Abstract
Background
Low-grade gliomas (LGGs) are slow-growing, infiltrative tumors frequently associated with seizures. Predicting which patients will develop early tumor recurrence based on clinical indicators following initial surgical intervention remains a challenge. Seizure recurrence following surgery may be an early indicator of tumor recurrence, especially in patients presenting with increase in seizure frequency.
Methods
This study analyzed 148 patients meeting inclusion criteria (age >18 years, LGG diagnosis, at least 1 seizure event recorded before and after initial surgical intervention). All patients were treated at the Brain and Spine Center at The University of Texas MD Anderson Cancer Center from January 2000 to March 2013. Seizure frequency in a 6-month period before and after tumor resection was categorized as none, 1, few (2 to 3 seizures) or several (>3 seizures). Immediately postoperative seizures (up to 48 hours from surgery) were not included in the analysis.
Results
A total of 116 (78.4%) patients had seizures at initial presentation and most (95%) were started on antiepileptic drugs (AEDs). We found 2 clinical variables with a significant impact on progression-free survival (PFS): Higher seizure frequency during the 6-month postoperative period and seizure frequency increase between the 6-month pre- and the 6-month postoperative periods were both correlated to higher risk of early tumor recurrence (P = .007 and P = .004, respectively).
Conclusion
Seizure frequency following surgical resection of LGGs and the seizure frequency change between the 6-month preoperative and postoperative periods may serve as clinical predictors of early tumor recurrence in patients with LGGs who are also afflicted by seizures.
Keywords: low-grade glioma, refractory epilepsy, seizures, tumor outcome, tumor-related epilepsy
Low-grade gliomas (LGGs) are slow-growing tumors of the central nervous system (CNS), characterized by an infiltrative growth pattern and cortical predilection. LGGs are frequently associated with seizures, more so than their high-grade counterparts.1,2 Up to 90% of LGG patients may have seizures as the initial presentation leading to tumor diagnosis.3,4 Although seizure control is successfully achieved in the majority of patients after surgical resection, the development of refractory epilepsy is seen in up to 15% of LGG patients, and is associated with poor quality of life and worse prognosis.5
Anatomically, LGGs infiltrate the cortex and subcortical white matter and slowly disrupt functional networks, causing dysfunction not only to the peritumoral area—the area of noninfiltrated brain tissue immediately surrounding the tumor mass, considered the epileptogenic zone—but also the CNS macroenvironment as a whole by compromising the function of the blood-brain and blood-cerebrospinal fluid barriers. Molecularly, mutation of the isocitrate dehydrogenase (IDH) isoenzyme is considered to be an early driver of oncogenesis in LGGs and such a mutation is found in the majority of these tumors. Its metabolite, 2-hydroxyglutarate (2-HG), is a highly epileptogenic compound chemically similar to glutamate, a potent excitatory neurotransmitter, and similarly activates N-methyl-D-aspartate receptors.6,7
Although there are several mechanisms to explain the development of seizures in the setting of a brain tumor,8,9 predicting whether a patient will develop refractory epilepsy or experience a more benign disease course remains a challenge.10 Good seizure control following tumor resection and adjuvant treatment, with or without the addition of antiepileptic drugs (AEDs), is usually seen.11 On the other hand, seizure recurrence may raise a concern for tumor recurrence and trigger further workup, especially in patients who have achieved a prolonged period of seizure freedom. Seizure recurrence or an increase in seizure frequency is commonly reported around the time of tumor progression in patients with tumor-related epilepsy.1 Nevertheless, there have been no studies focusing on early changes in seizure frequency and prediction of tumor recurrence in LGG patients.
In this retrospective study, we aimed to investigate the predictability of early tumor recurrence (<5 years) following maximal safe resection, based on change of seizure frequency in the 6-month period immediately prior to tumor resection compared to the 6-month period immediately following it. Immediately postoperative seizures (up to 48 hours from surgery) were not included in the analysis. As the reported median progression-free survival (PFS) of LGGs varies quite significantly (2 to 10+ years),12–14 owing to clinical factors and different molecular signatures driven by IDH and 1p/19q codeletion status, we defined a PFS of less than 5 years as early tumor recurrence. This information has the potential to inform management following surgery in LGG patients.
