Abstract
Background:
We recently reported a quantitative relationship between the degree of functional perturbation reported in the literature for 356 variants in the cardiac sodium channel gene SCN5A and the penetrance of resulting arrhythmia phenotypes. In the course of that work, we identified multiple SCN5A variants, including R1193Q, that are common in populations but are reported in HEK cells to generate large late sodium current (INa-L).
Objective:
To compare the functional properties of R1193Q to those of the well-studied LQT3 mutation ΔKPQ.
Methods:
We compared functional properties of SCN5A R1193Q to those of ΔKPQ in CHO cells at baseline and following exposure to intracellular PIP3, which inhibits INa-L generated by decreased PI3-kinase activity. We additionally used CRISPR/Cas9 editing to generate R1193Q in human induced pluripotent stem cells differentiated to cardiomyocytes (hiPSC-CMs).
Results:
Both R1193Q and ΔKPQ generated robust INa-L in CHO cells. PIP3 abrogated the late current phenotype in R1193Q cells, but had no effect on ΔKPQ. Homozygous R1193Q hiPSC-CMs displayed increased INa-L and long action potentials with frequent triggered beats, which were reversed with addition of PIP3.
Conclusions:
The consistency between the late current produced in HEK, CHO, and hiPSC-CMs suggests late current is a feature of the SCN5A R1193Q variant in human cardiomyocytes, but that the mechanism by which the late current is produced is distinct, and indirect, compared to the more highly penetrant ΔKPQ. These data suggest observing a late current in an in vitro setting does not necessarily translate to highly pathogenic LQT3 phenotype but depends on underlying mechanism.
Keywords: SCN5A/NaV1.5, human induced pluripotent stem cell cardiomyocytes hiPSC-CMs, Late/persistent current, LQT3, genetics
Introduction
Variants in SCN5A encoding the cardiac sodium channel NaV1.5 are associated with several inherited arrhythmias, including type 3 long QT (LQT3) and Brugada syndromes, arrhythmia disorders that predispose individuals to sudden cardiac arrest. We have recently shown SCN5A variant functional perturbation is quantitatively associated with the degree of clinical penetrance.1 However, we unexpectedly did not find that the magnitude of the late sodium current (INa-L) predicted penetrance in LQT3 and indeed some variants, notably R1193Q, are reported to generate large INa-L but are so common in certain populations that they cannot be invoked as causes of LQT3. Several studies initially implicated SCN5A R1193Q in sudden unexplained death, Brugada syndrome, and type 3 LQTS.2, 3 Functional assessment of R1193Q revealed a large late current,2, 4, 5 a feature previously associated with LQT3. However, aggregated exome sequencing results now estimate SCN5A R1193Q is present in 6.1% alleles in the East Asian population, with a 0.3% homozygous carrier rate (as reported in gnomAD, a large database of > 250,000 alleles from diverse ancestries).6, 7 Both homozygous and heterozygous carrier frequencies are much higher than the prevalence of LQT3, estimated at 0.005% in the general population (across all SCN5A mutations),8 suggesting little to no role of SCN5A R1193Q in disease presentation.9 These conflicting data—significant molecular phenotype, large late current, but high minor allele frequency—prompted us to test whether distinct mechanisms, some leading to high penetrance, are responsible for the ultimate expression of late current. In our previous assessment, the canonical ΔKPQ variant (the originally-described LQT3 mutant in which three codons in SCN5A coding for residues 1505-1507 thought to be critical for fast inactivation are deleted)10-12 displayed nearly 100% penetrance whereas penetrance was near zero for R1193Q.
We and others have recently shown that PI3-kinase inhibition generates late sodium current, which is reversed by the PI3K downstream effector phosphatidylinositol (3,4,5)-trisphosphate (PIP3).13, 14 We show here that the late current generated by R1193Q is inhibited by PIP3 whereas that generated by ΔKPQ is not. These differences in sensitivity implicate distinct mechanisms in late current generation. There are many intracellular processes that modulate INa-L, such as Ca2+ dependent pathways, PIP3 dependent pathways, etc.15 We therefore suggest structural variants directly inducing disruptions in inactivation and channel closure are more pathogenic in nature. The findings also demonstrate that screening novel genetic variants in SCN5A and other LQT-associated genes thought to be late current-inducing (such as CAV3 and syntactin) need to consider these diverse mechanisms.
