Abstract
Vincristine (VCR), a microtubule inhibitor that arrests the cell cycle by blocking metaphase of mitosis, is unique among the vinca alkaloids for causing polyneuropathy. Patients with increased risk of VCR neurotoxicity include the elderly and those with prior history of neuropathy-prone medical conditions. Identifying such risk factors prior to the development of neurotoxicity should be a goal prior to VCR administration. Clinicians should obtain a thorough medical and family history of neuropathies in any child scheduled to receive neurotoxic medications to avoid exacerbating an underlying disorder. We report a case of a young child with newly diagnosed medulloblastoma who started treatment on a VCR-containing chemotherapy regimen following surgery and craniospinal radiation. She subsequently developed severe peripheral polyneuropathy and new enhancement of the cranial and nerve roots following a relatively low cumulative dose of VCR and was diagnosed with previously unidentified Charcot–Marie–Tooth disease (CMTD) Type 1A. This case highlights that an evaluation of risk factors should be completed prior to initiation of neurotoxic chemotherapies and advocates for testing for inherited neuropathies such as CMTD even in asymptomatic patients when hereditary neuropathy is suspected.
Keywords: Charcot-Marie-Tooth disease, chromosome 17p11.2, hereditary motor and sensory neuropathy, medulloblastoma, neurotoxicity, PMP22 gene, vincristine
Vincristine (VCR), a microtubule inhibitor that arrests the cell cycle by blocking metaphase of mitosis, is unique among the vinca alkaloids for causing polyneuropathy, especially after a cumulative dose exceeding 6 mg/m2.1,2 In tumor cells and axons, VCR binds to the ß-subunit of tubulin and prevents the microtubule aggregation that leads to mitotic arrest and cell death in tumor cells and in axons, impaired transport, and degeneration.2 Risk factors for VCR-induced neurotoxicity include cumulative doses greater than 6 to 8 mg/m2, older age, hepatic dysfunction, concomitant medications metabolized by CYP3A4 including fluconazole and cyclosporine-A, and inherited genetic disorders that cause demyelinating neuropathies.1–3 Identifying such risk factors prior to initiating treatment with VCR is important to prevent debilitating neuropathy. Clinicians should also obtain thorough personal medical and family histories of neuropathies in any patient scheduled to receive neurotoxic medications to avoid exacerbating an underlying neuropathic condition.
Charcot–Marie–Tooth disease (CMTD) is the prototype inherited neurological disorder that is associated with severe VCR-induced peripheral neuropathy.1 The incidence of CMTD is 1 in 2500 worldwide with no known ethnic predisposition.4 Prevalence in Europe has been reported to be 10 to 28 in 100 000 individuals.4 Herein, we report a case of a young child with newly diagnosed medulloblastoma with no prior history of neuropathy, who started treatment on a VCR-containing chemotherapy regimen following surgery and craniospinal irradiation. She subsequently developed severe peripheral polyneuropathy after a relatively low cumulative dose of the drug and was later diagnosed with CMTD.