Methods
Of 1987 patients identified as undergoing a surgical intervention for a newly diagnosed brain tumor (excluding meningiomas) in the Department of Neurosurgery database at The University of Texas MD Anderson Cancer Center (MDACC) during the period of January 2000 through March 2013, a total of 150 patients met the inclusion criteria for this study (age 18 years, pathological confirmation of LGG—astrocytoma, oligodendroglioma, mixed oligoastrocytoma—at initial tumor diagnosis and at least 1 seizure event recorded before or after the initial surgical intervention). Of these, 148 patients were included in the analysis after 2 patients were excluded for lack of data regarding seizure history. Patients were followed up until January 2016 (median follow-up time 112 months (range: 37 to 178 months). This study was approved by the local institutional review board. The primary aim of this study was to examine the relationship of change in seizure frequency between the period 6 months prior to compared to the period 6 months posttumor resection, and time to tumor recurrence. The selection of a 6month pre- and postoperative period as a parameter to categorically quantify the change in seizure was based on the hypothesis that such a time frame would decrease recall bias in the preoperative period, as well as allowing postoperative changes to stabilize and medical seizure management to be consolidated. Additionally, with some LGGs recurring as early as 2 years (Radiation Therapy Oncology Group 0424), we chose this time frame as it represents 25% of this short PFS. Importantly, seizures occurring in the first 48 hours following tumor resection were not included in this study, as these could have been related to the surgical procedure itself. Pre- and postoperative seizure frequency was categorized as none (no seizures), 1 (single seizure), a few (2 to 3 seizures) or several (>3 seizures) to allow statistical comparison between the pre- and postoperative periods.
Seizures, classified as focal or generalized events,15 were assessed based on chart review of notes and procedures of internal (MDACC) as well as outside medical records. Seizure frequency assessment preoperatively was based on the number of seizures reported by the patient during the 6 months preceding surgical resection. Seizure frequency assessment postoperatively was based on the number of seizures reported by the patient during the 6 months following surgical resection, while on or off AEDs. Electroencephalographic (EEG) studies were not routinely used as a means to quantify seizure activity. Rather, the semiology of events was taken into consideration based on the patient’s best description.
Determination of progression of disease was made by the treating physician according to radiographic imaging (T1, T2/fluid-attenuated inversion recovery (FLAIR), postcontrast T1, diffusion-weighted imaging and/or diffusion tensor imaging sequences of brain MRI) and/or histopathological confirmation of recurrence in those cases submitted for a subsequent tumor resection.
Statistical Analysis
Descriptive statistical analysis such as mean, standard deviation, minimum, maximum, interquartile range, frequency, and percentage were used. Kaplan-Meier survival analysis including log-rank test and Cox regression analysis were used to assess the effect of categorical and continuous covariates on time to recurrence-free survival, respectively. All computations were carried out in SAS 9.4 (SAS Institute Inc, Cary, NC, USA) and SPlus (TIBCO Spotfire S+ 8.2).
Univariate Kaplan-Meier analysis on PFS was applied for categorical variables of interest, such as tumor location, surgery extension, seizure type prior to surgery, change in seizure frequency from the 6-month preoperative period to the 6-month postoperative period, and seizure frequency during the 6-month pre- and postoperative periods (based on 4 categories—none, single, few, several; and also on 2 categories, by combining none/single and few/several categories into 1). Multivariate Cox regression analysis on PFS was applied according to covariates demonstrating statistically significant results (P < .05) based on univariate Kaplan-Meier analysis.
Patients who had tumor recurrence, were lost to follow-up, or were deceased within 6 months postoperatively were excluded from analysis of the 6month postoperative period (n = 10).
Results
A total of 148 patients were included in this study. No gender predominance was found (male:female ratio 1:1).
Preoperative Analysis
Imaging features
Based on the brain MRI reports, T2/FLAIR change suggestive of tumor predominantly involved the frontal lobe (n = 88, 59.5%) followed by temporal lobe (n = 32, 21.6%), parietal lobe (n = 21, 14.2%), and insula (n = 7, 4.7%, Table 1). There was no hemispheric predominance (right:left ratio 1:1, n = 72 in each). The T2/FLAIR abnormalities demonstrated on brain MRI extended to eloquent cortical areas in 74 (52%) patients (Table 1).
Table 1.
