HR) of 0.70 (95% CI, 0.35–1.42). Uniquely, RECIST-defined disease progression was not statistically significantly different from stable disease in terms of overall survival (HR=1.65; 95% CI, 0.89–3.06).
Fig. 2.
Kaplan-Meier plots describing overall survival amongst patients that survived to obtain a follow-up scan, as per the different progression schema: RANO (2A), MacDonald (2B), and RECIST (2C).
Table 4.
Median survival as per response criteria outcomes
| RANO | MacDonald | RECIST | |
|---|---|---|---|
| Median Survival (Months) | |||
| Response (n) | 16.0 (8) | 16.0 (8) | 16.0 (8) |
| Stable (n) | 12.5 (27) | 12.0 (35) | 11.6 (37) |
| Pseudoprogression (n) | 13.0 (19) | 11.8 (25) | 9.9 (30) |
| True Progression (n) | 7.0 (41) | 6.3 (27) | 7.0 (20) |
RANO, Response Assessment in Neuro-Oncology; RECIST, Response Evaluation Criteria in Solid Tumours
Table 5.
Results from Cox models for overall survival
| Characteristics | Hazard Ratio (95% Confidence Interval) | ||
|---|---|---|---|
| RANO Deaths / N = 81 / 95 | MacDonald Deaths / N = 81 / 95 | RECIST Deaths / N = 81 / 95 | |
| Response Criteria Outcome | |||
| Response | 0.52 (0.20–1.35) | 0.51 (0.20–1.31) | 0.48 (0.19–1.23) |
| Pseudoprogression | 0.70 (0.35–1.42) | 0.86 (0.47–1.58) | 1.00 (0.57–1.78) |
| Progression | 2.01 (1.16–3.45) | 2.27 (1.28–4.02) | 1.65 (0.89–3.06) |
| Male Sex | 0.77 (0.48–1.24) | 0.84 (0.52–1.37) | 0.81 (0.51–1.31) |
| Age (every 10 years) | 1.02 (0.80–1.31) | 1.03 (0.81–1.31) | 1.01 (0.80–1.29) |
| Baseline KPS | 1.02 (0.86–1.22) | 1.04 (0.87–1.24) | 1.03 (0.86–1.23) |
| Disease Extent | |||
| Non-measurable | 0.60 (0.29–1.24) | 0.58 (0.27–1.22) | 0.67 (0.27–1.22) |
| Measurable | 0.85 (0.43–1.66) | 0.81 (0.41–1.61) | 0.76 (0.38–1.53) |
RANO, Response Assessment in Neuro-Oncology; RECIST, Response Evaluation Criteria in Solid Tumours
Discussion
There are numerous ways to define pseudoprogression in GBM but there are limited data comparing these methods. This study sought to determine which response criteria defined pseudoprogression in the most clinically relevant fashion and minimized incidence of this confounding diagnosis. Using information already collected routinely in a neuro-oncology clinic at a major Canadian academic center, all of the commonly studied response criteria (RANO, MacDonald, and RECIST) were applied to classify a patient’s response to therapy. To our knowledge, the comparison of all commonly used response criteria within the same cohort of GBM patients has not previously been done.
RANO criteria produced the lowest incidence of pseudoprogression in this study and in the literature.32,35 This lower incidence is an expected finding as RANO criteria contain the most variables that can signify progression. Thus, any patient with early progression is more likely to have progression subsequently detected again by RANO—qualifying as true progression, rather than pseudoprogression. When comparing this study to the literature, a caveat is that other studies using RANO also stipulated that a change in management qualified as true progression. In comparison, this study excluded patients from analysis if there was intervening surgical management.
In this study, all 3 definitions of pseudoprogression demonstrated a trend in survival similar to stable disease. A single study has shown that patients with MacDonald- and RANO-defined pseudoprogression do not have a survival rate different than patients without progression.32 This is congruent with a large meta-analysis that suggested progression by MacDonald or RANO criteria prognosticate similarly.36
This study investigated if a patient’s clinical status influenced survival by applying RECIST criteria (ie, criteria that did not account for a patient’s clinical status). Incidentally, in this study, survival of patients with RECIST-defined stable disease was not statistically significantly different from those with true progressive disease. Even though neurological assessment is a debated component of GBM assessment, it is not evaluated in numerous recent radiological studies of pseudoprogression.9,12,22,28,37,38 However, clinical changes were the only common feature differentiating RECIST from RANO and MacDonald criteria in this study. This suggests that evaluating a patient’s neurological status should be considered when classifying progression status.
