Abstract
Background
Re-irradiation may be considered for select patients with recurrent high-grade glioma. Treatment techniques include conformal radiotherapy employing conventional fractionation, hypofractionated stereotactic radiotherapy (FSRT), and single-fraction stereotactic radiosurgery (SRS).
Methods
A pooled, population-weighted, multiple linear regression analysis of publications from 1992 to 2016 was performed to evaluate the relationships between re-irradiation technique and median overall survival (OS) and radionecrosis outcomes.
Results
Seventy published articles were analyzed, yielding a total of 3302 patients. Across all studies, initial treatment was external beam radiotherapy to a median dose of 60 Gy in 30 fractions, with or without concurrent chemotherapy. On multivariate analysis, there was a significant correlation between OS and radiotherapy technique after adjusting for age, re-irradiation biologically equivalent dose (EQD2), interval between initial and repeat radiotherapy, and treatment volume (P < .0001). Adjusted mean OS was 12.2 months (95% CI, 11.8–12.5) after SRS, 10.1 months (95% CI, 9.7–10.5) after FSRT, and 8.9 months (95% CI, 8.4–9.4) after conventional fractionation. There was also a significant association between radionecrosis and treatment technique after adjusting for age, re-irradiation EQD2, interval, and volume (P < .0001). Radionecrosis rate was 7.1% (95% CI, 6.6–7.7) after FSRT, 6.1% (95% CI, 5.6–6.6) after SRS, and 1.1% (95% CI, 0.5–1.7) after conventional fractionation.
Conclusions
The published literature suggests that OS is highest after re-irradiation using SRS, followed by FSRT and conventionally fractionated radiotherapy. Whether this represents superiority of the treatment technique or an uncontrolled selection bias is uncertain. The risk of radionecrosis was low for all modalities overall. Re-irradiation is a feasible option in appropriately selected patients.
Keywords: glioma, radiosurgery, recurrent, re-irradiation, stereotactic radiotherapy
High-grade gliomas are World Health Organization (WHO) grade III and IV tumors and are the most common malignant primary central nervous system tumor in adults.1 Current standard treatment is maximal safe surgical resection followed by external beam radiotherapy of 59.4 to 60 Gy in 30 to 33 fractions with concurrent and/or adjuvant chemotherapy, depending on histology and molecular status.2–6 Despite advances in understanding the biological and molecular basis of disease, prognosis remains generally poor. For glioblastoma (grade IV), median survival is 14.6 months and 26.5% of patients are alive at 2 years. Local failure remains the most common mode of recurrence with 90% of tumors reccuring within the initial site of disease.7
At recurrence, high-quality data to inform management are lacking.1 Management options include repeat surgery, re-irradiation, cytotoxic chemotherapy, targeted agents, or best supportive care.8 For patients suitable for further treatment, median survival following first recurrence is approximately 6 to 12 months in patients receiving second-line systemic therapy with or without repeat surgery,9,10 and less than 12 months for patients receiving re-irradiation.11,12
The most appropriate patients with recurrent high-grade glioma suitable for re-irradiation have been suggested to be those at least 6 months from initial treatment, with a Karnofsky Performance Status score greater than 60 and lesion diameter less than 40 mm.13 A review of the re-irradiation tolerance of the brain reports a low rate of radionecrosis following cumulative doses up to 100 Gy, and smaller volumes may be treated to higher doses without significantly greater risk.14 A number of additional re-irradiation studies have been published since this review and a range of radiotherapy techniques have been employed including conformal or intensity-modulated radiotherapy using conventional fractionation (1.8 to 2 Gy per fraction), hypofractionated stereotactic radiotherapy (FSRT), and single-fraction stereotactic radiosurgery (SRS).1 Whether outcomes vary significantly according to dose, dose per fraction, or radiotherapy technique is not known.
We aimed to update the current literature on outcomes after re-irradiation for recurrent high-grade glioma and perform a pooled statistical analysis of published studies to assess differences in survival and rate of radionecrosis according to radiotherapy technique.
Methods
A systematic review was performed to identify relevant articles published in all languages in peer-reviewed journals between January 1, 1980 and December 31, 2016. Studies were eligible for inclusion if they reported outcomes in patients aged at least 18 years who received re-irradiation for recurrent WHO Grade III or IV glioma after having previously received conventionally fractionated radiotherapy. Studies employing re-irradiation techniques other than conventional radiotherapy, FSRT, or SRS (Gamma Knife® and linear accelerator-based)—such as brachytherapy or particle therapy—were excluded. Studies in which patients received re-irradiation in combination with repeat surgery and/or systemic therapies were not excluded because these patients were incorporated to varying degrees in the majority of studies. In the event of repeat publications or data arising from the same cohort more than once, efforts were made to use only the most recent for analysis. Publications with less than 20 patients or for which the full text was not available for review were excluded for statistical robustness. Conference abstracts, traditional reviews, editorials, and letters to the editor were also excluded.
MEDLINE, PubMed, and the Cochrane Database of Systematic Reviews databases were searched using relevant MeSH and non-MESH terms. The PubMed and Cochrane search strategy utilized “glioma,” “high-grade glioma,” “high grade glioma,” “recurrent,” “reirradiation,” “re-irradiation,” “radiotherapy,” “radiosurgery,” “Gamma Knife,” and “Cyber Knife.” The MEDLINE search strategy utilized algorithm terms “Glio*.tw” AND “Re-irrad*.tw” OR “reirrad*.tw” to source relevant articles. Results were screened initially using the title and abstract according to the inclusion and exclusion criteria. A secondary screen was then performed reviewing the full text of remaining articles and reference lists for additional potentially relevant articles.
Median overall survival (OS) and rate of radionecrosis were extracted from each study along with additional relevant clinical and technical information. Re-irradiation and total combined dose was calculated using the linear-quadratic model taking an α/β = 2 to determine an equivalent total dose in 2-Gy fractions (EQD2). A formal meta-analysis methodology was not employed because included studies were not comparative.15 A population-weighted linear regression analysis was performed to evaluate the relationships between OS and radionecrosis rate (primary outcome variables) and radiotherapy modality, median age, median interval between radiotherapy treatments, total cumulative EQD2, re-irradiation EQD2, median dose per fraction, and median planning target volume (PTV) (explanatory variables). Treatment modality was the primary explanatory variable of interest and was divided into 3 groups: conventionally fractionated radiotherapy (Group 1), SRS (Group 2), and FSRT (Group 3). All statistical analyses were performed using Statistical Analysis System (SAS) software (SAS Version 3.4, SAS Institute Inc., Cary, NC, USA). Univariate analyses were performed using a one-way analysis of variance (ANOVA) or Kruskal-Wallis test. Pairwise comparison of variables was performed using Tukey’s post-hoc test. A multiple linear regression analysis weighted by study size was performed incorporating the explanatory variables (treatment modality, age, interval, and PTV). Independent analyses for total cumulative EQD2, re-irradiation EQD2, and re-irradiation dose per fraction were performed to create 3 multivariate models each for OS and radionecrosis. A statistical significance of P < .05 was used for all analyses.
Explanatory variables that did not reach statistical significance were removed unless they were confounders of the primary relationship between treatment modality type and outcome. Valid confounders were defined as explanatory variables in the multivariate analysis whose exclusion changed the measure of effect of modality type on the outcome of interest by more than 10%. These were tested independently and variables that were not valid confounders and not statistically significant were excluded from the multivariate model. Studies with missing data were excluded from individual models as appropriate. Coefficients of multiple determination or R-squared values were assessed to determine the percentage of variability accounted for by explanatory variables.
