Abstract
People are constantly exposed to a wide variety of chemicals. Some of these compounds, such as parabens, bisphenols and phthalates, are known to have endocrine disrupting potencies. Over the years, these endocrine disrupting chemicals (EDCs) have been a rising cause for concern. In this study, we describe setup and validation of two methods to measure EDCs in human urine, using ultra-performance liquid chromatography tandem mass spectrometry. The phenol method determines methyl-, ethyl-, propyl-, n-butyl- and benzylparaben and bisphenol A, F and S. The phthalate method determines in total 13 metabolites of dimethyl, diethyl, diisobutyl, di-n-butyl, di(2-ethylhexyl), butylbenzyl, diiso-nonyl and diisodecyl phthalate. Runtime was 7 and 8 min per sample for phenols and phthalates, respectively. The methods were validated by the National Institute of Standards & Technology (NIST) for 13 compounds. In addition, EDCs were measured in forty 24-h urine samples, of which 12 EDCs were compared with the same samples measured in an established facility (Rigshospitalet, Copenhagen, Denmark). The intra-assay coefficient of variability (CV) was highest at 10% and inter-assay CV was highest at 12%. Recoveries ranged from 86 to 115%. The limit of detection ranged from 0.06 to 0.43 ng/mL. Of 21 compounds, 10 were detected above limit of detection in ≥93% of the samples. Eight compounds were in accordance to NIST reference concentrations. Differences in intercept were found for two compounds whereas slope differed for six compounds between our method and that used in the Danish facility. In conclusion, we set up and validated two high-throughput methods with very short runtime capable of measuring 5 parabens, 3 bisphenols and 13 different metabolites of 8 phthalates. Sensitivity of the phenol method was increased by using ammonium fluoride in the mobile phase.
Introduction
In daily life, people are constantly exposed to a wide variety of exogenous substances. Parabens are used as preservatives in cosmetics, creams, shampoos, pharmaceuticals and food. Bisphenol A (BPA) is a widely used high-production-volume chemical and is used in polycarbonate plastics and epoxy resins (1). Phthalate diesters are used as plasticizers and solvents in, e.g., cosmetics, printing inks, coatings of pharmaceuticals, cookware and food wrappers. Exposure to these compounds occurs through ingestion, dermal contact, inhalation and perinatal transmission (i.e., via placenta or breast milk) of every-day products (2–5). The general population living in the Western world is widely exposed to these chemicals (6–9). Yet, individuals who occupationally use these products are reported to be exposed to even higher concentrations (10). Still little is known about human exposure to the BPA analogs bisphenol F (BPF) and bisphenol S (BPS), which have been introduced to the market only recently (11–13). These chemicals are increasingly used as replacement of BPA, and are found in personal care products, food and paper (14–16).
Parabens, bisphenols and phthalates are endocrine disrupting chemicals (EDCs) (6, 17). Accumulating evidence supports the potency of these EDCs to interfere with various physiological processes including reproductive, metabolic and brain functions, and they are linked to multiple health complications and diseases (18–20). Due to observations on the adverse health effects of BPA, new BPA analogs such as BPF and BPS have been introduced as presumed safe replacements. Yet recent studies have shown that BPF and BPS may have an even stronger endocrine disrupting potency than BPA (21, 22).
While exposure to one EDC may be worrisome, people are in fact daily exposed to a complex mixture of chemicals. This means that exposure to multiple EDCs should be assessed at the same time (23, 24), which can be an analytical challenge. EDCs can be measured in urine by several different methods. The commonly used gas chromatography combined with mass spectrometry (GC–MS) often requires a derivatization step for sample volatility. Therefore, liquid chromatography tandem mass spectrometry (LC–MS-MS) is preferred, as this technique can measure multiple analytes without further derivations. Human exposure to these EDCs is often analyzed in urine samples, which is relatively easy to collect, non-invasive and available in large quantities and, thus, represents a biological matrix suitable for large epidemiological and human biomonitoring studies.
In this study, we used ultra-performance LC–MS-MS to improve runtime in two separate high-throughput isotope diluted methods, one for the simultaneous measurement of five parabens and three bisphenols, and the second method for measurement of 13 relevant metabolites of 8 different phthalate diesters. The methods were validated by comparison of data analyzed by classical LC–MS-MS methods.
Materials and Methods
Sample collection and preparation
Forty non-diabetic Dutch adults (37–59 years) were obtained from the Lifelines cohort study with available 24 h urine samples, as described in details elsewhere (25). The Lifelines study is a large population-based prospective study conducted in and representative for the north of the Netherlands (26, 27). Urine samples were collected between September 2008 and November 2010 from participants and stored at −80°C until analysis. The study protocol was approved by the medical ethics committee of the University Medical Center Groningen, and all participants provided written informed consent (26).
Materials
Five parabens, 3 bisphenols and 13 metabolites of 8 different phthalate diesters and their respective deuterated analogs were used as calibration material and internal standard (Table I). EtP, n-PrP, BzP and deuterated analogs of EtP (d4), n-PrP (d4), BzP (d7), MnHP (d4) and MiBP (d4) were purchased from Toronto Research Chemicals (Toronto, Canada). MeP, n-BuP, BPA, BPF, BPS, MMP, MiBP, MnBP, MEHP, MEHHP, MEOHP, MECPP, MBzP, MiNP, MHiNP and MiDP, and 13C analogs for MeP, n-BuP, BPA, BPF, BPS, MMP, MnBP, MEHP, MEHHP, MEOHP, MECPP, MBzP and MiNP were obtained from Cambridge Isotopes Laboratory (Tewksbury, MA, USA). β-glucuronidase originated from E. Coli K 12 (~140 U/mg at 37°C, at pH 7 with 4-nitrophenyl-β-d-glucuronide as substrate), sulfatase from Aerobacter aerogenes (10–20 units/mL) and ammonium fluoride (NH4F) were purchased from Sigma Aldrich (Schnelldorf, Germany). Sodium bicarbonate was obtained from Merck (Darmstadt, Germany). A 1-butanol HCl was purchased from Sigma-Aldrich (Schnelldorf, Germany) and methanol, acetonitrile, ethyl acetate and formic acid (all LC–MS grade) from Biosolve (Valkenswaard, The Netherlands). Hydrochloric acid, natrium hydroxide and buffers of sodium acetate and sodium chloride phosphate were provided by the UMCG apothecary (Groningen, Netherlands). Ultrapure water (18.2 MΩ) was obtained from a Milli-Q system (Millipore, Amsterdam, The Netherlands). For urine collection, 3 L containers (Becton Dickinson) and 6 mL Vacutainer Tubes (Becton Dickinson) were used, after which samples were stored in 2.0 mL vials (Sarstedt). All containers and vials that were used to collect and store the urine samples were tested for potential phenol and phthalate metabolite contamination before use. Stress tests were performed: six different containers/vials were filled with phosphate-buffered saline and incubated for 3 days at 37°C and subsequently aliquots were analyzed. No traces (<LOD) of the analyzed compounds were found. All chemicals, solutions, and lab wares were checked for contamination of phthalate metabolites and phenols before use. The solvents used for the mobile phase were checked by running the gradient without performing an injection. One phthalate was detectable above limit of detection (LOD), MnHP which was present in the acetonitrile used. This problem was circumvented by inserting a column just after the mixer of the UPLC gradient pump (Kinetex 5 μm XB-C18, 50 × 2.1 mm2, Phenomenex) which delayed the peak of MnHP originating from the solvent enough, to baseline separate it from MnHP detected in the sample. Furthermore, all glassware was rinsed and sonicated with methanol, after which it was allowed to air dry.
Table I.