Methods
Expression of SCN5A WT and variants in Chinese hamster ovary (CHO) cells.
Point mutations were introduced into SCN5A using the QuikChange Lightning kit (Agilent Technologies, Inc., Santa Clara, CA, USA). All recombinant cDNAs were sequenced to confirm the incorporation of R1193Q and ΔKPQ variants. CHO cells were transfected with wildtype or mutant SCN5A pIRES2-GFP mammalian expression plasmids using the FuGENE transfection system. Cells were incubated at 37 °C for 48 hours in DMEM supple mented with 10% FBS, 1% penicillin and streptomycin, and 5% CO2. Cells fluorescing green were selected for further electrophysiological studies. Ion currents in this study were recorded using the standard protocols as previous described.16 Additionally, 5 nM ATX17, 18 (Sigma-Aldrich, St. Louis, MO, USA) was used in the extracellular solution in some experiments where indicated.
Editing and differentiating human induced pluripotent stem cell cardiomyocytes (hiPSC-CMs).
We introduced R1193Q into exon 19 of hiPSCs derived from a healthy individual. Guide RNA was designed using the Webserver http://crispr.mit.edu/ and introduced into the Px459 vector coexpressing Cas9 and puromycin resistance. We used the following rescue template which includes the c.3578 G>A (bold) and the introduced silent variations, an NlaIII cut site and removal of the Protospacer-Adjacent Motif (PAM) site used in the guide RNA (both occupy the same site, underlined): CCGGCGCTGTCCCTGCTGTGCGGTGGACACCACACAGGCCCCAGGGAAGGTCTGGTGGC aGTTGCGCAAGACaTGCTACCACATCGTGGAGCACAGCTGGTTCGAGACATTCATCATCTT CATGATCCTACTCAGCAGTGGAGCGCTGGT. HiPSCs were grown to confluency and electroporated with the modified Px459 vector and recovery template using the Neon Transfection System (ThermoFisher Scientific, Waltham, MA). Resulting puromycin resistant colonies were isolated and assayed by restriction enzyme digestion and sanger sequenced for incorporation of the variant. For electrophysiologic characterization, cells homozygous for R1193Q were differentiated into cardiomyocytes by modulating Wnt signaling followed by glucose starvation to enrich the selection, as previously established.19 After 35 days or longer of differentiation, beating cells were dissociated from the Matrigel plate using TrypLE™ Select Enzyme (ThermoFisher Scientific, Waltham, MA) and passed through a 100 μm filter before electrophysiologic characterization.
Recordings of cardiac peak/late sodium currents and action potentials.
In whole-cell voltage clamp mode, we used previously described methods20, 21 to record peak and late sodium currents in SCN5A-transfected CHO cells and hiPSC-CMs by using an external K+- and Ca2+- free solution contained sodium concentration of 135 mM at room temperature (23°C). In current-clamp mode, we recorded action potentials (APs) from non-beating hiPSC-CMs by injection of a brief stimulus current (1-2 nA, 2-6 ms) at stimulation rates of 0.5 Hz and 0.1 Hz. Spontaneously-beating action potentials were also recorded without stimulation. For AP experiments, the bath (extracellular) solution was normal Tyrode’s, containing (in mM): NaCl 135, KCl 4.0, CaCl2 1.8, and MgCl2 1.0, HEPES 5.0, glucose 10, with a pH of 7.4. The pipette-filling (intracellular) solution contained (in mM): KCl 130, ATP-K2 5.0, MgCl2 1.0, CaCl2, 1.0, BAPTA 0.1, and HEPES 5.0, with a pH of 7.3 (adjusted by KOH). In some experiments, 1 μM PIP3 was added into the intracellular solution to observe its effect on action potentials.
Assessment of structural context of SCN5A ΔKPQ, R1193Q, and other INa-L-inducing variants.