Ultimately, no set of response criteria defined pseudoprogression in a way that could demonstrate a difference in median OS, relative to stable disease, by the time of the first follow-up MRI. Interestingly, this study and a study by Linhares et al demonstrate that MacDonald- or RANO-defined pseudoprogression does not suggest an improved prognosis compared to stable disease.32 This is in contrast to key data that preceded RANO assessment.33 While not a primary outcome in this study, it is worthwhile to note that no outcome defined by RECIST criteria had a median OS statistically significantly different from stable disease.
As pseudoprogression proposes a clinical quandary, it is most reasonable to consider supporting the response criteria that minimizes its incidence. The RANO criteria’s lower pseudoprogression incidence rate and ability to distinguish a difference in survival for true progressive disease, relative to stable disease, suggest that it is the most clinically relevant definition in this study’s population. MacDonald criteria are remarkably comparable with only a slightly higher incidence of pseudoprogression.
The limitations of this study relate to its retrospective nature, its size, and the study period. Changes in clinical status and T2/FLAIR signal are subjective evaluations that required further subjective evaluation to extract. These aspects were controlled to a reasonable extent and reviewed between authors when contentious. This study, while large for a GBM study, may lack power to detect statistically significant differences in survival. Although survival was assessed from a patient’s follow-up scan, in order to limit guarantee-time bias, there likely remains some influence of timing of the scan on overall survival.39
During the study period, there had been increasing evidence to contest aspects of the RANO criteria. More evidence supports that pseudoprogression may continue to occur outside of the 12-week window proposed by RANO.25,26. This study assessed outcomes in early pseudoprogressors. Patients with pseudoprogression past 12 weeks would not be captured in the definitions set by this study.
Presently, functional and volumetric studies are not incorporated into RANO. Thus, their contribution to patient assessment was not considered in this study. Examples with a degree of proven efficacy include relative cerebral blood flow volume,25,40,41 apparent diffusion coefficient,19,27,30 and volumetric analysis.42,43 Numerous centers include these modalities in their assessment of GBM progression, limiting the ability of these data to be applied in those settings.
Developing evidence regarding outcomes and assessment in high-grade gliomas will shape the next standardized evaluation of neuro-oncology patients. Clinical practice often represents the bridge between current standards and the state-of-the-art. Using the current and previous standards for assessment, this study demonstrated the prevalence and outcomes of pseudoprogression in a sizable cohort of GBM patients.
Funding
This work was supported by a research grant from the McMaster University’s Regional Medical Associates.
Acknowledgements
We thank Dr. Kimmen Quan for his assistance in obtaining the approval of our Research Ethics Board. Findings from this study were presented at the Canadian Association of Radiation Oncologists (CARO) and American Society for Radiation Oncology (ASTRO) annual scientific meetings.
Conflict of interest statement. None declared.