Names and abbreviations of components
| Compound | Abbreviation | Metabolite | Abbreviation | Internal standard |
|---|---|---|---|---|
| Methyl paraben | MeP | MeP (13C) | ||
| Ethyl paraben | EtP | EtP (d4) | ||
| Propyl paraben | PrP | n-PrP (d4) | ||
| n-Butyl paraben | n-BuP | n-BuP (13C) | ||
| Benzyl paraben | BzP | BzP (d7) | ||
| Bisphenol A | BPA | BPA (13C) | ||
| Bisphenol F | BPF | BPF (13C) | ||
| Bisphenol S | BPS | BPS (13C) | ||
| Di-methyl phthalate | DMP | Mono-methyl phthalate | MMP | MMP (13C) |
| Di-ethyl phthalate | DEP | Mono-ethyl phthalate | MEP | MEP (13C4) |
| Di-iso-butyl phthalate | DiBP | Mono-iso-butyl phthalate | MiBP | MiBP (d4) |
| Di-n-butyl phthalate | DnBP | Mono-n-butyl phthalate | MnBP | MnBP (13C) |
| Di-n-hexyl phthalate | DnHP | Mono-n-hexyl phthalate | MnHP | MnHP (d4) |
| Di-(2-ethyl-hexyl) phthalate | DEHP | Mono-(2-ethylhexyl) phthalate | MEHP | MEHP (13C) |
| Mono-(2-ethyl-5-hydroxyhexyl) phthalate | MEHHP | MEHHP (13C) | ||
| Mono-(2-ethyl-5-oxohexyl) phthalate | MEOHP | MEOHP (13C) | ||
| Mono-(2-ethyl-5-carboxypentyl) phthalate | MECPP | MECPP (13C) | ||
| Butylbenzyl phthalate | BBzP | Mono-benzyl phthalate | MBzP | MBzP (13C) |
| Di-iso-nonyl phthalate | DiNP | Mono-iso-nonyl phthalate | MiNP | MiNP (13C) |
| Mono-hydroxy-iso-nonyl phthalate | MHiNP | MEOHP (13C) | ||
| Di-iso-decyl phthalate | DiDP | Mono-iso-decyl phthalate | MiDP | MiNP (13C) |
Analytical procedures and offline solid phase extraction
Stock solutions of standards were prepared in methanol at a concentration of 10 μg/mL. A low and high working solution standard mixture (10 and 500 ng/mL) was prepared fresh from the stock solution in methanol on the day of analysis. The calibration curves consisted of eight points: 0, 0.5, 1.5, 5.0, 10, 50, 150, 50, 150, 500 and 1,000 ng/mL for the phenols and seven points for the phthalates: 0, 1, 2, 5, 10, 50, 100 and 500 ng/mL for the phthalates. The calibrators were treated the same as the samples, but without adding urine. The urine volume was replaced with buffer to make sure the same volume was used in the end. Internal standard working solutions were prepared in 50% MeOH and concentration was 100 ng/mL for the phenols and 40 ng/mL for the phthalates. Quality control (QC) samples were prepared by pooling several urine samples and were, when necessary, spiked to give two different concentrations. QC samples were stored in 0.5 mL aliquots and stored at −80°C until use. All urine samples were stored at −80°C until use. Solvent blank samples consisted of 0.5 M natrium acetate. Calibration curves, QC samples and blank samples were treated as described for samples below.
For the phenol analysis, 100 μL urine, 25 μL internal standard solution, 115 μL enzyme mix (0.5 M natrium acetate and 10 μL of 20% β-glucuronidase/aryl sulfatase) was added to wells of a 2.0 mL 96-deep well plate (Greiner Bio-One). After vortexing for 10 min, the plate was incubated at 37°C for 120 min. Successively, 200 μL methanol was added and vortexed for 1 min, where after 300 μL of the mix (i.e., sample internal standard, buffer, enzyme mix and methanol) was pipetted on a solid liquid extraction (SLE)-plate (Biotage). The sample was absorbed and incubated for 5 min. A 1,000 μL ethyl acetate was added to each well, and then eluted in a glass coated 96-well plate (Thermo Fisher Scientific). Elution solvent was evaporated under nitrogen flow at 60°C, and the residue was dissolved in 200 μL 50% methanol. After vortexing the samples for 10 min, 5 μL was injected on the LC–MS-MS system.
For the phthalate analysis, 100 μL urine, 25 μL internal standard solution, 115 μL enzyme mix (0.5 M sodium acetate pH 5.5, and 10 μL of 20% β-glucuronidase) were added to wells of a 2.0 mL 96-deep well plate (Greiner Bio-One). After vortexing for 10 min, the plate was incubated at 37°C for 120 min. After incubation, 150 μL 200 mM sodium bicarbonate was added to each well, after which 500 μL ultrapure water was added to the wells. Meanwhile, the solid phase extraction (SPE)-plate (Strong-anion exchange plate, Phenomenex) was conditioned with 500 μL methanol and 500 μL 20 mM sodium bicarbonate. Samples were extracted and the SPE plate was subsequently washed with 500 μL 20 mM sodium carbonate and 500 μL methanol. Elution was performed in a 96-well glass coated plate by adding 500 μL 5% formic acid in acetonitrile. Elution solvent was then evaporated under nitrogen flow at 60°C. The residue was dissolved in 200 μL 30% acetonitrile and vortexed for 10 min, where after 5 μL was injected on the LC–MS-MS system.
LC–MS-MS method
LC–MS-MS analysis of phenols and phthalates was performed on a Waters ACQUITY ultra-performance liquid chromatography (UPLC) system coupled to a Waters XEVO TQ-S triple quadrupole system using electrospray ionization. UPLC for both assays was performed on a Phenomenex Kinetex® Phenyl-Hexyl 2.1 × 100 mm2, 1.7 μm, kept at 40°C. For phenols, mobile phase consisted of A: 0.2 mM ammonium fluoride in 10% methanol in water; B: methanol. Gradient elution was performed with a flow of 0.4 mL/min and started at 15% B, with a linear increase to 80% B in 5 min. Gradient was increased to 100% B for 1 min, and was then returned to 15% B where it was equilibrated for 1 min until the next run, which resulted in a total runtime of 7 min. For phthalates, mobile phase consisted of A: 0.1% formic acid in 10% acetonitrile; B: 0.1% formic acid in acetonitrile. Gradient elution was performed with a flow of 0.4 mL/min and started at 5% B, with a linear increase to 65% B in 6.5 min. Gradient was increased to 90% B for 1 min, and was then returned to 5% B where it was equilibrated for 0.5 min until the next run, which resulted in a total runtime of 8 min. Target compounds were analyzed in negative electrospray ionization and selective reaction monitoring mode. For the phenols, the capillary voltage was 2.0 kV, desolvation temperature 650°C, cone gas 200 L/h, desolvation gas flow 1,000 L/h, and collision gas flow 0.2 mL/min. For the phthalates, the capillary voltage was 1.5 kV, desolvation temperature 650°C, cone gas 200 L/h, desolvation gas flow 1,000 L/h, and collision gas flow 0.2 mL/min. Cone voltage and collision energies were optimized for all m/z transitions and are listed in Supplementary Table S1a and b. Quantifier and qualifier m/z transitions were monitored for all compounds and their internal standards (Supplementary Table S1a and b). Quantitation was performed by using the peak-area response ratios of the quantifier transitions for the compounds and their respective internal standards, using Masslynx and Targetlynx software.
Analytical validation
Linearity of the calibration curves was assessed by analyzing the curves over 10 different days. The variation coefficient of the slope was calculated, and correlation coefficient were monitored, with a requirement of a maximal inaccuracy of 15% for the slope and R2 > 0.99 on each day, respectively. Potential matrix effects have been checked by mixing two different urine samples containing low and high concentrations of phenols, parabens and phthalates in different ratios (0, 25, 50, 75, 100%), (R2 > 0.99). Ion suppression was checked by performing post-column infusion experiments (28).