We used the recently released cryo-EM structure of the electric eel NaV1.4 (67% sequence identity to human NaV1.5) structure (PDBID: 5XSY)22 to assess the spatial relationship between variants in the structurally modellable region of NaV1.5 with high reported late current and high or low LQT3 penetrance. All variants assessed were previously reported to have late current at least 200% of WT and have been observed in at least five individuals. The low penetrance subset of this group had a penetrance < 20% (A185T, S216L, T353I, and R1193Q); the high penetrance subset had a penetrance > 20% (T1304M, N1325S, ΔKPQ, R1644H, V1763M, Y1767C, V1777M, and E1784K). We visualized the overall shape and construction of the channel molecules using the PyMOL molecular graphics suite.23 We then mapped onto this structure variants mentioned above to visualize which parts of the protein might be affected by these substitutions.
Results
Mechanism of late sodium current is distinct between SCN5A R1193Q and ΔKPQ.
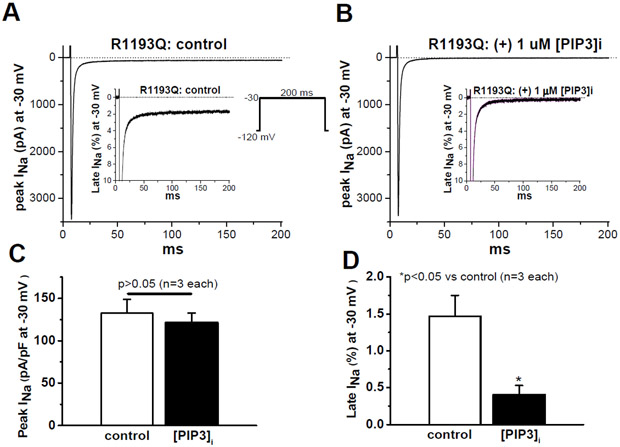
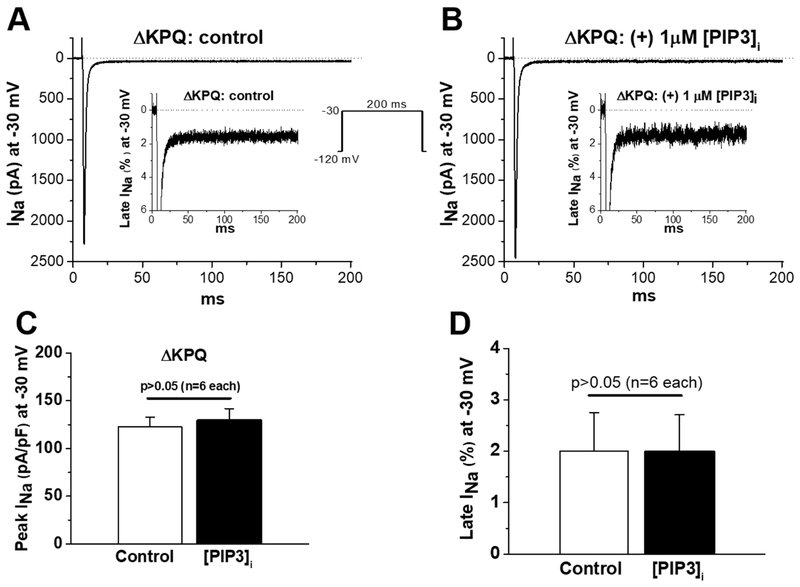
In CHO cells, both SCN5A R1193Q and ΔKPQ generated large late currents: 0.3 ± 0.01 % (WT), 1.24 ± 0.09 % (R1193Q) and 2.0 ± 0.8 % (ΔKPQ) of peak sodium current (Figures S1, 1 and 2) similar to what had been observed previously.2,4, 5,10-12 In addition, intracellular addition of 1 μM PIP3 significantly reduced SCN5A R1193Q late current from 1.5 ± 0.3 % to 0.4 ± 0.13 % (p < 0.05, Figure 1) without altering peak current, while PIP3 addition had no effect on ΔKPQ late current: 2.0 ± 0.7 % vs 2.0 ± 0.8 % (control, p > 0.05, Figure 2). These results suggested distinct mechanisms involved in late current generation between the two variants. Additionally, 5 nM ATX17, 18 generated late current recalcitrant to modulation by PIP3 (Figure S2). These data further suggest the mechanisms resulting in late current in the genetic context of the SCN5A ΔKPQ variant (or by the addition of ATX, which directly disrupts normal inactivation24) is distinct from R1193Q.