Case Presentation
A 6-year-old African American girl was diagnosed with average-risk medulloblastoma after presenting with headaches and ataxia. Her physical examination on presentation did not reveal any findings consistent with a preexisting neuropathy. MRI scan of the brain and spine at diagnosis did not reveal any thickening or enhancement of cranial nerves, spinal nerve roots, or brachial plexuses. An initial routine family history was negative for cancers and genetic disorders. She was enrolled on a multi-institutional treatment protocol (SJMB12 study [NCT 01878617]) and received craniospinal irradiation (23.4 Gy) with a focal boost (54 Gy) to the tumor bed using proton beams without concurrent weekly VCR followed by 2 cycles of adjuvant chemotherapy using VCR (1 mg/m2/dose IV given on days 1 and 8 of each cycle), cisplatin (75 mg/m2 on the first day of each cycle along with amifostine [Ethyol, Clinigen Pharmaceuticals, USA] 600 mg/m2/dose for 2 doses given just prior to and at 3 hours following commencement of the cisplatin infusion), and cyclophosphamide (1.5 gm/m2/day on days 1 and 2 of each cycle). MRI scan of the brain and spine 6 weeks after the completion of radiotherapy showed no evidence of recurrent tumor but were notable for symmetric enhancement and thickening of cranial nerves VII, VIII, and IX along with thickening and enhancement of anterior and posterior cervical, thoracic, and lumbar spinal nerve roots and bilateral brachial plexuses in neck and axillary regions. Cerebrospinal fluid (CSF) was negative for malignancy. The neuroimaging changes were initially attributed to radiotherapy-induced inflammation but with unusually extensive involvement. Approximately 4 weeks after initiating chemotherapy (cumulative VCR dose of 3 mg/m2) and 10 weeks following radiotherapy, she developed lower extremity weakness that ascended to include her upper extremities. Examination revealed absence of deep tendon reflexes, evolving sensorimotor changes, and bilateral foot-drop. MRI of the brain and spine showed persistent thickening and enhancement of the lower cranial nerves and the anterior and posterior spinal nerve roots (Fig. 1–6). CSF findings obtained at this time were notable for elevated protein without pleocytosis or malignant cells, leading to a presumptive diagnosis of Guillain-Barré syndrome. She received IV immunoglobulin (1 gm/kg/day) for 3 consecutive days with a limited subjective improvement following treatment. Nerve conduction studies of upper and lower extremities revealed absent response in the median, ulnar, peroneal, tibial motor, and sural sensory nerves of all extremities. Electromyography revealed severely reduced recruitment of the motor units in the forearm, hands, and distal extremity musculature. These findings were suggestive of a severe subacute sensorimotor axonal and demyelinating polyneuropathy. Suspecting an underlying inherited genetic disorder causing neuropathy, peripheral blood was sent for genetic analysis and revealed a duplication of exons 1 to 5 of the peripheral myelin protein 22 (PMP22) gene confirming the presence of CMTD Type 1A (CMT1A). On further specific questioning related to neurologic symptoms in family members, her father and paternal grandfather were reported to have hammertoes, suggesting that they are presumptive carriers of this mutation. A genetic consultation was obtained to test parents and her siblings. Parents have deferred genetic testing at this time. A decision was made to exclude not only VCR but cisplatin as well from the patient’s remaining 2 cycles of treatment because of the risk of possible exacerbation of underlying peripheral neuropathy from both agents. At the time of this report, our patient has remained without disease 15 months posttreatment. Her most recent neuroimaging continues to show persistent thickening but decreasing enhancement of the lower cranial and spinal nerve roots. She is now doing well academically, ambulates independently without orthotic braces, and has shown significant functional improvement in all extremities with intensive physical and occupational therapies.
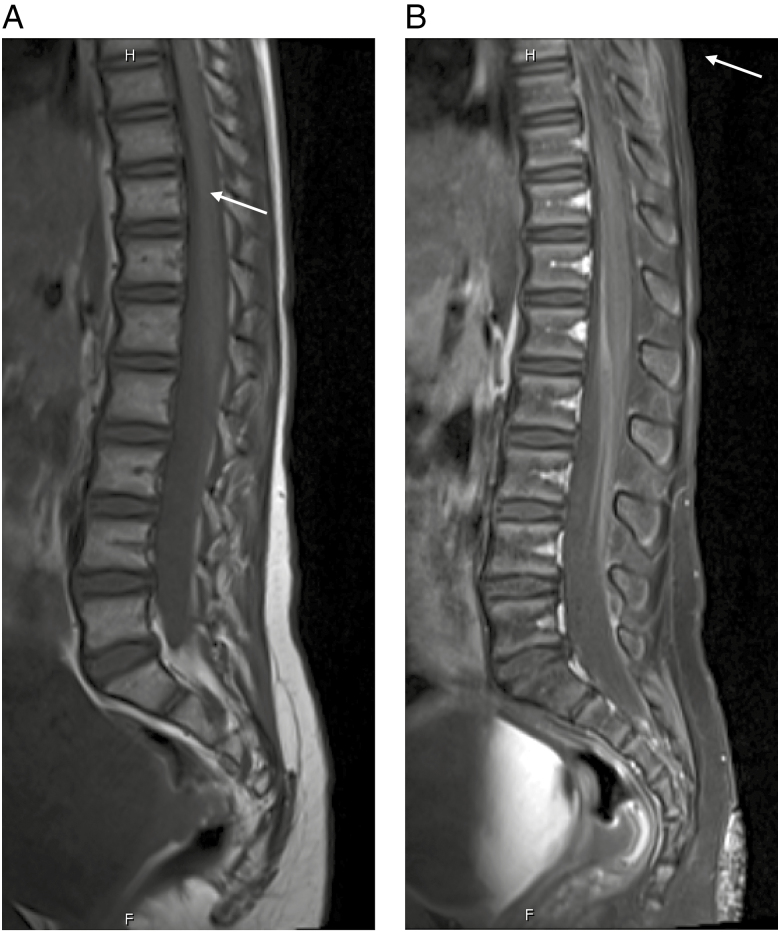
Fig. 1.