Intra-assay imprecision was determined by analyzing two urine pools on the same day in 10 replicates. Inter-assay imprecision was assessed by measuring two urine pools on 10 different days. Carry over was determined according CLSI guidelines (EP-10A) (29). Recovery was estimated by spiking two different urine samples with two different concentrations (100 and 250 ng/mL for phenols and 50 and 500 ng/mL for phthalates). Recovery percentage was calculated as follows: [(final concentration – initial concentration)/added concentration] * 100%. Recovery was considered acceptable within the range of 100 ± 15%.
The LOD for the phenols, and phthalates was calculated as 3.3 × S0/b, where S0 is the standard deviation of the response and b the slope of the calibration curve (30). S0 and b were determined by analyzing quintuplicate sets of the lowest five standards (0.1, 0.2, 0.4, 0.8 and 1.0 ng/mL) in different urine samples (n = 6) with very low to no phenols or phthalates detectable. Samples were screened before analysis. The Sy-intercept and slope of the best-fit line of this plot were used as S0 and b, respectively, and were calculated using the linear regression function in Graphpad Prism 5.0. The limit of quantification (LOQ) for each analyte was determined by analyzing six different samples with progressively lower concentrations of phenols, and phthalates on 6 different days. The LOQ was set where the imprecision was ≤20% and the signal to noise ratio was >10 on all 6 days (31).
Method comparison
The two presented methods were validated by analysis of reference material from the National Institute of Standards & Technology (NIST) (Gaithersburg, USA), SRM 3672. A NIST-to-Method-ratio <15% was considered as in excellent accordance, whereas a ratio 15–25% was considered similar, and a ratio >25% as different. Furthermore parabens, phenols and phthalate metabolites of the same 24 h urine samples of 40 subjects from the Lifelines cohort were measured with the present methods and compared with measurements performed by LC–MS-MS methods for phenols, parabens and phthalates at the Department of Growth and Reproduction, Rigshospitalet, Copenhagen University Hospital, Denmark (32–34). Compounds detected in <50% of the samples in one of the facilities were excluded from comparison. Medians were compared using Passing-Bablok regression for evaluation of the results using Rstudio (version: 1.1.383) (35).
Results
Assay performance
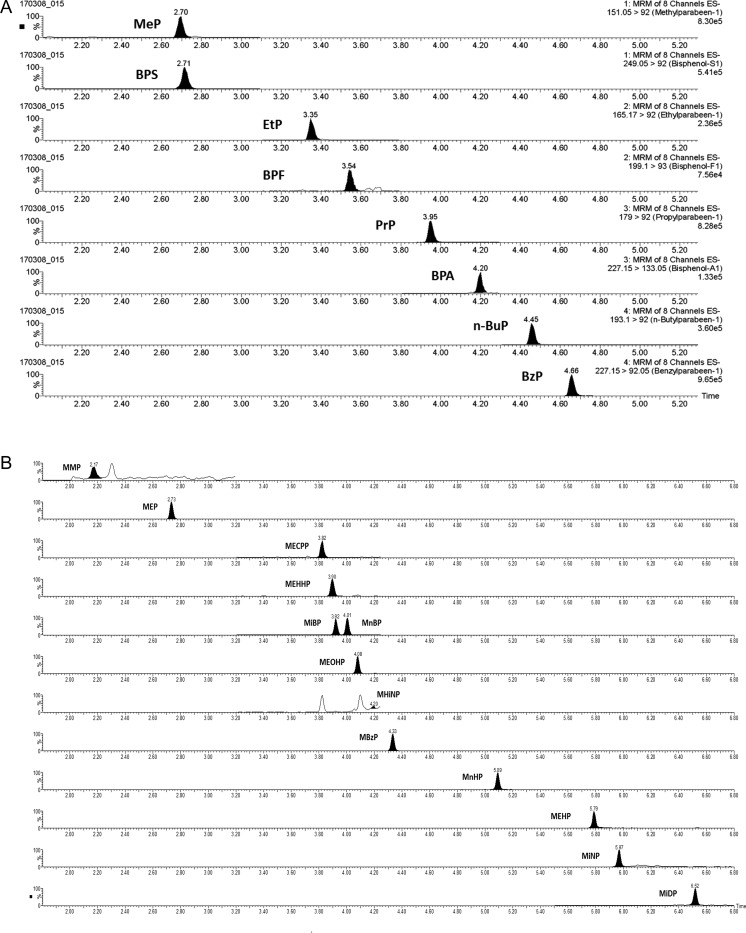
LC–MS-MS analysis time per sample was 7 min for phenols, and 8 min for phthalate metabolites. Chromatograms for analytes are presented in Figure 1a and b. For phthalates, the phenyl-hexyl column used was able to separate all metabolites at baseline, also the structural isomers MiBP and MnBP.
Figure 1.
Representative LC–MS-MS extracted ion chromatograms of a human urine sample fortified with phenols. Concentrations are MeP, 23 ng/mL; BPS, 4.8 ng/mL; EtP, 6.9 ng/mL; BPF, 5.2 ng/mL; PrP, 17 ng/mL; BPA, 7.5 ng/mL; n-BuP, 4.5 ng/mL; BzP, 5.2 ng/mL (A). Representative LC–MS-MS extracted ion chromatogram of a human urine sample fortified with phthalate metabolites. Concentrations are MMP, 2.3 ng/mL; MEP, 205 ng/mL; MECPP, 23 ng/mL; MEHHP, 21 ng/mL, MiBP, 30 ng/mL; MnBP, 28 ng/mL; MEOHP, 20 ng/mL; MHiNP, 0.13 ng/mL; MBzP, 34 ng/mL; MnHP, 6.9 ng/mL; MEHP, 16 ng/mL; MiNP, 6.5 ng/mL; MiDP, 4.8 ng/mL (B).
Calibration curves were linear over the calibration range for all compounds over all 10 days, with correlation coefficients (R2 > 0.99). The intra-assay coefficients of variability (CV) were ≤10% and inter-assay CV were ≤12% for all analytes at two QC levels. Recoveries ranged from 96 to 104% for bisphenols, from 101 to 113% in parabens and from 86 to 115% for phthalates (Tables IIa and IIb). No carry over was detected for any of the compounds in the calibration range. No significant ion suppression was found at the elution times of the compounds.
Table IIa.
Method validation for phenols: intra- and inter-assay controls and recoveries
| Intra-assay | Recovery | Inter-assay | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | CV (%) | Mean (%) | Mean | SD | CV (%) | ||
| 100 ng/mL | 250 ng/mL | |||||||
| MeP | ||||||||
| Low | 22.7 | 0.6 | 3 | 101 | 102 | 22.4 | 1.4 | 6 |
| High | 166 | 5.3 | 3 | 105 | 104 | 165 | 4.7 | 3 |
| EtP | ||||||||
| Low | 6.30 | 0.3 | 5 | 103 | 102 | 6.40 | 0.4 | 6 |
| High | 177 | 6.2 | 4 | 110 | 107 | 178 | 4.5 | 3 |
| n-PrP | ||||||||
| Low | 14.1 | 0.6 | 4 | 106 | 106 | 15.9 | 1.0 | 6 |
| High | 151 | 6.3 | 4 | 113 | 110 | 166 | 4.1 | 3 |
| n-BuP | ||||||||
| Low | 4.50 | 0.2 | 5 | 102 | 102 | 4.60 | 0.2 | 5 |
| High | 135 | 5.8 | 4 | 102 | 101 | 139 | 3.4 | 2 |
| BzP | ||||||||
| Low | 4.90 | 0.1 | 3 | 109 | 106 | 4.80 | 0.5 | 10 |
| High | 155 | 4.4 | 3 | 106 | 102 | 155 | 6.8 | 4 |
| BPA | ||||||||
| Low | 6.20 | 0.5 | 8 | 101 | 100 | 6.30 | 0.8 | 12 |
| High | 125 | 3.0 | 2 | 96 | 97 | 129 | 6.8 | 5 |
| BPF | ||||||||
| Low | 4.70 | 0.4 | 9 | 99 | 100 | 5.30 | 0.5 | 9 |
| High | 149 | 5.9 | 4 | 101 | 101 | 145 | 6.7 | 5 |
| BPS | ||||||||
| Low | 4.70 | 0.2 | 4 | 103 | 103 | 4.70 | 0.4 | 8 |
| High | 145 | 5.6 | 4 | 104 | 103 | 146 | 2.6 | 2 |
Names and abbreviations of all analytes are shown in Table I. SD, standard deviation; CV, coefficient of variability.
