Abstract
Brain metastases (BMs) have become increasingly prevalent and present unique considerations for patients, including neurocognitive sequelae and advanced disease burden. Therefore, assessing health-related quality of life (HRQoL) via patient-reported outcome measures (PROMs) is an important element of managing these patients. A systematic review of the literature was conducted with the aims of (1) assessing how PROMS used in BM patients were validated, (2) assessing PROM content, and (3) evaluating quality of PROM-results reporting. PROM validation and quality of reporting were assessed using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) grading criteria and International Society of Quality of Life (ISOQOL)-recommended PROM-reporting standards, respectively. Forty-seven studies reporting on 5178 patients with a range of primacy cancer types were included. Eight different PROMs were applied, ranging from general to brain-specific questionnaires. Weaknesses in the validation of these PROMs were assessed by the COSMIN criteria. Many of these PROMs were not developed for BM patients and contained little information on cognitive symptoms. The overall quality of PROM reporting was insufficient based on the ISOQOL scale. Given the unique clinical considerations in BM patients, our results indicate the need for a standardized, validated questionnaire to assess HRQoL in this population. Additionally, there is room for quality improvement with regard to reporting of PROM-related results.
Keywords: brain metastasis, patient reported outcomes, quality of life
Brain metastases (BMs) from systemic cancer represent an important health care problem with incidence rates and mortality rates higher than those of any individual primary brain malignancy.1,2 Approximately 20% to 40% of all cancer patients develop BMs, and incidence rates are still increasing, primarily because of improved therapies for systemic cancers and increased survival from systemic malignancies.1,2 Different treatment modalities are available for BMs, varying from surgical excision and stereotactic radiosurgery (SRS), to whole-brain radiation therapy (WBRT), systemic chemotherapy, and palliation.1 Choice of treatment strategy is dependent on a variety of clinical factors, including individual tumor characteristics such as histopathology, number, size, and location, and patient characteristics such as extracranial disease activity, age, and performance status.3–6 While aggressive treatment may prolong overall survival, it may also increase the risk of complications and treatment-related adverse events.5 Especially in palliative care, physicians aim to assess the potential clinical benefit of treatment in terms of morbidity and mortality, within the larger context of the patient’s health-related quality of life (HRQoL) and goals of care.7
Historically, the physician’s assessment of treatment-related adverse effects and performance status served as a surrogate for the patient’s HRQoL. Over the past decades, based on an understanding that HRQoL involves mainly subjective elements and are therefore best reported by the patients themselves, several patient-reported outcome measures (PROMs) have been developed to better capture HRQoL information.8–10 Notably, it has been shown that physician-reported symptoms do not accurately correlate with PROMs, emphasizing the importance of collecting HRQoL information directly from the patient.10 These PROMs, which may range from general to disease-specific questionnaires, aim to measure differences in HRQoL between patients or patient groups and to predict and evaluate changes in HRQoL over time.11 The use of PROMs to assess HRQoL can thereby facilitate optimal care management and even increase survival in metastatic cancer patients, compared to dependence solely on the physician’s evaluation of symptoms and patient’s HRQoL.8,12–15
Various PROMs have been applied in the care of BMs patients, including general cancer and brain-specific questionnaires. Focusing on PROMs that aim to measure HRQoL, the goal of this review was to assess the validation studies conducted on the PROMs, the domains covered by these instruments, and the quality of their reporting among BM patients in particular.
Methods
Study Selection
A systematic review of the literature was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.16 PubMed, Embase, and Cochrane databases were searched through December 2016 for studies that used PROMs in patients with BMs. Appropriate Medical Subject Headings and Emtree terms were used. The complete search strategy is listed in (Supplementary Table 1). Titles and abstracts were screened in duplicate followed by full-text screening in duplicate. Discrepancies were solved through discussion with senior authors. Screened articles were included if they met the following criteria: (1) contained original research; (2) were written in the English language; (3) reported on a patient population of at least 18 years of age who had a diagnosis of BMs, regardless of primary cancer type or treatment modality; and (4) used PROMs to determine patient HRQoL. If the population included in a study contained patients without BMs, the study was included for analysis only if the characteristics and outcomes of the BM subgroup were reported separately. PROMs were defined as a questionnaire completed by the patient, rather than a health care professional or family member, that reported on aspects of HRQoL.