Results
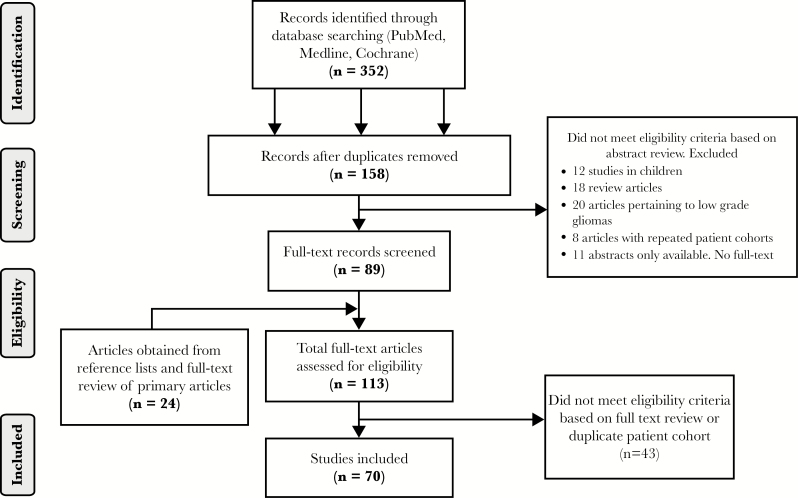
Initially 352 publications were identified. After screening, 70 were included, yielding 3302 patients (Figure 1 and Table 1). Patients initially received external beam radiotherapy to a dose of 48.3 to 60 Gy (median 60 Gy) using conventional fractionation, with or without concurrent chemotherapy. Regarding re-irradiation technique, conventional radiotherapy was employed in 20 studies (n = 1024), SRS in 23 studies (n = 1080), and FSRT in 27 studies (n = 1198). Overall baseline characteristics for outcome and explanatory variables are presented in Table 2. Across all studies, the median OS from recurrence was 10.8 months (range, 5.3–30) and the mean radionecrosis rate was 4.6% (range, 0–31.3%) (Table 2). For the individual groups, unadjusted mean OS was 10 months, 12.1 months, and 10.6 months and unadjusted mean radionecrosis rate was 0.9%, 10.6%, and 3.3% for the conventional, SRS, and FSRT groups, respectively (Table 3). OS, radionecrosis, and PTV were not reported in 2 (3%), 11 (15%), and 25 (35%) studies, respectively.
Fig. 1.
Search strategy and screening.
Table 1.
Studies Included for Analysis. *= Prospective Studies **=Randomized Trial
| Author | Year | Type | Sample size (n) Total / Grade IV | Age (years) | Initial XRT (Gy) | Re-irradiation (Gy) | Cumulative (Total) EQD2 (Gy) | Median interval (months) | Median PTV (ml) | Median Overall Survival (months) | Radionecrosis (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total dose | Dose per fraction | EQD2 | Total dose | Dose per fraction | EQD2 | |||||||||||
| Arcicasa 1 * | 1999 | Conventional | 24 | NR | 44 | 60 | 2 | 60 | 34.5 | 1.5 | 30.2 | 90.2 | 14 | NR | 8.5 | 0 |
| Neider 1 * | 1999 | Conventional | 32 | 21 | 44 | 58.5 | 1.3 | 48.3 | 45.5 | 1.3 | 37.5 | 85.8 | 20 | NR | 6.6 | 6.25 |
| Verninga 1 | 2001 | Conventional | 22 | 17 | 34 | 60 | 2 | 60 | 46 | 2 | 46 | 106 | 32.8 | NR | 11.3 | 4.54 |
| Combs 17 | 2005 | Conventional | 40 | 0 | 42 | 59.4 | 2 | 59.4 | 36 | 2 | 36 | 95.4 | 34.5 | 56.2 | 16 | 0 |
| Combs 1 | 2005 | Conventional | 101 | 59 | 50 | 60 | 2 | 60 | 36 | 2 | 36 | 96 | 19.14 | 49.3 | 11.32 | 1 |
| Combs 1 | 2005 | Conventional | 53 | 51 | 55 | 57 | 2 | 57 | 36 | 2 | 36 | 93 | 10 | 49 | 8 | 0 |
| Adkison 19 * | 2011 | Conventional | 76 | 0 | 45.5 | 59.4 | 2 | 59.4 | 50 | 2 | 50 | 109.4 | 18.2 | 369.2 | 10.4 | 0 |
| Maier-Hauff 1 * | 2011 | Conventional | 59 | 59 | 55.7 | NR | NR | - | 30 | 2 | 30 | - | NR | 46.5 | 13.4 | 0 |
| Niyazi 17 | 2012 | Conventional | 30 | 22 | 51.5 | 60 | 2 | 60 | 36 | 2 | 36 | 96 | NR | NR | NR | 0 |
| Niyazi 20 | 2012 | Conventional | 39 | 30 | 51 | 60 | 2 | 60 | 36 | 2 | 36 | 96 | 21.4 | NR | 7.7 | NR |
| Sholtyssek 21 | 2013 | Conventional | 64 | 11 | 53.4 | 60 | 2 | 60 | 36 | 2 | 36 | 96 | 13.4 | 110.4 | NR | 0 |
| Niyazi 22 | 2013 | Conventional | 58 | 46 | 52 | 60 | 2 | 60 | 36 | 2 | 36 | 96 | 21.4 | NR | 11.4 | 0 |
| Osman 23 | 2014 | Conventional | 29 | 20 | 42.1 | 60 | 2 | 60 | 36 | 2 | 36 | 96 | 13 | NR | 7.8 | 0 |
| Fleiger 24 * | 2014 | Conventional | 71 | 52 | 53 | 60 | 2 | 60 | 36 | 2 | 36 | 96 | NR | NR | 10.8 | 0 |
| Niyazi 25 | 2014 | Conventional | 31 | 25 | 51 | 60 | 2 | 60 | 36 | 2 | 36 | 96 | 34 | 118.1 | 11.5 | NR |
| Wick 26 ** | 2014 | Conventional | 91 | 91 | 57.6 | NR | NR | - | 36 | 2 | 36 | - | NR | NR | 6.9 | NR |
| Magnussen 27 | 2014 | Conventional | 23 | 23 | 53 | 60 | 2 | 60 | 54 | 2 | 54 | 114 | 11.8 | 424 | 14.7 | 0 |
| Aktan 28 | 2015 | Conventional | 21 | 18 | 48 | 60 | 2 | 60 | 54 | 2 | 54 | 114 | 39.4 | NR | 9 | 0 |
| Schnell 29 | 2016 | Conventional | 105 | 85 | 48.5 | 60 | 2 | 60 | 36 | 2 | 36 | 96 | 17.7 | 105.8 | 10.3 | 0 |
| Jeongshim 30 | 2016 | Conventional | 29 | 21 | 42.5 | 59.4 | 2 | 59.4 | 45 | 1.8 | 42.8 | 102.2 | 30.5 | NR | 13.7 | 10.34 |
| Alexander 1 | 1992 | SRS | 25 | 16 | 45 | 59.4 | 2 | 59.4 | 13 | 13 | 48.8 | 108.2 | 14 | 10 | 9 | 12 |
| Hall 17 | 1995 | SRS | 35 | 26 | 47 | 60 | 1.8 | 57 | 20 | 20 | 110 | 167 | 8 | 28 | 6.5 | 14 |
| Shreive 1 | 1995 | SRS | 86 | 86 | 46 | NR | NR | - | 13 | 13 | 48.8 | - | 10.3 | 10.1 | 10.2 | 22 |
| Larson 1 | 1996 | SRS | 93 | 66 | 49.9 | NR | NR | - | 16 | 16 | 72 | - | NR | 6.7 | 13 | NR |
| Kondziolka 1 | 1997 | SRS | 42 | 19 | 47.8 | 60 | 2 | 60 | 15.2 | 15.2 | 65.4 | 125.4 | 14.3 | 6 | 30 | 7.1 |
