Abstract
Atypical teratoid/rhabdoid tumor (AT/RT) is a rare CNS cancer that typically occurs in children younger than 3 years of age. Histologically, AT/RTs are embryonal tumors that contain a rhabdoid component as well as areas with primitive neuroectodermal, mesenchymal, and epithelial features. Compared to other CNS tumors of childhood, AT/RTs are characterized by their rapid growth, short symptomatic prodrome, and large size upon presentation, often leading to brain compression and intracranial hypertension requiring urgent intervention. For decades, the mainstay of care has been a combination of maximal safe surgical resection followed by adjuvant chemotherapy and radiotherapy. Despite advances in each of these modalities, the relative paucity of data on these tumors, their inherently aggressive course, and a lack of molecular data have limited advances in treatment over the past 3 decades. Recent large-scale, multicenter interdisciplinary studies, however, have significantly advanced our understanding of the molecular pathogenesis of these tumors. Multiple clinical trials testing molecularly targeted therapies are underway, offering hope for patients with AT/RT and their families.
Keywords: ATRT, epigenetics, infant, pediatric, rhabdoid
Clinical Case Presentation
A 1-year-old girl presented to her local emergency department with a 1-week history of vomiting following a fall. She was initially treated symptomatically but returned to the emergency department for persistent vomiting. A head CT was performed that demonstrated a left frontotemporal mass with a small amount of left anterior temporal calcification. She was referred to a quaternary referral hospital where she underwent further evaluation.
Initial Supportive Care
Medical Management
Atypical teratoid/rhabdoid tumors (AT/RTs) are 1 of the fastest-growing tumors of the CNS, often presenting after a relatively short prodrome of symptoms.1–3 Owing to the tendency for these tumors to obstruct cerebrospinal fluid (CSF) flow, patients often require emergent treatment of intracranial hypertension and/or hydrocephalus until surgical decompression can be achieved. In cases in which brain herniation is imminent, hyperosmolar therapy may be initiated with a loading dose of mannitol followed by redosing every 6 hours as tolerated by serum osmolality. Alternatively, hypertonic saline, either 10% or 23.4%, may be initiated to rapidly treat cerebral edema if central access is available. Additionally, although not commonly present in patients with these tumors, dexamethasone may also be initiated for symptomatic vasogenic edema.
Supratentorial lesions that are not large enough to cause symptomatic hydrocephalus may cause seizures secondary to cortical compression or direct brain involvement. Our initial approach to seizures is typically a levetiracetam load followed by twice-daily dosing. If this is insufficient to control seizure activity, a second agent, typically lacosamide or phenytoin, is added until seizure activity is controlled.
Cerebrospinal Fluid Diversion
Symptomatic hydrocephalus is the most dangerous acute finding in patients with AT/RT and is very common upon initial presentation. Rapid tumor growth often causes ventricular obstruction over a short period of time, leading to acute-onset obstructive hydrocephalus requiring external ventricular drainage. At our institution, if anatomy is favorable, we typically prefer placement of a right frontal external ventricular drain in the operating room under general anesthesia. However, if the patient is acutely declining neurologically, the procedure is performed at bedside in the pediatric intensive care unit with pediatric intensivists available for airway management and sedation as needed. Antibiotic prophylaxis with cefazolin or vancomycin is continued for the duration of CSF drainage.
Clinical Case Relevance
Because of clinical stability, the patient presented here did not require immediate treatment of seizures, intracranial hypertension, or hydrocephalus upon presentation to our institution. Complex partial seizures were noted on postoperative day 5, however, and she was initiated on a regimen of levetiracetam and phenytoin. She was gradually weaned from these medications in the months following surgery and remains seizure free.
Initial Diagnostics
A rapid-sequence MRI is the initial test of choice for young patients demonstrating signs and symptoms of intracranial hypertension. Assessment of hydrocephalus and lesion localization are absolutely necessary prior to any intervention in these patients. To minimize radiation exposure to young patients, we recommend against routine use of noncontrast head CT as a diagnostic test, although this may be utilized in emergent situations or if no MRI is available. Gadolidium-enhanced MRI remains the gold-standard diagnostic imaging test for AT/RT and should be acquired as soon as possible after clinical stabilization to facilitate diagnosis and timely surgical planning. The entire neuraxis should be imaged to facilitate early detection of leptomeningeal disease, which is present in 10% to 24% of patients at the time of diagnosis and may ultimately occur in up to 35% of patients.2,3
On MRI, AT/RTs are characterized by their large size, intratumoral heterogeneity, and variable degree of contrast enhancement. Anatomically, AT/RTs arise both in the supratentorial and infratentorial compartments with roughly equal frequency.2–4 These tumors are classically very large on initial imaging, uniformly cause cerebral compression, and are usually associated with some degree of hydrocephalus.1,2,4 One pediatric study reported a median patient age of 2.9 years and found the average presenting tumor diameter to be greater than 3.5 cm,3 a large size relative to the pediatric cranium. On CT, AT/RTs display heterogeneous hyperdensity and often contain numerous small areas of peripheral calcification.1,4 On MRI, these tumors do not have a specific pattern of MR signal but are generally hypointense on T1-weighted imaging, hyperintense on T2-weighted imaging, and often demonstrate restriction on diffusion-weighted imaging.1,3,4 Tumor-associated cysts, which usually arise at the periphery, are often hyperintense on T1-weighted MRI relative to CSF.1 On the whole, AT/RTs are contrast enhancing, although this feature is highly variable both within and between tumors.1,3,4 Despite the degree of mass effect caused by these tumors, AT/RTs are not classically associated with a significant amount of cerebral edema, perhaps because of their rapid growth and compression of surrounding blood vessels.1 Interestingly, unlike most other intramedullary brain tumors, there are multiple reports of AT/RTs invading dura and bone, indicating that these tumors do not uniformly respect natural anatomic planes.1,4 AT/RTs arising in the posterior fossa may at first resemble medulloblastomas, but AT/RTs are much more likely to contain intratumoral hemorrhage, which may be present in up to 72% of cases.2 Owing to the young age of affected patients and variable locations of AT/RTs, these tumors may resemble other high-grade tumors on initial imaging such as ependymoma, embryonal tumor with multilayered rosettes, choroid plexus carcinoma, and high-grade glioma. While anatomic location may narrow the differential diagnosis to tumors that tend to arise within a certain compartment (ie, intraventricular or intraparenchymal), there remain few distinguishing features that reliably differentiate AT/RTs from other intraaxial high-grade tumors on imaging.
Clinical Case Relevance
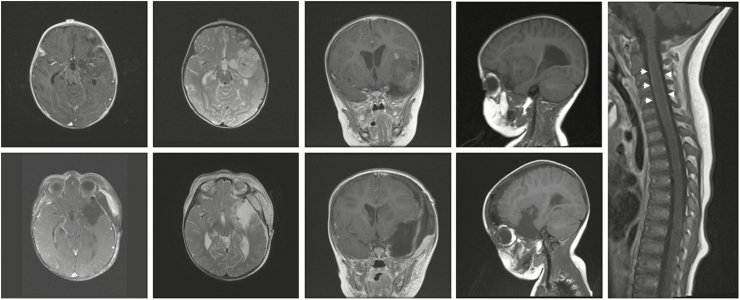
A brain MRI was obtained, which demonstrated a 4.4 × 2.4 × 4.2 cm left anterior temporal lobe mass extending into the insula and encasing the distal internal carotid and proximal middle cerebral arteries. The tumor demonstrated intrinsically low T1 and high T2 signal, patchy contrast enhancement, and minimal vasogenic edema. A full-spine MRI was obtained that showed diffuse leptomeningeal enhancement (Fig. 1).
Fig. 1.
Imaging Findings in a Patient With an Atypical Teratoid/Rhabdoid Tumor. Top row: Preoperative MRI demonstrated a heterogeneous, patchily enhancing left frontotemporal mass encasing branches of the middle cerebral artery with minimal surrounding cerebral edema. Right panel: Whole-spine MRI showed diffuse leptomeningeal enhancement of the spinal cord (arrowheads). Bottom row: Postoperative MRI demonstrated interval gross total resection of the mass of the tumor with improved mass effect.