Descriptive Statistics on Variables of Interest
| Covariate | Levels | N (%) |
|---|---|---|
| Age (N, mean ± SD, median (min, max) | 148, 42.2 ± 10.4, 42.5 (20, 72) | |
| Gender | Male | 78 (52.7%) |
| Female | 70 (47.3%) | |
| Tumor Location | Frontal | 88 (59.5%) |
| Insula | 7 (4.7%) | |
| Temporal | 32 (21.6%) | |
| Parietal/Occipital | 21 (14.2%) | |
| Surgery Type | Partial | 96 (64.9%) |
| Gross-total | 52 (35.1%) | |
| Histology | Astrocytoma | 85 (59%) |
| Oligodendroglioma | 48 (33.3%) | |
| Oligoastrocytoma | 11 (77.7%) | |
| No. of AEDs preop (N, mean ± SD, median (min, max) | 148, 0.9 ± 0.6, 1 (0, 3) | |
| 0 | 31 (21.0%) | |
| 1 | 102 (68.9%) | |
| >2 | 15 (10.2%) | |
| No. of AEDs Postop (N, mean ± SD, median (min, max) | 148, 1.1 ± 0.7, 1 (0, 3) | |
| 0 | 22 (14.9%) | |
| 1 | 91 (61.5%) | |
| >2 | 35 (23.6%) | |
| Seizure Frequency 6 Months Preop | None | 32 (21.6%) |
| Single | 54 (36.5%) | |
| Few | 23 (15.5%) | |
| Several | 39 (26.4%) | |
| Seizure Frequency 6 Months Postop | None | 98 (66.2%) |
| Single | 14 (9.5%) | |
| Few | 19 (12.8%) | |
| Several | 17 (11.5%) | |
| Seizure Frequency Change from 6 Months Prior To/After Surgery | None/Single → None/Single | 69 (46.6%) |
| None/Single → Few/Several | 17 (11.5%) | |
| Few/Several → None/Single | 43 (29.1%) | |
| Few/Several → Few/Several | 19 (12.8%) |
Abbreviations: AEDs, antiepileptic drugs; min, minimum; max, maximum; Postop, postoperatively; preop, preoperatively; SD, standard deviation.
Seizure management
A total of 116 patients (78.4%) had seizure as the initial presentation leading to tumor diagnosis. On average, the initial seizure event occurred 4.75 months prior to tumor resection (range 0.1 to 24.2 months). During the preoperative period, the majority of patients (n = 54, 36.5%) had a single seizure, while 23 patients (15.5%) had a few seizures, and 39 patients (26.4%) had several seizures. The most common seizure semiology was complex partial (44%), followed by generalized tonic-clonic (29.3%), and simple partial seizure (26.7%). No case of status epilepticus was documented.
After the initial seizure, most patients (n = 140, 95%) were started on AEDs. Phenytoin (n = 49, 34%), levetiracetam (n = 40, 28%), and carbamazepine (n = 17, 12%) were among the most commonly prescribed AEDs. Six patients required 2 AEDs for seizure control prior to surgery. Eight patients (5% of total study patients), all of whom had only 1 seizure prior to surgical resection, were not started on AEDs because of patient preference or based on the medical judgment of the treating physician.
Postoperative Analysis
Surgical management
All patients had maximal safe surgical resection via craniotomy. Ninety-six patients (64.9%) underwent partial resection of the tumor, whereas 52 patients (35.1%) underwent gross total resection based on MRI findings obtained within 72 hours following surgery. Forty-one patients (28%) developed postoperative neurological deficits (transient weakness, sensory deficits, visual field deficit, and aphasia being the most common), and in 14% of them (n = 6), the deficits lasted longer than 6 months. Two of the 6 patients (33%) with long-standing (>6 months) neurological deficits postoperatively had few or several seizures during the 6-month postoperative period, while 33 out of 128 patients (26%) with transient or no postoperative deficits had few or several seizures during the same time frame.
Histopathological findings
The LGG subtypes were as follows, according to World Health Organization 2016 classification of CNS tumors16: diffuse astrocytoma (including IDH-mutant [IDH-m], IDH-wildtype [IDH-wt], and not otherwise specified [NOS]): n = 85, 59%; oligoastrocytoma (NOS): n = 11, 7.7%; oligodendroglioma: n = 48, 33.3% (Table 1). All histopathological testing was completed or reviewed at MDACC. Tests for IDH and other molecular markers of glioma tumors were not routinely performed on the tumor samples analyzed in this manuscript based on the time period of this study.