| Van Kampen 17 * | 1998 | SRS | 27 | 27 | NR | 60 | 2 | 60 | 16 | 16 | 72 | 132 | 9.6 | 21 | 9 | 0 |
| Cho 1 | 1999 | SRS | 46 | 27 | 48 | 60 | 1.8 | 57 | 17 | 17 | 80.8 | 137.8 | 10 | 30 | 11 | 4.3 |
| Park 1 | 2000 | SRS | 23 | 23 | 53 | NR | NR | - | 15 | 15 | 63.8 | - | NR | 9.9 | 10.3 | NR |
| Larson 1 * | 2002 | SRS | 26 | 14 | 48.8 | NR | NR | - | 15 | 15 | 63.8 | - | NR | 12.3 | 12.95 | NR |
| Combs 1 | 2005 | SRS | 32 | 32 | 56 | 54 | 2 | 54 | 15 | 15 | 63.8 | 117.8 | 10 | 10 | 10 | 0 |
| Hseih 1 | 2005 | SRS | 26 | 26 | 58 | 60 | 2 | 60 | 12 | 12 | 42 | 102 | NR | 21.6 | 10 | 31.3 |
| Mahajan 1 * | 2005 | SRS | 41 | 41 | 54 | 60 | 2 | 60 | NR | NR | - | - | 10 | 4.7 | 11 | NR |
| Kong 1 * | 2008 | SRS | 114 | 65 | 49 | 60 | 2 | 60 | 16 | 16 | 72 | 132 | NR | 10.6 | 16.25 | 24.4 |
| Patel 1 | 2009 | SRS | 26 | 26 | 53 | 60 | 2 | 60 | 18 | 18 | 90 | 150 | 12.5 | 10.4 | 8.4 | NR |
| Villavicencio 17 | 2009 | SRS | 26 | 26 | 56.4 | 60 | 2 | 60 | 20 | 10 | 60 | 120 | 10 | 7 | 7 | NR |
| Pouratian 17 | 2009 | SRS | 26 | 26 | 60.1 | 60 | 2 | 60 | 17 | 17 | 80.8 | 140.8 | NR | 21.3 | 9.4 | 0 |
| Cuneo 31 | 2012 | SRS | 63 | 49 | 47 | 60 | 2 | 60 | 15 | 15 | 63.8 | 123.8 | NR | 4.8 | 10 | 10 |
| Skeie 32 | 2012 | SRS | 51 | 51 | 51 | 60 | 2 | 60 | 12.2 | 12.2 | 43.3 | 103.3 | 11 | 12.4 | 12 | 0 |
| Park 33 | 2012 | SRS | 44 | 44 | 64 | 60 | 2 | 60 | 15 | 15 | 63.8 | 123.8 | 16.8 | 9.5 | 12.2 | 0 |
| Elliott 34 | 2012 | SRS | 26 | 16 | 60.4 | 60 | 2 | 60 | 15 | 15 | 63.8 | 123.8 | 5.8 | 1.2 | 13.5 | 8 |
| Conti 35 * | 2012 | SRS | 23 | 23 | 58 | 60 | 2 | 60 | 20 | 10 | 60 | 120 | 7 | 13.8 | 12 | 4.3 |
| Martinez Carillo 36 | 2014 | SRS | 51 | 26 | 48 | 60 | 2 | 60 | 18 | 18 | 90 | 150 | 13.8 | 6 | 10 | 10 |
| Pinzi (sSRS)37 | 2015 | SRS | 42 | NR | 51 | 58 | 2 | 58 | 15 | 15 | 63.8 | 121.8 | 11 | 2 | 11.5 | 6 |
| Pinzi (mSRS)37 | 2015 | FSRT | 86 | NR | 51 | 58 | 2 | 58 | 23 | 7.66 | 55.5 | 113.5 | 11 | 11 | 11.5 | 6 |
| Glass 1* | 1997 | FSRT | 20 | 13 | 44 | NR | NR | - | 42 | NR | - | - | 8 | NR | 13.7 | 15 |
| Shepherd 1 | 1997 | FSRT | 33 | 0 | 37 | 55 | 1.8 | 52.25 | 35 | 5 | 61.25 | 113.5 | 29 | NR | 10.7 | 12 |
| Cho 17 | 1999 | FSRT | 25 | 15 | 53 | 60 | 1.8 | 57 | 37.5 | 2.5 | 42.1875 | 99.1875 | 19 | NR | 12 | 5 |
| Hudes 1* | 1999 | FSRT | 20 | 19 | 52 | 60 | 1.8 | 57 | 24–35 | 3–3.5 | 48.2 max | 105.2 max | 3.1 | NR | 10.5 | 0 |
| Selch 1 | 2000 | FSRT | 21 | 14 | 54 | 60 | 2 | 60 | 25 | 5.00 | 43.75 | 103.75 | 11 | NR | 6.7 | 0 |
| Lederman 1* | 2000 | FSRT | 88 | 88 | 56 | 60 | 1.8 | 57 | 24 | 6 | 48 | 105 | 7.8 | NR | 7 | 8 |
| Grosu 1* | 2005 | FSRT | 44 | 35 | 50 | 60 | 1.8–3 | 57–75 | 30 | 5 | 52.5 | 109.5–127.5 | 16 | NR | 8 | 0 |
| Wurm 1* | 2006 | FSRT | 25 | 20 | 45 | 60 | 1.8 | 57 | 25–30 | 5 | 43.75–52.5 | 100.75–109.5 | 12.8 | NR | 14.5 | 0 |
| Kohshi 1* | 2007 | FSRT | 25 | 11 | 46 | 60 | 2 | 60 | 22 | 2.8 | 26.1 | 86.1 | 11 | NR | 15.5 | 28 |
| Fokas 1* | 2009 | FSRT | 53 | 53 | 53 | 54 | 2 | 54 | 30 | 3 | 37.5 | 91.5 | NR | 35.01 | 9 | 0 |
| Gutin 1* | 2009 | FSRT | 25 | 20 | 56 | 59.4 | 1.8 | 56.4 | 30 | 6 | 60 | 116.4 | 14.5 | 34 | 12.5 | 0 |
| Henke 38 | 2009 | FSRT | 31 | 29 | 50 | 59 | 2 | 59 | 20 | 5 | 35 | 94 | 18 | NR | 5.3 | 0 |
| Fogh 1 | 2010 | FSRT | 147 | 105 | 53 | 60 | 2 | 60 | 35 | 3.5 | 48.1 | 108.1 | 8 | NR | 11 | 0 |
| Minniti 39* | 2011 | FSRT | 36 | 36 | 56 | 60 | 2 | 60 | 37.5 | 2.5 | 42.2 | 102.2 | 14 | 32.1 | 9.7 | 8 |
| Mckenzie 39* | 2013 | FSRT | 35 | 29 | 62 | 59.4 | 1.8 | 56.4 | 30 | 6 | 60 | 116.4 | NR | 8.54 | 8.6 | 9 |
| Shapiro 40 | 2013 | FSRT | 24 | 20 | 56 | 59.4 | 1.8 | 56.4 | 30 | 6 | 60 | 116.4 | 12.6 | 35 | 12.2 | 0 |
| Minniti 47 | 2015 | FSRT | 54 | 42 | 54 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | 14 | 33.1 | 9.6 | 5 |
| Ertas 41 | 2014 | FSRT | 42 | 30 | 52 | 60 | 2 | 60 | 18 | 6 | 36 | 96 | 19 | 42 | 8.85 | 4.7 |
| Miwa 42* | 2014 | FSRT | 21 | 21 | 53.9 | 60 | 2 | 60 | 30 | 6 | 60 | 120 | 12 | 27.4 | 11 | 9.5 |
| Yacizi 43* | 2014 | FSRT | 37 | 37 | NR | 60 | 2 | 60 | 30 | 6 | 60 | 120 | NR | NR | 10.6 | 2.7 |
| Palmer 44 | 2015 | FSRT | 231 | 208 | 54.125 | 60 | 2 | 60 | 35 | 3.5 | 48.1 | 108.1 | 10.2 | NR | 10.77 | 0 |
| Antoni 45 | 2015 | FSRT | 20 | 12 | 55.7 | 60 | 2 | 60 | 31.2 | 6.25 | 64.4 | 124.4 | 18.3 | 2.71 | 15.6 | NR |
| Scorsetti 46 | 2015 | FSRT | 21 | 21 | 50 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | 13 | NR | 11 | 0 |