Figure 1.
Internal addition of PIP3 reversed R1193Q variant-expressed late current in CHO cells. A) Control peak and late current traces recorded with the protocol shown as an insert. B) Peak and late current traces recorded with internally-added PIP3. C) Summary of control and peak INa recorded after two minutes with internal PIP3. D) Summary of control and late INa recorded after two minutes with internal PIP3.
Figure 2:
No effect of internal addition of PIP3 on ΔKPQ-expressed peak and late INa in CHO cells. A) control peak and late current traces recorded with the protocol shown as an insert. B) Peak and late current traces recorded with internally-added PIP3. C) Summary of control and peak INa recorded after two minutes with internal PIP3. D) Summary of control and late INa recorded after two minutes with internal PIP3. Note the observed late current is not modulated by addition of PIP3, in contrast to R1193Q.
hiPSC-CMs recapitulate late current phenotype and yield expected arrhythmogenic action potentials which are reversed by addition of PIP3.
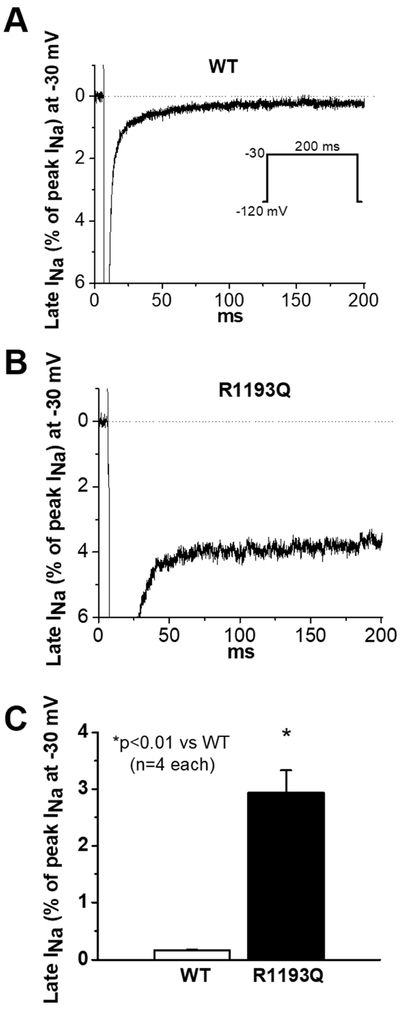
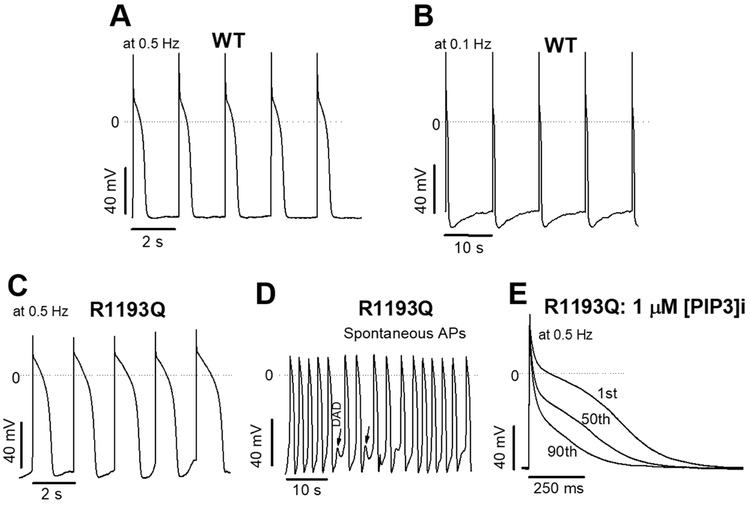
To further probe the late current phenotype of SCN5A R1193Q, we introduced the variant in human induced pluripotent stem cells. Five separate lines successfully incorporated the variant, three homozygous and two heterozygous (Figure S3). Endogenous late sodium current (INa-L) in cardiac cells is very small (~0.5% of peak sodium current) with a physiologic concentration of extracellular sodium.25, 26 Differentiated hiPS-CMs homozygous for the R1193Q edit also produced the large late current phenotype: 2.93 ± 0.4 % of peak INa vs WT 0.17 ± 0.01 % (p < 0.01, Figure 3). Measured action potentials had anomalous features of unusual prolongation and triggered activity (Figure 4): APD90 at 0.5 Hz was 370 ± 50 ms (WT) vs 920 ± 110 ms (R1193Q, p < 0.01). Such long APs in R1193Q cells were corrected by adding intracellular PIP3 (1 μM), to 380 ± 100 ms, which is indistinguishable from that seen in WT cells. R1193Q cells also displayed irregular spontaneous beating associated with delayed afterdepolarizations (DADs).
