Abstract
Rationale
There is evidence for a common genetic link between alcohol and nicotine dependence. Rodents selectively bred for high alcohol consumption/responsivity are also more likely to self-administer nicotine than controls.
Objectives
The experiments examined the systemic response to nicotine, the effects of nicotine within the drug reward pathway, and innate expression of nicotine related genes in a brain region regulating drug reward/self-administration in multiple lines of rats selectively bred for high and low alcohol consumption.
Methods
The experiments examined the effects of peripheral administration of nicotine on locomotor activity, the effects of nicotine administered directly into the (posterior ventral tegmental area; pVTA) on dopamine (DA) release in the nucleus accumbens shell (AcbSh), and innate mRNA levels of acetylcholine receptor genes in the pVTA were determined in 6 selectively bred high/low alcohol consuming rat lines with Wistar as controls.
Results
The high alcohol-consuming rat lines had greater nicotine-induced locomotor activity compared to low alcohol-consuming rat lines. Microinjections of nicotine into the pVTA resulted in DA release in the AcbSh with the dose response curves for high alcohol-consuming rats shifted leftward and upward. Genetic analysis of the pVTA indicated differences in nicotinic receptors (α2 and β4).
Conclusion
Selective breeding for high alcohol preference resulted in a genetically divergent behavioral and neurobiological sensitivity to nicotine. The observed behavioral and neurochemical differences between the rat lines would predict an increased likelihood of nicotine reinforcement. The data support the hypothesis of a common genetic basis for drug addiction and identifies potential receptor targets.
Keywords: alcohol-preferring P rats, locomotor activity, nicotine, high-alcohol-drinking HAD rats, ventral tegmental area, nucleus accumbens, dopamine
Introduction
The brain reward system regulates numerous motivationally relevant behaviors in an effort to attain natural rewards (i.e, food, sex, etc.). Drugs of abuse exert their effects within the brain reward system, exploiting natural processes, thereby increasing the propensity to consume drugs in the future. There is a strong genetic component determining the characteristics of the brain reward system (Hall et al., 2014; Parsons & Hurd, 2015). It is this genetically mediated development of the reward system that has lead researchers to suggest a common genetic predisposition to develop an addictive disorder (Grant et al., 2011; Uhl, 2004; Uhl et al., 2008a; 2008b). For instance, a family history of alcoholism predisposes individuals to exhibit eating, gambling and sexual disorders as well as an increased risk of alcoholism and addiction to other drugs of abuse (Grant et al., 2011; Kamarajan et al., 2015). The St. Louis Epidemiological Catchment Area (ECA) longitudinal study has clearly indicated that the highest rate of comorbidity observed was between gambling, alcohol use disorders and antisocial personality disorders (Cunningham-Williams et al., 1998). Thus, epidemiological evidence suggests that a genetic predisposition to develop alcoholism is associated with an altered natural brain reward system.
The two most common co-abused drugs are alcohol and nicotine. Individuals that are nicotine dependent (ND) are 10 times more likely to be diagnosed with alcoholism than non-smokers in their lifetime (DiFranza & Guerrera, 1990). In ND individuals, the rate of concurrent alcohol dependency (AD) is between 2.7 and 4.4 times higher than in non-smokers (Breslau, 1995; Grant et al., 2004). Preclinical data also indicate there is a possible genetic association between ethanol (EtOH) and nicotine (NIC) sensitivity. The alcohol-preferring (P) rat self-administered (i.v.) NIC at 4-fold lower concentrations than non-alcohol preferring (NP) rat, and self-administered approximately 3–4 fold more NIC than NP rats, and expressed greater drug-elicited (NIC primed) NIC seeking than NP rats (Le et al., 2006). The posterior ventral tegmental area (pVTA) is a neuroanatomical site supporting the reinforcing properties of EtOH (Rodd et al., 2005; Rodd-Henricks et al., 2000) and NIC (Ikemoto et al., 2006; Hauser et al., 2014). Rats will readily self-administer EtOH or NIC directly into the pVTA (Rodd-Henricks et al., 2000; Rodd et al., 2005; Ikemoto et al., 2006; Hauser et al., 2014; Rodd et al., 2004a) and the pVTA of P rats is more sensitive (requires a lower concentration of NIC to establish self-administration behaviors) to the reinforcing properties of NIC (Rodd et al., 2004a) than Wistar rats. Thus, the data suggest that selective breeding for high-EtOH preference results in a genetic phenotype that also possesses increased sensitivity to the rewarding effects of NIC which is, in part, mediated by the pVTA DA system.
The current experiment utilized 6 selectively bred rat lines to determine if a genetic predisposition for high alcohol preference, and intake, is associated with an alteration in response to systemic NIC (locomotor activity: LMA), the neurochemical response to NIC microinjected directly into the brain reward pathway, and innate expression of the acetylcholine receptor genes within the pVTA. Psychostimulant-induced LMA has been hypothesized as indicating the incentive motivational processes of these drugs (Pert et al., 1990). The development of LMA sensitization is thought to be indicative of neuroadaptations within the mesolimbic dopamine system that increases the likelihood to self-administer drugs and to develop dependence/addiction to these compounds (Thomas et al., 2008). It was hypothesized that rats selectively bred to prefer/consume alcohol would exhibit a greater LMA and neurochemical (extracellular dopamine) response in the nucleus accumbens shell (AcbSh) following microinjections of NIC into the pVTA than non-consuming or Wistar rats. Further, it was hypothesized that selective breeding for alcohol preference/intake alters the expression of acetylcholine receptor genes within the pVTA.
Materials and Methods
Subjects
Experiments utilized the alcohol-preferring (P) and non-preferring (NP) rat lines, Wistar rats, and both the High- (HAD) and Low-alcohol drinking (LAD) rat lines. All animal care was in accordance with the Guidelines for the Care and Use of Laboratory Animals (RIFLA, 2011) and experiments were approved by the Indiana University School of Medicine IACUC committee.
Nicotine
NIC hydrogen tartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% sterile saline (Exp 1) or artificial cerebral spinal fluid (aCSF; Exp 2). All doses/concentrations of NIC used is based upon salt, not free base. All solutions were pH adjusted to 7.3.
Experiment 1: Effects of NIC on LMA in Selectively Bred Rat Lines
Motor activity was assessed in arenas previously described (Bracken et al., 2011).
NIC induced locomotor activity in P and NP Rats
Adult male P and NP rats were tested during 2 hr sessions. After the 1st hr (daily habituation period), rats were removed from the activity monitors and injected (s.c., 1 mg/ml) with 0, 0.25, or 0.5 mg/kg (n = 8/group/line) NIC and then immediately placed back into the activity monitor. The doses used are from the literature and readily establish NIC-induced LMA in outbred rats following repeated injections (Bracken et al., 2011, Berg & Chambers, 2008). Activity from the 2nd hr was used to determine the effects of drug administration over 7 consecutive daily activity sessions. Two weeks after completing the injection series, all rats received a NIC challenge injection (0.5 mg/kg). P and NP rats are derived from an extinct stock of Wistar rats (c.f. Bell et al., 2016). Originally, the experiment was designed to include Harlan Wistar (Indianapolis IN) rats as a neutral control. During the time period of data collection, Harlan Wistar rats were not available (Parvo outbreak). Charles River Wistar (Wilmington MA) rats were purchased but were deemed to be sufficiently different from Harlan rats and not to be suitable for use. In our hands, the Charles River Wistar subjects never habituated to the injection procedure, displayed freezing behaviors during LMA testing (no injection given prior to freezing), and express a bimodal distribution for LMA (half very little activity, the other half displayed modest activity). Therefore, comparisons between P/NP and Wistar rats will rely on the established literature.
Nicotine induced locomotor activity in HAD and LAD Rats
Adult female HAD-1 (n = 28), HAD-2 (n = 23), LAD-1 (n = 16) and LAD-2 (n = 22) were tested during 2 hr sessions (identical to P/NP study). Rats were given 10 activity sessions. The additional 3 days of NIC exposure was given because of the possibility that the LAD lines would display sensitization to NIC-induced LMA with additional sessions. Activity sessions were conducted Monday-Friday for two consecutive weeks with a NIC challenge injection (0.5 mg/kg) 2 weeks later. Females were used for two reasons. First, it was desired to extend the male findings in the P/NP study to the opposite sex and because of availability. The HAD and LAD rats lines are maintained at low numbers, and HAD male rats are dedicated for a medication development contract.
Statistical Analysis
Data from day one of the injection series and the final challenge session were examined using a two-way ANOVA with independent factors of line and drug condition on the dependent measure of activity. The initial injection series was examined using a repeated measure ANOVA with days as the within subject measure. Post-hoc comparisons were made using the Tukey’s b analysis.