| Dincoglan 48 | 2015 | FSRT | 28 | 28 | 55.6 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | 11.2 | 36.5 | 10.3 | 11 |
| Zwirner 49 | 2016 | FSRT | 36 | NR | 55.9 | 60 | 2 | 60 | NR | NR | - | - | NR | 55.1 | 10.7 | 0 |
| Navarria 50 | 2016 | FSRT | 25 | 13 | 50 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | NR | 35 | 18 | 0 |
| Pinzi (mSRS)37 | 2015 | FSRT | 86 | NR | 51 | 58 | 2 | 58 | 23 | 7.66 | 55.5 | 113.5 | 11 | 11 | 11.5 | 6 |
| Glass 1* | 1997 | FSRT | 20 | 13 | 44 | NR | NR | - | 42 | NR | - | - | 8 | NR | 13.7 | 15 |
| Shepherd 1 | 1997 | FSRT | 33 | 0 | 37 | 55 | 1.8 | 52.25 | 35 | 5 | 61.25 | 113.5 | 29 | NR | 10.7 | 12 |
| Cho 17 | 1999 | FSRT | 25 | 15 | 53 | 60 | 1.8 | 57 | 37.5 | 2.5 | 42.1875 | 99.1875 | 19 | NR | 12 | 5 |
| Hudes 1 * | 1999 | FSRT | 20 | 19 | 52 | 60 | 1.8 | 57 | 24–35 | 3–3.5 | 48.2 max | 105.2 max | 3.1 | NR | 10.5 | 0 |
| Selch 1 | 2000 | FSRT | 21 | 14 | 54 | 60 | 2 | 60 | 25 | 5.00 | 43.75 | 103.75 | 11 | NR | 6.7 | 0 |
| Lederman 1 * | 2000 | FSRT | 88 | 88 | 56 | 60 | 1.8 | 57 | 24 | 6 | 48 | 105 | 7.8 | NR | 7 | 8 |
| Grosu 1 * | 2005 | FSRT | 44 | 35 | 50 | 60 | 1.8–3 | 57–75 | 30 | 5 | 52.5 | 109.5–127.5 | 16 | NR | 8 | 0 |
| Wurm 1 * | 2006 | FSRT | 25 | 20 | 45 | 60 | 1.8 | 57 | 25–30 | 5 | 43.75–52.5 | 100.75–109.5 | 12.8 | NR | 14.5 | 0 |
| Kohshi 1 * | 2007 | FSRT | 25 | 11 | 46 | 60 | 2 | 60 | 22 | 2.8 | 26.1 | 86.1 | 11 | NR | 15.5 | 28 |
| Fokas 1 * | 2009 | FSRT | 53 | 53 | 53 | 54 | 2 | 54 | 30 | 3 | 37.5 | 91.5 | NR | 35.01 | 9 | 0 |
| Gutin 1 * | 2009 | FSRT | 25 | 20 | 56 | 59.4 | 1.8 | 56.4 | 30 | 6 | 60 | 116.4 | 14.5 | 34 | 12.5 | 0 |
| Henke 38 | 2009 | FSRT | 31 | 29 | 50 | 59 | 2 | 59 | 20 | 5 | 35 | 94 | 18 | NR | 5.3 | 0 |
| Fogh 1 | 2010 | FSRT | 147 | 105 | 53 | 60 | 2 | 60 | 35 | 3.5 | 48.1 | 108.1 | 8 | NR | 11 | 0 |
| Minniti 39 * | 2011 | FSRT | 36 | 36 | 56 | 60 | 2 | 60 | 37.5 | 2.5 | 42.2 | 102.2 | 14 | 32.1 | 9.7 | 8 |
| Mckenzie 39 * | 2013 | FSRT | 35 | 29 | 62 | 59.4 | 1.8 | 56.4 | 30 | 6 | 60 | 116.4 | NR | 8.54 | 8.6 | 9 |
| Shapiro 40 | 2013 | FSRT | 24 | 20 | 56 | 59.4 | 1.8 | 56.4 | 30 | 6 | 60 | 116.4 | 12.6 | 35 | 12.2 | 0 |
| Minniti 47 | 2015 | FSRT | 54 | 42 | 54 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | 14 | 33.1 | 9.6 | 5 |
| Ertas 41 | 2014 | FSRT | 42 | 30 | 52 | 60 | 2 | 60 | 18 | 6 | 36 | 96 | 19 | 42 | 8.85 | 4.7 |
| Miwa 42 * | 2014 | FSRT | 21 | 21 | 53.9 | 60 | 2 | 60 | 30 | 6 | 60 | 120 | 12 | 27.4 | 11 | 9.5 |
| Yacizi 43 * | 2014 | FSRT | 37 | 37 | NR | 60 | 2 | 60 | 30 | 6 | 60 | 120 | NR | NR | 10.6 | 2.7 |
| Palmer 44 | 2015 | FSRT | 231 | 208 | 54.125 | 60 | 2 | 60 | 35 | 3.5 | 48.1 | 108.1 | 10.2 | NR | 10.77 | 0 |
| Antoni 45 | 2015 | FSRT | 20 | 12 | 55.7 | 60 | 2 | 60 | 31.2 | 6.25 | 64.4 | 124.4 | 18.3 | 2.71 | 15.6 | NR |
| Scorsetti 46 | 2015 | FSRT | 21 | 21 | 50 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | 13 | NR | 11 | 0 |
| Dincoglan 48 | 2015 | FSRT | 28 | 28 | 55.6 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | 11.2 | 36.5 | 10.3 | 11 |
| Zwirner 49 | 2016 | FSRT | 36 | NR | 55.9 | 60 | 2 | 60 | NR | NR | - | - | NR | 55.1 | 10.7 | 0 |
| Navarria 50 | 2016 | FSRT | 25 | 13 | 50 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | NR | 35 | 18 | 0 |
| Miwa 42 * | 2014 | FSRT | 21 | 21 | 53.9 | 60 | 2 | 60 | 30 | 6 | 60 | 120 | 12 | 27.4 | 11 | 9.5 |
| Yacizi 43 * | 2014 | FSRT | 37 | 37 | NR | 60 | 2 | 60 | 30 | 6 | 60 | 120 | NR | NR | 10.6 | 2.7 |
| Palmer 44 | 2015 | FSRT | 231 | 208 | 54.125 | 60 | 2 | 60 | 35 | 3.5 | 48.1 | 108.1 | 10.2 | NR | 10.77 | 0 |
| Antoni 45 | 2015 | FSRT | 20 | 12 | 55.7 | 60 | 2 | 60 | 31.2 | 6.25 | 64.4 | 124.4 | 18.3 | 2.71 | 15.6 | NR |
| Scorsetti 46 | 2015 | FSRT | 21 | 21 | 50 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | 13 | NR | 11 | 0 |
| Dincoglan 48 | 2015 | FSRT | 28 | 28 | 55.6 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | 11.2 | 36.5 | 10.3 | 11 |
| Zwirner 49 | 2016 | FSRT | 36 | NR | 55.9 | 60 | 2 | 60 | NR | NR | - | - | NR | 55.1 | 10.7 | 0 |
| Navarria 50 | 2016 | FSRT | 25 | 13 | 50 | 60 | 2 | 60 | 25 | 5 | 43.8 | 103.8 | NR | 35 | 18 | 0 |
Abbreviations: XRT, radiotherapy; EQD2, equivalent dose in 2Gy per fraction; PTV, planning target volume; SRS, stereotactic radiosurgery; FSRT, fractionated stereotactic radiotherapy.