Data Extraction
The following data were extracted from the articles: study design, publication year, patient characteristics, primary tumor pathology, treatment modality, PROM used, and schedule of PROM administration. Data extraction was performed by two investigators and discrepancies were resolved by discussion.
PROM Analysis
To analyze the content of the questionnaires, the individual questions from each of the PROMs used by the included articles were categorized into 1 of 4 domains: (1) Global Functioning, (2) Physical Well-Being, (3) Social and Emotional Well-Being, and (4) Cognitive Complaints. These comprehensive domains were chosen by the authors, who tried to reduce overlap among categories, and discrepancies were resolved via discussion. Categorization was performed to better represent the composition of the different questionnaires, focusing on domains especially important for BM patients. Global Functioning included all questions relating to a summative assessment of HRQoL, ability to work or drive, and outlook for the future. Physical Well-Being included questions pertaining to any physical symptoms related to disease or treatment, including pain, fatigue, and level of independence. Social and Emotional Well-Being included questions assessing physiological symptoms inherently related to the disease and the emotional repercussions of physical symptoms, as well as the effect of these factors on the patient’s relationships. For example, questions in this domain elicited symptoms of anxiety or depression, and touched on the effects of the disease or treatment on the patient’s relationship with friends or family. Cognitive Complaints included questions assessing clarity of thought, ability to communicate, memory, reading, and writing.
PROM Reporting
Quality of PROM-results reporting was determined using the International Society of Quality of Life (ISOQOL)-recommended PROM-reporting standards.17 In accordance with the previous literature, a modified version of the ISOQOL standards was used to accommodate both randomized and nonrandomized studies. The ISOQOL standards are based on the Consolidated Standards of Reporting Trials guidelines for randomized studies and are composed of a list of elements deemed to be important in every study reporting PROMs data. Some of the included elements include a PRO-specific hypothesis and a discussion of the generalizability of the PROM results. Scoring of each article was again performed in duplicate and discrepancies were resolved by discussion. In accordance with previous studies, a score of ≥11 out of 16 was considered to be a sufficient level of reporting for the purposes of this study.18 The Pearson Correlation and the Mann-Whitney U Test were used to assess relationships between ISOQOL scores with study design and length of follow up. P < .05 was considered significant.
Validation Assessment
Studies conducted to evaluate psychometric properties on each of the PROMs were identified via citation review and database search. Each PROM was then assessed using methods to assess measurement properties of the PROMs. This was modified from the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) grading criteria9 and this modification has been used previously in brain tumor patients.18 The COSMIN criteria were developed in a Delphi Study to evaluate PROMs for HRQoL. The modified version, used here, has previously been used in brain tumor patients (Supplementary Table 2). The domain “Criterion Validity” was graded as not accessible, because there is no clear gold standard.
Results
Study Characteristics
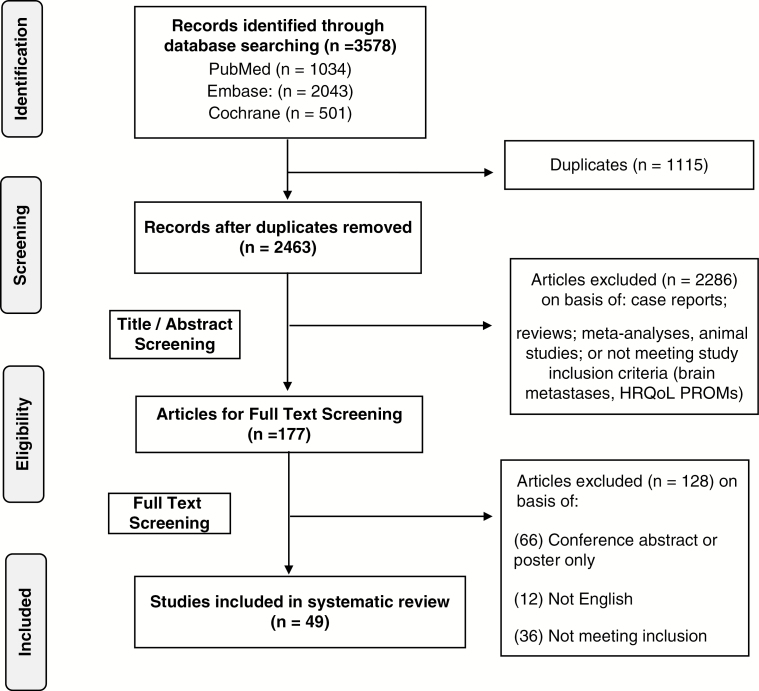
The search and screening identified 47 original studies that used a PROM to assess HRQoL in a total of 5178 patients with BMs (Fig. 1). The median of number of patients included in each study was 65 patients (interquartile range [IQR]: 38-151). The most common primary tumor types were lung cancer (58%, n = 2980) and breast cancer (14%, n = 728) (Supplementary Table 3). The remaining primary tumor types included renal, urogenital, colon, melanoma, liver, pancreas, and unknown primary. Seven studies included only patients with lung cancer (six of which were entirely non-small cell lung cancer) and 1 study described the results of only patients with breast cancer. All remaining studies included patients with a variety of different primary tumor pathologies. Treatment regimens included WBRT, SRS, surgery, chemotherapy, and supportive therapy, either independently or in combination.