Experiment 2: Effects of NIC microinfusions in the VTA on DA Efflux in the AcbSh
Detailed methodology can be obtained from previous publications (Deehan et al., 2013; Deehan et al., 2015). Naïve adult female P, Wistar, HAD-1, HAD-2, LAD-1 and LAD-2 (n = 7–9/line/ location/concentration) rats were used in this experiment. NP rats bleed excessively during surgeries which preclude NP rats from being used in experiments involving surgery. Instead, Wistar rats were used as a comparison for P rats. Wistar rats have been used previously for comparisons with P rats (Hauser et al., 2014; Rodd et al., 2004a; 2004b). In total, 269 rats were used in this experiment; 146 rats had placements within the pVTA and 123 rats had placements within the anterior VTA (Paxinos & Watson, 2005). The connections between the dorsal raphe and the VTA display a heterogeneous pattern. The transtegemental serotonin pathway, which originates in the DR and median raphe nuclei primarily innervate dopaminergic and non-dopaminergic cells in the interfascicular nucleus (IF) and posterior VTA with little indication of innervation in the anterior VTA (Herve et al., 1987). We defined the pVTA as the portion of the VTA that serotonin innervation (IF present) which occurs between −5.4 and −6.2 mm bregma. The anterior VTA was defined are the region of the VTA which occurs between −4.8 and −5.2 bregma.
The data collected in this experiment required >3200 hours of work and the costs exceeded $200,000 (US$). Males were not conducted in this portion of the experiment because of cost and work demands.
Microinjection/Microdialysis Procedure
The general procedures have been previously described (Deehan et al., 2013). Four to five 20-min baseline samples were collected prior to the start of microinjections. Microinjections were administered at a volume of 100 nl over a 5-sec period 3 times a minute (every 20 sec) during the first 10 min of the sample (30 microinjections total). This injection protocol mirrored self-administration for NIC into the pVTA (Hauser et al., 2014). Following microinjections, a minimum of 5 additional samples were collected. All lines were microinjected with aCSF, 10 or 100 μM NIC with additional groups of P and Wistar rats receiving 200 μM NIC.
Sample Analysis
Samples were analyzed for DA content using a reversed-phase high pressure liquid chromatography (HPLC) system as previously described (Engleman et al., 2006).
Statistical Analysis
The data are presented as a percent of baseline (average of final 3 baseline samples) value. Initial analysis consisted of a Rat line x Drug Concentration x Sample mixed analyses of variance (ANOVA). When significant effects (p<0.05) were observed using the mixed ANOVA’s, post hoc Tukey’s b or Student’s t-tests were utilized to determine differences between specific drug concentrations and across the time course of sample collection.
Experiment 3: Innate mRNA Levels in the pVTA of all Rat Lines
Subjects were naïve male P, NP, Wistar, HAD-1, HAD-2, LAD-1, and LAD-2 rats (n = 8/line).
Tissue Preparation and Micropunch for RT-PCR
All equipment and working surfaces were kept RNase free during dissection of the regions of interest. Frozen brains were sliced at 300 μm (coronal), using a Leica cryostat, and slices were placed onto microscope slides. The pVTA was dissected out of seven adjacent 300 μm coronal brain slices (from approximately −5.5 to −6.0 mm from bregma; Paxinos and Watson, 2005) using a 1.0-mm Harris Micro-punch (Electron Microscopy Sciences, Hatfeild, PA). The pVTA tissue was immediately placed into 75 μl of SurePrep™ TrueTotal™ RNA purification buffer (Fisher Scientific), and samples were vortexed and frozen on dry ice and kept at −80 °C overnight.
RNA was isolated using SurePrep™ TrueTotal™ RNA Purification Kit (Fisher Scientific). Total RNA was determined using Nanodrop 1000. Extracted RNA was then reverse transcribed using the GeneAmp Gold RNA PCR kit (Applied Biosystems) at the following reaction conditions: 2.5 μM Oligo-dT primer, 2.5 mM magnesium, 250 mM of each deoxynucleotide triphosphate, 0.5 U/ml of RNase inhibitor, and final concentration of 0.75 U/μl of MuLV reverse transcriptase. The reverse transcription conditions were 10 min at room temperature, 15 min at 42 °C, 10 min at 68 °C, and 5 min at 95 °C and produced approximately 25 μl of product.
The TaqMan® Low-Density Arrays (TMLDA) is a multiple rtPCR assay that quantifiably examines mRNA levels. We have previously used the TMLDA assay to assess the effects of EtOH and NIC microinjected into the pVTA on gene expression in the AcbSh (Truitt et al., 2015). The current TMLDA (Applied Biosystems) was an 18 acetylcholine gene array. The following acetylcholine related genes were assayed; Ache, Cdnf, Chat, Chrm1, Chrm2, Chrm3, Chrm4, Chrm5, Chrna2, Chrna3, Chrna4, Chrna5, Chrna6, Chrna7, Chrnb1, Chrnb2, Chrnb3, and Chrnb4. All subjects were adult males.
A cDNA sample (100 μl) isolated from the pVTA from each rat was added to an equal volume of TaqMan® universal PCR master mix (Applied Biosystems). After gentle mixing and centrifugation, the mixture was transferred to a loading port on a TMLDA card. The array was centrifuged twice for 1 min, each at 1,200 rpm, to distribute the samples from the loading port to each well. The card was then sealed and PCR amplification performed using an Applied Biosystems Prism 7900HT sequence detection system (equipped with a TaqMan® low-density array upgrade). Thermal cycler conditions were as follows: 2 min at 50 °C, 10 min at 94.5 °C, 30 s at 97 °C, and 1 min at 59.7 °C for 40 cycles. A common threshold was set for all genes using RQ manager software (ABI). The Ct values obtained from the RQ manager (ABI) were then imported into RealTime StatMiner v4.5 (Integromics) for further analyses.
Reference gene selection.
The TMLDAs included 18S, glyceraldehyde 3-phosphate dehydrogenase (Gapdh), hypoxanthine phosphoribosyltransferase 1 (Hprt1), ribosomal protein, large, P2 (Rplp2), and ubiquitin C (Ubc) as reference genes based on their proven role as housekeeping genes and their uniform expression in preliminary TMLDA rat endogenous control assays from rat pVTA(data not shown). The geNORM procedure, also known as the pairwise approach, included in the RealTime StatMiner (Integromics) software was utilized to determine the stability of the selected reference genes, as described previously (Vandesompele et al. 2002). Using this approach, the following genes were selected for normalization of gene expression levels: Gapdh, Hprt1, and Ubc.
Results
Experiment 1: Effects of NIC on LMA in Selectively Bred Rat Lines
Initial response to NIC
Methodological (male/female rats and the number of nicotine exposure days 7 vs 10) and behavioral (P/NP were more active than HAD/LAD rats) differences precluded performing an overall analysis on locomotor activity. In P and NP rats (data not shown) the analysis revealed a Dose x Line interaction (F2, 42 = 3.53; p = 0.038). Analyses revealed a significant effect of Dose in P (F2,21 = 4.36; p = 0.026) and NP (F2,21 = 4.73; p = 0.02) rats. Specifically, LMA was increased in P rats administered 0.5 mg/kg NIC compared to saline, whereas LMA was decreased in NP rats administered 0.25 or 0.5 mg/kg NIC. Analysis of the HAD/LAD data following an initial injection of NIC (data not shown) revealed a significant Line x Dose interaction (F6,77 = 4.0; p = 0.002). For HAD-1 and HAD-2 rats, injection of 0.5 mg/kg NIC increased LMA compared to saline and 0.25 mg/kg NIC groups (F values > 17.49; p values < 0.0001). There was no effect of NIC administration on the LMA of LAD-1 (F2,13 = 0.04; p = 0.96) or LAD 2 rats (F2,19 = 3.37; p = 0.07).
Response to repeated NIC injections
Repeated injections of NIC (Fig. 1) in P and NP rats produced a significant Day x Line x Dose interaction (F12,76 = 2.0; p = 0.031). P rats repeatedly treated with 0.5 mg/kg exhibited a significant increase in LMA across the days (F6,2 = 8.73; p = 0.029; specifically during the 4th and 7th days compared to the 1st day (t7 values > 3.0; p values < 0.019). In contrast, there were no effects of repeated NIC administration on LMA in the NP rats
Fig. 1.