A, Precontrast T1-weighted image of the sagittal lumbar spine. B, Postcontrast T1-weighted image of the sagittal lumbar spine with thickened, enhancing nerve roots anteriorly.
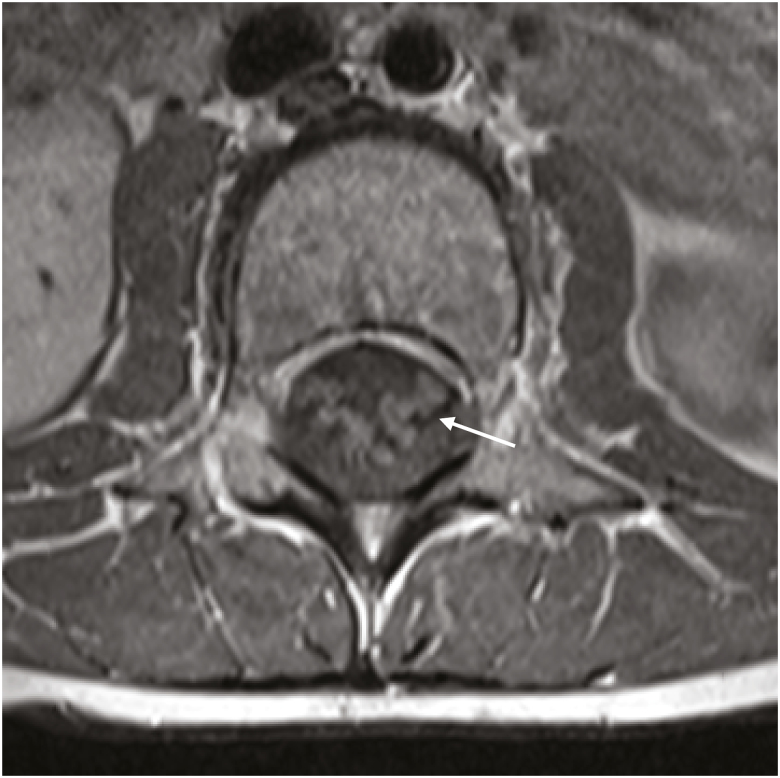
Fig. 2.
Axial postcontrast T1 image of the lumbar spine displaying enhancement of the nerve roots.