Fig. 1.
PRISMA Flow Diagram Showing the Study Selection Process. HRQoL indicates health-related quality of life; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROMs, patient-reported outcome measures.
HRQoL Reporting
Forty of the included studies assessed patient HRQoL using a single PROM, while 9 studies used multiple PROMs. A total of 8 different PROMs were used in this patient population. The most commonly used PROMs were the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) (n = 24 studies) and the Functional Assessment of Cancer Therapy-General (FACT-G) (n = 16 studies). The EORTC-QLQ-C30 questionnaire was used both with and without specific modules, including the brain module (BN20), lung cancer module (LC13), and breast cancer module (Br23). The FACT was used both with and without the brain module (Br). Other PROMs were used such as the abbreviated EORTC-C15-palliative (PAL) (n = 8 studies), the EuroQol-5D (n = 5 studies), Edmonton Symptom Assessment Scale (ESAS) (n = 4 studies), the Brain Symptom Impact Questionnaire (BASIQ) (n = 3 studies), the Short Form 36 (SF-36) (n = 2 studies), and the McGill Quality of Life Index (McGill QoL) (n = 1 study) (Supplementary Table 3).
Domains of Interest
The length of the 8 PROMs used in the BM population ranged from 5 to 36 questions, and the 2 brain-specific PROM extensions included 20-23 additional questions (Table 1). The most well-represented domain was Physical Well-Being, which comprised >50% of the questions in the 8 PROMs and 2 PROM extensions. This is followed by Emotional and Social Well-Being with an average of 5 questions, or 27% of the questions, in all PROMs (median: 21%, IQR: 18%-31%). Despite being used in patients with BMs, the McGill QoL and the EuroQoL-5D PROMS did not include questions regarding cognitive difficulties. When used without their respective brain-specific modules, the FACT-G did not include questions in the cognition domain either, while the EORTC-QLQ-C30 included 2.
Table 1.
Analysis of PROMs by Domain
| Questions by Domain, n (%) | ||||
|---|---|---|---|---|
| Questionnaire (N) | Global Functioning | Physical Well-Being | Emotional and Social Well-Being | Cognition |
| McGill QoL (N = 15) | 5 (36) | a1 (7) | 9 (64) | 0 (0) |
| EuroQoL 5D (N = 5) | 1 (20) | 4 (60) | 1 (20) | 0 (0) |
| BASIQ (N = 18) | 0 (0) | 9 (50) | 0 (0) | 9 (50) |
| FACT-G (N = 27) | 3 (11) | 8 (30) | 16 (59) | 0 (0) |
| FACT-Br (N = 23) | 2 (9) | 8 (35) | 4 (17) | 9 (39) |
| EORTC QLQ-C30 (N = 30) | 3 (10) | 19 (63) | 6 (20) | 2(7) |
| EORTC QLQ-BN20 (N = 20) | 1 (5) | 11 (55) | 5 (25) | 3 (15) |
| EORTC QLQ-C15-PAL (N = 15) | 1 (7) | 12 (80) | 2 (13) | 0 (0) |
| ESAS (N = 9) | 1 (11) | 6 (67) | 2 (22) | 0 (0) |
| SF-36 (N = 36) | 6 (17) | 18 (50) | 12 (33) | 0 (0) |
Abbreviations: BASIQ, Brain Symptom Impact Questionnaire; BN20, brain module; C15-PAL, C15-palliative; EuroQoL 5D, EuroQol 5 Dimension Questionnaire; EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; ESAS, Edmonton Symptom Assessment Scale; FACT-Br, Functional Assessment of Cancer Therapy-brain; FACT-G, Functional Assessment of Cancer Therapy-general; McGill QoL, McGill Quality of Life Index; N: total number of questions in questionnaire; PROMs, patient-reported outcome measures; QLQ, Quality of Life Questionnaire; SF-36, Short Form 36.
aPatients are instructed to list current physical symptoms and rate the degree to which they each affect functioning.