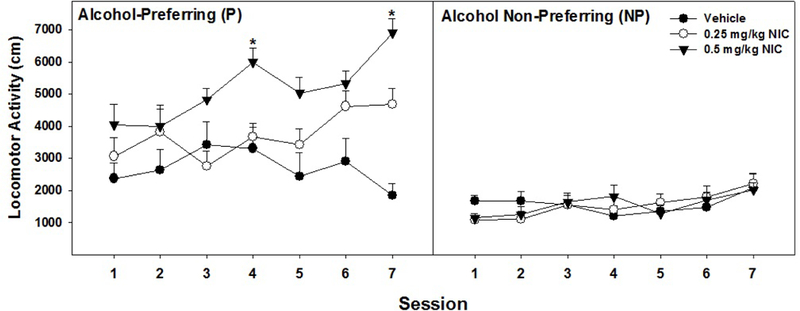
Displays the data for mean (+ SEMs) locomotor activity (LMA; cm traversed) by P and NP rats over the course of repeated (7 days) injections of 0.25, 0.5 mg/kg nicotine (NIC), or saline. * 0.5 mg/kg NIC injections in P rats caused significantly more LMA on days 4 and 7 compared to day 1 (p < 0.05). Sample size - P (n = 8) and NP (n = 8)
Similar to the P/NP study, the overall analysis in HAD/LAD rats indicated a significant Day x Line X Dose interaction (F 54, 1284 = 1.87; p < 0.001). Repeated injections of NIC (Fig. 2) produced a significant effect of Line on LMA for rats injected with 0.5 mg/kg NIC (F3,28 = 3.77; p = 0.022) as the HAD-1 and HAD-2 lines expressed greater 0.5 mg/kg NIC-induced LMA than LAD-1 rats. More specifically, for HAD-1 rats, 0.5 mg/kg NIC increased LMA compared to saline and 0.25 mg/kg NIC for all 10 sessions. For HAD-2 rats, repeated injections of 0.25 mg/kg significantly increased LMA during days 8–10 compared to day 1 as well as days 9 and 10 compared to day 2 (p values < 0.023). Additionally, injections of 0.5 mg/kg significantly increased LMA during days 8–10 compared to days 1 and 2 (p values < 0.037). In LAD-2 rats, repeated injections of saline produced a decrease in LMA (habituation) between sessions 1–3 compared to sessions 8–10 (t > 5.4; p values < 0.003). However, 0.5 mg/kg NIC increased LMA between the initial sessions compared to the last sessions (t values > 2.9; p values < 0.024) whereas 0.25 mg/kg NIC failed to alter LMA (p values > 0.09). Repeated injections of saline, 0.25 or 0.5 mg/kg NIC failed to dose dependently alter LMA in LAD-1 rats.
Fig. 2.
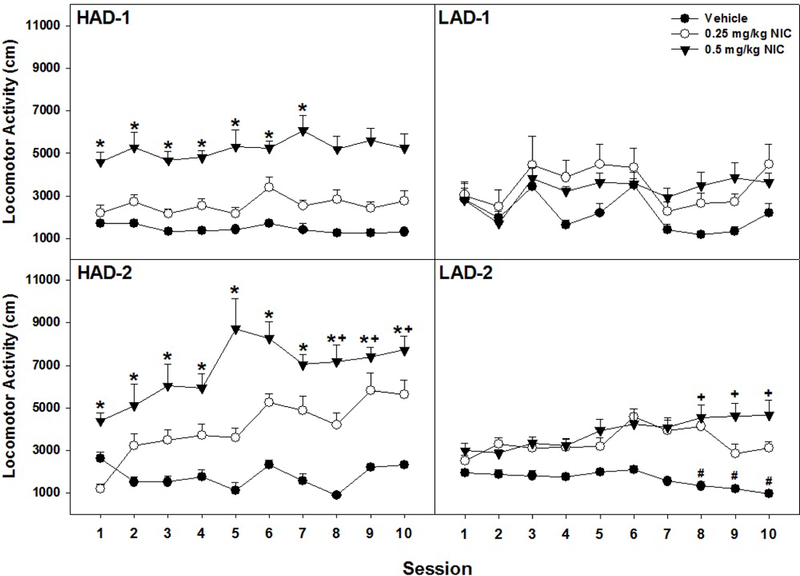
Shows the data for mean (+ SEMs) locomotor activity (LMA; cm traversed) by HAD-1 (upper left panel), LAD-1 (upper right panel), HAD-2 (lower left panel), and LAD-2 (lower right panel) rats during repeated (10 days) injections of saline, 0.25 mg/kg, or 0.5 mg/kg NIC. HAD-1: * 0.5 mg/kg NIC increased LMA in HAD-1 rats compared to LAD-1 and LAD-2 rats (p < 0.05). HAD-2: * 0.5 mg/kg NIC significantly enhanced LMA in HAD-2 compared to LAD-1 and LAD-2 rats. + repeated injections of 0.5 mg/kg NIC significantly increased LMA during sessions 8–10 compared to sessions 1 and 2 (p < 0.037). LAD-2: # repeated saline injections caused a significant decrease in LMA during sessions 8–10 compared to sessions 1–3 (p < 0.003). + repeated injections of 0.5 mg/kg NIC increased LMA in LAD-2 rats during sessions 8–10 compared to sessions 1–3 (p < 0.024). Sample size - HAD-1 (n = 28), HAD-2 (n = 23), LAD-1 (n = 16) and LAD-2 (n = 22).
Analysis across lines for animals receiving 0.5 mg/kg NIC yielded a significant effect of Line for all 10 Days (F3,28 values > 3.5, p values < 0.028). Post-hoc comparisons indicated that HAD-2 rats displayed significantly more LMA following injections of 0.5 mg/kg NIC than LAD-1 and LAD-2 rats across all 10 days. HAD-1 rats displayed significantly more LMA than LAD-1 rats for all 10 days and LAD-2 rats for 7 out of 10 days. There was no significant effect of Line in rats injected with 0.25 mg/kg for the 10 days (p values > 0.08). In rats injected with saline there was a significant effect of Line during days 3 and 5 (p values < 0.001), an effect driven by greater LMA in LAD-1 rats during those days.
Response to challenge injection of NIC
The challenge dose (0.5 mg/kg) of NIC administered 14 days after the last treatment session produced a significant Dose x Line interaction (F2, 42 = 4.36; p < 0.0001) in P/NP rats (Fig. 3; top panel). Basically, LMA in NP was still reduced following exposure to the NIC challenge dose, and the NIC challenge dose resulted in an increase in LMA in P rats with a previous history of exposure to 0.5 mg/kg NIC. In the HAD/LAD rats (Fig. 3; bottom panel), there was a significant Dose X Line interaction (F6,77 = 2.28; p = 0.045). Post-hoc comparisons indicated that HAD-1 and HAD-2 rats displayed significantly greater LMA during the challenge day than LAD-1 and LAD-2 rats. There was no significant effect of Line on LMA during the challenge day for rats previously injected with saline or 0.25 mg/kg. LAD-2 rats previously administered 0.25 or 0.5 mg/kg NIC displayed more LMA during the challenge day than saline controls. In contrast, there was no significant effect of previous Drug History on LMA during the challenge test in LAD-1 rats (F2,13 = 1.17; p = 0.34).
Fig. 3.