Table IIb.
Method validation for phthalates: intra- and inter-assay controls and recoveries
| Intra-assay | Recovery | Inter-assay | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | CV (%) | Mean (%) | Mean | SD | CV (%) | ||
| 50 ng/mL | 500 ng/mL | |||||||
| MMP | ||||||||
| Medium | 7.50 | 0.6 | 8 | 105 | 97 | 6.00 | 0.6 | 10 |
| High | 48.3 | 2.7 | 6 | 101 | 94 | 44.4 | 2.9 | 7 |
| MEP | ||||||||
| Low | 150 | 3.9 | 3 | 113 | 93 | 151 | 14 | 9 |
| High | 208 | 5.6 | 3 | 94 | 86 | 196 | 13 | 7 |
| MiBP | ||||||||
| Low | 34.4 | 1.9 | 6 | 103 | 101 | 29.0 | 1.8 | 6 |
| High | 167 | 7.9 | 5 | 115 | 96 | 152 | 19 | 12 |
| MnBP | ||||||||
| Low | 27.7 | 1.2 | 4 | 111 | 102 | 27.4 | 1.7 | 6 |
| High | 161 | 3.4 | 2 | 115 | 88 | 164 | 7.1 | 4 |
| MnHP | ||||||||
| Low | 4.80 | 0.2 | 4 | 105 | 95 | 4.80 | 0.2 | 4 |
| High | 46.1 | 1.0 | 2 | 104 | 93 | 45.0 | 2.0 | 5 |
| MEHP | ||||||||
| Low | 10.7 | 0.7 | 7 | 103 | 95 | 11.1 | 0.7 | 7 |
| High | 52.1 | 2.1 | 4 | 99 | 94 | 53.8 | 2.2 | 4 |
| MEHHP | ||||||||
| Low | 16.0 | 0.4 | 2 | 99 | 102 | 15.3 | 1.1 | 7 |
| High | 113 | 2.2 | 2 | 104 | 97 | 111 | 4.6 | 4 |
| MEOHP | ||||||||
| Low | 13.6 | 0.3 | 3 | 104 | 96 | 13.8 | 0.7 | 5 |
| High | 107 | 4.0 | 4 | 113 | 96 | 109 | 5.0 | 5 |
| MECPP | ||||||||
| Low | 17.9 | 0.7 | 4 | 103 | 99 | 18.0 | 1.1 | 6 |
| High | 116 | 3.0 | 3 | 105 | 97 | 119 | 7.6 | 6 |
| MBzP | ||||||||
| Low | 10.6 | 1.1 | 10 | 99 | 100 | 10.8 | 0.7 | 6 |
| High | 91.7 | 8.4 | 9 | 93 | 100 | 88.5 | 5.9 | 7 |
| MiNP | ||||||||
| Low | 5.50 | 0.2 | 3 | 111 | 92 | 5.30 | 0.2 | 4 |
| High | 54.7 | 1.7 | 3 | 100 | 89 | 54.6 | 2.0 | 4 |
| MHiNP | ||||||||
| Low | ND | ND | ND | |||||
| High | ND | ND | ND | |||||
| MiDP | ||||||||
| Low | 4.50 | 0.2 | 4 | 110 | 98 | 4.60 | 0.3 | 7 |
| High | 45.5 | 2.0 | 4 | 106 | 97 | 50.4 | 3.8 | 8 |
Names and abbreviations of all analytes are shown in Table I. SD, standard deviation; CV, coefficient of variability; ND, not determined.
LODs and LOQs are presented in Table III. LODs ranged between 0.06 ng/mL (n-BuP, BPS) and 0.43 ng/mL (MMP), and was 0.22 ng/mL for BPA. LOQs ranged between 0.5 ng/mL (EtP, MnHP, MiNP) and 2 ng/mL (MMP, MiBP, MnBP, MEHP, MHiNP), and was set at 1.4 ng/mL for BPA.
Table III.
Urinary levels of EDCs measured by new methods and established method at Department of Growth and Reproduction, Rigshospitalet, Copenhagen
| Dutch facility (ng/mL) | Danish facility (ng/mL) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | N > LOD (%) | LOQ | N > LOQ (%) | mean | q25 | q50 | q75 | max | LOD | N > LOD (%) | mean | q25 | q50 | q75 | max | |
| Parabens | ||||||||||||||||
| MeP | 0.14 | 39 (98) | 1 | 38 (95) | 58.7 | 5.32 | 53.3 | 79.5 | 200 | 0.08 | 40 (100) | 47.8 | 8.40 | 37.4 | 63.7 | 165 |
| EtP | 0.09 | 37 (93) | 0.5 | 31 (78) | 8.94 | 0.73 | 3.68 | 10.3 | 54.5 | 0.04 | 40 (100) | 8.39 | 0.63 | 3.18 | 8.83 | 56.5 |
| n-PrP | 0.07 | 31 (78) | 1 | 25 (63) | 20.0 | 0.09 | 3.72 | 29.4 | 141 | 0.05 | 37 (93) | 11.2 | 0.33 | 1.87 | 15.0 | 79.0 |
| n-BuP | 0.06 | 23 (58) | 1 | 14 (35) | 2.39 | <LOD | 0.15 | 2.05 | 19.3 | 0.05 | 26 (65) | 2.4 | <LOD | 0.16 | 2.03 | 20.8 |
| BzP | 0.07 | 6 (15) | 1 | 0 (0) | 0.05 | <LOD | <LOD | <LOD | 0.48 | 0.05 | 15 (38) | 0.07 | <LOD | <LOD | 0.02 | 0.52 |
| Bisphenols | ||||||||||||||||
| BPA | 0.22 | 15 (38) | 1.4 | 8 (20) | 1.47 | <LOD | <LOD | 1.22 | 27.3 | 0.12 | 40 (100) | 2.06 | 0.95 | 1.69 | 2.34 | 14.3 |
| BPF | 0.23 | 14 (35) | 1.5 | 7 (18) | 1.40 | <LOD | <LOD | 0.93 | 27.3 | |||||||
| BPS | 0.06 | 3 (8) | 0.8 | 1 (3) | 0.11 | <LOD | <LOD | <LOD | 3.28 | |||||||
| Phthalates | ||||||||||||||||
| MMP | 0.43 | 19 (48) | 2 | 7 (18) | 1.06 | <LOD | <LOD | 1.31 | 7.28 | 0.53 | 3 (8) | 0.32 | <LOD | <LOD | <LOD | 7.32 |
| MEP | 0.35 | 40 (100) | 1 | 40 (100) | 114 | 18.9 | 28.8 | 169 | 965 | 0.79 | 40 (100) | 103 | 17.2 | 28.4 | 169 | 767 |
| MiBP | 0.33 | 40 (100) | 2 | 40 (100) | 34.2 | 13.3 | 21.1 | 36.1 | 196 | 0.44 | 40 (100) | 33.3 | 13.2 | 21.1 | 35.5 | 199 |
| MnBP | 0.22 | 40 (100) | 2 | 40 (100) | 35.7 | 13.1 | 18.2 | 33.6 | 307 | 0.68 | 40 (100) | 30.7 | 11.3 | 17.6 | 27.0 | 273 |
| MnHP | 0.07 | 23 (58) | 0.5 | 0 (0) | 0.10 | <LOD | 0.08 | 0.18 | 0.47 | 0.33 | 1 (3) | 0.4 | <LOD | <LOD | <LOD | <LOD |
| MEHP | 0.12 | 40 (100) | 2 | 25 (63) | 3.30 | 1.54 | 2.54 | 3.38 | 14.0 | 0.42 | 40 (100) | 3.16 | 1.53 | 2.34 | 3.44 | 13.5 |
| MEHHP | 0.11 | 40 (100) | 1 | 40 (100) | 13.5 | 5.66 | 8.36 | 15.6 | 52.7 | 0.44 | 40 (100) | 15.7 | 7.18 | 10.5 | 18.5 | 62.5 |
| MEOHP | 0.09 | 40 (100) | 1 | 40 (100) | 8.84 | 3.72 | 5.58 | 9.71 | 32.5 | 0.46 | 40 (100) | 9.44 | 3.71 | 6.39 | 11.5 | 38.6 |
| MECPP | 0.25 | 40 (100) | 1 | 40 (100) | 12.8 | 5.34 | 10.2 | 15.0 | 59.2 | 0.28 | 40 (100) | 10.0 | 4.51 | 7.79 | 11.9 | 47.8 |
| MBzP | 0.22 | 40 (100) | 1 | 40 (100) | 8.63 | 2.89 | 4.8 | 10.8 | 41.6 | 0.50 | 40 (100) | 5.47 | 1.96 | 3.03 | 6.66 | 28.5 |
| MiNP | 0.10 | 0 (0) | 0.5 | 0 (0) | <LOD | <LOD | <LOD | <LOD | 0.39 | 13 (33) | 0.28 | <LOD | <LOD | 0.48 | 2.17 | |
| MHiNP | 0.29 | 0 (0) | 2 | 0 (0) | <LOD | <LOD | <LOD | <LOD | ||||||||
| MiDP | 0.31 | 0 (0) | 1 | 0 (0) | <LOD | <LOD | <LOD | <LOD | ||||||||
Names and abbreviations of all analytes are shown in Table I. LOQ, limit of quantification; q25, 25th quartile; q75, 75th quartile; q95, 95th quartile; LOD, limit of detection.