Tumor management
During the 6-month period following tumor resection and pathological confirmation of LGG, the majority of patients (n = 121, 83%) underwent some type of adjuvant treatment. Seventy patients (48%) underwent adjuvant radiotherapy (RT). Fifty-nine patients (41%) received sequential RT followed by chemotherapy. In total, 114 patients (79%) underwent adjuvant chemotherapy, either alone (n = 55) or following RT (n = 59). All oligodendroglioma cases were submitted to adjuvant chemotherapy combined or not with RT. The most common chemotherapy regimens were a combination of procarbazine, lomustine (CCNU), and vincristine (PCV), procarbazine and CCNU (PC), and temozolomide (TMZ) alone. Twenty-three patients (16%) did not undergo adjuvant treatment because of gross total resection (n = 13), history of medication noncompliance, and/or loss to follow-up (n = 3), patient preference (n = 4), refractory alcohol abuse (n = 1), or unknown reason (n = 2). Tumor recurrence was noted in 91 patients (61.5% of the cohort, 27 of whom had oligodendroglioma histology) with a median time to recurrence of 77.4 months (95% confidence interval [CI]: 62 to 88.8 months). Median follow-up time was 112 months (range 37 to 178 months).
Seizure management
Following surgical resection (and excluding the perioperative period), most patients were seizure free (n = 98, 66.2%) during the 6-month postoperative period, while 14 patients (9.5%) had a single seizure, 19 patients (12.8%) had a few seizures, and 17 patients (11.5%) had several seizures. Ninety-one patients (61.5%) continued on 1 AED during the postoperative period, 35 patients required 2 or more AEDs, and 22 patients were not on an AED during this period. The number of AEDs during this time frame was based on the number of AEDs the patient was maintained on for more than 50% of this 6-month time frame. The 3 most frequently prescribed AEDs were levetiracetam (n = 83), phenytoin (n = 27), and valproic acid (n = 13). The most frequent combination AED therapy was levetiracetam and carbamazepine (n = 11), levetiracetam and phenytoin (n = 9), and levetiracetam and valproic acid (n = 6).
The seizure frequency change between the 6-month pre- and postoperative periods (according to the following qualitative categories (Table 1): None/single event → none/single event [N1→N1]; none/single event → few/several events [N1→FS]; few/several events → none/single events [FS→N1]; few/several events → few/several events [FS→FS]) were: N1→N1, n = 69 (46.6%), N1→FS, n = 17 (11.5%), FS→N1, n = 43 (29.1%) and FS→FS, n = 19 (12.8%).
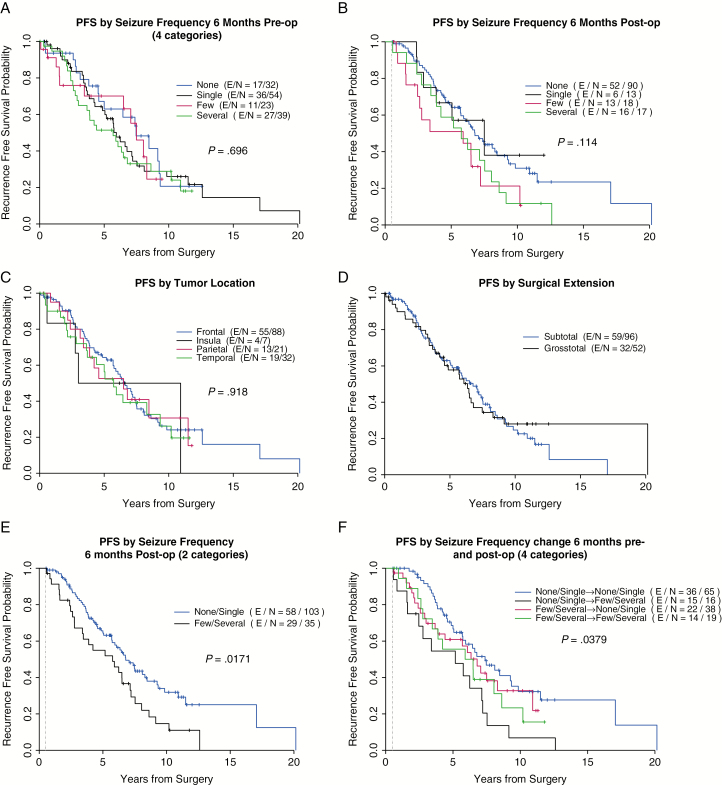
Univariate analysis of seizure frequency change between the 6-month pre- and postoperative periods (Table 2a; Fig. 1F) revealed that patients with none or 1 seizure during the 6-month preoperative period who continued to be seizure free or had only 1 seizure in the 6-month postoperative period had significantly lower risk of tumor recurrence at 2 and 5 years following surgery (2-PFS and 5-PFS) (P = .038, hazard ratio [HR] 0.984 and 0.667 [CI 95%, 0.889 to 0.998 and 0.528 to 0.773], respectively), whereas those who had no or a single seizure preoperatively and developed few or several seizures in the 6-month postoperative period had the lowest 2-PFS and 5-PFS rate (HR 0.750 and 0.545 [CI 95%, 0.463 to 0.898 and 0.274 to 0.753], respectively). Univariate analysis comparing only 2 categorical subgroups of seizure frequency (by combining the “none” and “1” subgroups of seizure frequency into 1 category: “none/1,” and “few” and “several” into another category: “few/several”) also demonstrated a significant correlation between seizure frequency in the 6-month postoperative period and 2- and 5-PFS (P = .017, Table 2a; Fig. 1E).