References
- 1. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii93–101. [DOI] [PubMed] [Google Scholar]
- 4. Greenspoon JN, Sharieff W, Hirte H, et al. Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: a prospective cohort study. Onco Targets Ther. 2014;7:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–384. [DOI] [PubMed] [Google Scholar]
- 6. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 7. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 8. Sanghera P, Perry J, Sahgal A, et al. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci. 2010;37(1):36–42. [DOI] [PubMed] [Google Scholar]
- 9. Van Mieghem E, Wozniak A, Geussens Y, et al. Defining pseudoprogression in glioblastoma multiforme. Eur J Neurol. 2013;20(10):1335–1341. [DOI] [PubMed] [Google Scholar]
- 10. Easaw JC, Mason WP, Perry J, et al. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol. 2011;18(3):e126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hygino da Cruz LC, Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roldan GB, Scott JN, McIntyre JB, et al. Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can J Neurol Sci. 2009;36(5):617–622. [DOI] [PubMed] [Google Scholar]
- 13. Topkan E, Topuk S, Oymak E, Parlak C, Pehlivan B. Pseudoprogression in patients with glioblastoma multiforme after concurrent radiotherapy and temozolomide. Am J Clin Oncol. 2012;35(3):284–289. [DOI] [PubMed] [Google Scholar]
- 14. McDonald MW, Shu HK, Curran WJ, Jr, Crocker IR. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;79(1):130–136. [DOI] [PubMed] [Google Scholar]
- 15. Petrecca K, Guiot MC, Panet-Raymond V, Souhami L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J Neurooncol. 2013;111(1):19–23. [DOI] [PubMed] [Google Scholar]
- 16. Sherriff J, Tamangani J, Senthil L, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;86(1022):20120414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tejada S, Aldave G, Marigil M, Gallego Perez-Larraya J, Dominguez PD, Diez-Valle R. Factors associated with a higher rate of distant failure after primary treatment for glioblastoma. J Neurooncol. 2014;116(1):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young RJ, Gupta A, Shah AD, et al. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology. 2011;76(22):1918–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoo RE, Choi SH, Kim TM, et al. Independent poor prognostic factors for true progression after radiation therapy and concomitant temozolomide in patients with glioblastoma: subependymal enhancement and low ADC value. AJNR Am J Neuroradiol. 2015;36(10):1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27(23):3861–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–410. [DOI] [PubMed] [Google Scholar]
- 22. Gerstner ER, McNamara MB, Norden AD, Lafrankie D, Wen PY. Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol. 2009;94(1):97–101. [DOI] [PubMed] [Google Scholar]
- 23. Chaskis C, Neyns B, Michotte A, De Ridder M, Everaert H. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol. 2009;72(4):423–428. [DOI] [PubMed] [Google Scholar]
- 24. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 25. Nasseri M, Gahramanov S, Netto JP, et al. Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question. Neuro Oncol. 2014;16(8):1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radbruch A, Fladt J, Kickingereder P, et al. Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol. 2015;17(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song YS, Choi SH, Park CK, et al. True progression versus pseudoprogression in the treatment of glioblastomas: a comparison study of normalized cerebral blood volume and apparent diffusion coefficient by histogram analysis. Korean J Radiol. 2013;14(4):662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suh CH, Kim HS, Choi YJ, Kim N, Kim SJ. Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. AJNR Am J Neuroradiol. 2013;34(12):2278–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi YJ, Kim HS, Jahng GH, Kim SJ, Suh DC. Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiol. 2013;54(4):448–454. [DOI] [PubMed] [Google Scholar]
- 30. Cha J, Kim ST, Kim HJ, et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol. 2014;35(7):1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chu HH, Choi SH, Ryoo I, et al. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology. 2013;269(3):831–840. [DOI] [PubMed] [Google Scholar]
- 32. Linhares P, Carvalho B, Figueiredo R, Reis RM, Vaz R. Early pseudoprogression following chemoradiotherapy in glioblastoma patients: the value of RANO evaluation. J Oncol. 2013;2013:690585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 34. Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 35. Young RJ, Gupta A, Shah AD, et al. MRI perfusion in determining pseudoprogression in patients with glioblastoma. Clin Imaging. 2013;37(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han K, Ren M, Wick W, et al. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: a literature-based meta-analysis from 91 trials. Neuro Oncol. 2014;16(5)696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan DT, Ng RY, Siu DY, et al. Pseudoprogression of malignant glioma in Chinese patients receiving concomitant chemoradiotherapy. Hong Kong Med J. 2012;18(3):221–225. [PubMed] [Google Scholar]
- 38. Pouleau HB, Sadeghi N, Baleriaux D, Melot C, De Witte O, Lefranc F. High levels of cellular proliferation predict pseudoprogression in glioblastoma patients. Int J Oncol. 2012;40(4):923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31(23):2963–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kong DS, Kim ST, Kim EH, et al. Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: the role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. AJNR Am J Neuroradiol. 2011;32(2):382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gahramanov S, Muldoon LL, Varallyay CG, et al. Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology. 2013;266(3):842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neal ML, Trister AD, Ahn S, et al. Response classification based on a minimal model of glioblastoma growth is prognostic for clinical outcomes and distinguishes progression from pseudoprogression. Cancer Res. 2013;73(10):2976–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gladwish A, Koh ES, Hoisak J, et al. Evaluation of early imaging response criteria in glioblastoma multiforme. Radiat Oncol. 2011;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]