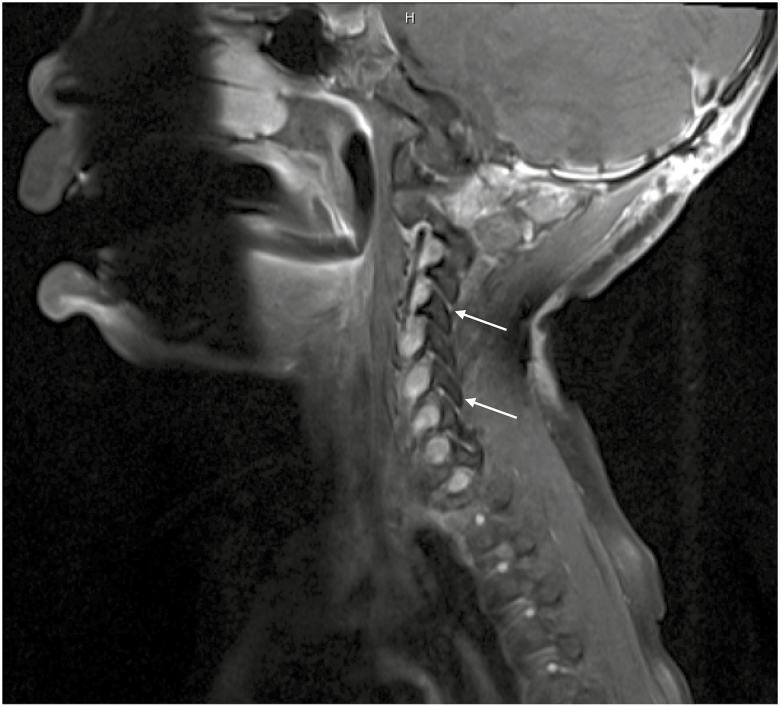
Fig. 3.
Postcontrast T1-weighted cervical spine sagittal image showing thickened, enhancing nerve roots in the neural foramina at multiple levels.
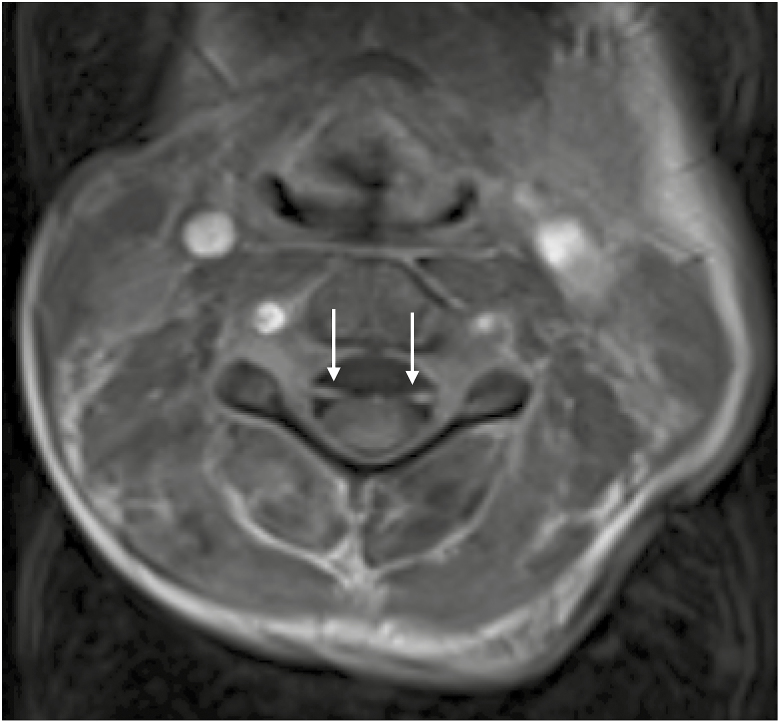
Fig. 4.
Axial postcontrast cervical spine image exhibiting enhancement of the anterior and posterior nerve roots.
Fig. 5.