Level of PROMs Reporting
The studies included in this analysis scored an average of 9 points out of a possible 16 points on the ISOQOL scale (median: 9, IQR: 6-11). This indicates a relatively low-quality level of reporting of PROM results in the literature on BM (Supplementary Table 3). Only 14 of the 47 (30%) included studies scored equal to or greater than 11/16 points, which is used as the benchmark for sufficient reporting in previous studies on brain tumors.18 A total of 22 studies used the EORTC questionnaires, of which only 18% (4/22) achieved an ISOQOL score ≥11 (Supplementary Table 3). Similarly, the FACT questionnaires were used in 18 studies, of which 33% achieved the ISOQOL benchmark of ≥11. Though less frequently used, studies using BASIQ (2/3), ESAS (2/4), and McGill (1/1) were more likely to meet the ISOQOL benchmark. The studies performed particularly poorly on 3 of the ISOQOL criteria: (1) PRO-specific hypothesis is clearly stated (78% failed to meet standards), (2) evidence of appropriate statistical analysis is provided for each hypothesis (82% failed to meet standards), and (3) statistical approaches for the handling of missing data are explicitly stated (73% failed to meet standards) (Supplementary Table 4). Neither length of follow-up (R = –0.125, P = .476), randomized vs observational study (U = 148.5, P = .341), nor prospective as compared to retrospective study design (U = 117.5, P = .274) were associated with ISOQOL scores.
Development and Validation of PROMs
Three of the 8 identified PROMs questionnaires used in patients with BMs (BASIQ, EORTC QLQ-C30 [with BN20], and FACT-G [with FACT-Br]) have been specifically validated in this population (Table 2). The remaining 5 instruments have been validated in general cancer patients (ESAS, McGill), cancer palliative care patients (EORTC-QLQ-C15-PAL), and a general population of patients (SF-36, EuroQOL). The COSMIN criteria9 (Supplementary Table 2) were developed to assess the measurement properties of PROMs, and contain 9 domains, all of which can be scored as positive (“+”), negative (“–”), not included (“0”), and questionable (“?”). None of the PROMs completely fulfilled all the COSMIN validation criteria (Table 2). EORTC-QLQ-C30 with the BN-20 supplement received the most “+” ratings (3 total), indicating that some criteria were addressed and fulfilled. The BASIQ followed closely behind, with 2 “+” ratings. The validation of the PROMS was most likely to be positively rated on the criteria of content validity, followed by internal consistency and construct validity. PROM validation was the weakest in 2 criteria: responsiveness, or floor and ceiling effects.
Table 2.
Validation of PROMs with the COSMIN Criteria
| PROM | PROM Designed for | PROM Validated in BM? | Validation Studies Used in Scoring | COSMIN Criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Content Validity | 2. Internal Consistency | 3. Criterion Validity | 4. Construct Validity | 5.1 Reproducibility: Agreement | 5.2 Reproducibility: Reliability | 6. Responsiveness | 7. Floor and Ceiling Effects | 8. Interpretability | Total number of “+” | ||||
| McGill QoL | Advanced cancer at the end of life | No | Advanced cancer19 | – | + | NA | + | 0 | 0 | 0 | 0 | ? | 2 |
| EuroQoL 5D | General | No | Range of health interventions20,21 | – | 0 | NA | + | – | + | – | – | ? | 2 |
| BASIQ | BM | Yes | BM22,23 | + | ? | NA | ? | 0 | + | ?b | 0 | ? | 2 |
| FACT-G (and FACT-Br) |
Assessment of cancer therapy (brain-specific therapy) | Yes | Brain tumors,24 BM,25 Cancer26 | + | – | NA | ? | ? | – | 0 | 0 | ? | 1 |
| EORTC QLQ-C30 (and BN20) |
Cancer (brain cancer) |
Noa | Variation validated in BM patients,27 brain tumor,28,29 cancer26 | + | + | NA | + | 0 | ? | ? | – | ? | 3 |
| EORTC-QLQ- C15-PAL | Palliative cancer care | No | Palliative cancer care,30 cancer31 | + | + | NA | 0 | 0 | ? | ? | 0 | 0 | 2 |
| ESAS | Cancer | No | Cancer32 | ? | 0 | NA | 0 | – | ? | 0 | 0 | ? | 0 |
| SF-36 | General | No | General,33 chronic pain34 | ? | –c | NA | – | 0 | 0 | 0 | – | + | 2 |