Epidemiology
AT/RTs are rare cancers and are most commonly diagnosed in children aged 3 years or younger. According to data compiled from multiple large North American disease databases, AT/RTs account for 1.6% of all CNS tumors diagnosed in children and 10.1% of all CNS tumors diagnosed in children aged 1 year or younger.5 In an analysis utilizing an AT/RT-specific billing code, 586 cases of AT/RT were diagnosed in children between 2001 and 2010, yielding an age-adjusted incidence rate of 0.07 per 100,000 children.5 Greater than 80% are diagnosed at age 3 or younger, and the median age at diagnosis is 1 year.5,6 They are slightly more common in males and are most often diagnosed in children of European descent, although there is not a consistently significant difference in incidence by conventional demographic variables.5,6 Infratentorial location is more common in children 3 years of age or younger.5–7 These tumors may present as a primarily spinal lesion, especially in younger patients: According to 1 analysis, 4.6% of AT/RTs were documented as having primarily arisen in the spinal cord or cauda equina.5,7Metastatic disease was present upon diagnosis in 27% of cases in 1 large study.7
The majority of patients with AT/RT present without a personal or family history of cancer. However, a significant proportion of patients with rhabdoid tumors such as AT/RT will ultimately present with a second rhabdoid tumor as part of a rhabdoid tumor predisposition syndrome (RTPS). Patients with RTPS typically present within the first year of life with a spectrum of tumors including AT/RT, malignant rhabdoid tumor of the kidney (RTK or MRTK), and/or extrarenal rhabdoid tumors of the head, neck, liver, bladder, or retroperitoneum, among other locations.8,9 Roughly one-third of patients with a rhabdoid tumor will be diagnosed with a germline mutation that portends RTPS.10,11 Most patients with RTPS have the syndrome as a result of a de novo mutation,10,11 but when inherited, RTPS-associated mutations are inherited in an autosomal-dominant fashion with incomplete penetrance.12 Patients with AT/RT who have a germline mutation consistent with RTPS tend to present at a younger age, have more extensive disease, and are more likely to die from progressive disease compared to patients with sporadic rhabdoid tumors.12 Despite this, favorable outcome has been reported in a few patients with RTPS who have undergone aggressive treatment, and the consensus in the field is that a multimodal approach involving surgery, chemotherapy, and radiotherapy is important in extending life in these patients.12,13 Because of the relatively common presence of germline mutations in patients with apparently sporadic rhabdoid tumors, germline mutation assessment is performed in virtually all patients.10,14 Early body imaging with a screening ultrasound or MRI of the head, abdomen, and pelvis as well as a comprehensive assessment by an experienced medical genetics team is necessary upon initial assessment.9 Additionally, as germline mutations may be shared among siblings and across generations, it is necessary to initiate an early discussion with patients’ families regarding RTPS and potentially screening family members for germline mutations for the purpose of risk assessment and family planning.9 The molecular genetics of RTPS have been increasingly well defined over the last decade, and this is discussed in the context of the molecular pathogenesis of AT/RT below.
Surgery
Surgical resection of AT/RTs is often a matter of necessity because of intracranial hypertension, mass effect, hydrocephalus, and the need for diagnostic tissue. Surgery for AT/RT has remained a mainstay of treatment since this tumor was first described, and all published registries of patients with AT/RT regard surgical resection as standard of care. Surprisingly, the question of whether degree of surgical resection is associated with improved progression-free (PFS) or overall survival (OS) has been infrequently addressed in the literature, in large part because of the rarity of AT/RT and therefore the small size of published series.
Retrospective data from large AT/RT registries suggest that gross total resection (GTR) is associated with an increase in PFS and OS, although direct comparisons typically have either not quite reached statistical significance or sample sizes precluded adequate statistical power. In a large, multicenter, retrospective study, Hilden et al found that patients who underwent a GTR experienced a longer event-free survival (EFS) than those who had a biopsy or partial resection only (median EFS 14 months vs 9.25 months, respectively), although no statistical comparisons were made.15 Of note, in this study, 71% of long-term disease-free survivors (median EFS 42 months) underwent GTR, compared to 35% of the remainder of the cohort. Similarly, a single-center retrospective study of 31 patients found that EFS did not correlate with extent of resection by Kaplan–Meier analysis (P = .096), but when stratified by patient age, a known survival variable in AT/RT, there was a trend toward an increase in OS based on extent of resection (P = .053).7 Most recently, in an international multicenter study including clinical data from 109 patients, Torchia et al demonstrated that GTR is associated with improved PFS and OS, both in patients who receive combination chemotherapy and radiotherapy and in those who receive chemotherapy alone.16
Taken together, these data provide some evidence that degree of resection affects survival in AT/RT. Although there are no randomized studies demonstrating that surgical resection significantly improves patient prognosis, surgery remains a necessity for the purpose of obtaining diagnostic tissue, relieving mass effect, treating hydrocephalus and, in emergent situations, saving life. Given that the available data on surgical outcomes are relatively limited, we recommend that maximum safe surgical resection be pursued in all instances at a center adequately experienced and equipped to handle all the patient’s intraoperative as well as postoperative needs. Tumor location and the patient’s preoperative level of functioning should be taken into careful consideration when deciding whether to pursue GTR.
Clinical Case Relevance
The patient was taken to the operating room for a left frontotemporal craniotomy for tumor resection. Postoperatively, the patient experienced a dense upper and lower extremity right hemiparesis, although this improved somewhat with time. An MRI was obtained on postoperative day 2 that demonstrated GTR of the tumor (Fig. 1). As described above, her postoperative course was notable for complex partial seizures of the right upper extremity that resolved with levetiracetam and phenytoin.
Pathology
History and Histologic Features
Malignant rhabdoid tumors of the kidney were first described in 1978 in a large centralized pathology review of 427 cases of Wilms’ tumor.17 A small subset of Wilms’ tumors displayed a “rhabdomyosarcomatoid” pattern of differentiation consisting of sheets of cells with features suggestive of myoblastic differentiation, ultimately giving rise to the designation MRT.17,18 Brain tumors that closely resembled primitive neuroectodermal tumors (PNETs) but contained MRT-like components were first described in 1987 and were initially termed “atypical teratoid tumors of infancy.”19 These tumors were distinguished by the presence of rhabdoid cells, which are characterized by eccentrically round nuclei, open chromatin, small-to-medium-sized (and sometimes multiple) nucleoli, and a large cell body with characteristic inclusions (Fig. 2). The morphologic features of the cells themselves are highly variable, ranging from small, spindle-shaped cells to large cells with an atypical, wrinkled border. These often-heterogeneous tumors were usually initially diagnosed as PNETs but all shared the features of rhabdoid cells and “teratoid” differentiation along neuroepithelial, epithelial, and mesenchymal cell lineages at different stages of development but without widely divergent tissue development.20,21 In fact, initial pathologic series describing these tumors found that roughly two-thirds of tumors contained fields that were indistinguishable from previously described PNETs, although up to 13% of tumors contained only rhabdoid cells. Roughly one-third of tumors contained a significant mesenchymal component, and a lower fraction exhibited some form of epithelial differentiation, typically in an adenomatous or papillary pattern resembling choroid plexus papilloma.21
Fig. 2.
A, Histological sections show a highly cellular tumor composed of primitive cells with scant cytoplasm (hematoxylin and eosin; 200× magnification, scale 50 µm). Numerous mitotic figures are identified in even this small field. B, There is loss of SWItch/Sucrose Non-Fermentable–related, matrix-associated, actin-dependent regulator of chromatin, subfamily b, member 1 (SMARCB1) nuclear expression in the tumor cells (200× magnification, scale 50 µm). In comparison, SMARCB1 expression is retained in normal endothelial cells, which serve as internal controls. C, These tumors may have variable populations of rhabdoid cells, characterized by relatively abundant, eosinophilic cytoplasm and eccentrically placed nuclei (arrowheads; hematoxylin and eosin; 400× magnification, scale 20 µm).
The differential diagnosis of AT/RT pathology is largely narrowed to other embryonal tumors of the CNS. While pathologic diagnosis was previously supported by differential expression of cell lineage markers such as vimentin and neurofilament protein, AT/RT is now almost exclusively diagnosed using a combination of the above histologic features combined with loss of nuclear expression of SMARCB1 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily b, member 1; also known as integrase interactor 1 [INI1], SNF5, and Brahma-related gene 1 associated factor 47 [BAF47]), which is discussed further below. Other tumors such as schwannomas and poorly differentiated chordomas may demonstrate loss of nuclear SMARCB1 expression, but these are distinguished from AT/RT by demographic and anatomic differences. Schwannomas that present as part of a genetic syndrome tend to display SMARCB1 loss, whereas sporadic schwannomas retain SMARCB1 expression.22 Poorly differentiated chordomas also display SMARCB1 loss, but these tumors arise in remnant structures of the notochord, not in the brain or spinal cord proper.23
Molecular Genetics
Over the past 30 years, the growth of widely available, cost-effective molecular analytic techniques has revolutionized our understanding of the molecular basis of AT/RT, and multiple reviews have summarized application of recent discoveries to novel therapeutics.24,25 Throughout the 1990s, multiple cytogenetic studies demonstrated that chromosome 22q11.2 deletions were a consistent finding both in MRTs and AT/RTs.26–31 Positional cloning approaches led to the identification of SMARCB1 as the gene in 22q11.2 inactivated in MRTs.32 Germline mutations in SMARCB1 that predisposed patients with AT/RT as well as MRT to cancer were subsequently reported, consistent with a role for SMARCB1 as a tumor suppressor gene.8 The most recent studies have demonstrated that nearly all rhabdoid tumors, including AT/RT, contain biallelic mutations in SMARCB1.33,34 Furthermore, the vast majority of patients with synchronous, multiple primary AT/RTs and/or MRTs have an underlying genetic alteration in SMARCB1, and the frequency of germline mutations in SMARCB1 in all patients with rhabdoid tumors may be as high as 35%10,11,35,36 A second rhabdoid tumor locus, SMARCA4, another key member of the SWI/SNF complex, is inactivated in a small percentage of patients with rhabdoid tumors who do not have mutations in SMARCB1.37
Further cementing the role of SMARCB1 in MRTs, recent in-depth molecular studies using whole-exome and whole-genome sequencing and copy number variation (CNV) analysis failed to demonstrate additional nonrandom copy number alterations or mutations across the genome. These findings demonstrated that MRTs not only lack mutations in other canonical oncogenic pathways but also contain virtually no consistent mutations or CNVs outside the SMARCB1 gene.33,34,38,39 In fact, rhabdoid tumors contain 1 of the lowest mutation rates among all human cancers.34,40 In some cases, SMARCB1 mutation or deletion is the only detectable genetic alteration throughout the entire region of the genome under study.34,38 Of note, the mutation rate in AT/RTs is higher in noncoding regions of the genome, indicating that noncoding mutations may be important in the pathogenesis of these tumors.41 These data demonstrate SMARCB1 loss is not only a specific feature of rhabdoid tumors but also a critical event in their pathogenesis.