Figure 3:
SCN5A WT- and R1193Q-expressed late INa from CRISPR/Cas9 edited hiPS-CMs. A-B) Normalized late INa traces from WT (A) and R1193Q cells with a single-pulse voltage protocol shown as an insert. C) Comparison of average late INa between WT and R1193Q.
Figure 4:
Action potentials of SCN5A WT and R1193Q in hiPS-CMs. A-B) Action potentials generated by stimulating hiPS-CMs derived from an unaffected individual at 0.5 and 0.1 Hz. C) Action potentials from SCN5A R1193Q edited otherwise isogenic control stimulated at 0.5 Hz as in A. Action potentials are much longer for R1193Q than the unedited control line. D) Cells could not be stimulated at 0.1 Hz due to spontaneous beats. Arrhythmogenic DADs were also observed in the absence of AP stimulation. E) Long action potential phenotype observed in R1193Q hiPS-CMs can be rescued by addition of PIP3, a known late current modulator.
Discussion
Although there is a clear correlation between SCN5A variant perturbation measured by heterologous expression and clinical presentation,1 there are variants where relatively high minor allele frequency (as reported in gnomAD) conflicts with the severity of functional perturbation, especially variants characterized by a large “late current” gain-of-function phenotype. SCN5A R1193Q was shown previously to generate large late currents, a well-established mechanism leading to type 3 LQTS, by heterologous expression in HEK cells.27-31 However, the prevalence of this variant in ostensibly unaffected populations, 6.1% in East Asian alleles, argues strongly that it cannot play a significant role in disease presentation.9 This conflict prompted us to assess whether underlying mechanisms which induce late current may be identifiably distinct between high and low penetrant variants with similar late current phenotypes. We additionally examined the response in the more native like hiPS-CM context closer to the native context. Here we showed that the SCN5A R1193Q late current phenotype is produced in both CHO cells and hiPS-CMs and that the mechanism of this late current is distinct from the ΔKPQ variant.