Table 2.
Overall Outcome and Explanatory Variable Characteristics
| Variable (n = number of patients) | Median | Min | Max | Standard Deviation | IQR |
|---|---|---|---|---|---|
| Median overall survival (months) (n = 3190) |
10.8 | 5.3 | 30 | 3.2 | 2.5 |
| Radionecrosis (%) (n = 2860) | 0 (Mean = 4.6%) | 0 | 31.3 | 7.26 | 8 |
| Median age (years) (n = 3302) |
52 | 34 | 64 | 4.7 | 5.6 |
| Median interval (months) (n = 2494) | 13 | 3.1 | 39.4 | 6.5 | 8 |
| Total EQD2 (Gy) (n = 2827) | 108.1 | 80 | 167 | 15.5 | 20.4 |
| Re-irradiation EQD2 (Gy) (n = 3205) |
48.1 | 20 | 110 | 15.6 | 24 |
| Dose per fraction (Gy) (n = 3205) | 3.5 | 1.3 | 20 | 5.6 | 11 |
| Median PTV (ml) (n = 1961) |
21.6 | 1.22 | 424 | 81.0 | 39 |
Abbreviations: XRT, radiotherapy; EQD2, equivalent dose in 2Gy per fraction; PTV, planning target volume; SRS, stereotactic radiosurgery; FSRT, fractionated stereotactic radiotherapy.
Table 3.
Unadjusted Outcome Variable Characteristics by Treatment Technique
| Variable (n = number of patients) | Type | Median (Mean) | Min | Max | Standard Deviation | IQR | Overall P value | Pairwise P value |
|---|---|---|---|---|---|---|---|---|
| Median Overall Survival (months) (n = 3190) |
Conventional | 10.4 (10) | 5.3 | 16 | 2.6 | 3.6 | <.0001 | <.01 vs SRS <.01 vs FSRT |
| SRS | 11.5 (12.1) | 6.5 | 30 | 4.3 | 3.0 | <.01 vs FSRT | ||
| FSRT | 10.8 (10.6) | 6.7 | 18 | 2.14 | 1.4 | - | ||
| Radionecrosis (%) (n = 2860) | Conventional | 0 (0.9) | 0 | 10.3 | 2.1 | 1.0 | <.0001 | <.01 vs SRS <.01 vs FSRT |
| SRS | 8.0 (10.6) | 0 | 31.3 | 9.1 | 17.7 | <.01 vs FSRT | ||
| FSRT | 0 (3.3) | 0 | 28.0 | 5.5 | 5.0 | - |
Abbreviations: IQR, interquartile range; SRS, stereotactic radiosurgery; FSRT, Fractionated stereotactic radiotherapy.
Median re-irradiation EQD2 was 48.1 Gy (range, 20–110 Gy) and median total EQD2 was 108.1 Gy (range, 80–167 Gy). The median of the median PTV volume was 21.6 ml (range, 1.22–424 ml). Associations between the explanatory variables according to re-irradiation technique are summarized in Table 4. There was a statistically significant difference in cumulative EQD2, re-irradiation EQD2, dose per fraction, and median PTV between treatment groups (P < .0001).
Table 4.
Unadjusted Explanatory Variable Characteristics by Treatment Technique
| Variable (n = patients) | Type | Median | Min | Max | IQR | Overall P value | Pairwise P value |
|---|---|---|---|---|---|---|---|
| Median age (years) (n = 3302) |
Conventional | 50.0 | 34 | 57.6 | 6.9 | .06 | .18 vs SRS <.01 vs FSRT |
| SRS | 49.9 | 45 | 64.0 | 5.0 | <.01 vs FSRT | ||
| FSRT | 53.9 | 37 | 62.0 | 2.1 | - | ||
| Median interval (months) (n = 2494) | Conventional | 19.1 | 10 | 39.4 | 7.4 | <.0001 | <.01 vs SRS <.01 vs FSRT |
| SRS | 11 | 5.8 | 16.8 | 2.5 | <.01 vs FSRT | ||
| FSRT | 10.6 | 3.1 | 29.0 | 6.0 | |||
| Cumulative EQD2 (Gy) (n = 2827) | Conventional | 96 | 80 | 114 | 0 | <.0001 | <.01 vs SRS <.01 vs FSRT |
| SRS | 123.8 | 102 | 167 | 14.3 | <.01 vs FSRT | ||
| FSRT | 108.1 | 86.1 | 103.8 | 4.4 | - | ||
| Re-irradiation EQD2 (Gy) (n = 3205) |
Conventional | 36 | 20 | 54 | 0 | <.0001 | <.01 vs SRS <.01 vs FSRT |
| SRS | 63.8 | 42 | 110 | 16.5 | <.01 vs FSRT | ||
| FSRT | 48.1 | 26.1 | 64.4 | 4.4 | - | ||
| Dose per fraction (Gy) (n = 3205) | Conventional | 2.0 | 1.3 | 2 | 0 | <.0001 | <.01 vs SRS <.01 vs FSRT |
| SRS | 15.0 | 7.7 | 20 | 3 | <.01 vs FSRT | ||
| FSRT | 5 | 2.5 | 6.3 | 2.5 | |||
| Median PTV (ml) (n = 1961) |
Conventional | 105.8 | 46.5 | 424 | 61.1 | <.0001 | <.01 vs SRS <.01 vs FSRT |
| SRS | 10.1 | 1.22 | 30 | 4.3 | <.01 vs FSRT | ||
| FSRT | 35 | 2.7 | 55.1 | 9.9 | - |
Abbreviations: IQR, interquartile range; SRS, stereotactic radiosurgery; FSRT, fractionated stereotactic radiotherapy; EQD2, equivalent dose in 2Gy per fraction; PTV, planning target volume.