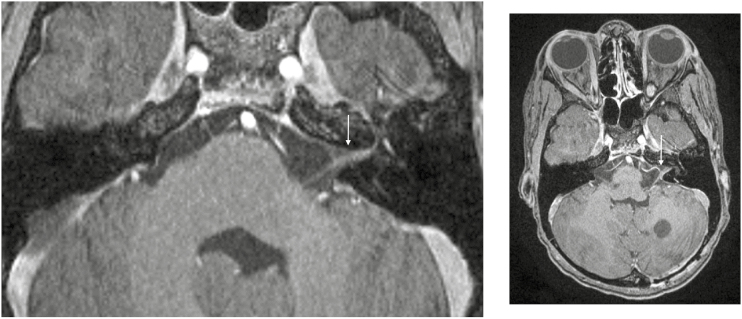
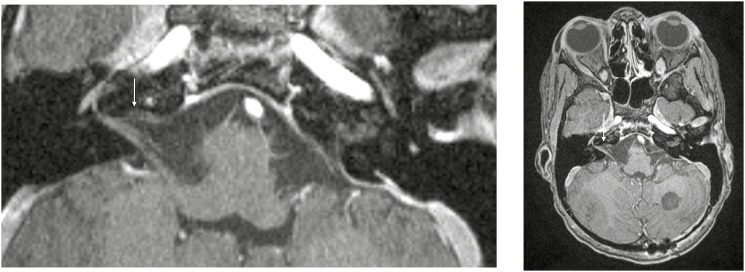
Postcontrast T1-weighted high-resolution images demonstrating left-sided enhancement of the seventh and eighth nerve complexes.
Fig. 6.
Postcontrast T1-weighted high-resolution images demonstrating right-sided enhancement of the seventh and eighth nerve complexes.
Discussion
The CMT diseases are the most common inherited degenerative disorders of the peripheral nervous system.4,5 While the disease can be caused by more than 70 mutations,5 90% of patients have mutations in 1 of the following 5 genes: PMP22, gap junction protein, beta 1 (GJB1), myelin protein zero (MPZ), mitochondrial fusion protein mitofusin 2 (MFN2), or ganglioside-induced differentiation-associated protein-1 (GDAP1).4 The 2 major subtypes of CMTD—CMT1 (demyelinating type) and CMT2 (axonal type)—are autosomal dominant and can be distinguished based on electrophysiologic findings and nerve biopsies.5,6 CMT1 is the most common (80% of cases) with subtypes that include CMT1A to E.5 CMT1A is associated with duplication of exons 1 to 5 of the PMP22 gene on chromosome 17p11.2–p12.5,7 Patients with CMT1A have motor nerve conduction delays that clinically manifest most frequently in the second decade of life as mild to moderate disability although the disease can be quite severe in some patients.5 Lower extremities are more affected than upper extremities, resulting in a steppage gait, sensory deficits, distal muscle atrophy with subsequent development of claw hands and hammertoes, and an “inverted champagne bottle” appearance of the distal limbs.5
Cases of CMTD and exacerbation of VCR toxicity have been reported in children with leukemia, lymphoma, Wilms tumor, Ewing sarcoma, and medulloblastoma who have received cumulative doses ranging from 3 to 9 mg/m2 prior to onset of neurotoxic symptoms.7–14 Similar to the reported cases, our patient was asymptomatic for CMTD prior to the diagnosis of medulloblastoma and experienced rapidly evolving quadriparesis following a relatively low cumulative dose of VCR. However, our patient is unique in that she is the youngest patient with medulloblastoma and CMTD to have developed this complication following VCR exposure. The extensive cranial and nerve root enhancement and thickening on neuroimaging postradiotherapy (and prior to VCR exposure) were initially considered to be radiation-induced inflammatory changes15 yet unusually more extensive than what is expected following craniospinal irradiation. In retrospect, these changes were more suggestive of the presence of CMT1A, which has been previously reported to cause hypertrophic neuropathy due to concentric layers of myelin around degenerating axons caused by repeated cycles of myelination and demyelination producing the “onion bulb” appearance of the nerve on histologic examination,5 even in patients with CMTD without prior radiation exposure.6,15,16 To our knowledge, this is the first description of extensive cranial and nerve root enhancement and thickening on imaging of a pediatric patient with CMTD after radiotherapy and VCR exposure. Aghajan et al did report similar spinal MRI findings in a 16-year-old girl with previously undiagnosed CMTD; however, the patient had leptomeningeal disease at diagnosis and imaging was more consistent with tumor involvement of the spinal cord.7
Electromyography typically reveals a demyelinating neuropathy in CMT1A disease.5 The presence of a predominantly axonal pattern in our patient despite having CMT1A might be due to possible confounding effects of direct axonal damage due to VCR and cisplatin.17 While VCR is the chemotherapeutic agent classically associated with exacerbation of neuropathy in CMTD, the role of cisplatin in exacerbating the neuropathy has also been reported and it was therefore prudent to avoid this drug as well in our patient.18 The exact mechanism of VCR-induced deterioration in these patients with CMTD is unclear. The gene duplication in CMT1A causes excess PMP22 accumulation in Schwann cells, demyelination, and subsequent axonal degeneration.19,20 Additive axonal damage by VCR might be the cause of worsening neuropathy.2 Although CMT1A is the most common form of CMTD associated with exacerbation of VCR toxicity, other mutations associated with CMTD involving the glycyl-TRNA synthetase (GAR) and early growth response 2 (EGR2) genes have also been implicated in VCR toxicity.21,22 Interestingly, VCR at a cumulative dose of 45 mg/m2 was tolerated without any neurotoxicity in a female child with Wilms tumor and a type of CMTD referred to as CMT1X caused by a point mutation on the GJB1 gene that codes for connexin 32 protein,23 suggesting that the spectrum of CMT mutations may have varying tolerance to neurotoxic agents with VCR-associated neurotoxicity being most prominent in CMT1A.