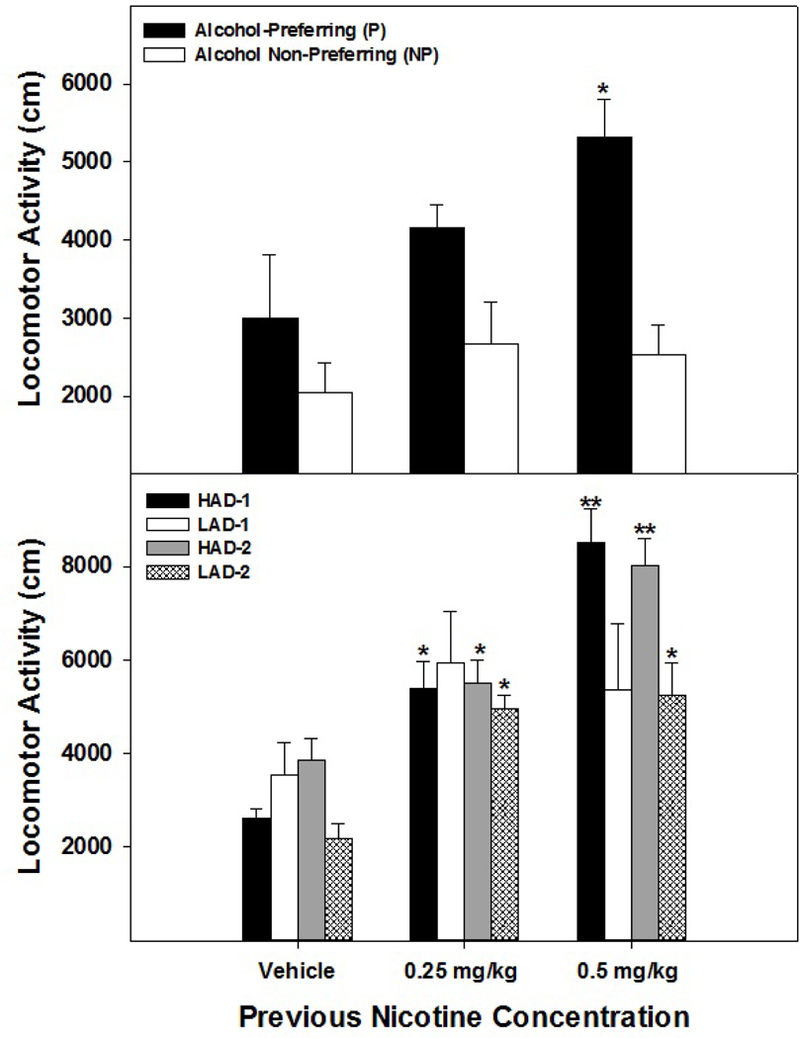
Represents data for mean (+ SEMs) locomotor activity (LMA; cm traversed) by P and NP (top panel) and HAD-1,2 and LAD-1,2 (bottom panel) rats following a challenge injection of 0.5 mg/kg NIC given approximately 14 days after repeated injections of 0.25, 0.5 mg/kg NIC or saline. Top panel: * the challenge injection of 0.5 mg/kg NIC significantly elevated LMA in P rats compared to their NP counterparts (p < 0.001). Bottom panel: * challenge injection of NIC significantly elevated LMA compared to saline controls (p < 0.0001). ** challenge injection of 0.5 mg/kg NIC significantly elevated LMA in HAD-1 and HAD-2 rats compared to LAD-1 and LAD-2 rats (p < 0.001).
Experiment 2: Effects of NIC microinfusions in the VTA on DA Efflux in the AcbSh
Extracellular basal dopamine estimates in the AcbSh for each rat line were as follows: P – 1.36 + 0.15, Wistar – 1.23 + 0.13, HAD-1 – 1.06 + 0.24, HAD-2 – 0.99 + 0.27, LAD-1 – 1.13 + 0.20, and LAD-2 – 1.05 + 0.22 μM. Probe recovery heavily influences estimated basal levels under standard microdialysis procedures (accurate basal levels can only be assessed utilizing a No-Net-Flux protocol).
The overall analysis (all 6 lines; Figs. 4 & 5) indicated a significant Time x Line x Location x NIC concentration interaction (F90,1854 = 1.35; p = 0.018). Analyses in which location was held constant revealed that there was a significant Time x Line x NIC concentration (F90,1854 = 2.83; p < 0.0001) in rats with cannula placements in the pVTA, but no significant effects for rats with placements in the aVTA (p values > 0.26; data not shown). To reduce redundancy in statistical analysis, the significant 3-way interaction term has been decomposed into an examination between high alcohol drinking rat line and the corresponding low alcohol drinking parallel line.
Fig. 4.
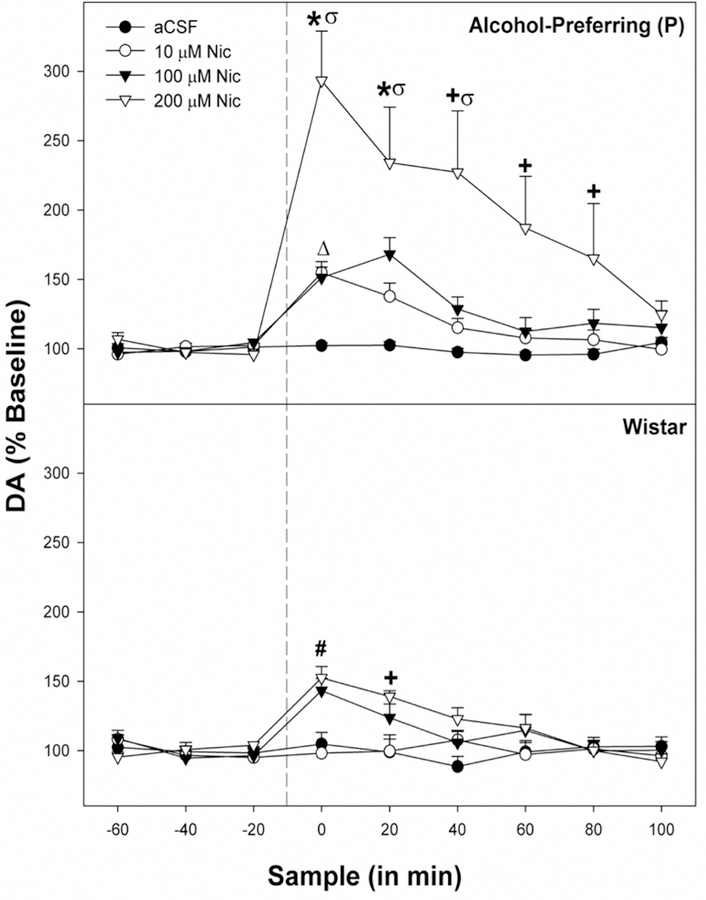
Displays the mean (+ SEMs) extracellular levels of DA for P (top panel) and Wistar rats (bottom panel) in the nucleus accumbens shell (AcbSh) following pulse microinjections of aCSF, 10 uM, 100 uM, or 200 uM NIC into the posterior ventral tegmental area (pVTA; n = 7–9/line/concentration). * indicates that in P rats DA levels are higher in the 10 and 100 μM Nic groups compared to baseline, and rats administered 200 μM Nic have higher DA levels than all other groups . + indicates that rats administered 200 μM Nic have higher DA levels than all other groups. # indicates that in Wistar rats administration of 100 μM Nic resulted in elevated DA compared to aCSF controls, and 200 μM Nic administered rats had higher DA levels compared to aCSF and 10 μM Nic rats. Δ indicates that increase in DA% in the AcbSh in P rats microinjected with 10 μM NIC into the pVTA was significantly greater than that observed with Wistar rats. σ indicates that increase in DA% in the AcbSh in P rats microinjected with 200 μM Nic into the pVTA was significantly greater than that observed with Wistar rats.
Fig. 5.
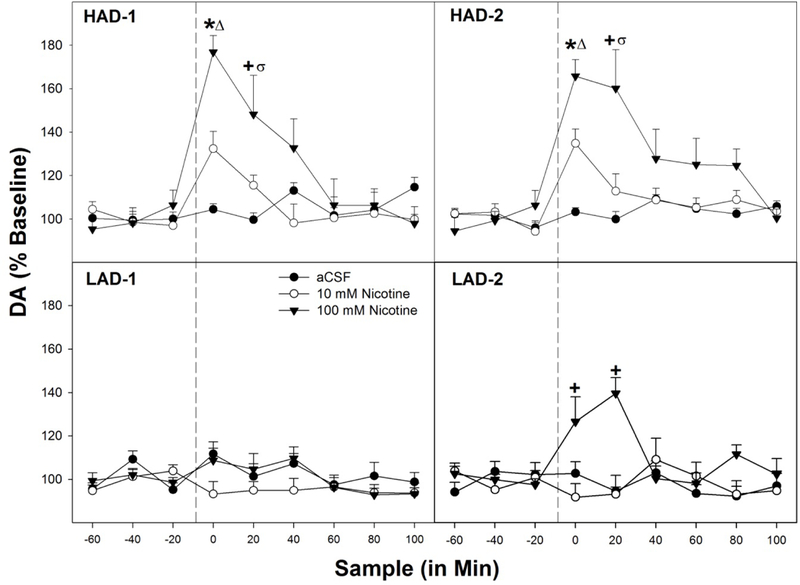
Displays the mean (+ SEMs) extracellular levels of DA for HAD (top two panels) and LAD rats (bottom two panels) in the nucleus accumbens shell (AcbSh) following microinjections of aCSF, 10 μM, or 100 μM NIC into the posterior ventral tegmental area (pVTA; n = 7–9/line/concentration). * indicates that all groups are different from each other (p’s < 0.02). + indicates that rats administered 100 μM Nic have higher DA levels than those receiving 10 μM NIC or aCSF (p’s < 0.019). Δ indicates that increase in DA% in the AcbSh in HAD-1 and HAD-2 rats microinjected with 10 or 100 μM NIC into the pVTA was significantly greater than that observed LAD-1 or LAD-2 (corresponding with selective rat line) rats. σ indicates that increase in DA% in the AcbSh HAD-1 and HAD-2 rats microinjected with 100 μM NIC into the pVTA was significantly greater than that observed LAD-1 or LAD-2 (corresponding with selective rat line) rats.