Table 2a.
Univariate Kaplan-Meier Analysis on Tumor Progression-Free Survival for Categorical Variables
| Covariate | Levels | PFS (years) | Survival | 95% Lower | 95% Upper | P-value |
|---|---|---|---|---|---|---|
| Tumor Location | Frontal | 2 | .903 | .816 | .950 | .918 |
| 5 | .643 | .526 | .738 | |||
| Insula | 2 | .833 | .273 | .975 | ||
| 5 | .500 | .111 | .804 | |||
| Parietal | 2 | .900 | .656 | .974 | ||
| 5 | .526 | .284 | .721 | |||
| Temporal | 2 | .829 | .637 | .925 | ||
| 5 | .563 | .358 | .725 | |||
| Extent of Resection | Subtotal | 2 | .898 | .814 | .946 | .835 |
| 5 | .616 | .503 | .712 | |||
| Gross-total | 2 | .858 | .725 | .930 | ||
| 5 | .578 | .424 | .704 | |||
| Seizure Frequency 6 Months Preop | None | 2 | .935 | .766 | .983 | .697 |
| 5 | .630 | .418 | .783 | |||
| Single | 2 | .899 | .774 | .957 | ||
| 5 | .621 | .467 | .742 | |||
| Few | 2 | .759 | .513 | .892 | ||
| 5 | .701 | .448 | .854 | |||
| Several | 2 | .893 | .738 | .958 | ||
| 5 | .514 | .344 | .660 | |||
| Seizure Frequency 6 Months Postopa | None | 2 | .931 | .852 | .968 | .114 |
| 5 | .642 | .526 | .736 | |||
| Single | 2 | 1.00 | . | . | ||
| 5 | .667 | .337 | .860 | |||
| Few | 2 | .765 | .488 | .904 | ||
| 5 | .510 | .254 | .718 | |||
| Several | 2 | .882 | .606 | .969 | ||
| 5 | .588 | .325 | .778 | |||
| Seizure Frequency 6 Months Postop (Categorized Into 2 Groups)a | None/Single | 2 | .939 | .870 | .972 | .017 |
| 5 | .644 | .537 | .733 | |||
| Few/Several | 2 | .824 | .650 | .917 | ||
| 5 | .549 | .367 | .699 | |||
| Seizure Frequency Change from 6 Months Preop to Postopa | None/Single → None/ Single | 2 | .984 | .889 | .998 | .038 |
| 5 | .667 | .528 | .773 | |||
| None/Single → Few/ Several | 2 | .750 | .463 | .898 | ||
| 5 | .545 | .274 | .753 | |||
| Few/Several → None/ Single | 2 | .865 | .805 | .941 | ||
| 5 | .609 | .430 | .747 | |||
| Few/Several → Few/ Several | 2 | .889 | .624 | .971 | ||
| 5 | .556 | .305 | .748 |
Abbreviations: min, minimum; max, maximum; PFS, progression-free survival; Postop, postoperatively; preop, preoperatively.
aPatients with tumor recurrence, lost to follow-up or deceased within 6 months postoperatively were excluded (n = 10).
Fig. 1.
Kaplan-Meier curves for tumor progression-free survival (PFS) according to the following categorical variables: seizure-frequency preoperatively (preop), seizure-frequency postoperatively (postop), tumor location, surgery extension, seizure type before surgery, and seizure frequency change from 6 months prior to surgery to 6 months after surgery.
Multicovariate Cox regression analysis on PFS comparing patients with few/several seizures during the 6-month postoperative period to those with none/single seizure event in the same time frame revealed the former had a higher risk for early recurrence (P = .007, HR 1.879 [CI 95% 1.193 to 2.961]). Similarly, multicovariate Cox regression analysis based on the seizure frequency change between the 6-month preoperative and postoperative periods revealed that patients who had none/single seizure preoperatively but experienced an increase in seizure frequency to few/several seizures during the postoperative period also had a significantly higher risk of early tumor recurrence, compared to patients who initially had no/single seizure preoperatively and continued to have no/one seizure postoperatively (HR 2.431 [95% CI: 1.318 to 4.482], P = .004).