Abbreviations: BASIQ, Brain Symptom Impact Questionnaire; BM, brain metastases; BN20, brain module; C15-PAL, C15-palliative; COSMIN, COnsensus-based Standards for the selection of health Measurement INstruments; EuroQoL 5D, EuroQol 5 Dimension Questionnaire; EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; ESAS, Edmonton Symptom Assessment Scale; FACT-Br, Functional Assessment of Cancer Therapy-brain; FACT-G, Functional Assessment of Cancer Therapy-general; McGill QoL, McGill Quality of Life Index; SF-36, Short Form 36; +, the criteria are addressed and are met statistically through validation; –, the criterion was included in the development of the questionnaire but the questionnaire failed to meet the criteria in practice; 0, the criteria were not included or addressed; ?, there was doubtful design or methods, or a major component of the criteria was not addressed; NA, not accessible;9 PROMs, patient-reported outcome measures.
aWhile the original tool was not validated in patients with BMs, a variation of this tool has been studied in this patient population.
bNo significant change over 1 month’s time, verified by other FACT-G and FACT-Br.
cCriteria for + were almost fully met.
Discussion
Among the 47 studies found on HRQoL assessment using PROMs in BM patients, 8 different general and brain-specific PROMs were used. Based on the ISOQOL scale, the overall quality of reporting of PROM results was insufficient. Many of the PROMs used in patients with BMs were extensive, yet the development and validation of the PROMs inadequately addressed several aspects of the COSMIN criteria.
Patient HRQoL is increasingly recognized as an essential treatment goal. For this reason, PROMs have become an integral part of clinical trial design. In addition, these instruments are gradually adopted into clinical care outside the realm of trials. Studies show that the use of PROMs in clinic can help facilitate physician-patient communication35 and even improve survival12 in oncology patients, thus adding value to clinical practice. Especially in BM patients, HRQoL is often highlighted as an important measure, because this patient population generally has limited life expectancy and palliative treatments focus on improving or maintaining HRQoL.36 Our results show that the PROMs used in patients with BMs are questionnaires largely developed for a general cancer population, which highlights an opportunity to improve reporting and monitoring of HRQoL in BM patients. Specifically, improvement can be made in how PROMs for patients with BM are (1) developed and validated, and (2) implemented and reported.
Only 1 PROM (BASIQ) was developed specifically for patients with BMs, and 3 PROMs or variations of the PROMs have been validated in patients with BMs. However, PROM development and validation were lacking several of the criteria that were determined to be important by the experts who developed the COSMIN criteria. Most PROMs did not fulfill the “Responsiveness” criterion, which assesses how well the questionnaire can detect changes over time. In other words, it is unclear how sensitive these PROMs are to changes in HRQoL over time, associated with treatment or progression of disease in the BM population. For PROMs to be effective in informing management of care in BM patients, improvement of development and validation of PROMs in this population is required. Additionally, most studies did not assess the “Floor and Ceiling Effects” criterion in the process of validation. This COSMIN criterion aims to characterize the distribution of the studied population by making sure that only a low percentage of respondents achieve the highest or lowest possible values. While the omission of “Floor and Ceiling Effects” criterion consideration technically represents a gap in the validation of these PROMs, this may be unavoidable given the heterogeneity in primary pathology and treatment modalities in the BM population, and thus should not necessarily indicate a fundamental weakness in the validation.
BM patients are a heterogeneous population encompassing differences in underlying pathology, treatment regimens, symptoms, and life expectancy. Regardless of this heterogeneity in patient characteristics, the presence of BM puts these patients at high risk for cognitive dysfunction. Moreover, postchemotherapy cognitive impairment (PCCI) and cognitive dysfunction secondary to WBRT are recognized complications of therapy.37–41 These cognitive impairments might affect the accuracy and completeness of the PROM data. Additionally, given that cognition is a particular concern in BMs, it is important that the PROMs used by these patients address cognitive complaints. The BASIQ, EORTC QLQ-C30 and specific brain modules (FACT-Br and EORTC QLQ-BN20) are the only questionnaires that assess cognition.