P vs Wistar Analysis
For the P line, there was a significant Time x NIC concentration interaction (F27,63 = 2.34; p = 0.003; Fig. 4). Individual ANOVAs revealed a significant effect of NIC concentration during the 1st – 3rd sample period following microinjection (F3,27 values > 5.0; p values < 0.007). During the 1st and 2nd sample following NIC microinjection DA levels in the AcbSh were higher in P rats administered 10 and 100 μM NIC compared to the aCSF. Additionally, during the 1st – 3rd sample following administration of 200 μM NIC in P rats, DA levels in the AcbSh were significantly greater than all other groups. Wistar rats also exhibited a significant Time x NIC concentration interaction (F27,63 = 3.14; p < 0.001). Analyses revealed a significant effect of NIC concentration during the 1st and 2nd post-injection samples (F3,27 values > 7.159; p values < 0.001). Specifically, during the 1st post-injection sample, 100 μM NIC produced significantly higher DA efflux than aCSF. Animals receiving 200 μM NIC exhibited significantly higher DA levels than aCSF and 10 μM groups during the 1st and 2nd post-injection samples (p < 0.05). Between line comparisons (P vs Wistar) yielded significant elevations in DA release for P rats administered 10 μM NIC during the 1st post-injection sample (F1,13 = 4.78; p = 0.041). Microinjections of 200 μM NIC, elevated DA extracellular levels during the 1st – 3rd post-injection samples (F1,13 values > 4.95; p = 0.037) compared to the Wistar groups.
HAD-1 vs LAD-1 Analysis
For HAD-1 rats (Fig. 5), there was a significant Time x NIC concentration interaction term (F18,24 = 2.1; p = 0.041). Analyses revealed a significant increase in extracellular DA levels within the AcbSh during the 1st and 2nd post-injection samples (F2,19 values > 4.855; p values < 0.02). Post-hoc comparisons revealed that, for the 1st post-injection sample, DA levels in the AcbSh of the groups were significantly different from each other. During the 2nd post-injection sample, 100 μM NIC significantly elevated DA levels compared to the aCSF group. Between line comparison (HAD-1 vs LAD-1) yielded significant elevations in DA levels for HAD-1 rats following microinjections of 10 or 100 μM NIC during the 1st post-injection sample (p values < 0.004) and following microinjections of 100 μM NIC during the 2nd post-injection sample (p < 0.001) compared to their LAD-1 counterparts.
HAD-2 vs LAD-2 Analysis
For HAD-2 rats (Fig. 5) there was also a significant Time x NIC concentration interaction (F18,24 = 2.14; p = 0.041). Individual ANOVAs indicated that there was a significant effect of NIC concentration administered on extracellular DA levels in the AcbSh in HAD-2 rats during the 1st and 2nd time period following microinjections (F2,19 values > 4.91; p values < 0.019). Post-hoc comparisons indicated that during these two time periods all groups (aCSF, 10 and 100 μM NIC) were statistically different from each other. Unlike the LAD-1 rats, LAD-2 rats displayed a significant Time x NIC concentration interaction (F18,18 = 2.77; p = 0.023). Follow-up analyses determined there was a significant effect of NIC concentration administered on DA levels in the AcbSh in LAD-2 rats during the 1st and 2nd time periods following microinjections (F2,17 values > 8.59; p values < 0.003). Post-hoc comparisons indicated that during these two time periods the 100 μM NIC group was statistically different from the aCSF and 10 μM NIC groups. Between line comparisons (HAD-2 and LAD-2) yielded significant elevations in DA levels in for HAD-2 rats 10 or 100 μM NIC during the 1st post-injection sample (p values < 0.007) and following microinjections of 100 μM NIC during the 2nd post-injection sample (p < 0.019) compared to their LAD-2 counterparts.
Area under the Curve Analysis – All Rat Lines
A standard Area under the Curve (AUC) analysis was performed in all rats (6 lines) microinjected with 100 μM NIC directly into the pVTA with linear value derived from extracellular DA levels in the AcbSh (data not shown). The AUC analysis (y = f(x)) is an x-axis functional analysis that provides a definite integral of a value across a certain independent variable (e.g., time) in which the trapezoidal rule is applied to obtain a quantitative value (AUC). An ANOVA indicated a significant effect of Line (F5,46 = 36.8; p < 0.0001). Post-hoc comparisons (Tukey’s b) indicated that P, HAD-1, and HAD-2 were significantly higher than Wistar, LAD-1, and LAD-2 (no other differences).
Experiment 3: Innate mRNA Levels in the pVTA of all Rat Lines
P – Wistar- NP Comparison
All TMLDA data were normalized to a log10 base for analysis (Fig. 6 and Table 1). Statistically, there was a significant effect of rat line for the expression of the muscarinic 4 receptor (F2, 15 = 8.97; p = 0.03) and the α2, α3, α5, and β4 subunits of the nicotinic receptor (F2, 15 values > 13.29; p values = 0.0.0001, 0.002, 0.007, 0.0001, respectively). Post-hoc comparisons (SNK) indicated that expression of chrm4 was significantly reduced in the NP rats (different from all other groups). There were two genes in which expression was reduced in both the P and NP rat lines compared to the Wistar controls (chrna3 and chrna5). Of particular interest were the two nicotinic receptor subunits that were divergently expressed (P rat increased, NP rats decreased) compared to the Wistar rats, and each other (chrna2 and chrb4).
Fig. 6.
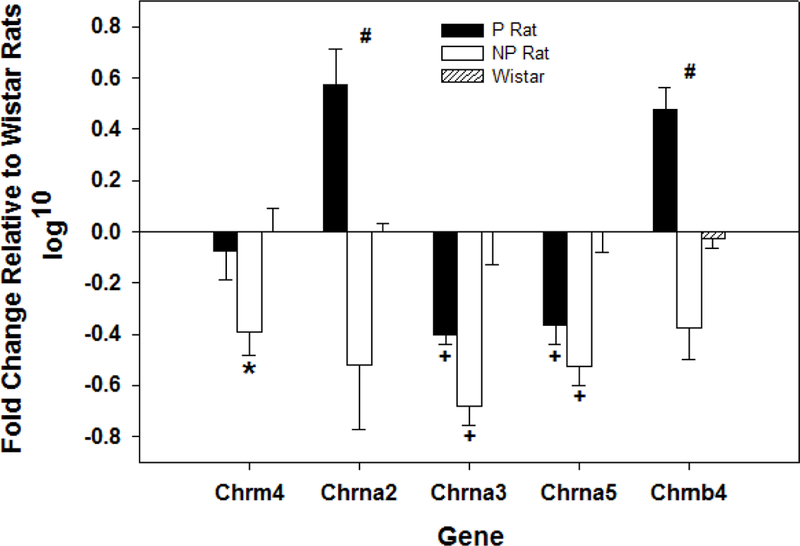
Displays the mean (+ SEMs) of the log10 transformation of the RT-PCR detection of acetylcholine related genes that were significantly different in P, NP, and Wistar (controls) rat lines in the posterior VTA using the TMLDA platform (n = 8/line). * indicates significantly different from all other groups. # indicates that all groups are statistically different from each other. + indicates that both the P and NP rats are statistically different from Wistar.
Table 1.
Exhibits the gene change data (with SEM) for all genes measured within the pVTA of P, Wistar and NP rats. All data is presented as fold-change compared to Wistar gene expression. Sig indicates statistically significant results which are portrayed in Figure 6 (n = 8/line).