Interlaboratory comparison
Analysis of NIST reference material showed for a majority of the analytes agreeable: ≤15% for MeP, EtP, BPA, MEP and MnBP; and ≤25% for PrP, n-BuP and MEHP. Only MiBP, MEHHP, MEOHP, MECPP and MBzP deviated more (84, 62, 27, 34 and 40%, respectively). Data is provided in Supplementary Table SII.
24 h Urine measurements
The concentrations of phenols and phthalate metabolites from forty 24 h urine samples are presented in Table III. The parabens MeP, and EtP, and the phthalate metabolites MEP, MiBP, MnBP, MEHP, MEHHP, MEOHP, MECPP and MBzP were detected in ≥93% of the samples. MMP and MnHP were detected in respectively 48 and 58% of the samples. MHiNP, MiNP and MiDP were not detectable above LOD in any of the samples measured by the present method. The parabens n-PrP, n-BuP and BzP were detected in 78, 58 and 15% of the samples, respectively. The bisphenols were detected in 38, 35 and 8% of the samples (BPA, BPF and BPS, respectively).
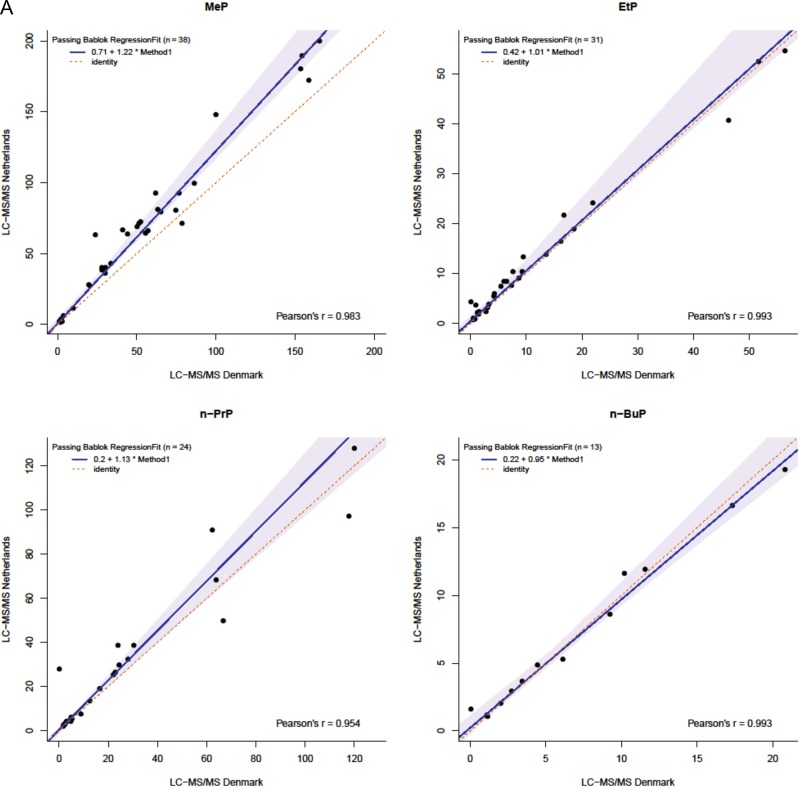
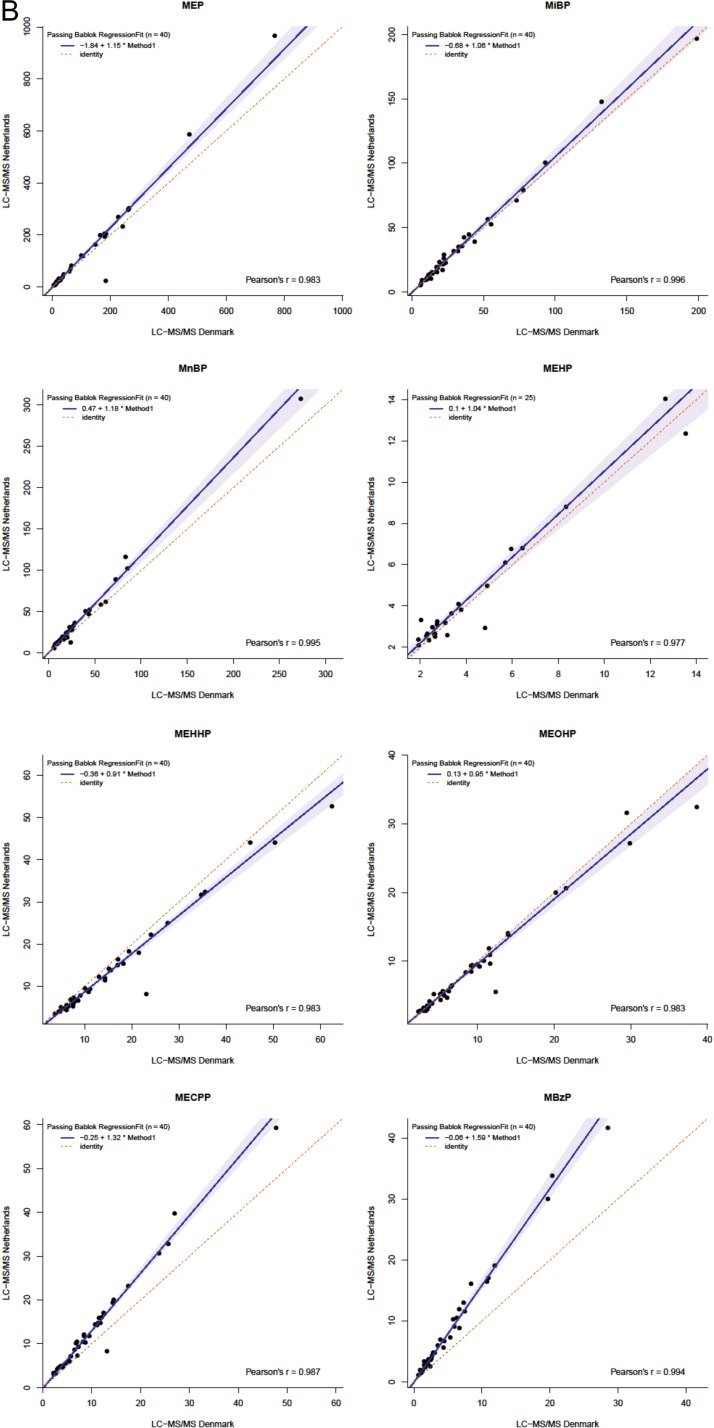
The results of measurement of these forty 24 h urine samples performed by the present Dutch method were compared with equal measurements performed by Danish LC–MS-MS methods (Table III). Both methods were compared for the 17 compounds measured in the same set of 40 samples by the two laboratories. The Dutch method detected more samples above LOD for MMP, whereas the Danish methods measured more cases for MeP, EtP, n-PrP, n-BuP, BzP, BPA and MiNP. Since BPA, BzP and MiNP were detected in <50% of the samples by the Dutch method, as was MMP by the Danish method, these compounds were excluded from the method comparison. Passing Bablok regression showed slopes and intercepts of 1.22 and 0.71 (MeP), 1.01 and 0.42 (EtP), 1.13 and 0.20 (n-PrP), 0.95 and 0.22 (n-BuP), 1.15 and −1.84 (MEP), 1.06 and −0.68 (MiBP), 1.18 and 0.47 (MnBP), 1.04 and 0.10 (MEHP), 0.91 and −0.36 (MEHHP), 0.95 and 0.13 (MEOHP), 1.32 and −0.25 (MECPP), and 1.59 and −0.06 (MBzP), respectively (Figure 2). Supplementary Table S3 displays 95% confidence intervals of slopes and intercepts. Intercepts were similar between both methods for all EDCs, except for EtP and MEP. Slopes differed for MeP, MEP, MnBP, MECPP, MEHHP and MBzP, suggesting proportional differences between methods. Yet, when including all samples >LOD in the analyses, the difference for EtP at intercept disappeared. All other observed differences remained.