The results of the multivariate analysis are summarized in Table 5. Re-irradiation EQD2 and re-irradiation dose per fraction were independently substituted for total cumulative EQD2 in the multivariate models for OS and radionecrosis because these distinctions are clinically relevant. There were no concerns for collinearity between explanatory variables. There was a statistically significant difference in OS after re-irradiation according to treatment technique after adjusting for median age, total EQD2, median interval, and median PTV. This relationship remained when assessing re-irradiation EQD2 alone (P < .0001). The adjusted mean OS was 12.2 months (95% CI, 11.8–12.5) after SRS, 10.1 months (95% CI, 9.7–10.5) after FSRT, and 8.9 months (95% CI, 8.4–9.4) after conventional radiotherapy (P < .0001). Improved OS after re-irradiation was also associated with a greater interval between initial and repeat radiotherapy (OS gain of 0.25 months [~8 days] per month interval) (P < .0001).
Table 5.
Multivariate Analysis
| Median age | Median interval (months) | Total EQD2 (Gy) | Re-irradiation EQD2 (Gy) | Dose per fraction (Gy) | PTV (ml) | Type | |
|---|---|---|---|---|---|---|---|
| Model 1 (MOS) | 0.08-month decreased survival [95% CI, 0.03–0.13 months less] P = .0019 |
0.25-month increased survival [95% CI, 0.02–0.3 months greater] P < .0001 |
0.03-month increased survival [95% CI, 0.01–0.04 months greater] P = .0004 |
- | - | 0.014-month decreased survival [95% CI, 0.01–0.02 months less] P < .0001 |
*Conventional as reference SRS 3.8 month increased survival [95%CI, 3.0–4.6 months greater] FSRT 1.5 month increased survival [95%CI, 0.9–2.2 months greater] P < .0001 |
| Model 2 (MOS) | 0.02-month decreased survival [95% CI, 0.02–0.07 months less] P = .30 [Non-confounder] |
0.28-month increased survival [95% CI, 0.24–0.32 months greater] P < .0001 |
- | 0.014-month decreased survival [95% CI, 0.0003 months greater–0.03 less] P = .055 [Confounder] |
- | 0.014-month decreased survival [95% CI, 0.01– 0.02 months less] P < .0001 |
*Conventional as reference SRS 3.2 months increased survival [95%CI, 2.5–4.0 months greater] FSRT 1.2 months increased survival [95%CI 0.5–1.8 months greater] P < .0001 |
| Model 3 (MOS) | 0.02-month decreased survival [95% CI, 0.07 months less–0.02 greater] P = .28 [Non-confounder] |
0.28-month increased survival [95% CI, 0.24–0.32 months greater] P < .0001 |
- | - | 0.04-month decreased survival [95% CI, 0.1 months less– 0.03 greater P = .34 [Confounder] |
0.014-month decreased survival [95% CI, 0.01–0.02 months less] P < .0001 |
*Conventional as reference SRS 3.2 months increased survival [95%CI, 2.1–4.3 months greater] FSRT 1.1 months increased survival [95%CI, 0.5–1.8 months greater] P < .0001 |
| Model 4 (RN) | 0.37% decreased rate [95% CI, 0.3–0.41 less] P < .0001 |
0.23% decreased rate [95% CI, 0.19–0.27 less] P < .0001 |
0.10% increased rate [95% CI, 0.09–0.11 greater] P < .0001 |
- | - | 0.01% decreased rate [95%CI 0.009–0.013 less] P < .0001 |
*Conventional as reference SRS 0.4% increased rate [95%CI, 0.2–1.0% greater] FSRT 4% increased rate [95%CI, 3.4 to 4.4 greater] P < .0001 |
| Model 5 (RN) | 0.78% decreased rate [95% CI, 0.73–0.84 less] P < .0001 |
0.48% decreased rate [95% CI, 0.42–0.55 less] P < .0001 |
- | 0.03% increased rate [0.01– 0.05% greater] P ≤.0005 |
- | 0.01% decreased rate [95%CI 0.007–0.014 less] P < .0001 |
*Conventional as reference SRS 5.0% increased rate [95%CI, 4.1–5.9 greater] FSRT 6.0% increased rate [95% CI, 5.3–6.8 greater] P < .0001 |
| Model 6 (RN) | 0.78% decreased rate [95% CI, 0.72–0.84 less] P < .0001 |
0.48% decreased rate [95% CI, 0.42–0.54 less] P < .0001 |
- | - | 0.03% decreased rate [95% CI, 0.13% less-0.06% greater] P = .43 [Confounder] |
0.01% decreased rate [95% CI, 0.009–0.015 less] P < .0001 |
*Conventional as reference SRS 4.5% increased rate [95%CI, 3.2–5.9% greater] FSRT 5.8% increased rate [95%CI, 5.0–6.6% greater] P < .0001 |
There was a statistically significant association between radionecrosis rate and radiotherapy technique when adjusted for median age, total cumulative EQD2, median interval, and median PTV. The adjusted mean radionecrosis rate was 7.1% (95% CI, 6.6–7.7) for FSRT, 6.1% (95% CI, 5.6–6.6) for SRS, and 1.1% (95% CI, 0.5–1.7) for conventional radiotherapy. Radionecrosis rate after re-irradiation increased by 0.1% per Gy increase in total EQD2. Radionecrosis rate decreased with increasing interval between initial and repeat radiotherapy (reduction of 0.23% to 0.48% per month interval) (P < .0001).
There was no significant association between re-irradiation dose per fraction and OS (P = .34) or between re-irradiation dose per fraction and radionecrosis (P = .43); however, it was a valid confounder of the relationship between radiotherapy technique and the 2 outcome variables in their respective multivariate analyses. Increasing PTV volume at re-irradiation was associated with shorter OS (reduction of 0.014 months per ml PTV) (P < .0001) and a lower radionecrosis rate (reduction of 0.01% per ml PTV) (P < .0001). Age was not associated with OS but was associated with radionecrosis rate (rate decreased by 0.37% to 0.78% for each year older in age) (P < .0001). Explanatory variables accounted for 27% to 30% of the variability in OS and 55% to 59% in radionecrosis rate.