Lastly, the results of this analysis showed a low quality of PROMs reporting in the included studies, as measured by ISOQOL.42 The more commonly used PROMs (EORTC and FACT) showed variable quality of reporting and met the ISOQOL benchmark in a mere 18% and 33% of the relevant studies, respectively. There are several factors that may contribute to low-quality reporting, some of which are inherent to the studied population, and therefore it should not be inferred that there was low quality of study design. Only 11 of the 49 studies explicitly stated the PRO hypothesis that was being tested or the relevant outcome domain that was applicable in the context of their study. This precludes the possibility of robust statistical analysis to test PRO hypotheses. It is therefore important to apply PROMs in a hypothesis-driven manner to assess various factors, including how PROMs relate to symptom burden, communication with physicians, or need for auxiliary services and counseling. Additionally, given the poor prognosis and rapid clinical deterioration in many patients with BMs, reporting bias is a major factor in studies reporting on HRQoL in this population. This may lead to significant dropout and missing data. Unfortunately, only 13 studies fulfilled the ISOQOL criteria for stating and addressing the extent of missing data. Two included studies compared PROM data to PROMs filled out by proxies. While this is a potential solution for patients with low cognitive function, the data do not come directly from the patient and thus these results need to be used with that limitation in mind.
One suitable alternative in patients who cannot complete longer questionnaires is the use of computerized adaptive testing (CAT), which is currently available for the QLQ-C30.43,44 These are instruments that adapt the type and number of questions asked based on the patients’ earlier responses, and could therefore reduce the response burden and increase the precision of measurement. Another potential approach that may become available in the coming years is the use of smartphone-based data collection of general digital phenotypes, which can track patients’ mobility, social interaction, and even cognitive functioning by the way they interact with their phones.45,46 The use of shorter questionnaires and/or the use of passively collected smartphone-based data may decrease selection bias, dropout, and the potential for reporting bias of PROMs reporting, thereby improving the quality of reported data. Improvement in the reporting of PROMs will advance our understanding of how patient HRQoL is affected in the BM population and thereby facilitate the integration of these lessons learned into clinical practice. Naturally, in situations in which patient condition is too poor to complete any type of PROM, questionnaires may be filled out by patients’ proxies, or lastly, physician assessment of HRQoL can be used.
Given the variability in validation methods employed, it remains unclear which PROM is best correlated with clinical outcomes including survival. Nevertheless, the use of HRQoL PROMs designed with BM patients in mind is likely useful beyond correlation (or lack thereof) with survival, as tracking HRQoL may help inform symptom management and identify patients who may benefit from additional psychosocial support services. The only 2 PROMs that were validated in the BM population specifically were the BASIQ and the FACT (G and Br). Though not formally validated in the BM population, EORTC-QLQ-C30 administered alongside its respective brain module (BN20) performed the best on the COSMIN scoring in its validation and was comprehensive in the domains covered, and a variation of the brain module (EORTC QLQ-BN20 + 2) has been validated for patients with BMs. Its primary shortcomings are 2-fold. First, the quality of reporting in studies using the EORTC tools as assessed by ISOQOL criteria was suboptimal, though this is more so an issue of study design than the characteristics of the questionnaire itself. Second, when administered in the fixed length traditional manner, its length (50 questions) may very well be prohibitive for some patients; however, this may be addressed by using the aforementioned CAT approach. The shorter PROMs (BASIQ, EuroQOL, ESAS, and EORTC QLQ-C15-PAL) are unbalanced in their HRQoL domain representation and should be used with this limitation in mind if used in isolation. The use of several questionnaires in combination, such as the BASIQ + EuroQoL 5D, is another alternative approach to ensuring a well-rounded interrogation of HRQoL. Overall, there is a need for validated PROMs that accurately track HRQoL in this population.