| Rat Line | |||||||
|---|---|---|---|---|---|---|---|
| P | SEM | Wistar | SEM | NP | SEM | ||
| Gene | |||||||
| Ache | 0.05 | 0.04 | 0 | 0.03 | −0.04 | 0.03 | |
| Cdnf | −0.002 | 0.05 | 0 | 0.07 | 0.003 | 0.063 | |
| Chat | −0.23 | 0.17 | 0 | 0.137 | −0.112 | 0.186 | |
| Chrm1 | 0.069 | 0.064 | 0 | 0.155 | −0.018 | 0.074 | |
| Chrm2 | 0.005 | 0.004 | 0 | 0.225 | 0.046 | 0.057 | |
| Chrm3 | 0.046 | 0.006 | 0 | 0.09 | −0.034 | 0.039 | |
| Chrm4 | sig | sig | sig | sig | sig | sig | |
| Chrm5 | −0.059 | 0.052 | 0 | 0.095 | −0.079 | 0.052 | |
| Chrna2 | sig | sig | sig | sig | sig | sig | |
| Chrna3 | sig | sig | sig | sig | sig | sig | |
| Chrna4 | −0.078 | 0.023 | 0 | 0.085 | −0.074 | 0.036 | |
| Chrna5 | sig | sig | sig | sig | sig | sig | |
| Chrna6 | −0.024 | 0.077 | 0 | 0.078 | −0.116 | 0.08 | |
| Chrna7 | −0.048 | 0.033 | 0 | 0.058 | −0.055 | 0.029 | |
| Chrnbl | −0.183 | 0.059 | 0 | 0.113 | −0.006 | 0.303 | |
| Chrnb2 | −0.016 | 0.059 | 0 | 0.063 | −0.077 | 0.054 | |
| Chrnb3 | −0.015 | 0.05 | 0 | 0.083 | −0.022 | 0.036 | |
| Chrnb4 | sig | sig | sig | sig | sig | sig | |
HAD-LAD Comparison
Statistically, there was a significant effect of rat line for the expression of the muscarinic 2 receptor and the α3 and α7 receptor subunit of the nicotinic receptor (F3, 22 values > 13.3; p values = 0.0001, 0.002, and 0.01, respectively; Fig. 7 and Table 2). Post-hoc comparisons (SNK) indicated that the expression of the chrm2 was significantly reduced in the HAD-1 rats compared to all other rats. The HAD-2 rat lines had a significant reduction in the expression of the chrna3 compared to the other rat lines. Expression of the chrna7 receptor subunit was increased in both HAD-1 and HAD-2 rat lines compared to both the LAD-1 and LAD-2 rat lines.
Fig. 7.
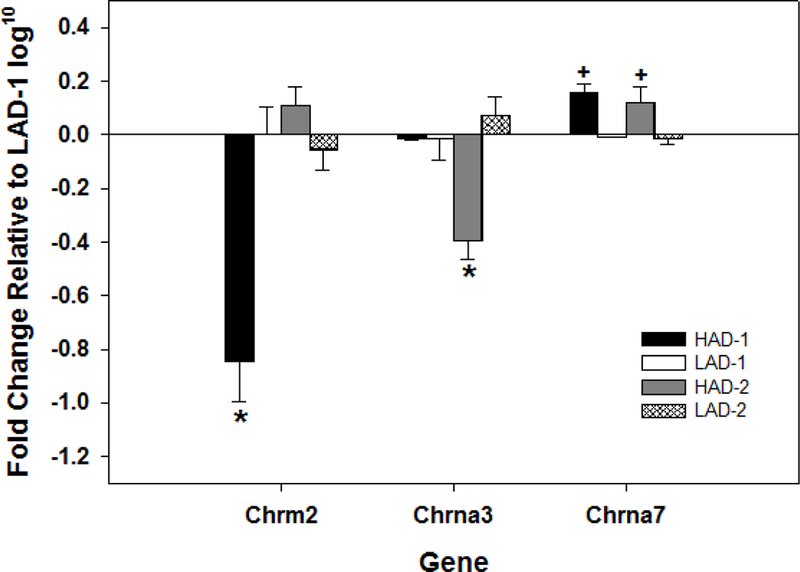
Displays the mean (+ SEMs) of the log10 transformation of the RT-PCR detection of acetylcholine related genes that were significantly different in HAD-1, LAD-1 (controls), HAD-2, and LAD-2 rat lines in the posterior VTA using the TMLDA platform (n = 8/line). * indicates significantly different from all other groups. + indicates that the HAD-1 and HAD-2 rats were significantly different from LAD-1 and LAD-2 rats.
Table 2.
Displays gene change data (with SEM) for all genes measured within the pVTA of both replicate lines of HAD and LAD rats. All data is presented as fold-change compared to gene expression in the LAD-1 rat line. Sig indicates significant results which are portrayed in Figure 7 (n = 8/line).
| Rat Line | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| HAD-1 | SEM | LAD-1 | SEM | HAD-2 | SEM | HAD-2 | SEM | ||
| Gene | |||||||||
| Ache | −0.057 | 0.025 | 0 | 0.057 | −0.042 | 0.075 | −0.014 | 0.046702 | |
| Cdnf | −0.023 | 0.112 | 0 | 0.131 | −0.033 | 0.085 | −0.046 | 0.120046 | |
| Chat | −0.058 | 0.145 | 0 | 0.191 | 0.057 | 0.065 | −0.085 | 0.154241 | |
| Chrm1 | −0.033 | 0.073 | 0 | 0.171 | −0.061 | 0.77 | −0.044 | 0.053875 | |
| Chrm2 | sig | sig | sig | sig | sig | sig | sig | sig | |
| Chrm3 | 0.009 | 0.066 | 0 | 0.078 | −0.078 | 0.059 | −0.078 | 0.048333 | |
| Chrm4 | −0.068 | 0.045 | 0 | 0.064 | −0.039 | 0.116 | 0.031 | 0.160217 | |
| Chrm5 | −0.035 | 0.096 | 0 | 0.108 | −0.015 | 0.07 | −0.017 | 0.085844 | |
| Chrna2 | −0.019 | 0.059 | 0 | 0.293 | −0.078 | 0.055 | −0.069 | 0.31085 | |
| Chrna3 | sig | sig | sig | sig | sig | sig | sig | sig | |
| Chrna4 | −0.012 | 0.11 | 0 | 0.078 | −0.045 | 0.03 | −0.075 | 0.038402 | |
| Chrna5 | −0.015 | 0.165 | 0 | 0.082 | −0.071 | 0.067 | −0.055 | 0.07058 | |
| Chrna6 | −0.053 | 0.119 | 0 | 0.039 | −0.038 | 0.033 | −0.11272 | 0.096165 | |
| Chrna7 | sig | sig | sig | sig | sig | sig | sig | sig | |
| Chrnb1 | −0.061 | 0.096 | 0 | 0.064 | −0.048 | 0.075 | −0.118 | 0.088 | |
| Chrnb2 | −0.063 | 0.067 | 0 | 0.057 | −0.046 | 0.066 | −0.098 | 0.0464 | |
| Chrnb3 | −0.073 | 0.161 | 0 | 0.069 | −0.064 | 0.13 | −0.165 | 0.112 | |
| Chrnb4 | −0.057 | 0.059 | 0 | 0.169 | −0.039 | 0.164 | −0.083 | 0.047 | |
Discussion
High alcohol-consuming rat lines were more responsive to nicotine than the low alcohol-consuming rat lines or unselected Wistar rats. The pleiotropic association between genetic predisposition to consume/prefer ethanol and enhanced responsiveness to the effects of nicotine was evident during systemic assessment (Figs. 1–3) and in specific neuroanatomical sites mediating drug reward (Figs. 4–5; for full summary see Table 3). Specifically, acute nicotine increased LMA in all 3 selectively bred rat lines compared to the low alcohol-consuming lines. Utilizing an identical experimental protocol, Wistar rats required 6, while Sprague-Dawley rats required 5, daily injections to observe a NIC-induce increase in LMA (Bracken et al., 2011, Berg & Chambers, 2008). In other experimental protocols, unselected rat lines do not express an NIC-induced LMA following the initial exposure to NIC (Bracken et al., 2011, Berg & Chambers, 2008). P and HAD-2 rats rapidly develop sensitization to NIC-induced LMA (day 4 and 7). Thus, in outbred rats, NIC sensitization is the expression of NIC-induced LMA, while in the P and HAD rats NIC sensitization is a significant increase to an already established behavior. These two observations could be different effects. A limitation in this assertion is that male rats were used for the P/NP comparison while female rats were used for the HAD/LAD component.
Table 3.
Summarizes the significant changes to locomotor activity (Exp. 1) and DA efflux in the AcbSh (Exp. 2) between the different rat lines examined as well as the direction of change following NIC administration. Comparison groups are included. For time course changes in DA efflux (Exp. 2) please refer to figures 5 and 6.