Discussion
This study demonstrates that survival after re-irradiation for recurrent high-grade glioma is similar to that observed following other treatments and that the risk of radionecrosis is low overall. There was a significant association between re-irradiation radiotherapy technique and both OS and radionecrosis. OS was longest in the SRS group, followed by the FSRT and conventional radiotherapy groups. FSRT and SRS were associated with higher rates of radionecrosis whether assessing total EQD2, median re-irradiation EQD2, or median re-irradiation dose per fraction and after adjustment for median age, median interval between initial and repeat radiotherapy, and median PTV. In the multivariate analysis, increasing total EQD2 and decreasing PTV were associated with improved OS. Although these reached statistical significance, the clinical relevance is uncertain because the magnitude of potential benefit is limited within the range of explanatory data analyzed. Assessment for effects in the PTV range 10 to 106 ml (median SRS and conventional values) is likely to be most reliable and is a clinically common range. The impact of re-irradiation EQD2 and re-irradiation dose per fraction were not significantly associated with improved OS after adjusting for radiotherapy technique and median PTV. This suggests further dose escalation is unlikely to be of clinical benefit, which is consistent with data in the front-line setting.16
Prolonged interval between initial and repeat radiotherapy was associated with improved OS, which may indicate a more favorable disease biology. Larger PTV was associated with inferior OS and may reflect a more advanced stage of recurrence. The superior survival of patients in studies pertaining to SRS were likely reflective of healthier patients with smaller lesions who may have received additional chemotherapy and surgical options. Our inclusion criteria permitted studies in which patients received other treatment modalities and the heterogeneity in cases of systemic treatment, interval surgery, and other comorbidities are likely to have a significant but unquantifiable contribution to OS.
SRS and FSRT were associated with a higher risk of radionecrosis than conventional radiotherapy. The magnitude of the range in total EQD2 observed in the data (80–167 Gy) could equate to a clinically meaningful difference in radionecrosis between the re-irradiation techniques of approximately 8.7%. Associations between radionecrosis and PTV and age were clinically small and potentially confounded by expected inferior OS because individual patient data is lacking. Accounting for the expected increased conformality of SRS and FSRT compared to conventional radiotherapy, their greater re-irradiation, and total EQD2 values, we speculate that total dose carries greater weight in the risk for radionecrosis than PTV or fraction size. This has been asserted in previous studies where cumulative doses of 100 Gy were associated with a greater rate of radionecrosis.14,17 In this analysis, 11 studies did not report radionecrosis data and only 5 of 28 (18%) studies observing radionecrosis included patients treated to a median total EQD2 dose below 100 Gy.
There are certain limitations to this analysis, which lacks individual patient data15 and is based on predominantly retrospective and small prospective studies. Explanatory variables account for approximately 27% and 55% of the observed variability in OS and radionecrosis data, respectively, suggesting that additional uncontrolled factors may confound these associations such as performance status, co-morbidities, and additional treatments including interval surgery and/or systemic therapy. Combining WHO grade III and IV recurrent gliomas in this analysis is justifiable because initial radiotherapy dose and treatment options at recurrence are similar, but OS at recurrence may differ according to histological and molecular information including IDH mutation and MGMT promoter methylation status.7 The validity of the linear quadratic model when applied to high doses per fraction is uncertain,18 but was a practical requirement for data aggregation and analysis. While survival outcomes are important, data relating to effects on functional independence, quality of life, and steroid dependence is lacking. Whether the differences in survival identified in this analysis represent true differences between treatment techniques or underlying selection biases is unclear. The diagnostic accuracy of differentiating between radiation necrosis and tumor recurrence is challenging.51 Conventional MRI is unable to differentiate between recurrence, early progression, or treatment effect; however, it is often the most common diagnostic modality used in practice in combination with clinical assessment. Readers are directed to Parvez et al (2014), which comprehensively discusses various diagnostic parameters and modalities that can be utilized to more accurately discern between radiological radiation necrosis and recurrence. Variability in the definition of radionecrosis used among studies used in this analysis could impact on the interpretability of the data presented. Prospective studies did not perform survival analyses of time-dependent radionecrosis development following completion of re-irradiation and these would be useful for future studies.
Although a potential survival benefit was associated with SRS re-irradiation when adjusting for confounding factors, cautious clinical judgement is required to select suitable patients for treatment in the context of tumor volume. Despite adjustments for PTV in the multivariate analysis, this comparison is only valid within a limited range of volumes where significant overlap for the treatment modalities exists in this analysis (ie, SRS PTV range 1.2 to 30 ml, median 10.1 ml). Patients with large volumes would not be considered appropriate for SRS due to an expected increased risk of toxicity. The median PTV values for each re-irradiation technique given in Table 4 may be considered an approximate guide for suitability based on the published literature. While there was an association between increasing treatment volume and reduced OS in the multivariate analysis, this represents an overall small magnitude of change; 0.014-month decreased survival with each ml increase in PTV.
SRS re-irradiation dose and treatment volume must both be considered with respect to the risk of radionecrosis. The median unadjusted radionecrosis rate for SRS was 8% for a median treatment volume of 10.1 ml and median fractional dose of 15 Gy. These values are consistent with those observed in the initial re-irradiation dose-finding SRS study for patients receiving doses ranging between 15 Gy for tumor volumes up to 33 ml (~40 mm diameter) and 24 Gy for tumor volumes less than 4 ml (~20 mm maximal diameter).52
Overall, all 3 re-irradiation techniques are reasonable options for appropriately selected patients, with an acceptable and low rate of radionecrosis. Re-irradiation technique selection may be influenced by fixed factors such as size of recurrence and PTV volume. However, in the absence of randomized data and where genuine clinical equipoise exists, hypofractionated or SRS approaches may be preferred for appropriate tumor volumes, particularly if the slightly higher risk of radionecrosis is deemed acceptable.
In conclusion, this population-weighted, pooled, multiple regression analysis demonstrates that re-irradiation is a feasible treatment option for select patients with recurrent high-grade glioma and the rate of radionecrosis is acceptable. Overall, all 3 re-irradiation techniques have clinical utility based on tumor size and PTV. Future studies should incorporate histological, molecular, and patient performance data and focus on comparison of re-irradiation to other treatments for recurrent high-grade glioma. Studies should also report data describing patient-reported outcomes and quality of life after treatment.
Funding
No sources of funding declared.
Acknowledgements
We acknowledge the assistance of Dr Anne Bernard, Senior Biostatistician at the Queensland Facility for Advanced Bioinformatics (QFAB), for guidance and review of all statistical methodology used in this study.
Conflict of interest statement. None declared.