Given the remarkably bland genetic background of AT/RTs, recent studies have employed a combination of whole-genome sequencing, gene expression, copy number analysis, and DNA and histone methylation analysis to further probe the molecular underpinnings of these tumors. The results of these studies provide a comprehensive illustration of the diverse molecular landscape of AT/RT and demonstrate a clear clinicopathologic correlation among molecular subtype, clinical presentation, and outcome. Torchia et al16 initially delineated 2 broad molecular classes of AT/RT based on gene expression: One group of tumors (“Group 1”) was enriched for loci in the Notch signaling pathway, and genes associated with neural development, and a second group (“Group 2”) was enriched for genes associated with mesenchymal differentiation and the bone morphogenic protein (BMP) signaling pathway.16 Subsequent analyses by 2 study groups further delineated 3 distinct molecular subclasses of AT/RT based on gene expression and epigenetic profiles (Table 1). Differences in nomenclature preclude a direct comparison among the 3 subgroups identified by different studies, and for the sake of clarity, we will use the nomenclature used by Johann et al here.33 Briefly, AT/RT-Sonic Hedgehog (SHH)/group 1 tumors contain focal mutations or deletions in SMARCB1, display a hypermethylated phenotype, and are enriched for genes in the Notch and SHH pathways, particularly achaete-scute complex homolog-like 1 (ASCL1), glioma-associated oncogene family zinc finger 2 (GLI2), hes family bHLH transcription factor 5/6 (HES5/6), and delta-like canonical Notch ligand 1/3 (DLL1/3).33,41 AT/RT-TYR/group 2A tumors often contain broad deletions of SMARCB1 or chr22q11.2, are hypermethylated, and are characterized by a melanosomal gene signature including micropthalmia-associated transcription factor (MITF) and tyrosinase (TYR).33,41 Finally, AT/RT-MYC/group 2B tumors contain focal SMARCB1 deletions, have a hypomethylated phenotype, and are enriched for MYC and HOX family genes.33,41 Given the role of SMARCB1 in cell lineage determination (described below), it is hypothesized that alterations in SMARCB1 cooperate with subgroup-specific enhancers, ie, gene regulatory elements that coordinate the recruitment of chromatin remodeling complexes (CRCs) and transcription factors to DNA and thus determine cell identity (reviewed in Shlyueva et al42), to yield the molecular diversity seen in AT/RTs. The mechanisms by which different mutations in SMARCB1 differentially affect enhancer activity are not yet fully understood and remain an active area of investigation.
Table 1.
Molecular Subgroups of Atypical Teratoid/Rhabdoid Tumors (AT/RT) and Their Clinical Features
| AT/RT-SHH/Group 1 | AT/RT-TYR/Group 2A | AT/RT-MYC/Group 2B | |
|---|---|---|---|
| Location | Supratentorial > infratentorial | Infratentorial >> supratentorial | Supratentorial >> infratentorial |
| Mutation type | Focal SMARCB1 mutations | Broad SMARCB1 deletions (ie, 22q loss). | Focal SMARCB1 deletions |
| DNA methylation status | Hypermethylated | Hypermethylated | Hypomethylated |
| Dysregulated molecular pathways | NOTCH, SHH, EZH2, CDK6 | MITF, OTX2, CCND1, VEGFA | HOX, EZH2 |
| BMP, PDGFRB, MYC, ERBB2 | |||
| Published representative cell lines | CHLA02, CHLA04, CHLA05 | BT12, BT16, SH, CHLA06, CHLA266 | |
Abbreviations: BMP, bone morphogenic protein; CCND1, cyclin D1; MITF, micropthalmia-associated transcription factor; PDGFRB, platelet-derived growth factor receptor beta; SHH, Sonic Hedgehog; SMARCB1, SWItch/Sucrose Non-Fermentable–related, matrix-associated, actin-dependent regulator of chromatin, subfamily b, member 1; VEGFA, vascular endothelial growth factor A.
In summary, studies of MRT and AT/RT in humans have clearly defined a role for SMARCB1 as a bona fide tumor suppressor gene. Compared to the genetic uniformity of AT/RTs, the epigenetic landscape and secondary alterations in gene expression are variable across molecular subtypes of AT/RTs. Most important, these epigenetically defined subgroups have significant implications for treatment and overall prognosis, as discussed below.
Function of SWI/SNF Complex in Development and Cancer
SMARCB1 is a member of the SWI/SNF complex, a ubiquitous CRC that is critical in normal embryonic development and implicated in numerous cancers. Mutations in at least 1 SWI/SNF subunit are found in 20% of all cancers, making it the most commonly mutated CRC in human cancer.43,44 As targeted therapies for AT/RT are critically dependent on an accurate understanding of the molecular biology of the SWI/SNF complex, the role of this CRC in development and cancer biology is briefly reviewed here.
The SWI/SNF chromatin remodeling complex is composed of a combination of core subunits, including SMARCB1, an ATPase subunit (either SMARCA4/BRG1 or SMARCA2/BRM), and several other subunits whose exact composition facilitates binding to specific regions of the genome and is thought to contribute to cell lineage determination (reviewed in Wilson and Roberts45). The SWI/SNF CRC functions via nucleosome repositioning, opening neighboring stretches of DNA and thus allowing a host of transcription factors and associated complexes to either activate or repress target genes (reviewed in Wilson and Roberts45 and Saha et al46). Although it is not yet clear how SWI/SNF binding activates cell lineage specification programs, recent evidence implicates SMARCB1 in the dynamic activation of genes associated with cell differentiation via antagonism of the polycomb repressor complex 2 (PRC2) at enhancers that determine cell identity and differentiation via trimethylation of lysine 27 at histone 3 (H3K27me3).47–49 Embryonic stem cells contain a unique combination of SWI/SNF subunits that facilitate expression of transcription factors that maintain pluripotency, and SMARCB1 loss leads to upregulation of these stem cell gene expression programs.49,50
Although the specific function of the SMARCB1 subunit in cell fate determination is not entirely clear, abundant research implicates this protein in the regulation of numerous pathways of development and cancer. SMARCB1 regulates the cell cycle at the G1-S transition via P16INK4a,51–54 and SMARCB1 loss leads to cell cycle progression through a mechanism that requires changes in DNA methylation.55,56 SMARCB1 directly regulates transcription of Aurora Kinase A, a serine-threonine kinase that controls cell cycle proliferation via chromatid segregation, and this kinase is necessary for rhabdoid tumor cell survival.57 Interestingly, multiple studies have indicated that SMARCA4 is necessary for the cell-cycle arrest function of retinoblastoma,58,59 and the SWI/SNF complex is necessary for tumor protein 53 (TP53)-mediated transcription.60 Multiple studies indicate that SMARCB1 loss may synergize with TP53 loss to promote tumor development,52,61 although clinicopathologic studies do not clearly indicate TP53 dysfunction in SMARCB1-mutant tumors. In addition to cell cycle control, mutations in SMARCB1 have also been shown to enhance cellular invasion: SMARCB1 loss increases cell migration and facilitates loss of cellular adhesions via disinhibition of RhoA-dependent changes in the actin cytoskeleton.62,63 SMARCB1 also regulates several other pathways implicated in pediatric cancer, and SMARCB1 loss may serve to cooperate with these pathways to facilitate tumor development: SMARCB1 loss results in downstream activation of the SHH,64 WNT/β-catenin,65,66 and c-MYC pathways.67,68 Although the SWI/SNF complex has been implicated in DNA repair,69–72 SMARCB1 loss does not appear to promote tumorigenesis via genomic instability.73 These studies demonstrate diverse roles of the SWI/SNF complex in normal development and specifically highlight the importance of the SMARCB1 subunit in cell lineage determination, cell identity, cell cycle control, and migration by orchestrating numerous pathways via chromatin remodeling (Fig. 3). This has numerous implications for the treatment of SMARCB1-mutant cancers, which are further detailed below.
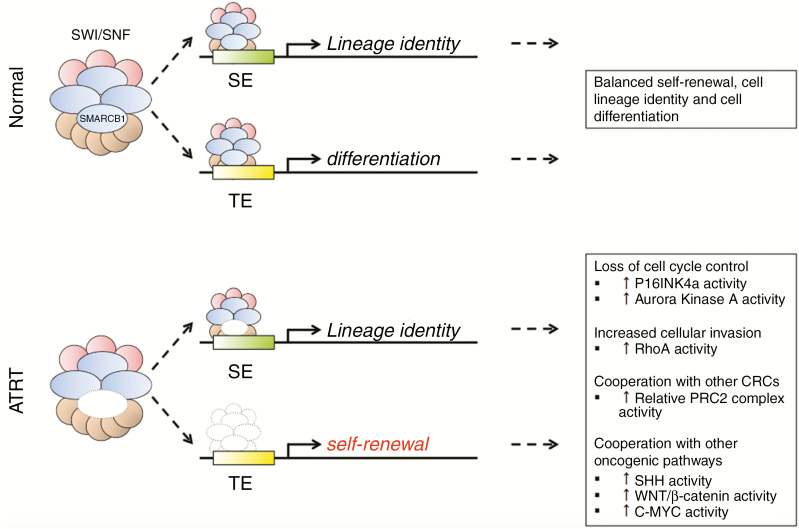
Fig. 3.