To our knowledge, this is the only study on the use of PROMs for the purpose of HRQoL assessment in patients with BMs. Thus, this review fills a gap in the literature and points to areas of concern by highlighting some of the methodological lapses in the development and use of PROMs for patients with BMs. Undoubtedly, patients with BMs represent a diverse patient population, and therefore their primary malignancy-specific HRQoL concerns are similarly varied. Despite the heterogeneity of this population, this review focused on the overlap among the subgroups as it relates to BMs. In addition, most included studies had exclusion criteria based on cognitive functioning, which precludes more frail patients from undergoing formalized HRQoL surveillance. A limitation of our study includes the use of various tools of assessment, including the COSMIN criteria and ISOQOL. These tools were chosen because they were created by experts in the field and have been used elsewhere in the literature. Nevertheless, they may reflect the specific priorities of their authors and are not the only quality assessment systems that exist. Unfortunately, the heterogeneity in patient characteristics and in the domains included in the PROMs, as well as the lack of unified reporting in the included studies made it methodologically unfeasible to pool results and directly compare the functionality of these, and we would encourage prospective investigation on the subject to further explore this question. With this scope in mind, this systematic review describes an important clinical problem and can help guide future studies of patients with BMs as well as the clinical care of these patients using PROMs.
In conclusion, HRQoL is of vital importance in the management of patients with BMs, and there is room for improvement in the development and use of PROMs to assess HRQoL in a way that captures the unique characteristics of this specific population. An awareness of the limitations in the administration of the currently available PROM questionnaires is essential to patient-centered practice and the optimization of care management in BM patients. This study highlights the need for prospective validation studies of PROMs in BM patients that could improve our understanding of HRQoL in this population and help to identify a “gold-standard” PROM in this population.
Supplementary Data
Supplementary material is available at Neuro-Oncology online.
Funding
None
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. Tsao MN, Rades D, Wirth A et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sperduto PW, Chao ST, Sneed PK et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. [DOI] [PubMed] [Google Scholar]
- 3. Mehta MP, Paleologos NA, Mikkelsen T et al. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Linskey ME, Andrews DW, Asher AL et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalkanis SN, Kondziolka D, Gaspar LE et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaspar LE, Mehta MP, Patchell RA et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chow R, Lao N, Popovic M et al. Comparison of the EORTC QLQ-BN20 and the FACT-Br quality of life questionnaires for patients with primary brain cancers: a literature review. Support Care Cancer. 2014;22(9):2593–2598. [DOI] [PubMed] [Google Scholar]
- 8. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622–629. [DOI] [PubMed] [Google Scholar]
- 9. Terwee CB, Bot SD, de Boer MR et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. [DOI] [PubMed] [Google Scholar]
- 10. Pakhomov SV, Jacobsen SJ, Chute CG Roger VL. Agreement between patient-reported symptoms and their documentation in the medical record. Am J Manag Care. 2008;14(8):530–539. [PMC free article] [PubMed] [Google Scholar]
- 11. Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chronic Dis. 1985;38(1):27–36. [DOI] [PubMed] [Google Scholar]
- 12. Basch EM, Deal AM Dueck AC et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017; 318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quinten C, Maringwa J, Gotay CC et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst. 2011;103(24):1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mercieca-Bebber R, Rouette J, Calvert M et al. ; International Society for Quality of Life Research (ISOQOL) Best Practice for PROs—Reporting Taskforce Preliminary evidence on the uptake, use and benefits of the CONSORT-PRO extension. Qual Life Res. 2017;26(6):1427–1437. [DOI] [PubMed] [Google Scholar]
- 18. Zamanipoor Najafabadi AH, Peeters MCM, Dirven L et al. Impaired health-related quality of life in meningioma patients—A systematic review. Neuro Oncol. 2017;19(7):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen SR, Mount BM, Strobel MG, Bui F. The McGill quality of life questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliat Med. 1995;9(3):207–219. [DOI] [PubMed] [Google Scholar]
- 20. Brooks R. EuroQol: the current state of play. Health policy. 1996;37(1):53–72. [DOI] [PubMed] [Google Scholar]