Univariate analysis of the following categorical variables did not show a statistically significant correlation with PFS: seizure frequency (categorized into the 4 subgroups) in the 6-month pre- and postoperative period, tumor location, and extent of surgery (Fig. 1A, B, C and D, respectively; Table 2a). Of note, the recurrent tumors associated with change in seizure frequency that underwent re-resection had not transformed to a higher histologic grade. Finally, with respect to overall survival (OS) outcomes, univariate analysis of this cohort failed to demonstrate any significant correlation among the categorical variables, including gender, tumor location, surgery type, number of AEDs before surgery, seizure type before and after surgery, seizure frequency before and after surgery, and seizure frequency change between the 6-month pre- and postoperative periods (Supplementary Material, Table 3).
Discussion
In this study, we investigated the potential of seizure frequency change in the pre- and postoperative periods in predicting early tumor recurrence in LGGs. We identified 2 clinical factors significantly correlated with early tumor recurrence: (1) high seizure frequency (few/several events) during the 6-month postoperative period and (2) increase in seizure frequency from none/single events in the 6-month preoperative period to few/several events in the 6-month postoperative period. Both factors were statistically correlated with higher rates of early tumor recurrence in LGG patients (P = .007 and P = .004, respectively, Table 2b). These findings support the hypothesis that change in seizure frequency and early tumor recurrence may be correlated. Validation from large prospective studies is needed, as prior LGG trials have not consistently described seizure features, management, and outcome.14,17,18
Table 2b.
Multicovariate Cox Regression Analysis on Tumor Progression-Free Survival
| Covariate | Levels | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|
| Seizure Frequency 6 Months Postop (Categorized into 2 Groups)a | Few/Several vs None/Single | 1.879 (1.193, 2.961) | .007 |
| Seizure Frequency from 6 Months Preop to Postopa | None/Single → Few/Several vs Non/Single → None/Single | 2.431 (1.318, 4.482) | .004 |
| Few/Several → None/Single vs Non/Single → None/Single | 1.362 (0.795, 2.334) | .261 | |
| Few/Several → Few/Several vs Non/Single → None/Single | 1.824 (0.968, 3.438) | .063 |
Abbreviations: CI, confidence interval; postop, postoperatively; preop, preoperatively.
aPatients with tumor recurrence, lost to follow-up or deceased within 6 months postoperatively were excluded (n = 10).
Seizures are the most common presenting symptom of newly diagnosed LGGs,10,19 and are a result of excessive synchronized neuronal electric discharges that may remain focal or generalize, and may therefore manifest with various clinical presentations.20 Seizure control has major implications for quality of life in patients with tumor-related epilepsy, as intractable seizures are associated with significant morbidity.21 The possibility that a change in seizure frequency may correlate with early LGG recurrence, particularly in patients with an increase in seizure frequency following surgery, corroborates the importance of careful evaluation and documentation of seizure features, management, and outcome, both in the pre- as well as post-surgery periods in the LGG population. A recent publication by Avila et al that provides tools to better report seizures in clinical trials could also be applied to clinical practice.2
It is hypothesized that electrophysiologic alterations may be present even before structural lesions are radiographically identifiable, especially in cases of tumor-related epilepsy.8,22 In fact, lesions on structural MRI are perceptible only after the anatomic abnormality reaches a certain volumetric threshold, depending on its resolution. Interestingly, 40% to 60% of the pathological specimens of nonlesional epilepsy patients with localizing EEG abnormalities who underwent surgery demonstrated microscopic abnormalities such as focal cortical dysplasia, microdysgenesis, and sclerosis.23 Hence, an abnormal EEG—and a seizure—may serve as a reliable clinical predictor of early tumor progression, as it may result from a structural abnormality not sizable enough to be identified by anatomy-based neuroimaging tests.
As in prior studies, the majority of our patients (n = 96, 64.9%) underwent partial resection, often because the tumor was located close to or within functional parenchymal areas, based on surgical reports using intraoperative stimulation and cortical mapping.24–26 Residual tumor mass, especially in cortical locations, may increase the risk for seizure recurrence and intractable epilepsy3 while the extent of the resection may directly affect both tumor and seizure outcomes, although no consensus exists because of the lack of large prospective studies.3,24,27–29 In this same line, growing attention has been given to the concept of “supratotal” resective surgery, which uses electrophysiologic procedures such as electrocorticography, subdural/depth electrodes, and electric intraoperative stimulation, among others, to combine a functional assessment of the tumor and peritumoral area with the more conventional anatomic-based approach in neuro-oncologic surgery.30–32 This study did not identify OS or PFS differences based on extent of resection (P = .835, Table 2a).