Normal Function of the SWItch/Sucrose Non-Fermentable (SWI/SNF) Complex and Mechanisms of Tumorigenesis. SWItch/Sucrose Non-Fermentable–related, matrix-associated, actin-dependent regulator of chromatin, subfamily b, member 1 (SMARCB1) is a subunit of the SWI/SNF complex, a dynamic chromatin remodeling complex that is necessary for normal cell differentiation and lineage determination. The SWI/SNF complex functions by binding specific chromatin-bound enhancers, elongating neighboring stretches of DNA and exposing them to transcription factors that guide normal cell differentiation and regulate self-renewal. In the absence of a functional SMARCB1 subunit, less SWI/SNF complex is available, the complex is not appropriately recruited to typical enhancers, and the remaining complex is recruited to super enhancers. In the absence of a functional SMARCB1 subunit, control of cell lineage determination is lost and multiple oncogenic pathways are disinhibited.
Mouse Models/Experimental Therapeutics
Although the genetic landscape of AT/RTs has been well studied, genetically engineered mouse models (GEMM) have only recently reliably reproduced an AT/RT-like tumor phenotype. Knockout of the initiation codon of Smarcb1 is lethal to the developing embryo by at least embryonic day 7.5.74–76 However, about 12% of mice with only a single functional copy of Smarcb1 develop soft-tissue rhabdoid tumors of the first branchial arch early in life but do not develop brain, kidney, or other soft-tissue tumors.76 Using a Cre-lox conditional knockout model, Roberts et al found that partial preservation of a mutant Smarcb1 allele led to T-cell lymphomas in the vast majority of mice studied, with only a minority of mice developing rhabdoid tumors.76 These early studies demonstrated the importance of Smarcb1 and Smarca4 in the normal differentiation of many cell types and supported the notion that Smarcb1 is a tumor suppressor gene but also painted a conflicting picture of its role in rhabdoid tumorigenesis.
Recent studies targeting specific cell lineages at different time points have lent insight into the role of SMARCB1 in the development of AT/RT. In an effort to create a murine model that specifically recapitulates AT/RT, Moreno et al77 developed a cell lineage-specific model with either Smarcb1 or Smarca4 in cerebellar granule neurons. While these mice developed hypoplastic cerebelli, none of them developed tumors, effectively ruling out these cells as the cell-of-origin for AT/RT.77 Using a tamoxifen-induced Cre-lox model, Han and colleagues found that partial knockout of Smarcb1 resulted in brain tumors (and to a lesser extent spine tumors) with a rhabdoid phenotype and penetrance of 96%, but this occurred only when knockout occurred between embryonic days 6 and 7.78 Notably, this study did not restrict Smarcb1 knockout to any particular progenitor cell and Smarcb1 knockout cast broad downstream effects affecting multiple cell lineages. Interestingly, familial mutations in SMARCB1 are associated with familial schwannomatosis, a form of neurofibromatosis with a phenotype very similar to neurofibromatosis type 2 but without vestibular schwannomas.14,79–81 Vitte et al82 therefore hypothesized that neural crest cells are a cell of origin for AT/RTs and selectively knocked out Smarcb1 expression in cells expressing P0, a highly specific gene for developing neural crest cells. When Smarcb1 knockout was restricted to P0-expressing cells at E9.5, about 65% of mice developed rhabdoid-type tumors of the cranial nerves, meninges, spinal nerve roots, and the eye; intracranial tumors often demonstrated brain invasion.82 Moreover, gene expression patterns from these tumors could be divided into 3 unique molecular subgroups based on gene expression, and these groups demonstrated significant molecular overlap with the molecular subgroups of AT/RTs.33,82
Compared to GEMMs, patient-derived orthotopic xenografts (PDXs) of AT/RTs offer a more readily available platform for preclinical testing, and several widely used cell lines are available for routine laboratory use. Intracranial injection of serially passaged AT/RT cell lines not only yields brain tumors in immunocompromised mice; these tumors also display a similar pattern of immunohistochemical markers to the primary AT/RT, often demonstrate CSF invasion and are capable of metastasizing through the length of the CNS.83,84 Moreover, these tumors predictably respond to molecular inhibitors of known mutant SMARCB1 targets, including CDK4/6.83 Reflecting the molecular heterogeneity of AT/RTs, recent studies have further expanded PDX models to include cell lines from different molecular subgroups. Using the nomenclature of Torchia et al, published AT/RT cell lines BT12 and BT16, along with SH, CHLA06, and CHLA266, belong to Group 2; CHLA02, CHLA04, and CHLA05 belong to Group 1.41
There are relatively few studies assessing the efficacy of experimental therapeutics in AT/RT, both in AT/RT cell lines and in PDXs. Most studies using PDX models utilize the cell lines BT12 and BT16, so most of the available literature on preclinical testing is heavily biased toward Group 2 tumors. Studies utilizing an intracranial injection PDX model have thus far demonstrated in vivo efficacy of cabazitaxel (a second-generation taxane that is relatively resistant to efflux by P-glycoprotein pumps),85 paclociclib (an orally available CDK4/6 inhibitor),86 and volasertib (an inhibitor of PLK1, a target of retinoblastoma).87 Temozolomide had no clinical efficacy against AT/RT in this model.83 Lapatinib, a human epidermal growth factor receptor 2 (HER2)-Neu and epidermal growth factor receptor (EGFR) inhibitor, was identified as a potentially efficacious drug in a screen of 129 small molecular inhibitors on 3 AT/RT cell lines (2 of which were Group 2 cell lines) and was shown to slow tumor growth in an extracranial PDX model.88 Other studies have demonstrated in vitro efficacy of selumetinib (a mitogen-activated protein kinase [MEK] inhibitor),89 3-deazaneplanocin A (an enhancer of zeste homolog 2 [EZH2] inhibitor),90 and the histone deacetylase inhibitor SNDX-27591 against AT/RT cell lines.
Following their comprehensive molecular analysis of AT/RTs, Torchia et al performed a screen of 8 compounds targeting multiple pathways on both Group 1 and Group 2 cell lines.92 In their study, dasatinib, a commercially available multi-tyrosine kinase inhibitor (MTKI) designed for treatment of chronic myelogenous leukemia, decreased cell viability in vitro and prolonged OS in an intracranial injection PDX model. Interestingly, their screen identified a differential response of Group 1 and Group 2 cell lines to different molecular therapies in vitro. Another MTKI, nilotinib, and dorsomorphin, an inhibitor of BMP signaling, decreased viability of Group 2 cells in vitro but had minimal effect on Group 1 cell lines. Conversely, the viability of Group 1 cell lines was decreased in response to multiple drugs targeting chromatin remodeling including UNC0638 (a multihistone methyltransferase inhibitor), JQ1 (a bromodomain containing [BRD]2/3/4 inhibitor), UNC1999 (an orally available EZH2 inhibitor), and LAQ824 (a multihistone deacetylase inhibitor) as well as DAPT, a gamma-secretase inhibitor and indirect inhibitor of the Notch pathway.41 These early data demonstrate tumor subtype-specific responses to small molecular inhibitors and highlight the importance of molecular screening for tumor genotype when testing novel therapeutics in animal models.
In addition to small-molecule inhibitors, there are a few studies in the literature that assess the role of other modalities in the treatment of AT/RT. Double-deleted vaccinia virus,93 oncolytic measles virus,94 herpes virus,95 stomatitis virus, and myxoma virus96 have demonstrated both in vitro and in vivo efficacy in an intracranial injection PDX model using Group 2 cell lines. Two recent studies have also assessed the role of immunotherapy in AT/RT. One study that treated 7 patients with AT/RT with an autologous tumor-lysate-loaded dendritic cell vaccine achieved a median OS of 41.8 months, including 2 patients who survived 138 and 143 months after diagnosis.97,98 In an intracranial injection PDX model, treatment of mice with a humanized antibody to CD47, a surface protein that facilitates immune escape in cancer, dramatically halted tumor growth and effectively prevented animal death from tumor burden over the study period.99 While there are relatively few data regarding the efficacy of oncolytic virotherapy and immunotherapy in AT/RT, their use in preclinical models has provided some of the most promising translational data yet.
Clinical Case Relevance
In the case presented here, the surgical specimen was hemorrhagic and gritty. Histopathologic analysis demonstrated a hypercellular tumor composed of primitive-appearing, small, round blue cells (Fig. 2A). Mitotic activity was brisk, and numerous apoptotic bodies were identified throughout. There was uniform loss of SMARCB1 expression in tumor cells by immunohistochemistry, confirming the diagnosis of AT/RT (Fig. 2B). Although not abundantly present in this case, AT/RTs classically contain “rhabdoid” cells with relatively abundant, eosinophilic cytoplasm and eccentrically placed nuclei (Fig. 2C). Multimodal molecular analysis was performed on the tumor and peripheral blood lymphocytes. The tumor contained a single-base point mutation in SMARCB1, c.472C>T, and displayed copy-neutral loss of heterozygosity. No mutation was detected in peripheral blood, consistent with sporadic AT/RT.