| Rat Line | |||||||
|---|---|---|---|---|---|---|---|
| Dose: | Wistar | P | NP | HAD1 | LAD1 | HAD2 | LAD 2 |
| Acute NIC (i.p.) | Peripheral Injections (s.c.) of NIC - Locomotor Activity | ||||||
| Saline (Sal) | --- | --- | --- | --- | --- | --- | --- |
| 0.25 mg/kg | --- | ↑ NP | ↓ Sal | --- | --- | --- | --- |
| 0.5 mg/kg | --- | ↑ Sal; NP | ↓ Sal | ↑Sal, 0.25; LAD1 | --- | ↑Sal, 0.25; LAD2 | --- |
| Repeated NIC (i.p.) | |||||||
| Saline | --- | --- | --- | --- | --- | --- | ↓ Day 8–10 vs. 1–3 |
| 0.25 mg/kg | --- | --- | --- | --- | --- | --- | --- |
| 0.5 mg/kg | --- | ↑ Day 4, 7 T vs. 1; NP | --- | ↑ Sal, 0.25; Day 1–10; LAD1&2 | --- | ↑ Sal, 0.25; Day 8–10 vs. 1–2 LAD1&2 | ↑ Day 8–10 vs. 1–3 |
| Challenge NIC (i.p.) | |||||||
| Saline | --- | --- | --- | -- | --- | --- | --- |
| 0.25 mg/kg | --- | --- | --- | ↑ SAL | --- | ↑ SAL | ↑ SAL |
| 0.5 mg/kg | --- | ↑ NP | --- | ↑ SAL, 0.25; LAD1&2 | --- | ↑ SAL, 0.25 LAD1&2 | ↑ SAL |
| Concentration: | Microinjections of NIC in the pVTA - DA efflux in the AcbSh | ||||||
| aCSF | --- | --- | -- | --- | --- | --- | -- |
| 10 uM NIC | --- | ↑ Base; aCSF; Wistar | --- | ↑ Base; aCSF; LAD1 | --- | ↑ Base; aCSF; LAD2 | --- |
| 100 uM NIC | ↑ Base; aCSF | ↑ Base; aCSF | --- | ↑ Base; aCSF, 10; LAD1 | --- | ↑ Base; aCSF,10; LAD2 | ↑ Base; aCSF, 10 |
| 200 uM MC | ↑ Base; aCSF, 10 | ↑ Base; aCSF, 10, 100; Wistar | --- | --- | --- | --- | --- |
The data clearly indicate that selective breeding for high alcohol preference has resulted in an innate reward system that is more responsive to NIC (Figs. 4–5). The pVTA supports the self-administration of ethanol and/or NIC (Rodd-Henricks et al., 2000, Rodd et al., 2004a, Truitt et al., 2015). Previous data have indicated that P rats self-administer NIC at lower concentrations and at a higher rate than Wistar rats into the pVTA (Hauser et al., 2014). Convergently, extracellular DA levels in the P rats are increased by microinjections of 10 μM NIC in the pVTA compared to Wistar rats (Fig. 4). The data suggest that the willingness of P rats to self-administer lower concentrations of NIC into the pVTA may be based upon a corresponding increase in DA in the AcbSh. Similar data (Fig. 5) were observed in the HAD-1/HAD-2 rat lines compared to their selective counterparts (LAD-1/LAD-2). Of note, the anterior VTA does not support NIC self-administration (Ikemoto et al., 2006), and there is no alteration in extracellular DA levels in the AcbSh following NIC microinjected into this region (data not shown).
The pleiotropic association between ethanol and NIC has been reported in other rodent models. Mice selected for high EtOH-induced LMA are more sensitive to the locomotor stimulatory effects of morphine, cocaine, methamphetamine, and nicotine than mice selected for low EtOH-induced LMA (Bergstrom et al., 2003). Furthermore, reverse selection of these mice indicated that there was a parallel loss in responsiveness to EtOH and other drugs of abuse (Bergstrom et al., 2003). Long-sleep mice are more sensitive than short-sleep mice to the actions of nicotine in several behavioral paradigms (De Fiebre et al., 1987). This effect is also observed in rats (cross species effect) since long-sleep rats are more responsive to NIC compared to short-sleep rats (De Fiebre et al., 2002). Therefore, multiple lines of research indicate that a phenotypic trait associated with selective breeding for high responsiveness to EtOH results in a corresponding augmented response to NIC.
Examining the innate expression of acetylcholine related genes in the pVTA between P, NP, and Wistar rats indicated potential biological basis for the divergent behavioral and neurochemical response between the rat lines (Fig. 6). NP rats have a reduction in the expression of M4 AchRs. Alterations in the M4 receptor have been linked to abnormal social behaviors, altered prepulse inhibition, and a reduced cocaine-induced LMA (Koshimizu et al., 2012; Stein & Hell, 2010). Acute NIC-induced LMA sedation may be related to M4 expression. The chrna2 gene was divergently expressed in the P (increased) and NP (decreased) rat lines compared to Wistar controls. The α2 nAchR has been associated with alcohol dependence and the much broader antisocial drug dependence in adolescence (Corley et al., 2008). Increased expression of chrna2 is linked with heavy cigarette use (Cannon et al., 2014). The α5, α3, β4 receptor subunits are clustered on the same chromosome and have been linked with amount of daily NIC use (Furberg et al., 2010). In addition, genetic expression of the CHRNA5/A3/B4 cluster is linked to various nicotine phenotypes including likelihood to relapse and craving (Shmulewitz et al., 2016; Ware et al., 2011) and (in particular variants of the β4 receptor) associated with a genetic predisposition to alcoholism (Hallfors et al., 2013). Alterations in the α5, α3, β4 receptor subunits have been shown to modify the response of VTA DA neurons to drugs of abuse (Morel et al., 2014), which could be the biological basis for the neurochemical response difference between P and Wistar rats.
Contrasting the innate expression of acetylcholine related genes between HAD-1, HAD-2, LAD-1, and LAD-2 rats revealed significant differences (Fig. 7). HAD-1 rats had significantly lower expression of the M2 acetylcholine receptor than all other groups. The M2 receptor is associated with an increased risk of substance disorders, major depressive disorder, and college binge drinking (Bauer & Ceballos, 2014). Only P and HAD-2 rats had a reduction in the genetic expression of the α3 subunit in the pVTA (HAD-1 did not). The α3 subunit mediates the development of sensitization to NIC-induced and EtOH-induced LMA (Kamens et al., 2009). Only P and HAD-2 rats expressed the development of sensitization to NIC-induced LMA (Figs. 2 and 3). Therefore, in rats predispose to consume high amounts of alcohol, a reduction in the expression of the α3 subunit in the pVTA is correlated with a propensity to develop sensitization to NIC-induced LMA. Enthusiasm for the α3 subunit finding is reduced because the NP rat line (no evidence of NIC-induced LMA) had the largest significant reduction in expression (Fig. 6).
Both HAD-1 and HAD-2 rats had increased expression of the α7 receptor subunit in the pVTA. The α7 receptors are located presynaptically on glutamate neurons that innervate DA neurons in the pVTA (Mansvelder et al., 2002) which may have contributed to the observed increase in dopaminergic response to NIC. Yet, the increase in α7 receptor subunit was not observed in P rats (Table 1). Recent unpublished work from our laboratory (Knight et al) has indicated that microinjection of an α7 receptor subunit into the pVTA stimulates DA release in the AcbSh. Therefore, it is possible that increase in sensitivity and response to NIC in the HAD-1 and HAD-2 rats may be linked to this receptor. Overall, the PCR data sets indicate similarities between all selectively bred high alcohol consuming rats (selective enhancement in expression of nicotine receptors compared to control rats), but lack a single consistent target for the behavioral and biochemical effects. These findings could be predicated upon the rat lines being derived from different founding stock (Wistars – P/NP, NIH HGS – HAD/LAD) and from the genetic pressure from selection. It is possible that selective breeding for high alcohol consumption predispose an ‘enhance’ response to NIC compared to outbred stock of rats, and that selective breeding for low alcohol consumption has produced animals insensitive to NIC (NP and LAD-1 specifically). This bidirectional effect of selection may be the cause of the muddled genetic findings. It important to note that there was no single, consistent genetic factor in the pVTA in the rat lines selected for high alcohol consumption. This could the result of 2 factors; 1) there are multiple manners to alter a system that would have a similar behavioral effect and/or 2) given the limited number of genes assessed, the current study failed to examine the consistent genetic factor in all lines selectively bred for high alcohol consumption.
Alterations within the reward pathway may predispose an individual to exhibit addictive disorder(s) toward natural rewards and drugs of abuse (Hall et al., 2014). The current experiments extend and verify the overall consensus in the literature that selective breeding for an alcohol phenotype produces animals with an enhanced response to NIC. Recent meta-analyses have indicated that the CHRNA5/A3/B4 and the α2 subunit are associated with this common genetic predisposition to addiction (Buhler et al., 2015). These critical genes were altered in the P rat compared to both Wistar and NP rats (β4 and α2). Yet, it may not be specific genes but classes of genes that are more important, as the current results reflect this convergence of genetic selection on the nAchR system within the pVTA. Therefore, it appears that genetic associated differences in, and/or drug-induced changes of the pVTA-nAChR system may provide molecular targets for pharmacogenetic medications screening/development to treat AD and/or ND.
Acknowledgments:
Supported by NIAAA grants: AA07611, AA07462, AA020908, AA024612, AA019366, and AA012262
Footnotes
Disclosure of conflict of interests: All authors listed herein have no conflicts of interest to disclose.