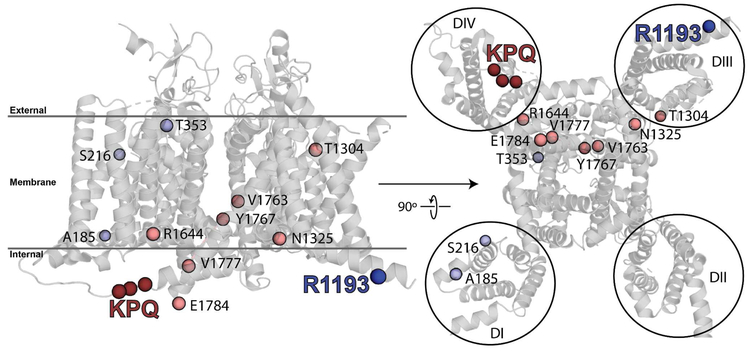
Given the differences in physical location, and without locations proposed common to both, we suspected these variants perturb channel function by distinct mechanisms (Figure 5). Residue 1193 is located on the “S0” helix which precedes the voltage-sensing, four-helix bundle of Domain III known to be partly responsible for activation of voltage-gated sodium channels,32 whereas ΔKPQ is located on the DIII-DIV linker (residues 1481 to 1515). Residue 1193, as for residues 185 and 216, both with late current greater than 200% of WT and estimated LQT3 penetrance < 20%, lies peripherally on the NaV1.5 molecule and is likely indirectly involved in inactivation (Figure 5). The molecular mechanism of voltage-gated sodium channel inactivation or how late current is generated are unknown, however the DIII-DIV linker is a key intracellular component. In fact, substituting three key residues (I1488, F1489, and M1490) near the ΔKPQ site (K1505-Q1507) with glutamines removes all inactivation.33 Additionally, the DIII-DIV linker is dynamic along most its length34 which may be necessary to exhibit the multiple conformations observed in recently determined structures of sodium channels;22, 35 this property is likely perturbed in the context of ΔKPQ (Figure 5). As opposed to residue 1193, residues 1505-1507 are centrally located within NaV1.5 as are other highly penetrant, high late current variants (Figure 5), which we believe suggests a more direct role in perturbing inactivation of the channel.
Figure 5:
Cartoon representation of a model of NaV1.5 highlighting the membrane spanning segments as well as R1193 and residues 1505-1507 (KPQ, removed in ΔKPQ). Rightmost image is from the perspective of inside the cell looking out through the channel and membrane. R1193 is in the amphipathic helix preceding the transmembrane domain of domain III while ΔKPQ is in the domain III-IV linker, the segment of SCN5A known to influence inactivation.33 The model is based on electric eel NaV1.4 (67% sequence identity to human NaV1.5) structure (PDBID: 5XSY).22 The four helix voltage-sensing modules of each domain (I-IV) are noted by black circles. Residues where variants with at least five carriers and late current greater than 200% that of WT are known are shown as spheres. Light blue spheres are variants where the predicted penetrance is < 20%; light red spheres are variants where predicted penetrance is > 20%. The high-penetrance variants are spatially proximal to the inactivation machinery, in contrast with low-penetrance variants.
Variants with high late current and high penetrance localize most often near the region of the NaV1.5 molecular responsible for inactivation. Of the higher penetrance variants modeled (Figure 5, light red), all but one lies on the intracellular side of the NaV1.5 channel. Furthermore, all higher penetrance variants lie toward the DIII-DIV half of the molecule that houses the inactivation machinery, including the DIII-DIV linker. The lower penetrance variants appear distributed more peripherally, suggesting distinct roles structurally between these classes of variants in SCN5A. In fact, of the 15 variants with low LQT3 penetrance identified in our earlier work, 9 lie outside the modellable region (R458C, L619F, R689H, P1177L, E1901Q, S1904L, R1913H, F2004L, and P2006A), mostly in interdomain linkers and the C-terminus. However, none of the 11 higher penetrance variants lie outside the modellable region. These data imply a subset of variants with direct impact on inactivation and late current lead to a higher penetrance than other more peripherally located SCN5A variants which influence the channel more indirectly.