Standard-of-Care Treatment
Owing to the relative rarity of the tumor and heterogeneity in clinical practice, there is no clearly defined standard treatment for AT/RT. This is complicated by the fact that AT/RT is a relatively new distinct pathologic entity, making it difficult to interpret older studies of PNETs. With more recent studies focusing on AT/RT as a separate disease, the largest studies show the best outcomes with a combination of surgery, radiation, and chemotherapy.100,101 While the degree of surgical resection is associated with improved outcome, there is no universally agreed-upon standard regimen for radiotherapy or chemotherapy.
Radiation
Multiple studies have demonstrated improved outcomes in patients with AT/RT treated with craniospinal radiation (CSI) with a focal tumor bed boost, typically 54 Gy with lower doses in selected patients.7,100–107 Concerns regarding the effect of radiation on the developing brain have previously prohibited the use of radiation in children with brain tumors, although recent evidence suggests this may allow early tumor progression. One retrospective study of children with AT/RT demonstrated that delayed CSI (ie, more than 3 months following surgery) with tumor bed boost was associated with a higher rate of both local and metastatic disease progression,107 and multiple studies have shown that disease progression is proportional to delay in radiotherapy.103,107 Similarly, multiple studies have demonstrated improved outcomes with earlier timing of radiation,103,106 although 1 study found no change in OS.105 Owing to increased use of radiation in younger children with AT/RT, proton-beam therapy may be offered as stand-alone therapy or as an adjunct to CSI: One study of proton-beam therapy in 31 patients with AT/RT demonstrated a median PFS of 20.8 months and median OS of 34.3 months.92 Five of these patients developed delayed white-matter radiation changes, all of which improved with steroids or bevacizumab. Taken together, these studies suggest that radiotherapy, either CSI or proton-beam therapy, is part of the optimal treatment regimen in AT/RT, although the long-term sequelae of radiation on the developing nervous system remain to be fully elucidated. Minimizing local radiation dose and using proton-beam therapy may mitigate effects on the developing nervous system and should be implemented on a case-by-case basis.
Chemotherapy
Given the rarity of AT/RT, there are few clinical trials that assess the efficacy of specific chemotherapeutic regimens on clinical outcome. Most of the current literature on the role of multimodal therapy is based on small registries and single-arm studies, so clinicians have relatively few data to guide clinical decision making. Most of the published chemotherapeutic approaches consist of a sarcoma-like therapy with high-dose chemotherapy and hematopoietic stem cell rescue (HDSCT) with or without intrathecal chemotherapy as an adjunct. Table 1 summarizes the major studies of traditional chemotherapeutic regimens in AT/RT.
Data from early studies, including American and German HIT trials, used a variety of different chemotherapeutic regimens and demonstrated the role of aggressive multimodal therapy in improving survival outcomes, often with significant adverse effects including transverse myelitis, radiation recall, chemotherapeutic toxicity, and infections.7,15,108,109 High-dose chemotherapy with stem cell rescue is considered as a means to postpone or spare radiation for younger children but carries significant toxicity and a risk of death. Although multiple studies have assessed the role of HDSCT in AT/RT,15,105,110–114 small sample size, heterogeneity in high-dose chemotherapy regimens between different studies, and lack of clear inclusion criteria preclude a definitive recommendation regarding the role of HDSCT in AT/RT. A multicenter French study of 58 patients receiving adjuvant chemotherapy included 11 patients who received HDSCT; the median OS for all patients in this study was 9 months.111 The first Head Start (HS) trials (HS-I, HS-II) included HDSCT as part of their protocol and reported better overall outcomes but at the expense of high toxicity, including 1 death due to Staphylococcal meningitis and another from a secondary leukemia (median OS 10.25 months and >36 months for HS-I and HS-II, respectively).112 HS-III reported a 3-year median OS of 26%.114 A retrospective Canadian Pediatric Brain Tumor Consortium study with 50 patients included 18 patients who underwent HDSCT and reported improved outcomes in patients who underwent HDSCT compared to those who did not (median 2-year OS 47.9% for HDSCT vs 27.3% with conventional chemotherapy).105 Perhaps most surprisingly, the results from a University of Vienna study demonstrated a 100% 5-year OS in patients treated with a combination of a dose-dense chemotherapeutic regimen of doxorubicin, cyclophosphamide, vincristine, ifosfamide, cisplatin, etoposide and methotrexate, intrathecal chemotherapy, focal radiation, and HDSCT.113 In a second cohort from this study that did not receive HDSCT or intrathecal chemotherapy, 5-year median OS was 28.5%.113 The interim results of the European Rhabdoid Registry, which included 19 patients treated with HDSCT and autologous stem cell transplant, included a 2-year EFS and OS of 29% and 50%, respectively.115 An updated analysis of 31 patients in this study demonstrated a 6-year OS of 46% and a 6-year EFS of 45%.110
The Children’s Oncology Group study ACNS0333 is closed to accrual, and although the final results have not yet been published, preliminary data have been made available. This study included a regimen of surgery followed by 2 cycles of induction chemotherapy (methotrexate, vincristine, cyclophosphamide, etoposide, and cisplatin) followed by 3 cycles of HDSCT (thiotepa and carboplatin) and local radiation therapy. The order of the consolidation and radiation was dependent on patient age as well as extent and location of disease. Preliminary results on 65 patients included 24-month EFS and OS of 42% and 53%, respectively. For 54 patients <3 years of age, the 24-month EFS and OS were 39% and 48%. Failures were uncommon after 2 years, and there were 2 treatment-related deaths during therapy.116
While multiple layers of heterogeneity preclude a simple interpretation of these results, these data suggest that aggressive chemotherapeutic regimens, radiotherapy, and HDSCT are likely associated with a survival benefit in patients with AT/RT, albeit with the risk of significant toxicity. While some studies demonstrating prolonged survival have utilized intrathecal chemotherapy, its role remains unclear in AT/RT: A recent individual pooled-data analysis showed no significant difference in median EFS between patients who received intrathecal chemotherapy compared to those who did not.101 A summary of available clinical registries of patients with AT/RT is provided in Table 2. There are many ongoing clinical trials assessing conventional chemotherapy and radiotherapy in AT/RT, which are summarized in Table 3. As evidenced by the sample size of studies from around the globe, continued international collaborative efforts are essential to determining the optimal treatment regimen for AT/RT.
Table 2.
Major Registries of Patients With Atypical Teratoid/Rhabdoid Tumors (AT/RT) Treated With Conventional Chemotherapy and Radiotherapy
| Reference | Demographics | Treatment | Outcomes |
|---|---|---|---|
| Hilden et al, 200415 | N = 42; median age 24 months (range, 3-62 months) | Surgery + chemotherapy (all patients; numerous regimens, IT chemotherapy in 16 patients) + HDSCT (13 patients) + radiation (13 patients; CSI N = 4, tumor bed only N = 9) | Median OS 16.75 months; median EFS 10 months |
| Tekautz et al, 20057 | N = 31; <3 years of age N = 22, ≥ 3 years of age N = 9 | Surgery + chemotherapy ± radiation ≥ 3 years: one of numerous regimens, radiation (N = 3, local in 2, CSI in 1). <3 years: ICE, SJMB96 or none; CSI (N = 7) |
≥ 3 years: 2-year EFS 78%, OS 89% <3 years: 2-year EFS 11%; OS 17% |
| Gardner et al, 2008112 | N = 13 HS-I: N = 6, median age 36 months HS-II: N = 7, median age 28 months |
Surgery + chemotherapy + HDSCT (HS-I/II protocol: induction with cisplatin, vincristine, cyclophosphamide, etoposide ± methotrexate; consolidation with carboplatin, thiotepa, etoposide) | HS-I: median OS 10.25 months; median EFS 4.25 months HS-II median OS > 36 months months; median EFS > 10.5 months (3 patients alive at time of publication) |
| Chi et al, 2009108 | N = 20; median age 26 months (range, 2.4 months- 19.5 years) | Surgery + modified IRS-III regimen: chemotherapy (vincristine, dactinomycin, cyclophosphamide, cisplatin, doxorubicin, imidazole, temozolomide ± IT methotrexate, cytarabine, hydrocortisone) + radiation (focal ± CSI depending on age) | 2-year EFS 53%; 2-year OS 70% |
| Von Hoff et al, 2011109 | N = 56; median age 1.2 years (range, 0.1-4.0 years) | Surgery + chemotherapy (HIT 2000 protocol, N = 18; HIT-SKK-92, N = 9; HIT-91, N = 6, and HIT-SKK-87, N = 6; CWS-96, SIOP-93-01 and others, N = 17) + radiation (N = 29) | 3-year EFS 13%; 3-year OS 22% |
| Dufour et al, 2012111 | N = 58; median age 1.4 years (range, 14 days-8.5 years) | Surgery + chemotherapy (N = 47; BB-SFOP, N = 9; PNET-HR; N = 11; AT/RT-04, N = 24; other, N = 3) + HDSCT (N = 11) + radiation (N = 16) | Median OS 9 months |
| Lafay- Cousin et al, 2012105 | N = 50; median age 16.7 months | Surgery + chemotherapy (IRS-III-like; ICE; HS; or carboplatin, thiotepa ± methotrexate) + HDSCT (N = 18) + radiation (N = 21) | Median OS 13.5 months |
| Slavc et al, 2014113 | N = 22 Cohort A (N = 9), median age 24 months (range, 9 months-17 years) Cohort B (N = 13), median age 30 months (range, 2 months-22 years) |
Surgery + chemotherapy + radiotherapy depending on timing Cohort A: chemotherapy (MUV-AT/RT: doxorubicin, cyclophosphamide, vincristine, ifosfamide, cisplatin, etoposide, methotrexate + IT chemotherapy) + HDSCT + focal radiation Cohort B: chemotherapy (HIT-SKK-92, HIT-91, PEI, HIT-2000 or MUV-ATRT) + radiation (N = 7) |
Cohort A: 5-year OS 100% and EFS of 88.9% Cohort B: 5-year OS and EFS 28.8% |
| Zaky et al, 2014114 | N = 19, median age 14 months (range, 0 months-32 months) | Surgery + chemotherapy (HS-III (cisplatin, vincristine, etoposide, cyclophosphamide, methotrexate, temozolomide, thiotepa, etoposide, carboplatin) + HDSCT + radiation (N = 5, all patients at progression) | 3-year EFS 21%; 3-year OS 26% |
| Bartelheim et al, 2016110 | N = 31; median age 20 months | Surgery + chemotherapy (Rhabdoid 2007: vincristine, cyclophosphamide, doxorubicin, ifosfamide, carboplatin, etoposide ± IT methotrexate, cytarabine, hydrocortisone) + HDSCT (N = 8) + radiotherapy (N = 23); also see Benesch et al, 2014.115 | 6-year OS 46% (±0.10) 6-year EFS 45% (±0.09) |
| Reddy et al, 2016116; (abstract only) | N = 65 | Surgery + chemotherapy (ACNS0333) + HDSCT + radiation | 24-month EFS 42%; 24-month OS 53% |
Abbreviations: BB-SFOP, Baby Brain-French Society of Pediatric Oncology; CSI, craniospinal radiation; CWS, Cooperative Soft Tissue Sarcoma trial; EFS, event-free survival; HDSCT, hematopoietic stem cell rescue; HIT-SKK-92, Therapieprotokoll für Säuglinge und Kleinkinder mit Hirntumoren; HS, Head Start; ICE, ifosfamide, platinum, etoposide; IRS-III, Intergroup Rhabdomyosarcoma Study; IT, intrathecal; MUV, Medical University of Vienna; N, number; OS, overall survival; PEI, cisplatin, etoposide, ifosfamide; PFS, progression-free survival; PNET, primitive neuroectodermal tumor; SIOP, International Society of Pediatric Oncology; SJMB96, St. Jude Children’s Research Hospital Medulloblastoma protocol 96.