Figure 2.
Passing Bablok regression plots comparing parabens (A) and phthalate metabolites (B) in 40 human urine samples measured by the present method (y-axis) and by LC–MS-MS methods at Department of Growth and Reproduction, Rigshospitalet, Copenhagen (x-axis). The line of identity (x = y) represents a perfect match.
Discussion
In this study we developed and validated two LC–MS-MS methods which are able to measure 5 parabens, 3 bisphenols and 13 different metabolites of eight phthalates.
By implementing the measurements of both parabens and bisphenols in one run, as well as using UPLC, we reduced the LC–MS-MS runtime to 7 min for phenols and 8 min for phthalates. This is much shorter than other methods, which report retention times from 8.9 to 17 min for phenol methods (13, 36, 37), and 10.2–27 min for phthalate methods (37–40). Although the runtime was reduced, chromatographic resolution was more than sufficient to separate isomers, which is important, especially in phthalate analysis. MiBP and MnBP were separated at baseline by using acetonitrile as eluent, instead of methanol. Furthermore, sensitivity of the phenol analysis was increased by the addition of ammonium fluoride in the mobile phase. It was previously shown that ammonium fluoride enhances ionization of estrogens, and this is also the case for the analysis of phenols with electrospray ionization, ranging from 12 times for MeP to 70 times for BPA with respect to peak area (supplemental Figure 1) (41). As there is 200 μL of sample available for analysis at the end of the SPE of which only 5 μL is injected, we tried to further improve chromatographic resolution by increasing the injecting volume. Yet neither method showed a better signal-to-noise ratio when injecting a volume of 10 μL, the maximum loading volume.
For method validation, we measured NIST reference material and found that 5 out of the 13 compared analytes were in excellent accordance (i.e., NIST-to-Method-ratio <15%) to the concentrations given by NIST (i.e., MeP, EtP, BPA, MEP and MnBP), whereas PrP, n-BuP and MEHP showed similar values (i.e., NIST-to-Method-ratio: 15–25%). Yet the concentrations measured for the phthalates MiBP, MEHHP, MEOHP, MECPP and MBzP showed differences (i.e., NIST-to-Method-ratio >25%), which might be due to the difference in analytical methods and calibration. The levels of EDCs in the NIST standard were established using a GC–MS-MS method for BPA and online SPE-LC–MS-MS for parabens and phthalates, whereas the present method uses offline SPE in combination with UPLC–MS-MS (42). Although Sigma-Aldrich states the NIST samples are suitable for HPLC, this is not known for UPLC. Notably, for the four out of five phthalates (i.e., MiBP, MEHHP, MEOHP and MECPP) which showed the largest differences, our method measures lower concentrations, which could indicate better separation of possible interferences, due to higher resolution of UPLC in comparison to HPLC.
Furthermore, we compared measurements of forty 24 h urine samples from our present Dutch UPLC–MS-MS methods with established Danish LC–MS-MS methods (32–34). These methods show very similar results. Discrepancies found in intercept for EtP and MEP suggest systematic differences between methods. Yet, when including samples measured above LOD by the Dutch method this difference disappears. Although samples with a concentration <LOQ cannot be accurately quantified, this implies that this systematic difference is due to the different cut-off levels used. Differences in slope were minor (9–22%) for all compounds but for MECPP and MBzP, and can be explained by a few outliers at high concentrations, and minor differences in calibration. Additionally, as phthalate monoesters are found in a wide variety of products, analysis is prone to variation due to possible external contamination (43). Yet MECPP is a secondary metabolite for DEHP, and therefore has to be metabolized in the human body, which makes external contamination not possible (44). By thoroughly checking all materials used for contamination, as well as including blank samples in every analysis, potential contamination is closely monitored and minimized. For one urine sample, we detected consistently lower concentrations in multiple phthalate measurements (i.e., MEP, MEOHP, MEHP, MEHHP, MECPP), suggesting a problem at sample level (e.g., sample contamination), rather than in the LC–MS-MS method. In a comparison of median concentrations of compounds measured by both countries, the Dutch methods reported significantly higher concentrations for one paraben and four phthalate metabolites (i.e., MeP, MEP, MnBP, MECPP, MBzP). As this could be explained by using a higher LOQ as cut-off in the Dutch method, Passing Babloks were compared using all samples measured above LOD by the Dutch method. This resulted in similar outcomes for all but EtP, implying that the difference between methods is due to a higher cut-off used in the Dutch method.
As EDCs have only recently been introduced in human biomonitoring, interlaboratory comparisons are often difficult. In a recent study, the European (DEMO)COPHES project aimed to develop analytical methods for the human biomonitoring of environmental pollutants in urine and generate comparable data across Europe (43). This study included five phthalate metabolites of which three are also included in this study, and BPA. Yet even the laboratories chosen as reference because of their expertise showed a relatively high interlaboratory imprecision (relative standard deviations ranging from 6 to 39% for phthalates, and 11 to 20% for BPA). Of the participating laboratories, only 37 and 38% were able to qualify for the measurements of phthalates and BPA, respectively, by passing one interlaboratory comparison investigation and one external quality assessment scheme exercise. Taking the above results into account our results show a high level of agreement with the NIST and Danish method.
The samples investigated were collected between 2008 and 2010, after which they have been stored at −80°C for a maximum of eight years before analyses. During this time, EDCs of interest could have been subject to degradation. Parabens, BPA, and phthalate metabolites are described to remain stable for at least six months to one year at −70°C (45, 46). Another study shows that, although for a timespan of one week, additional preservatives do not improve stability (47). Yet, degradation over multiple years has not yet been investigated. Various freeze-thaw cycles may negatively influence the quality of the samples. In this study, both facilities received their own aliquot. Therefore, samples did not have to be thawed to be distributed, and were thawed twice in total (i.e., for phthalate method, for phenol method) at both facilities.