No known specific treatment exists for CMTD. A Cochrane review identified 38 clinical trials exploring various pharmacological agents and supportive care with none reporting significant benefit.24 The mainstays of treatment in such patients include intensive physical and occupational therapies, orthotics, and symptomatic treatment.5 It was gratifying that our patient made significant recovery with just these measures following cessation of chemotherapy. However, since even a single dose of VCR can cause debilitating neurotoxicity in the setting of CMTD, preventing such instances should be a priority for clinicians prescribing VCR. Obtaining a thorough clinical history, including existence of inherited neuropathies in the patient and family members, is paramount prior to initiating VCR-containing chemotherapy regimens. Genetic studies for CMTD should be considered even in asymptomatic patients when hereditary neuropathy is suspected to avoid exposing vulnerable patients to the drugs that exacerbate neuropathy.1
Funding
The authors have no financial relationships to disclose relevant to this case report.
Conflict of interest statement. None declared.
References
- 1. Weimer LH, Podwall D. Medication-induced exacerbation of neuropathy in Charcot Marie Tooth disease. J Neurol Sci. 2006;242(1-2):47–54. [DOI] [PubMed] [Google Scholar]
- 2. Mora E, Smith EM, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416–2430. [PMC free article] [PubMed] [Google Scholar]
- 3. Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pareyson D, Saveri P, Pisciotta C. New developments in Charcot–Marie–Tooth neuropathy and related diseases. Curr Opin Neurol. 2017;30(5):471–480. [DOI] [PubMed] [Google Scholar]
- 5. Tazir M, Hamadouche T, Nouioua S, Mathis S, Vallat JM. Hereditary motor and sensory neuropathies or Charcot–Marie–Tooth diseases: an update. J Neurol Sci. 2014;347(1-2):14–22. [DOI] [PubMed] [Google Scholar]
- 6. Tatewaki Y, Nishiyama S, Kato Y, et al. MR imaging of the cauda equina in Charcot–Marie–Tooth disease. Poster no. C-0741. Paper presented at: European Congress of Radiology, March 4-8, 2015, Vienna, Austria. [Google Scholar]
- 7. Aghajan Y, Yoon JM, Crawford JR. Severe vincristine-induced polyneuropathy in a teenager with anaplastic medulloblastoma and undiagnosed Charcot–Marie–Tooth disease. BMJ Case Rep. 2017;2017. Accessed January 20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olek MJ, Bordeaux B, Leshner RT. Charcot–Marie–Tooth disease type I diagnosed in a 5-year-old boy after vincristine neurotoxicity, resulting in maternal diagnosis. J Am Osteopath Assoc. 1999;99(3):165–167. [DOI] [PubMed] [Google Scholar]
- 9. Chauvenet AR, Shashi V, Selsky C, et al. ; Pediatric Oncology Group Study Vincristine-induced neuropathy as the initial presentation of Charcot–Marie–Tooth disease in acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Pediatr Hematol Oncol. 2003;25(4): 316–320. [DOI] [PubMed] [Google Scholar]