Table 3.
Ongoing Clinical Trials of Conventional Radio- and Chemotherapy in Atypical Teratoid/Rhabdoid Tumors
| Clinicaltrials. gov ID | Recruitment Status | Phases | Intervention |
|---|---|---|---|
| NCT00003141 | Completed | Phase 1 | HSCT; carboplatin, cisplatin, cyclophosphamide, etoposide, thiotepa, vincristine |
| NCT00007813 | Unknown | Phase 1 | HSCT; carboplatin, cyclophosphamide, etoposide |
| NCT00047177 | Completed | Phase 2 | Oxaliplatin |
| NCT00053118 | Completed | Phase 1 | HSCT; carboplatin |
| NCT00084838 | Completed | Phase 2 | Radiation; cisplatin, cyclophosphamide, cytarabine, dexrazoxane, doxorubicin, etoposide, methotrexate, temozolomide, hydrocortisone, vincristine, dactinomycin |
| NCT00085202 | Active, not recruiting | Phase 3 | HSCT; radiation; cisplatin, cyclophosphamide, vincristine |
| NCT00100880 | Completed | Phase 1 | Lenalidomide |
| NCT00112619 | Terminated | Phase 1 | Topotecan |
| NCT00138216 | Completed | Phase 1 | Irinotecan, temoxolomide, vincristine |
| NCT00392886 | Unknown | Phase 3 | HSCT; radiation; carboplatin, cisplatin, cyclophosphamide, etoposide, methotrexate, temozolomide, thiotepa, vincristine |
| NCT00602667 | Active, not recruiting | Phase 2 | Risk-stratified combination of methotrexate, vincristine, cisplatin, cyclophosphamide, carboplatin, etoposide, topotecan, erlotinib, vinblastine |
| NCT00623077 | Terminated | Phase 1 | Radiation; busulfan, etoposide, ifosfamide, melphalan, thiotepa |
| NCT00653068 | Active, not recruiting | Phase 3 | HSCT; methotrexate, etoposide, cyclophosphamide, cisplatin, carboplatin, thiotepa, vincristine |
| NCT00983398 | Recruiting | Phase 1/2 | Carboplatin, mannitol, melphalan |
| NCT01331135 | Active, not recruiting | Phase 1 | Sirolimus |
| NCT01505569 | Recruiting | Phase 1 | HSCT; radiation; etoposide, busulfan, melphalan, thiotepa, carboplatin, paclitaxel |
| NCT01737671 | Completed | Phase 1 | IT methotrexate |
| NCT02684071 | Recruiting | Phase 2 | IT methotrexate, topotecan, cyclophosphamide |
Abbreviations: HSCT, hematopoietic stem cell transplantation; ID, identification; IT, intrathecal.
Novel Therapeutic Approaches
While surgery, radiation, conventional chemotherapy, and HDSCT have been shown to improve outcomes in AT/RT, their efficacy remains limited. Additionally, the price of therapeutic success comes at the cost of significant toxicities, limiting further escalation of conventional therapeutics. Recent insights into the molecular genetics and biology of AT/RT have opened the door to novel molecular therapeutics as well as immunotherapy, and evidence from phase 1 and 2 clinical trials demonstrates disease efficacy with multiple novel treatments.
While the numerous downstream targets of the SWI/SNF CRC comprise a broad spectrum of targets for novel therapeutics, many novel small-molecule inhibitors exist that target the downstream pathways affected by mutant SMARCB1, and clinical trials are currently underway to assess their efficacy. In a study of 4 children with AT/RT, single-agent alisertib, a novel Aurora Kinase A inhibitor, was well tolerated. All children had stable disease or regression after 3 cycles of therapy, and 2 patients had durable responses at 1 to 2 years following treatment initiation.117 There is an ongoing phase 2 clinical trial utilizing alisertib as part of age- and risk-adapted chemotherapy both in newly diagnosed and recurrent AT/RT (NCT02114229). Tazemetostat, an EZH2 inhibitor, has been shown to inhibit self-renewal and induce radiation sensitivity in AT/RT in vitro;90 there are 2 ongoing clinical trials assessing its efficacy in humans (NCT02601937, NCT03213665). A recent phase 1 clinical trial of ribociclib, an oral CDK4/6 inhibitor, enrolled 32 patients, including 15 with AT/RT, and demonstrated an acceptable safety profile and disease efficacy.118 At the study endpoint, however, stable disease was achieved in only 2 patients with AT/RT, although outcomes were better for patients with neuroblastoma.118 There is an ongoing study using ribociclib and everolimus in the treatment of recurrent pediatric brain tumors, including AT/RT, ependymomas, and malignant gliomas (NCT03387020). As described above, there are phase 1 clinical data that demonstrate safety and perhaps efficacy of a dendritic-cell vaccine in children with AT/RT.97,98Table 4 gives a summary of ongoing clinical trials utilizing novel therapeutics in the form of biologics or small-molecule inhibitors.
Table 4.
Ongoing Clinical Trials of Novel Therapeutics in Atypical Teratoid/Rhabdoid Tumors
| NCT Number | Subtype | Recruitment | Phases | Type of intervention / target |
|---|---|---|---|---|
| NCT00089245 | Biologic | Recruiting | Phase 1 | I-131 monoclonal antibody 8H9 |
| NCT00445965 | Biologic | Active, not recruiting | Phase 2 | I-131 monoclonal antibody 3F8 |
| NCT02444546 | Biologic | Suspended | Phase 1 | Wild-type reovirus |
| NCT02962167 | Biologic | Recruiting | Phase 1 | Modified measles virus (MV-NIS) |
| NCT00003469 | SMI | Terminated | Phase 2 | Atengenal, astugenal (antineoplastons) |
| NCT00015899 | SMI | Completed | Phase 1 | Lonafarnib (Farnesyltransferase inhibitor) |
| NCT00217412 | SMI | Completed | Phase 1 | Vorinostat (HDAC inhibitor) |
| NCT00303940 | SMI | Completed | Phase 1 | Talabostat (dipeptidyl peptidase inhibitor / immune modulator) |
| NCT00326664 | SMI | Completed | Phase 1 | Cediranib (VEGF inhibitor) |
| NCT00572182 | SMI | Terminated | Phase 1 | MK-0752 (Gamma secretase, Notch inhibitor) |
| NCT00788125 | SMI | Active, not recruiting | Phase 1 / 2 | Dasatinib (multi-TK inhibitor) |
| NCT00939770 | SMI | Active, not recruiting | Phase 1 / 2 | Crizotinib (ALK, ROS1 inhibitor) |
| NCT00946335 | SMI | Completed | Phase 1 | Veliparib (PARP inhibitor) |
| NCT01076530 | SMI | Completed | Phase 1 | Vorinostat (HDAC inhibitor) |
| NCT01088763 | SMI | Terminated | Phase 1 | RO4929097 (Gamma-secretase, Notch inhibitor) |
| NCT02114229 | SMI | Recruiting | Phase 2 | Alisertib (aurora Kinase A inhibitor) |
| NCT03387020 | SMI | Not yet recruiting | Phase 1 | Ribociclib (CDK4/6 inhibitor) |
| NCT03434262 | SMI | Recruiting | Phase 1 | Ribociclib (CDK4/6 inhibitor) |
Abbreviations: ALK, anaplastic lymphoma kinase; CDK, cyclin-dependent kinase; SMI, small molecule inhibitor; HDAC, histone deacetylase; HSCT, hematopoietic stem cell transplantation; ID, identification; IT, intrathecal; MV-NIS, measles virus encoding human thyroidal sodium iodide symporter; PARP, poly (adenosine diphosphate-ribose) polymerase; ROS1, proto-oncogene tyrosine-protein kinase; SMI, submucosal invasion; TK, tyrosine kinase; VEGF, vascular endothelial growth factor.