Timing of surgery is also important, as early surgical intervention has been shown to improve survival outcomes in LGGs, compared to watchful waiting.33,34 Likewise, the recognition and early management of tumor recurrence may have a positive impact on survival outcomes in this population. With this in mind, identifying reliable clinical predictors of tumor recurrence would be a valuable tool. The majority of patients with tumor-related epilepsy have an identifiable structural lesion on MRI, although in some cases the epileptogenic zone, as well as tumor cells, may localize beyond the structurally defined areas of abnormality, creating a fertile environment for seizure—and tumor—recurrence.11,35–37 Therefore, identifying patients who may be at higher risk for early tumor recurrence, based on seizure outcome soon after surgical intervention, could help guide more thorough surveillance with imaging studies and time-efficient tumor management with earlier surgical and medical interventions.
Several limitations apply to this study. These data were gathered retrospectively, and the total number of cases analyzed was relatively small. The median follow-up period was relatively short, which could underestimate tumor recurrence rate and OS. Additionally, our method of categorical classification of seizure frequency before and after surgery, the risk of recall bias based on the way seizure frequency was reported by patients, the lack of standardized reporting and confirmation of seizure events, and the lack of positive confirmatory EEG studies in this cohort may have culminated in underreporting of seizures both pre- and postoperatively. In parallel, the lack of molecular stratification of tumors and the lack of pathological confirmation of tumor progression in all cases may also affect our findings in regard to survival associations. For instance, oligodendrogliomas are more often associated with seizures yet carry a better prognosis than IDH-wt astrocytomas.27,38,39 Most of our patients underwent pathological investigation prior to 2014, when molecular testing was not routinely performed, which could affect PFS and OS in this study since, for instance, IDH-m tumors have longer PFS and OS than IDH-wt based on increased susceptibility to alkylating chemotherapy.17,18 Owing to lack of molecular information, lack of power in each adjuvant therapy subgroup, and the fact that the majority of patients (83%) were underwent chemotherapy and/or radiation, we did not report the effect of adjuvant therapy on PFS and OS in this cohort, as the results would be of limited value and it was not the focus of this study. Nonetheless, our findings support an association between change in seizure frequency and early LGG recurrence. Further studies focusing on this relationship are needed to validate these findings.
Funding
None declared.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1. Kahlenberg CA, Fadul CE, Roberts DW, et al. Seizure prognosis of patients with low-grade tumors. Seizure. 2012;21(7):540–545. [DOI] [PubMed] [Google Scholar]
- 2. Avila EK, Chamberlain M, Schiff D, et al. Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro Oncol. 2017;19(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108(2):227–235. [DOI] [PubMed] [Google Scholar]
- 4. Van Breemen MS, Wilms EB, Vecht CJ. Seizure control in brain tumors. Handb Clin Neurol. 2012;104:381–389. [DOI] [PubMed] [Google Scholar]
- 5. Meguins LC, Adry RA, Silva Júnior SC, et al. Gross-total resection of temporal low grade gliomas is a critically important factor in achieving seizure-freedom. Arq Neuropsiquiatr. 2015;73(11):924–928. [DOI] [PubMed] [Google Scholar]
- 6. Wang ZF, Chen HL. Relationship between IDH1 mutation and preoperative seizure in low-grade gliomas: a meta-analysis. Clin Neurol Neurosurg. 2016;148:79–84. [DOI] [PubMed] [Google Scholar]
- 7. Zhong Z, Wang Z, Wang Y, You G, Jiang T. IDH1/2 mutation is associated with seizure as an initial symptom in low-grade glioma: a report of 311 Chinese adult glioma patients. Epilepsy Res. 2015;109:100–105. [DOI] [PubMed] [Google Scholar]
- 8. Politsky JM. Brain tumor-related epilepsy: a current review of the etiologic basis and diagnostic and treatment approaches. Curr Neurol Neurosci Rep. 2017;17(9):70. [DOI] [PubMed] [Google Scholar]
- 9. Maschio M. Brain tumor-related epilepsy. Curr Neuropharmacol. 2012;10(2):124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Englot DJ, Berger MS, Barbaro NM, et al. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg. 2011;115(2):240–244. [DOI] [PubMed] [Google Scholar]