References
- Bauer LO & Ceballos NA (2014) Neural and genetic correlates of binge drinking among college women. Biol Psychol 97: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Hauser S, Rodd ZA, Liang T, Sari Y, McClintick J, Rahman S, Engleman EA (2016) A genetic animal model of alcoholism for screening medications to treat addiction. Int Rev Neurobiol 126: 179–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg SA & Chambers RA (2008) Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharmacology 54: 1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, Palmer AA, Wood RD, Burkhart-Kasch S, Mckinnon CS & Phillips TJ (2003) Reverse selection for differential response to the locomotor stimulant effects of ethanol provides evidence for pleiotropic genetic influence on locomotor response to other drugs of abuse. Alcohol Clin Exp Res 27: 1535–1547. [DOI] [PubMed] [Google Scholar]
- Bracken AL, Chambers RA, Berg SA, Rodd ZA & Mcbride WJ (2011) Nicotine exposure during adolescence enhances behavioral sensitivity to nicotine during adulthood in Wistar rats. Pharmacol Biochem Behav 99: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N (1995) Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet 25: 95–101. [DOI] [PubMed] [Google Scholar]
- Buhler KM, Gine E, Echeverry-Alzate V, Calleja-Conde J, De Fonseca FR & Lopez-Moreno JA (2015) Common single nucleotide variants underlying drug addiction: more than a decade of research. Addict Biol 20: 845–871. [DOI] [PubMed] [Google Scholar]
- Cannon DS, Mermelstein RJ, Hedeker D, Coon H, Cook EH, Mcmahon WM, Hamil C, Dunn D & Weiss RB (2014) Effect of neuronal nicotinic acetylcholine receptor genes (CHRN) on longitudinal cigarettes per day in adolescents and young adults. Nicotine Tob Res 16: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley RP, Zeiger JS, Crowley T, Ehringer MA, Hewitt JK, Hopfer CJ, Lessem J, Mcqueen MB, Rhee SH, Smolen A, Stallings MC, Young SE & Krauter K (2008) Association of candidate genes with antisocial drug dependence in adolescents. Drug Alcohol Depend 96: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fiebre CM, Medhurst LJ & Collins AC (1987) Nicotine response and nicotinic receptors in long-sleep and short-sleep mice. Alcohol 4: 493–501. [DOI] [PubMed] [Google Scholar]
- De Fiebre NC, Dawson R Jr. & De Fiebre CM (2002) The selectively bred high alcohol sensitivity (HAS) and low alcohol sensitivity (LAS) rats differ in sensitivity to nicotine. Alcohol Clin Exp Res 26: 765–772. [PubMed] [Google Scholar]
- Deehan GA Jr., Engleman EA, Ding ZM, Mcbride WJ & Rodd ZA (2013) Microinjections of acetaldehyde or salsolinol into the posterior ventral tegmental area increase dopamine release in the nucleus accumbens shell. Alcohol Clin Exp Res 37: 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA Jr., Hauser SR, Waeiss RA, Knight CP, Toalston JE, Truitt WA, Mcbride WJ & Rodd ZA (2015) Co-administration of ethanol and nicotine: the enduring alterations in the rewarding properties of nicotine and glutamate activity within the mesocorticolimbic system of female alcohol-preferring (P) rats. Psychopharmacology (Berl) 232: 4293–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difranza JR & Guerrera MP (1990) Alcoholism and smoking. J Stud Alcohol 51: 130–135. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, Mcbride WJ, Lumeng L & Murphy JM (2006) Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol 38: 5–12. [DOI] [PubMed] [Google Scholar]
- Furberg H, Ostroff J, Lerman C & Sullivan PF (2010) The public health utility of genome-wide association study results for smoking behavior. Genome Med 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS & Dawson DA (2004) Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 61: 1107–1115. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MN, Weinstein A, Gorelick DA (2011) Introduction to behavioral addictions. Am J Drug Alcohol Abuse 36: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GB, Milne AM & Macqueen GM (2014) An fMRI study of reward circuitry in patients with minimal or extensive history of major depression. Eur Arch Psychiatry Clin Neurosci 264: 187–198. [DOI] [PubMed] [Google Scholar]
- Hallfors J, Loukola A, Pitkaniemi J, Broms U, Mannisto S, Salomaa V, Heliovaara M, Lehtimaki T, Raitakari O, Madden PA, Heath AC, Montgomery GW, Martin NG, Korhonen T & Kaprio J(2013) Scrutiny of the CHRNA5-CHRNA3-CHRNB4 smoking behavior locus reveals a novel association with alcohol use in a Finnish population based study. Int J Mol Epidemiol Genet 4: 109–119. [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Bracken AL, Deehan GA Jr., Toalston JE, Ding ZM, Truitt WA, Bell RL, Mcbride WJ & Rodd ZA (2014) Selective breeding for high alcohol preference increases the sensitivity of the posterior VTA to the reinforcing effects of nicotine. Addict Biol 19: 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D, Pickel VM, Joh TH, Beaudet A (1987) Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res 435:71–83. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Qin M & Liu ZH (2006) Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci 26: 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Pandey AK, Chorlian DB, Manz N, Stimus AT, Bauser LO, Hesselbrock VM, Schuckit MA, Kuperman S, Kramer J, Porjesz B (2015) Reward processing deficits and impulsivity in high-risk offspring of alcoholics: A study of event-related potentials during a onetary gambling task. Int J Psychophysiol 98: 182–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Mckinnon CS, Li N, Helms ML, Belknap JK & Phillips TJ (2009) The alpha 3 subunit gene of the nicotinic acetylcholine receptor is a candidate gene for ethanol stimulation. Genes Brain Behav 8: 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu H, Leiter LM & Miyakawa T (2012) M4 muscarinic receptor knockout mice display abnormal social behavior and decreased prepulse inhibition. Mol Brain 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK & Shaham Y (2006) Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci 26: 1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR & Mcgehee DS (2002) Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33: 905–919. [DOI] [PubMed] [Google Scholar]
- Morel C, Fattore L, Pons S, Hay YA, Marti F, Lambolez B, De Biasi M, Lathrop M, Fratta W Maskos U & Faure P (2014) Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol Psychiatry 19: 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH & Hurd YL (2015) Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16: 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 2005. The Rat Brain in Stereotaxic Coordinates, 5th ed. [DOI] [PubMed] [Google Scholar]
- Pert A, Post R, Weiss SR (1990) Conditioning as a critical determinant of sensitization induced by psychomotor stimulants. NIDA Res Monogr 97: 208–41. [PubMed] [Google Scholar]
- Research RIFLA 2011. Guide for the Care and Use of Laboratory Animals. 8th ed. [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li TK, Murphy JM & Mcbride WJ (2004a) Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring and Wistar rats. Alcohol Clin Exp Res 28: 1212–1219. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, Li TK & Mcbride WJ (2005) Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology 30: 330–338. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM & Mcbride WJ (2004b) Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci 24: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Mckinzie DL, Crile RS, Murphy JM & Mcbride WJ (2000) Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 149: 217–224. [DOI] [PubMed] [Google Scholar]
- Shmulewitz D, Meyers JL, Wall MM, Aharonovich E, Frisch A, Spivak B, Weizman A, Edenberg HJ, Gelernter J & Hasin DS (2016) CHRNA5/A3/B4 Variant rs3743078 and Nicotine-Related Phenotypes: Indirect Effects Through Nicotine Craving. J Stud Alcohol Drugs 77: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein IS & Hell JW (2010) CaMKII hunkers down on the muscarinic M4 receptor to help curb cocaine-induced hyperlocomotion. EMBO J 29: 1943–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y (2008) Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol 154: 327–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Hauser SR, Deehan GA Jr., Toalston JE, Wilden JA, Bell RL, Mcbride WJ & Rodd ZA (2015) Ethanol and nicotine interaction within the posterior ventral tegmental area in male and female alcohol-preferring rats: evidence of synergy and differential gene activation in the nucleus accumbens shell. Psychopharmacology (Berl) 232: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR (2004) Molecular genetic underpinnings of human substance abuse vulnerability: likely contributions to understanding addiction as a mnemonic process. Neuropharmacology 47 Suppl 1: 140–147. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Fatusin OO, Liu QR, Contoreggi C, Li CY, Buck K & Crabbe J (2008) “Higher order” addiction molecular genetics: convergent data from genome-wide association in humans and mice. Biochem Pharmacol 75: 98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, Naiman D & Liu QR (2008) Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify “connectivity constellation” and drug target genes with pleiotropic effects. Ann N Y Acad Sci 1141: 318–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paipe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PSR data be geometric averaging of multiple internal control genes. Geneom Biol 18; 3(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JJ, Van Den Bree MB & Munafo MR (2011) Association of the CHRNA5-A3-B4 gene cluster with heaviness of smoking: a meta-analysis. Nicotine Tob Res 13: 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]