Clinical Case Relevance
Following surgery, the patient was enrolled in the Children’s Oncology Group clinical trial ACNS0333 and received 2 cycles of induction chemotherapy followed by 3 cycles of HDSCT and craniospinal proton-beam radiation with tumor-bed boost. During induction chemotherapy, she experienced vincristine-associated peripheral neuropathy and vocal cord paralysis, both of which improved but did not resolve with completion of treatment. She experienced multiple infections during chemotherapy, none of which were life-threatening and all of which were treated successfully.
Follow-Up
Although there are no consensus guidelines on follow-up and surveillance imaging, brain and spine MRIs are typically acquired every 3 months for the first 2 years after diagnosis, every 6 months for the following 5 years, and annually thereafter. Given the long-term toxicities associated with therapy, surveillance should include a comprehensive follow-up with experts in endocrinology, ophthalmology, audiology, and neuropsychology as needed, as well as regular evaluations for physical, occupational, and speech therapy needs. When regimens utilizing agents that carry significant cardio- and pulmonary toxicity are utilized, long-term surveillance should include echocardiograms and pulmonary function tests. Survivors with germline mutations or deletions of SMARCB1 may also develop additional primary tumors as long as 15 years after the initial diagnosis,119,120 and patients should be counseled about their risk appropriately.
In addition to these screening guidelines, patients with RTPS deserve very close clinical follow-up with comprehensive imaging to detect asymptomatic tumors as early as possible. The most recent screening guidelines9 recommend monthly physical exams and ultrasounds of the head for patients with AT/RT and of the abdomen and pelvis for patients with MRTK during the first year of life; brain and spine MRI every 2 to 3 months is a suitable alternative for patients with a closed fontanelle. Children aged 1 to 4 years with AT/RT should undergo a brain and whole-spine MRI every 3 months, and those with MRTK should have an abdomen and pelvis ultrasound every 3 months. At any of these time points, a whole-body MRI is a suitable screening alternative but is often not feasible because of access or cost. Patients 5 years and older should undergo twice-yearly physical examination at a center experienced in caring for patients with RTPS with targeted imaging at the practitioner’s discretion. Prenatal testing and in utero screening guidelines are also available but are beyond the scope of this manuscript.9
Clinical Case Relevance
Following initiation of induction chemotherapy, the patient underwent a baseline MRI of the brain and whole spine. Surveillance MRIs were obtained every 3 months for 2 years. After 2 years of clinical stability, MRIs of the brain and spine were obtained every 6 months. She was regularly assessed by audiology while undergoing chemotherapy to screen for chemotherapy-associated ototoxicity. Following completion of chemotherapy, she underwent annual neuropsychological assessments. Throughout her therapy, she underwent regular assessments by physical and occupational therapy, and her needs were addressed as needed. She did not experience any heart or lung toxicity from chemotherapy.
Prognosis and Survivorship
The overall prognosis of AT/RT remains poor, although there are long-term disease survivors, and intensive multimodal treatments have significantly prolonged life in many patients, as detailed above. A study from the largest available epidemiologic database reported OS at 6 months, 1 year, and 5 years is 65.0%, 46.8% and 28.3%, respectively.5 This study included patients who underwent a wide array of therapies, and while these data reflect the heterogeneous treatment approaches to AT/RT, they largely reflect patient prognosis in the early 2000s. Heterogeneity in data reporting makes it difficult to extrapolate similar figures from more recent cancer registries, although the available data suggest that prognosis is improving with more recent therapies. When reported, median PFS rates range from 4.25 to 10 months, and median OS rates range from 10.25 to 36 months; total 2-year PFS rates range from 42% to 53%, and 2-year OS rates range from 53% to 70%.6,7,15,105,108–112,114,116 As just described, 1 study with an intense integrated treatment regimen reported a 100% 5-year OS and 88.9% 5-year PFS rate.113 Younger age, infratentorial location, and presence of metastatic disease upon diagnosis are associated with poorer prognosis, and survival is closely related to interventions received.6,7
Recent studies demonstrate that molecular genetics has a significant impact on not only the clinical manifestation of these tumors but also prognosis and treatment susceptibility. Group 1 tumors, which correspond to the AT/RT-SHH subgroup, have a propensity to arise in the supratentorial compartment and have the oldest median age at diagnosis of the 3 subgroups at 24 months.33,41 Group 1 tumors had a better overall prognosis with a 5-year PFS and OS of 28% and 34%, respectively.16 Group 2A tumors, the molecular equivalent to the AT/RT-TYR subgroup, are found in the youngest patients with a median age at diagnosis of 12 months and are more likely to be infratentorial.33,41 Group 2B tumors are clinically heterogeneous; this subgroup included all spinal tumors and accounted for most of the patients older than 3 years.41 Group 2 tumors as a whole have a worse prognosis with a 5-year PFS and OS of 12% and 9%, respectively.16 Consistent with MYC overexpression in AT/RT-myc/group 2B tumors, this finding was foreshadowed in an earlier study by Birks et al, who found that high expression of BMP-pathway genes in a subset of AT/RTs was associated with poorer prognosis.121 Using multivariate logistic regression analyses, Torchia and colleagues delineated 3 clinical subgroups based on ASCL1 expression (ie, tumors enriched for SHH signaling), tumor location, disease stage, and extent of resection.16 “Average-risk” patients, who harbored ASCL1-positive, nonmetastatic supratentorial tumors that underwent a GTR, had a 5-year PFS and OS of 60%. “Very high-risk” patients had ASCL1-negative infratentorial tumors that were incompletely resected and had a 5-year PFS and OS of 8% and 6%, respectively. The high-risk group, which included patients in the remaining areas of the spectrum, had a 5-year PFS and OS of 29% and 32%, respectively. These early molecular data demonstrate that clinical manifestation and outcomes largely reflect the underlying molecular genetics of the tumor and emphasize the importance of molecularly targeted therapy in AT/RT.
Clinical Case Relevance
As of this writing, the patient completed her therapy 4 years and 8 months ago and remains recurrence free. She has ongoing physical, occupational, and speech therapy needs. Owing to residual chemotherapy-associated neuropathies, she has bilateral hearing aids and requires ankle-foot orthoses for ambulation. Although the right-sided hemiparesis improved after surgery, it did not resolve entirely, and she periodically receives botulinum toxin injections for spasticity. As expected, her growth rate is slower than average, and she is regularly evaluated by an endocrinology team to determine optimal timing of growth hormone initiation.
Conclusion
AT/RTs are CNS cancers that most commonly arise in children 3 years of age or younger. The standard of care for the treatment of these tumors remains maximal safe surgery followed by a combination of radiation and intense chemotherapy, often with HDSCT with or without intrathecal chemotherapy. Current therapies can be extremely toxic, so there is a great need for more targeted, efficacious therapeutics. Recent studies on the molecular genetics of AT/RT, coupled with the use of murine models for preclinical testing, have crucially advanced our understanding of and ability to treat molecular pathways that are deregulated in these tumors. There are clinical trials underway assessing the efficacy both of conventional therapies and novel therapeutics, and the demand for novel treatments for this lethal childhood cancer remains high.
Funding
None declared.
Acknowledgment
This work’s originating institution is the Department of Neurologic Surgery, Mayo Clinic, Rochester, MN, USA (to C.L.N. and D.J.D.).
Conflict of interest statement. None declared.