- 11. Englot DJ, Han SJ, Berger MS, et al. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012;70(4):921–928; discussion 928. [DOI] [PubMed] [Google Scholar]
- 12. Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30(25):3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van den Bent MJ, Afra D, de Witte O, et al. ; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 15. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676–685. [DOI] [PubMed] [Google Scholar]
- 16. Komori T. Updated 2016 WHO classification of tumors of the CNS: turning the corner where molecule meets pathology. Brain Tumor Pathol. 2017;34(4):139–140. [DOI] [PubMed] [Google Scholar]
- 17. Fisher B, Chen H, Macdonald D, et al. MR-009. RTOG 0424: Phase II study of a temozolomide (TMZ)-based chemo-radiotherapy (RT) regimen for high risk low-grade gliomas (LGGs) preliminary results including quality of life (QOL) and neurological function (NCF). Neuro-Oncology. 2013;15(Suppl 3):77. [Google Scholar]
- 18. Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30(25):3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kemerdere R, Yuksel O, Kacira T, et al. Low-grade temporal gliomas: surgical strategy and long-term seizure outcome. Clin Neurol Neurosurg. 2014;126:196–200. [DOI] [PubMed] [Google Scholar]
- 20. Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470–472. [DOI] [PubMed] [Google Scholar]
- 21. You G, Sha Z, Jiang T. The pathogenesis of tumor-related epilepsy and its implications for clinical treatment. Seizure. 2012;21(3):153–159. [DOI] [PubMed] [Google Scholar]
- 22. Lhatoo SD, Moghimi N, Schuele S. Tumor-related epilepsy and epilepsy surgery. Epilepsia. 2013;54(Suppl 9):1–4. [DOI] [PubMed] [Google Scholar]
- 23. Carne RP, O’Brien TJ, Kilpatrick CJ, et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127(Pt 10):2276–2285. [DOI] [PubMed] [Google Scholar]
- 24. Pallud J, Audureau E, Blonski M, et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 2014;137(Pt 2):449–462. [DOI] [PubMed] [Google Scholar]
- 25. Kim SS, McCutcheon IE, Suki D, et al. Awake craniotomy for brain tumors near eloquent cortex: correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery. 2009;64(5):836–845; discussion 345–346. [DOI] [PubMed] [Google Scholar]
- 26. Capelle L, Fontaine D, Mandonnet E, et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013;118(6):1157–1168. [DOI] [PubMed] [Google Scholar]
- 27. Xu DS, Awad AW, Mehalechko C, et al. An extent of resection threshold for seizure freedom in patients with low-grade gliomas. J Neurosurg. 2018;128(4):1084–1090. [DOI] [PubMed] [Google Scholar]
- 28. Gorlia T, Wu W, Wang M, et al. New validated prognostic models and prognostic calculators in patients with low-grade gliomas diagnosed by central pathology review: a pooled analysis of EORTC/RTOG/NCCTG phase III clinical trials. Neuro Oncol. 2013;15(11):1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pignatti F, van den Bent M, Curran D, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. [DOI] [PubMed] [Google Scholar]
- 30. Duffau H. Long-term outcomes after supratotal resection of diffuse low-grade gliomas: a consecutive series with 11-year follow-up. Acta Neurochir (Wien). 2016;158(1):51–58. [DOI] [PubMed] [Google Scholar]
- 31. Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. Clinical article. J Neurosurg. 2011;115(2):232–239. [DOI] [PubMed] [Google Scholar]
- 32. Yordanova YN, Duffau H. Supratotal resection of diffuse gliomas—an overview of its multifaceted implications. Neurochirurgie. 2017;63(3):243–249. [DOI] [PubMed] [Google Scholar]
- 33. Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–1888. [DOI] [PubMed] [Google Scholar]
- 34. Jakola AS, Skjulsvik AJ, Myrmel KS, et al. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28(8):1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mittal S, Barkmeier D, Hua J, et al. Intracranial EEG analysis in tumor-related epilepsy: evidence of distant epileptic abnormalities. Clin Neurophysiol. 2016;127(1):238–244. [DOI] [PubMed] [Google Scholar]
- 36. Pallud J, Varlet P, Devaux B, et al. Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology. 2010;74(21):1724–1731. [DOI] [PubMed] [Google Scholar]
- 37. Duffau H. Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochir (Wien). 2012;154(4):575–584; discussion 584. [DOI] [PubMed] [Google Scholar]
- 38. Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ. The role of surgery in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015;125(3):503–530. [DOI] [PubMed] [Google Scholar]
- 39. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018;20(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.