Compared to another Dutch study which analyzed exposure to EDCs, some of the phthalate metabolites measured were comparable (median (ng/mL) [inter-quartile range]: MiBP: 21 [22,83] versus 22 [36,36]; MnBP: 18 [20,55] versus 16 [24,21]), although in general we measured lower concentrations in our LifeLines cohort (12). Differences could be explained by the difference in study population (pregnant women versus general population), as several of these EDCs have sex-specific concentrations (48). Also, the study location differed (Rotterdam versus the north of the Netherlands), which could influence the consumer products used. Although differences in EDC exposure between urban, metropolitan and rural areas have been shown in a study conducted in Italy, a Danish study showed similar exposure to EDCs (49, 50). Lastly, the method of analysis was different. Although Philips et al. (12) use a validated method, methods have not been compared. Furthermore, the Rotterdam study collected urine samples in 2004 and 2005, whereas Lifelines urine samples were collected between 2008 and 2010. Changes in regulation, consumer awareness or the introduction of alternatives have led to a change of EDC production and exposure through time. This has been shown in different studies reporting change in EDC exposure through time (i.e., calendar year). For example, higher concentrations of BPF, BPS, MiBP and DiNP have been detected over the years, whereas BPA, MnBP, MBzP and DEHP concentrations have decreased over time (22, 51–53). Furthermore, the method of urine collection (spot urine versus 24 h urine) may play an important factor. Although spot-urine samples are a good estimate for population studies, 24 h urine samples are required for assessing individual daily exposure due to the quick metabolism and excretion of these compounds (54, 55). Lastly, due to high inter-individual variation in concentrations, epidemiological studies with large sample sizes are required. Keeping in mind the limited sample size of this study comparisons should be made with caution.
In conclusion, we showed that our newly developed fast high throughput UPLC–MS-MS methods are capable of reproducible and sensitive analysis of 5 parabens and 3 bisphenols, and 13 different metabolites of 8 different phthalates in human urine. Sensitivity of the phenol method including both bisphenols and parabens was increased by using ammonium fluoride in the mobile phase.
Supplementary Material
Acknowledgments
The authors thank Robbert Noordkamp, Remke Bijma, Sewara Khalilova, Lotus Westerhof and Irene van der Kooij-Wijbenga for their contribution to the development and validation of the methods. This work was supported by a Diabetes Funds Junior Fellowship from the Dutch Diabetes Research Foundation (to JVvVO, project no. 2013.81.1673), and by the Danish Center on Endocrine Disrupters and the International Center for Research and Research Training in Endocrine Disruption of Male Reproduction and Child Health (EDMaRC).
Author contribution statement
T.P.v.d.M. performed the analysis, interpreted data and wrote the article; M.v.F. coordinated and performed the measurements and contributed to writing the article; H.F. coordinated and performed the measurements and contributed to writing the article; B.H.R.W. acquired data and/or provided study materials; A.P.v.B. acquired data and/or provided study materials; I.P.K. contributed to interpretation of the data and analyses; and J.V.v.V.O. conceived, designed and implemented the study, was involved in data acquisition and contributed to writing the article. All authors reviewed and approved the final article.
References
- 1. Jalal N., Surendranath A.R., Pathak J.L., Yu S., Chung C.Y. (2018) Bisphenol A (BPA) the mighty and the mutagenic. Toxicology Reports, 5, 76–84. 10.1016/j.toxrep.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heffernan A.L., Baduel C., Toms L.M., Calafat A.M., Ye X., Hobson P., et al. (2015) Use of pooled samples to assess human exposure to parabens, benzophenone-3 and triclosan in Queensland, Australia. Environmental International, 85, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. North E.J., Halden R.U. (2013) Plastics and environmental health: the road ahead. Reviews on Environmental Health, 28, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vandenberg L.N., Hauser R., Marcus M., Olea N., Welshons W.V. (2007) Human exposure to bisphenol A (BPA). Reproductive Toxicology, 24, 139–177. [DOI] [PubMed] [Google Scholar]
- 5. Main K.M., Mortensen G.K., Kaleva M.M., Boisen K.A., Damgaard I.N., Chellakooty M., et al. (2006) Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environmental Health Perspectives, 114, 270–276. 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frederiksen H., Jensen T.K., Jorgensen N., Kyhl H.B., Husby S., Skakkebaek N.E., et al. (2014) Human urinary excretion of non-persistent environmental chemicals: an overview of Danish data collected between 2006 and 2012. Reproduction (Cambridge, England), 147, 555–565. [DOI] [PubMed] [Google Scholar]
- 7.CDC Fourth National report on human exposure to environmental chemicals, updated tables. 1–1075. 20. [PubMed]
- 8. Kasper-Sonnenberg M., Koch H.M., Wittsiepe J., Bruning T., Wilhelm M. (2014) Phthalate metabolites and bisphenol A in urines from German school-aged children: results of the Duisburg birth cohort and Bochum cohort studies. International Journal of Hygiene and Environmental Health, 217, 830–838. [DOI] [PubMed] [Google Scholar]
- 9. Cutanda F., Koch H.M., Esteban M., Sanchez J., Angerer J., Castano A. (2015) Urinary levels of eight phthalate metabolites and bisphenol A in mother-child pairs from two Spanish locations. International Journal of Hygiene and Environmental Health, 218, 47–57. [DOI] [PubMed] [Google Scholar]
- 10. Heinälä M., Ylinen K., Tuomi T., Santonen T., Porras S.P. (2017) Assessment of occupational exposure to bisphenol A in five different production companies in Finland. Annals of Work Exposures and Health, 61, 44–55. 10.1093/annweh/wxw006. [DOI] [PubMed] [Google Scholar]
- 11. Andrianou X.D., Gängler S., Piciu A., Charisiadis P., Zira C., Aristidou K., et al. (2016) Human exposures to bisphenol A, bisphenol F and chlorinated bisphenol A derivatives and thyroid function. PLoS One, 11, e0155237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Philips E.M., Jaddoe V.W.V., Asimakopoulos A.G., Kannan K., Steegers E.A.P., Santos S., et al. (2018) Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004–5. Environmental Research, 161, 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou X., Kramer J.P., Calafat A.M., Ye X. (2014) Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 944, 152–156. [DOI] [PubMed] [Google Scholar]
- 14. Liao C., Kannan K. (2014) A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Archives of Environmental Contamination and Toxicology, 67, 50–59. [DOI] [PubMed] [Google Scholar]
- 15. Liao C., Liu F., Kannan K. (2012) Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environmental Science and Technology, 46, 6515–6522. 10.1021/es300876n. [DOI] [PubMed] [Google Scholar]
- 16. Liao C., Kannan K. (2013) Concentrations and profiles of bisphenol a and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. Journal of Agricultural and Food Chemistry, 61, 4655–4662. 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- 17. Calafat A.M., Ye X., Wong L.Y., Reidy J.A., Needham L.L. (2008) Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental Health Perspectives, 116, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song Y., Chou E.L., Baecker A., You N.C., Sun Q., Liu S. (2016) Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: a systematic review and meta-analysis. Journal of Diabetes, 8, 516–532. [DOI] [PubMed] [Google Scholar]
- 19. Heindel J.J., Blumberg B., Cave M., Machtinger R., Mantovani A., Mendez M.A., et al. (2017) Metabolism disrupting chemicals and metabolic disorders. Reproductive Toxicology, 68, 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., et al. (2015) EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocrine Reviews, 36, E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andra S.S., Charisiadis P., Arora M., van Vliet-Ostaptchouk J.V., Makris K.C. (2015) Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environmental International, 85, 352–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rochester J.R., Bolden A.L. (2015) Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environmental Health Perspectives, 123, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heindel J.J., vom Saal F.S., Blumberg B., Bovolin P., Calamandrei G., Ceresini G., et al. (2015) Parma consensus statement on metabolic disruptors. Environmental Health, 14, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Konkel L. (2017) Compound interest: assessing the effects of chemical mixtures in vivo. Environmental Health Perspectives, 125, 124001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walaszczyk E., Luijten M., Spijkerman A.M.W., Bonder M.J., Lutgers H.L., Snieder H., et al. (2018) DNA methylation markers associated with type 2 diabetes, fasting glucose and HbA1c levels: a systematic review and replication in a case–control sample of the Lifelines study. Diabetologia, 61, 354–368. 10.1007/s00125-017-4497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scholtens S., Smidt N., Swertz M.A., Bakker S.J., Dotinga A., Vonk J.M., et al. (2015) Cohort Profile: LifeLines, a three-generation cohort study and biobank. International Journal of Epidemiology, 44, 1172–1180. [DOI] [PubMed] [Google Scholar]
- 27. Klijs B., Scholtens S., Mandemakers J.J., Snieder H., Stolk R.P., Smidt N. (2015) Representativeness of the LifeLines Cohort Study. PLoS One, 10, e0137203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matuszewski B.K., Constanzer M.L., Chavez-Eng C.M. (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Analytical Chemistry, 75, 3019–3030. 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 29. Lynch K.L. (2016) CLSI C62-A: a new standard for clinical mass spectrometry. Clinical Chemistry, 62, 24–29. 10.1373/clinchem.2015.238626. [DOI] [PubMed] [Google Scholar]
- 30. ICH. Validation of Analytical Procedures: Text and Methodology Q2(R1). 2005.