- 21. Kontodimopoulos N, Aletras VH, Paliouras D, Niakas D. Mapping the cancer-specific EORTC QLQ-C30 to the preference-based EQ-5D, SF-6D, and 15D instruments. Value Health. 2009;12(8):1151–1157. [DOI] [PubMed] [Google Scholar]
- 22. Thavarajah N, Ray S, Bedard G, et al. Psychometric validation of the Brain Symptom and Impact Questionnaire (BASIQ) version 1.0 to assess quality of life in patients with brain metastases. CNS Oncol. 2015;4(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bedard G, Ray S, Zhang L, et al. Validation of the Brain Symptom and Impact Questionnaire (BASIQ) to assess symptom and quality of life in brain metastases. CNS Oncol. 2014;3(4):275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weitzner MA, Meyers CA, Gelke CK, et al. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151–1161. [DOI] [PubMed] [Google Scholar]
- 25. Thavarajah N, Bedard G, Zhang L, et al. Psychometric validation of the functional assessment of cancer therapy--brain (FACT-Br) for assessing quality of life in patients with brain metastases. Support Care Cancer. 2014;22(4):1017–1028. [DOI] [PubMed] [Google Scholar]
- 26. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen J, Zhang L, Clemons M, et al. Content validation of the EORTC QLQ-BN20 + 2 with patients and health care professionals to assess quality of life in brain metastases. J Radiat Oncol. 2012;1(4):397–409. [Google Scholar]
- 28. Taphoorn MJ, Claassens L, Aaronson NK, et al. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 29. Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5(1):139–150. [DOI] [PubMed] [Google Scholar]
- 30. Groenvold M, Petersen MA, Aaronson NK, et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer. 2006;42(1):55–64. [DOI] [PubMed] [Google Scholar]
- 31. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 32. Chang VT, Hwang SS, Feuerman M. Validation of the edmonton symptom assessment scale. Cancer. 2000;88(9):2164–2171. [DOI] [PubMed] [Google Scholar]
- 33. Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 34. Fredheim OM, Borchgrevink PC, Saltnes T, Kaasa S. Validation and comparison of the health-related quality-of-life instruments EORTC QLQ-C30 and SF-36 in assessment of patients with chronic nonmalignant pain. J Pain Symptom Manage. 2007;34(6):657–665. [DOI] [PubMed] [Google Scholar]
- 35. Detmar SB, Muller MJ, Schornagel JH Wever LD Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288(23):3027–3034. [DOI] [PubMed] [Google Scholar]
- 36. Pulenzas N, Ray S, Zhang L et al. The Brain Symptom and Impact Questionnaire in brain metastases patients: a prospective long-term follow-up study. CNS Oncol. 2016;5(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown PD, Jaeckle K, Ballman KV et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. López Zunini RA, Scherling C, Wallis N et al. Differences in verbal memory retrieval in breast cancer chemotherapy patients compared to healthy controls: a prospective fMRI study. Brain Imaging Behav. 2013;7(4):460–477. [DOI] [PubMed] [Google Scholar]
- 39. Conroy SK, McDonald BC, Smith DJ et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;137(2):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whitney KA, Lysaker PH, Steiner AR Hook JN Estes DD Hanna NH. Is “chemobrain” a transient state? A prospective pilot study among persons with non-small cell lung cancer. J Support Oncol. 2008;6(7):313–321. [PubMed] [Google Scholar]
- 41. Vearncombe KJ, Rolfe M, Wright M Pachana NA Andrew B Beadle G. Predictors of cognitive decline after chemotherapy in breast cancer patients. J Int Neuropsychol Soc. 2009;15(6):951–962. [DOI] [PubMed] [Google Scholar]
- 42. Brundage M, Blazeby J, Revicki D et al. Patient-reported outcomes in randomized clinical trials: development of ISOQOL reporting standards. Qual Life Res. 2013;22(6):1161–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petersen MA, Groenvold M, Aaronson N et al. ; European Organisation for Research and Treatment of Cancer Quality of Life Group Multidimensional computerized adaptive testing of the EORTC QLQ-C30: basic developments and evaluations. Qual Life Res. 2006;15(3):315–329. [DOI] [PubMed] [Google Scholar]
- 44. Petersen MA, Aaronson NK, Arraras JI et al. ; EORTC Quality of Life Group The EORTC computer-adaptive tests measuring physical functioning and fatigue exhibited high levels of measurement precision and efficiency. J Clin Epidemiol. 2013;66(3):330–339. [DOI] [PubMed] [Google Scholar]
- 45. Torous J, Onnela JP, Keshavan M. New dimensions and new tools to realize the potential of RDoC: digital phenotyping via smartphones and connected devices. Transl Psychiatry. 2017;7(3):e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Torous J, Kiang MV, Lorme J, Onnela JP. New tools for new research in psychiatry: a scalable and customizable platform to empower data driven smartphone research. JMIR Ment Health. 2016;3(2):e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.