We tried a pharmacological approach to assess differences between the late current found in R1193Q compared to the more highly penetrant ΔKPQ. PIP3 contributes to regulation of several intracellular pathways including PDK1, SGK, and Akt. The mechanism by which NaV1.5 is regulated by PIP3 is not known; however, certain QT prolonging drugs that downregulate PI3Kα, an upstream regulator of PIP3, are known to modulate cardiac sodium late current, an effect rescued by addition of PIP3 intracellularly.13, 21 We therefore tested the hypothesis of distinct mechanisms resulting in late current between R1193Q and ΔKPQ by observing the response to intracellular PIP3. The sensitivity of R1193Q to PIP3, but not ΔKPQ or the ATX-NaV1.5 complex, suggests distinct mechanisms inducing late current have distinct pathologies which need to be considered when interpreting the severity of the excess late current functional defect in NaV1.5. This distinction may in part explain the heterogeneity in LQT3 penetrance among variants with similarly large late currents. In addition, our data suggest a PIP3-influenced compensatory pathway leading to susceptibility to QT prolongation in other contexts beyond lesions in SCN5A such as drug challenges, other genetic variants, electrolyte abnormalities or other processes known to modulate late current, Ca2+ concentration, β subunit interaction, oxidative stress, and phosphorylation.15
Conclusion
We propose that late current is a functional perturbation that arises from heterogeneous mechanisms of NaV1.5 dysfunction, one direct and structu (ΔKPQ) and another indirect (R1193Q). The consistency between the SCN5A R1193Q late current produced in CHO and hiPS-CM cells suggest late current is a feature of this variant in human cardiomyocytes, but that the mechanism by which the late current is produced is distinct compared to ΔKPQ. We believe this mechanistic distinction gives rise to differences in penetrance, near 0% for R1193Q and near 100% for ΔKPQ, and suggest certain mechanisms resulting in additional late current can be compensated for in the human myocardium.
Supplementary Material
Acknowledgements
The authors would like to thank Marcia Blair, Lynn Hall, and Laura Short for help in preparing CHO and hiPS cells.
Funding: K99 HL135442 (BMK), P50 GM115305 (DMR), R01 HL49989 (DMR), R01 HL118952 (DMR)
Footnotes
Conflict of interest: the authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brett M. Kroncke, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN..
Tao Yang, Department of Medicine and Pharmacology, Vanderbilt University Medical Center, Nashville, TN..
Dan M. Roden, Department of Medicine, Biomedical Informatics and Pharmacology, Vanderbilt University Medical Center, Nashville, TN..
References
- 1.Kroncke BM, Glazer AM, Smith DK, Blume JD, Roden DM. SCN5A (NaV1.5) Variant Functional Perturbation and Clinical Presentation: Variants of a Certain Significance. Circ Genom Precis Med 2018;11:e002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vatta M, Dumaine R, Varghese G, et al. Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Hum Mol Genet 2002;11:337–345. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, Zhao J, Barrane FZ, Champagne J, Chahine M. Nav1.5/R1193Q polymorphism is associated with both long QT and Brugada syndromes. Can J Cardiol 2006;22:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Chen S, Chen Q, Wan X, Shen J, Hoeltge GA, Timur AA, Keating MT, Kirsch GE. The common SCN5A mutation R1193Q causes LQTS-type electrophysiological alterations of the cardiac sodium channel. J Med Genet 2004;41:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan BH, Valdivia CR, Rok BA, Ye B, Ruwaldt KM, Tester DJ, Ackerman MJ, Makielski JC. Common human SCN5A polymorphisms have altered electrophysiology when expressed in Q1077 splice variants. Heart Rhythm 2005;2:741–747. [DOI] [PubMed] [Google Scholar]
- 6.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiffin N, Minikel E, Walsh R, O'Donnell-Luria AH, Karczewski K, Ing AY, Barton PJR, Funke B, Cook SA, MacArthur D, Ware JS. Using high-resolution variant frequencies to empower clinical genome interpretation. Genet Med 2017;19:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm 2005;2:507–517. [DOI] [PubMed] [Google Scholar]
- 9.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett PB, Yazawa K, Makita N, George AL Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature 1995;376:683–685. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, Schwartz PJ, Keating MT. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet 1995;4:1603–1607. [DOI] [PubMed] [Google Scholar]