- 31. Clinical and Laboratory Standards Institute. Protocols for Determination of Limits of Detection and Limits of Quantitation; Approved Guideline. CLSI Document EP17-A.
- 32. Frederiksen H., Aksglaede L., Sorensen K., Nielsen O., Main K.M., Skakkebaek N.E., et al. (2013) Bisphenol A and other phenols in urine from Danish children and adolescents analyzed by isotope diluted TurboFlow-LC-MS/MS. International Journal of Hygiene and Environmental Health, 216, 710–720. 10.1016/j.ijheh.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 33. Frederiksen H., Jørgensen N., Andersson A.M. (2011) Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Journal of Exposure Science and Environmental Epidemiology, 21, 262–271. 10.1038/jes.2010.6. [DOI] [PubMed] [Google Scholar]
- 34. Frederiksen H., Jørgensen N., Andersson A.M. (2010) Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. Journal of Analytical Toxicology, 34, 400–410. [DOI] [PubMed] [Google Scholar]
- 35. Team R.D.C. R: A Language and Environment for Statistical Computing 2017. https://www.r-project.org/.
- 36. Ye X., Kuklenyik Z., Needham L.L., Calafat A.M. (2005) Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Analytical Chemistry, 77, 5407–5413. [DOI] [PubMed] [Google Scholar]
- 37. Heffernan A.L., Thompson K., Eaglesham G., Vijayasarathy S., Mueller J.F., Sly P.D., et al. (2016) Rapid, automated online SPE-LC-QTRAP-MS/MS method for the simultaneous analysis of 14 phthalate metabolites and 5 bisphenol analogues in human urine. Talanta, 151, 224–233. [DOI] [PubMed] [Google Scholar]
- 38. Kato K., Silva M.J., Needham L.L., Calafat A.M. (2005) Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Analytical Chemistry, 77, 2985–2991. [DOI] [PubMed] [Google Scholar]
- 39. Koch H.M., Gonzalez-Reche L.M., Angerer J. (2003) On-line clean-up by multidimensional liquid chromatography-electrospray ionization tandem mass spectrometry for high throughput quantification of primary and secondary phthalate metabolites in human urine. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 784, 169–182. [DOI] [PubMed] [Google Scholar]
- 40. Silva M.J., Samandar E., Preau J.L., Reidy J.A., Needham L.L., Calafat A.M. (2007) Quantification of 22 phthalate metabolites in human urine. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 860, 106–112. [DOI] [PubMed] [Google Scholar]
- 41. Fiers T., Casetta B., Bernaert B., Vandersypt E., Debock M., Kaufman J.M. (2012) Development of a highly sensitive method for the quantification of estrone and estradiol in serum by liquid chromatography tandem mass spectrometry without derivatization. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 893–894, 57–62. [DOI] [PubMed] [Google Scholar]
- 42. Schantz M.M., Benner B.A., Heckert N.A., Sander L.C., Sharpless K.E., Vander Pol S.S., et al. (2015) Development of urine standard reference materials for metabolites of organic chemicals including polycyclic aromatic hydrocarbons, phthalates, phenols, parabens, and volatile organic compounds. Analytical and Bioanalytical Chemistry, 407, 2945–2954. 10.1007/s00216-014-8441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schindler B.K., Esteban M., Koch H.M., Castano A., Koslitz S., Cañas A., et al. (2014) The European COPHES/DEMOCOPHES project: towards transnational comparability and reliability of human biomonitoring results. International Journal of Hygiene and Environmental Health, 217, 653–661. 10.1016/j.ijheh.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 44.CID 148386. https://pubchem.ncbi.nlm.nih.gov/compound/148386 (2 October 2018).
- 45. Ye X., Bishop A.M., Reidy J.A., Needham L.L., Calafat A.M. (2007) Temporal stability of the conjugated species of bisphenol A, parabens, and other environmental phenols in human urine. Journal of Exposure Science and Environmental Epidemiology, 17, 567–572. 10.1038/sj.jes.7500566. [DOI] [PubMed] [Google Scholar]
- 46. Samandar E., Silva M.J., Reidy J.A., Needham L.L., Calafat A.M. (2009) Temporal stability of eight phthalate metabolites and their glucuronide conjugates in human urine. Environmental Research, 109, 641–646. 10.1016/j.envres.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 47. Hoppin J.A., Ulmer R., Calafat A.M., Barr D.B., Baker S.V., Meltzer H.M., et al. (2006) Impact of urine preservation methods and duration of storage on measured levels of environmental contaminants. Journal of Exposure Science and Environmental Epidemiology, 16, 39–48. 10.1038/sj.jea.7500435. [DOI] [PubMed] [Google Scholar]
- 48. Moore J.X., Chaudhary N., Akinyemiju T. (2017) Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Preventing Chronic Disease, 14, E24 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. La Rocca C., Tait S., Guerranti C., Busani L., Ciardo F., Bergamasco B., et al. (2015) Exposure to endocrine disruptors and nuclear receptors gene expression in infertile and fertile men from Italian areas with different environmental features. International Journal of Environmental Research and Public Health, 12, 12426–12445. 10.3390/ijerph121012426.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Frederiksen H., Nielsen J.K., Mørck T.A., Hansen P.W., Jensen J.F., Nielsen O., et al. (2013) Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. International Journal of Hygiene and Environmental Health, 216, 772–783. [DOI] [PubMed] [Google Scholar]
- 51. LaKind J.S., Naiman D.Q. (2015) Temporal trends in bisphenol A exposure in the United States from 2003–2012 and factors associated with BPA exposure: spot samples and urine dilution complicate data interpretation. Environmental Research, 142, 84–95. [DOI] [PubMed] [Google Scholar]
- 52. Zota A.R., Calafat A.M., Woodruff T.J. (2014) Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environmental Health Perspectives, 122, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koch H.M., Rüther M., Schütze A., Conrad A., Pälmke C., Apel P., et al. (2017) Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. International Journal of Hygiene and Environmental Health, 220, 130–141. 10.1016/j.ijheh.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 54. Sun Q., Bertrand K.A., Franke A.A., Rosner B., Curhan G.C., Willett W.C. (2017) Reproducibility of urinary biomarkers in multiple 24-h urine samples. The American Journal of Clinical Nutrition, 105, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Christensen K.L., Lorber M., Koch H.M., Kolossa-Gehring M., Morgan M.K. (2012) Population variability of phthalate metabolites and bisphenol A concentrations in spot urine samples versus 24- or 48-h collections. Journal of Exposure Science and Environmental Epidemiology, 22, 632–640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.