- 10. Sy A, Cheng J, Cooper R, Mueller L. Heterozygosity for CMT Type 4 predicts a severe vincristine-induced polyneuropathy phenotype: a case report and review of literature. J Pediatr Hematol Oncol. 2019;41(1):41–e43. [DOI] [PubMed] [Google Scholar]
- 11. Neumann Y, Toren A, Rechavi G, et al. Vincristine treatment triggering the expression of asymptomatic Charcot–Marie–Tooth disease. Med Pediatr Oncol. 1996;26(4):280–283. [DOI] [PubMed] [Google Scholar]
- 12. Dickerhoff R, Lindner W, Scheiber W. Severe vincristine neurotoxicity in a patient with Charcot–Marie–Tooth disease. Pediatr Hematol Oncol. 1988;5(1):61–64. [DOI] [PubMed] [Google Scholar]
- 13. Schiavetti A, Frascarelli M, Uccini S, et al. Vincristine neuropathy: neurophysiological and genetic studies in a case of Wilms tumor. Pediatr Blood Cancer. 2004;43(5):606–609. [DOI] [PubMed] [Google Scholar]
- 14. Trobaugh-Lotrario AD, Smith AA, Odom LF. Vincristine neurotoxicity in the presence of hereditary neuropathy. Med Pediatr Oncol. 2003;40(1):39–43. [DOI] [PubMed] [Google Scholar]
- 15. Kontzialis M, Poretti A, Michell H, Bosemani T, Tekes A, Huisman TA.. Spinal nerve root enhancement on MRI scans in children: a review. J Neuroimaging. 2016;26(2):169–179. [DOI] [PubMed] [Google Scholar]
- 16. Aho TR, Wallace RC, Pitt AM, Sivakumar K.. Charcot–Marie–Tooth disease: extensive cranial nerve involvement on CT and MR imaging. AJNR Am J Neuroradiol. 2004;25(3):494–497. [PMC free article] [PubMed] [Google Scholar]
- 17. Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, Balayssac D.. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Front Pharmacol. 2017;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yerushalmi R, Levi I, Wygoda M, Ifergane G, Wirguin I.. Are platinum-based chemotherapeutic drugs safe for patients with Charcot–Marie–Tooth disease? J Peripher Nerv Syst. 2007;12(2):139–141. [DOI] [PubMed] [Google Scholar]
- 19. Li J. Caveats in the established understanding of CMT1A. Ann Clin Transl Neurol. 2017;4(8):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SM, Lee J, Yoon BR, Kim YJ, Choi BO, Chung KW.. Severe phenotypes in a Charcot–Marie–Tooth 1A patient with PMP22 triplication. J Hum Genet. 2015;60(2):103–106. [DOI] [PubMed] [Google Scholar]
- 21. Nakamura T, Hashiguchi A, Suzuki S, Uozumi K, Tokunaga S, Takashima H.. Vincristine exacerbates asymptomatic Charcot–Marie–tooth disease with a novel EGR2 mutation. Neurogenetics. 2012;13(1):77–82. [DOI] [PubMed] [Google Scholar]
- 22. Holloway MP, DeNardo BD, Phornphutkul C, et al. An asymptomatic mutation complicating severe chemotherapy-induced peripheral neuropathy (CIPN): a case for personalised medicine and a zebrafish model of CIPN. NPJ Genom Med. 2016;1:16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ajitsaria R, Reilly M, Anderson J. Uneventful administration of vincristine in Charcot–Marie–Tooth disease type 1X. Pediatr Blood Cancer. 2008;50(4):874–876. [DOI] [PubMed] [Google Scholar]
- 24. Young P, De Jonghe P, Stögbauer F, Butterfass-Bahloul T. Treatment for Charcot–Marie–Tooth disease. Cochrane Database Syst Rev. 2008;(1):CD006052. [DOI] [PMC free article] [PubMed] [Google Scholar]