- 12.Wang DW, Yazawa K, George AL Jr., Bennett PB. Characterization of human cardiac Na+ channel mutations in the congenital long QT syndrome. Proc Natl Acad Sci U S A 1996;93:13200–13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang T, Meoli DF, Moslehi J, Roden DM. Inhibition of the alpha-Subunit of Phosphoinositide 3-Kinase in Heart Increases Late Sodium Current and Is Arrhythmogenic. J Pharmacol Exp Ther 2018;365:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Z, Wu CY, Jiang YP, Ballou LM, Clausen C, Cohen IS, Lin RZ. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci Transl Med 2012;4:131ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makielski JC. Late sodium current: A mechanism for angina, heart failure, and arrhythmia. Trends in cardiovascular medicine 2016;26:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang P, Kanki H, Drolet B, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation 2002;105:1943–1948. [DOI] [PubMed] [Google Scholar]
- 17.Isenberg G, Ravens U. The effects of the Anemonia sulcata toxin (ATX II) on membrane currents of isolated mammalian myocytes. The Journal of physiology 1984;357:127–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol 2004;44:192–199. [DOI] [PubMed] [Google Scholar]
- 19.Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods 2014;11:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang T, Atack TC, Stroud DM, Zhang W, Hall L, Roden DM. Blocking Scn10a channels in heart reduces late sodium current and is antiarrhythmic. Circ Res 2012;111:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang T, Chun YW, Stroud DM, Mosley JD, Knollmann BC, Hong C, Roden DM. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation 2014;130:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Z, Zhou Q, Wang L, Wu J, Zhao Y, Huang G, Peng W, Shen H, Lei J, Yan N. Structure of the Nav1.4-beta1 Complex from Electric Eel. Cell 2017;170:470–482 e411. [DOI] [PubMed] [Google Scholar]
- 23.Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.82015. [Google Scholar]
- 24.Schreibmayer W, Kazerani H, Tritthart HA. A mechanistic interpretation of the action of toxin II from Anemonia sulcata on the cardiac sodium channel. Biochim Biophys Acta 1987;901:273–282. [DOI] [PubMed] [Google Scholar]
- 25.Saint DA, Ju YK, Gage PW. A persistent sodium current in rat ventricular myocytes. The Journal of physiology 1992;453:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju YK, Saint DA, Gage PW. Inactivation-resistant channels underlying the persistent sodium current in rat ventricular myocytes. Proc Biol Sci 1994;256:163–168. [DOI] [PubMed] [Google Scholar]
- 27.Moreau A, Krahn AD, Gosselin-Badaroudine P, Klein GJ, Christe G, Vincent Y, Boutjdir M, Chahine M. Sodium overload due to a persistent current that attenuates the arrhythmogenic potential of a novel LQT3 mutation. Frontiers in pharmacology 2013;4:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumaine R, Wang Q Keating MT, Hartmann HA, Schwartz PJ, Brown AM, Kirsch GE. Multiple mechanisms of Na+ channel--linked long-QT syndrome. Circ Res 1996;78:916–924. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Millat G, Rodriguez-Lafrasse C, Rousson R, Kugener B, Chevalier P, Chahine M. Biophysical characterization of a new SCN5A mutation S1333Y in a SIDS infant linked to long QT syndrome. FEBS Lett 2009;583:890–896. [DOI] [PubMed] [Google Scholar]
- 30.Kato K, Makiyama T, Wu J, Ding WG, Kimura H, Naiki N, Ohno S, Itoh H, Nakanishi T, Matsuura H, Horie M. Cardiac channelopathies associated with infantile fatal ventricular arrhythmias: from the cradle to the bench. J Cardiovasc Electrophysiol 2014;25:66–73. [DOI] [PubMed] [Google Scholar]
- 31.Bankston JR, Yue M, Chung W, Spyres M, Pass RH, Silver E, Sampson KJ, Kass RS. A novel and lethal de novo LQT-3 mutation in a newborn with distinct molecular pharmacology and therapeutic response. PloS one 2007;2:e1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capes DL, Goldschen-Ohm MP, Arcisio-Miranda M, Bezanilla F, Chanda B. Domain IV voltage-sensor movement is both sufficient and rate limiting for fast inactivation in sodium channels. The Journal of general physiology 2013;142:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A 1992;89:10910–10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohl CA, Boeckman FA, Baker C, Scheuer T, Catterall WA, Klevit RE. Solution structure of the sodium channel inactivation gate. Biochemistry 1999;38:855–861. [DOI] [PubMed] [Google Scholar]
- 35.Shen H, Zhou Q, Pan X, Li Z, Wu J, Yan N. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 2017;355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.