References
- 1. Amichetti M, Amelio D. A review of the role of re-irradiation in recurrent high-grade glioma (HGG). Cancers (Basel). 2011;3(4):4061–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niyazi M, Brada M, Chalmers AJ et al. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother Oncol. 2016;118(1):35–42. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. van den Bent MJ, Baumert B, Erridge SC et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Bent MJ, Brandes AA, Taphoorn MJ et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 6. Cairncross G, Wang M, Shaw E et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brandes AA, Tosoni A, Franceschi E et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27(8):1275–1279. [DOI] [PubMed] [Google Scholar]
- 8. Seystahl K, Wick W, Weller M. Therapeutic options in recurrent glioblastoma–an update. Crit Rev Oncol Hematol. 2016;99:389–408. [DOI] [PubMed] [Google Scholar]
- 9. Stupp R, Wong ET, Kanner AA et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 10. Taal W, Oosterkamp HM, Walenkamp AM et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 11. Brada M, Stenning S, Gabe R et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol. 2010;28(30):4601–4608. [DOI] [PubMed] [Google Scholar]
- 12. Gallego O. Nonsurgical treatment of recurrent glioblastoma. Curr Oncol. 2015;22(4):e273–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhermain F, de Crevoisier R, Parker F et al. Role of radiotherapy in recurrent gliomas. Bull Cancer. 2004;91(11):883–889. [PubMed] [Google Scholar]
- 14. Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys. 2008;70(5):1350–1360. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Green S.. Cochrane Handbook for Systematic Reviews of Interventions. Vol 4 John Wiley & Sons; Chichester, UK; 2011. [Google Scholar]
- 16. Cho KH, Hall WA, Lo SS et al. Stereotactic radiosurgery versus fractionated stereotactic radiotherapy boost for patients with glioblastoma multiforme. Technol Cancer Res Treat. 2004;3(1):41–49. [DOI] [PubMed] [Google Scholar]
- 17. Sminia P, Mayer R. External beam radiotherapy of recurrent glioma: radiation tolerance of the human brain. Cancers (Basel). 2012;4(2):379–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Seminars in Radiation Oncology 2008; 18(4):240–243. [DOI] [PubMed] [Google Scholar]
- 19. Adkison JB, Tomé W, Seo S et al. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(3):835–841. [DOI] [PubMed] [Google Scholar]
- 20. Niyazi M, Söhn M, Schwarz SB et al. Radiation treatment parameters for re-irradiation of malignant glioma. Strahlenther Onkol. 2012;188(4):328–333. [DOI] [PubMed] [Google Scholar]
- 21. Scholtyssek F, Zwiener I, Schlamann A et al. Reirradiation in progressive high-grade gliomas: outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol. 2013;8:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niyazi M, Karin I, Söhn M et al. Analysis of equivalent uniform dose (EUD) and conventional radiation treatment parameters after primary and re-irradiation of malignant glioma. Radiat Oncol. 2013;8:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Osman MA. Phase II trial of temozolomide and reirradiation using conformal 3D-radiotherapy in recurrent brain gliomas. Ann Transl Med. 2014;2(5):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flieger M, Ganswindt U, Schwarz SB et al. Re-irradiation and bevacizumab in recurrent high-grade glioma: an effective treatment option. J Neurooncol. 2014;117(2):337–345. [DOI] [PubMed] [Google Scholar]
- 25. Niyazi M, Jansen NL, Rottler M et al. Recurrence pattern analysis after re-irradiation with bevacizumab in recurrent malignant glioma patients. Radiat Oncol. 2014;9:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wick W, Fricke H, Junge K et al. A phase II, randomized, study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res. 2014;20(24):6304–6313. [DOI] [PubMed] [Google Scholar]
- 27. Magnuson W, Ian Robins H, Mohindra P et al. Large volume reirradiation as salvage therapy for glioblastoma after progression on bevacizumab. J Neurooncol. 2014;117(1):133–139. [DOI] [PubMed] [Google Scholar]
- 28. Aktan M, Koc M, Kanyilmaz G. Survival following reirradiation using intensity-modulated radiation therapy with temozolomide in selected patients with recurrent high grade gliomas. Ann Transl Med. 2015;3(20):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schnell O, Thorsteinsdottir J, Fleischmann DF et al. Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol. 2016;130(3):591–599. [DOI] [PubMed] [Google Scholar]
- 30. Jeongshim L, Lee I, Chang J et al. Re-irradiation for recurrent gliomas: treatment outcomes and prognostic factors. Int J Radiat Oncol Biol Phys. 2013;87(2):S251. [Google Scholar]
- 31. Cuneo KC, Vredenburgh JJ, Sampson JH et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82(5):2018–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skeie BS, Enger PØ, Brøgger J et al. γ knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg. 2012;78(6):658–669. [DOI] [PubMed] [Google Scholar]
- 33. Park KJ, Kano H, Iyer A et al. Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol. 2012;107(2):323–333. [DOI] [PubMed] [Google Scholar]
- 34. Elliott RE, Parker EC, Rush SC et al. Efficacy of gamma knife radiosurgery for small-volume recurrent malignant gliomas after initial radical resection. World Neurosurg. 2011;76(1–2):128–140; discussion 61. [DOI] [PubMed] [Google Scholar]
- 35. Conti A, Pontoriero A, Arpa D et al. Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir (Wien). 2012;154(2):203–209. [DOI] [PubMed] [Google Scholar]
- 36. Martínez-Carrillo M, Tovar-Martín I, Zurita-Herrera M et al. Salvage radiosurgery for selected patients with recurrent malignant gliomas. Biomed Res Int. 2014;2014:657953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinzi V, Orsi C, Marchetti M et al. Radiosurgery reirradiation for high-grade glioma recurrence: a retrospective analysis. Neurol Sci. 2015;36(8):1431–1440. [DOI] [PubMed] [Google Scholar]
- 38. Henke G, Paulsen F, Steinbach JP et al. Hypofractionated reirradiation for recurrent malignant glioma. Strahlenther Onkol. 2009;185(2):113–119. [DOI] [PubMed] [Google Scholar]
- 39. McKenzie JT, Guarnaschelli JN, Vagal AS et al. Hypofractionated stereotactic radiotherapy for unifocal and multifocal recurrence of malignant gliomas. J Neurooncol. 2013;113(3):403–409. [DOI] [PubMed] [Google Scholar]
- 40. Shapiro LQ, Beal K, Goenka A et al. Patterns of failure after concurrent bevacizumab and hypofractionated stereotactic radiation therapy for recurrent high-grade glioma. Int J Radiat Oncol Biol Phys. 2013;85(3):636–642. [DOI] [PubMed] [Google Scholar]
- 41. Ertas G, Guney YY, Altungag MB et al. Survival following stereotactic radiotherapy for recurrent high grade gliomas. Int J Hematol Oncol. 2014;27(1):233–238. [Google Scholar]
- 42. Miwa K, Matsuo M, Ogawa S et al. Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol. 2014;9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yazici G, Cengiz M, Ozyigit G et al. Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J Neurooncol. 2014;120(1):117–123. [DOI] [PubMed] [Google Scholar]
- 44. Palmer JD, Siglin J, Yamoah K et al. Re-resection for recurrent high-grade glioma in the setting of re-irradiation: more is not always better. J Neurooncol. 2015;124(2):215–221. [DOI] [PubMed] [Google Scholar]
- 45. Antoni D, Jastaniah Z, Haoming QC et al. Patterns of relapse in patients with high grade glioma receiving combined treatments including stereotactic re-irradiation for a first relapse. Cancer Radiother. 2016;20(4):282–291. [DOI] [PubMed] [Google Scholar]
- 46. Scorsetti M, Navarria P, Pessina F et al. Multimodality therapy approaches, local and systemic treatment, compared with chemotherapy alone in recurrent glioblastoma. BMC Cancer. 2015;15:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Minniti G, Scaringi C, De Sanctis V et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol. 2013;111(2):187–194. [DOI] [PubMed] [Google Scholar]
- 48. Dincoglan F, Beyzadeoglu M, Sager O et al. Management of patients with recurrent glioblastoma using hypofractionated stereotactic radiotherapy. Tumori. 2015;101(2):179–184. [DOI] [PubMed] [Google Scholar]
- 49. Zwirner K, Paulsen F, Schittenhelm J et al. Prognostic parameters and outcome after re-irradiation for progressive glioblastoma. Acta Neurol Scand. 2017;136(3):239–245. [DOI] [PubMed] [Google Scholar]
- 50. Navarria P, Ascolese AM, Tomatis S et al. Hypofractionated stereotactic radiation therapy in recurrent high-grade glioma: a new challenge. Cancer Res Treat. 2016;48(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014;15(7):11832–11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shaw E, Scott C, Souhami L et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2008;47(2):291–298. [DOI] [PubMed] [Google Scholar]