References
- 1. Arslanoglu A, Aygun N, Tekhtani D, et al. . Imaging findings of CNS atypical teratoid/rhabdoid tumors. AJNR Am J Neuroradiol. 2004;25(3):476–480. [PMC free article] [PubMed] [Google Scholar]
- 2. Koral K, Gargan L, Bowers DC, et al. . Imaging characteristics of atypical teratoid-rhabdoid tumor in children compared with medulloblastoma. AJR Am J Roentgenol. 2008;190(3):809–814. [DOI] [PubMed] [Google Scholar]
- 3. Meyers SP, Khademian ZP, Biegel JA, Chuang SH Korones DN Zimmerman RA.. Primary intracranial atypical teratoid/rhabdoid tumors of infancy and childhood: MRI features and patient outcomes. AJNR Am J Neuroradiol. 2006;27(5):962–971. [PMC free article] [PubMed] [Google Scholar]
- 4. Han L, Qiu Y, Xie C, et al. . Atypical teratoid/rhabdoid tumors in adult patients: CT and MR imaging features. AJNR Am J Neuroradiol. 2011;32(1):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ostrom QT, Chen Y, M de Blank P, et al. . The descriptive epidemiology of atypical teratoid/rhabdoid tumors in the United States, 2001-2010. Neuro Oncol. 2014;16(10):1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lau CS, Mahendraraj K, Chamberlain RS. Atypical teratoid rhabdoid tumors: a population-based clinical outcomes study involving 174 patients from the Surveillance, Epidemiology, and End Results database (1973-2010). Cancer Manag Res. 2015;7:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tekautz TM, Fuller CE, Blaney S, et al. . Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol. 2005;23(7):1491–1499. [DOI] [PubMed] [Google Scholar]
- 8. Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM,Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59(1):74–79. [PubMed] [Google Scholar]
- 9. Nemes K, Bens S, Bourdeaut F, et al. . Rhabdoid tumor predisposition syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. eds. GeneReviews®. Seattle, WA: University of Washington, Seattle;1993. [Google Scholar]
- 10. Bourdeaut F, Lequin D, Brugières L, et al. . Frequent hSNF5/INI1 germline mutations in patients with rhabdoid tumor. Clin Cancer Res. 2011;17(1):31–38. [DOI] [PubMed] [Google Scholar]
- 11. Eaton KW, Tooke LS, Wainwright LM, Judkins AR, Biegel JA. Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer. 2011;56(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruggers CS, Bleyl SB, Pysher T, et al. . Clinicopathologic comparison of familial versus sporadic atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system. Pediatr Blood Cancer. 2011;56(7):1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kordes U, Bartelheim K, Modena P, et al. . Favorable outcome of patients affected by rhabdoid tumors due to rhabdoid tumor predisposition syndrome (RTPS). Pediatr Blood Cancer. 2014;61(5):919–921. [DOI] [PubMed] [Google Scholar]
- 14. Sestini R, Bacci C, Provenzano A, Genuardi M Papi L. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Hum Mutat. 2008;29(2):227–231. [DOI] [PubMed] [Google Scholar]
- 15. Hilden JM, Meerbaum S, Burger P, et al. . Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004;22(14):2877–2884. [DOI] [PubMed] [Google Scholar]
- 16. Torchia J, Picard D, Lafay-Cousin L, et al. . Molecular subgroups of atypical teratoid rhabdoid tumours in children: an integrated genomic and clinicopathological analysis. Lancet Oncol. 2015;16(5):569–582. [DOI] [PubMed] [Google Scholar]
- 17. Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms’ Tumor Study. Cancer. 1978;41(5):1937–1948. [DOI] [PubMed] [Google Scholar]
- 18. Haas JE, Palmer NF, Weinberg AG, Beckwith JB. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Hum Pathol. 1981;12(7):646–657. [DOI] [PubMed] [Google Scholar]
- 19. Lefkowitz IB, Rorke LB Packer RJ Sutton LN Siegel KR Katnick RJ. Atypical teratoid tumor of infancy: definition of an entity. Ann Neurol. 1987;22:448–449. [Google Scholar]
- 20. Rorke LB, Packer R, Biegel J. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood. J Neurooncol. 1995;24(1):21–28. [DOI] [PubMed] [Google Scholar]
- 21. Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg. 1996;85(1):56–65. [DOI] [PubMed] [Google Scholar]
- 22. Patil S, Perry A, Maccollin M, et al. . Immunohistochemical analysis supports a role for INI1/SMARCB1 in hereditary forms of schwannomas, but not in solitary, sporadic schwannomas. Brain Pathol. 2008;18(4):517–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasselblatt M, Thomas C, Hovestadt V, et al. . Poorly differentiated chordoma with SMARCB1/INI1 loss: a distinct molecular entity with dismal prognosis. Acta Neuropathol. 2016;132(1):149–151. [DOI] [PubMed] [Google Scholar]
- 24. Nemes K, Frühwald MC. Emerging therapeutic targets for the treatment of malignant rhabdoid tumors. Expert Opin Ther Targets. 2018;22(4):365–379. [DOI] [PubMed] [Google Scholar]
- 25. Frühwald MC, Biegel JA, Bourdeaut F, Roberts CW, Chi SN. Atypical teratoid/rhabdoid tumors—current concepts, advances in biology, and potential future therapies. Neuro Oncol. 2016;18(6):764–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Douglass EC, Valentine M, Rowe ST, et al. . Malignant rhabdoid tumor: a highly malignant childhood tumor with minimal karyotypic changes. Genes Chromosomes Cancer. 1990;2(3):210–216. [DOI] [PubMed] [Google Scholar]
- 27. Fort DW, Tonk VS, Tomlinson GE, Timmons CF, Schneider NR. Rhabdoid tumor of the kidney with primitive neuroectodermal tumor of the central nervous system: associated tumors with different histologic, cytogenetic, and molecular findings. Genes Chromosomes Cancer. 1994;11(3):146–152. [DOI] [PubMed] [Google Scholar]
- 28. Shashi V, Lovell MA, von Kap-herr C, Waldron P, Golden WL. Malignant rhabdoid tumor of the kidney: involvement of chromosome 22. Genes Chromosomes Cancer. 1994;10(1):49–54. [DOI] [PubMed] [Google Scholar]
- 29. Biegel JA, Allen CS, Kawasaki K, Shimizu N, Budarf ML, Bell CJ. Narrowing the critical region for a rhabdoid tumor locus in 22q11. Genes Chromosomes Cancer. 1996;16(2):94–105. [DOI] [PubMed] [Google Scholar]
- 30. Schofield DE, Beckwith JB, Sklar J. Loss of heterozygosity at chromosome regions 22q11-12 and 11p15.5 in renal rhabdoid tumors. Genes Chromosomes Cancer. 1996;15(1):10–17. [DOI] [PubMed] [Google Scholar]
- 31. Rosty C, Peter M, Zucman J, Validire P, Delattre O, Aurias A. Cytogenetic and molecular analysis of a t(1;22)(p36;q11.2) in a rhabdoid tumor with a putative homozygous deletion of chromosome 22. Genes Chromosomes Cancer. 1998;21(2):82–89. [PubMed] [Google Scholar]
- 32. Versteege I, Sévenet N, Lange J, et al. . Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394(6689):203–206. [DOI] [PubMed] [Google Scholar]
- 33. Johann PD, Erkek S, Zapatka M, et al. . Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Cancer Cell. 2016;29(3):379–393. [DOI] [PubMed] [Google Scholar]
- 34. Lee RS, Stewart C, Carter SL, et al. . A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122(8):2983–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sévenet N, Sheridan E, Amram D, Schneider P, Handgretinger R, Delattre O. Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers. Am J Hum Genet. 1999;65(5):1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Biegel JA, Tan L, Zhang F, Wainwright L Russo P Rorke LB.. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res. 2002;8(11):3461–3467. [PubMed] [Google Scholar]
- 37. Schneppenheim R, Frühwald MC, Gesk S, et al. . Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet. 2010;86(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasselblatt M, Isken S, Linge A, et al. . High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes Chromosomes Cancer. 2013;52(2):185–190. [DOI] [PubMed] [Google Scholar]
- 39. Kieran MW, Roberts CW, Chi SN, et al. . Absence of oncogenic canonical pathway mutations in aggressive pediatric rhabdoid tumors. Pediatr Blood Cancer. 2012;59(7):1155–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawrence MS, Stojanov P, Polak P, et al. . Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Torchia J, Golbourn B, Feng S, et al. . Integrated (epi)-genomic analyses identify subgroup-specific therapeutic targets in CNS rhabdoid tumors. Cancer Cell. 2016;30(6):891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shlyueva D, Stampfel G, Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet. 2014;15(4):272–286. [DOI] [PubMed] [Google Scholar]
- 43. Kadoch C, Hargreaves DC, Hodges C, et al. . Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45(6):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shain AH, Pollack JR. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS One. 2013;8(1):e55119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11(7):481–492. [DOI] [PubMed] [Google Scholar]
- 46. Saha A, Wittmeyer J, Cairns BR. Chromatin remodelling: the industrial revolution of DNA around histones. Nat Rev Mol Cell Biol. 2006;7(6):437–447. [DOI] [PubMed] [Google Scholar]
- 47. Nakayama RT, Pulice JL, Valencia AM, et al. . SMARCB1 is required for widespread BAF complex-mediated activation of enhancers and bivalent promoters. Nat Genet. 2017;49(11):1613–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X, Lee RS, Alver BH, et al. . SMARCB1-mediated SWI/SNF complex function is essential for enhancer regulation. Nat Genet. 2017;49(2):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson BG, Wang X, Shen X, et al. . Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell. 2010;18(4):316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ho L, Jothi R, Ronan JL, Cui K, Zhao K, Crabtree GR. An embryonic stem cell chromatin remodeling complex, esBAF, is an essential component of the core pluripotency transcriptional network. Proc Natl Acad Sci U S A. 2009;106(13):5187–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Betz BL, Strobeck MW, Reisman DN, Knudsen ES, Weissman BE. Re-expression of hSNF5/INI1/BAF47 in pediatric tumor cells leads to G1 arrest associated with induction of p16ink4a and activation of RB. Oncogene. 2002;21(34):5193–5203. [DOI] [PubMed] [Google Scholar]
- 52. Isakoff MS, Sansam CG, Tamayo P, et al. . Inactivation of the Snf5 tumor suppressor stimulates cell cycle progression and cooperates with p53 loss in oncogenic transformation. Proc Natl Acad Sci U S A. 2005;102(49):17745–17750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oruetxebarria I, Venturini F, Kekarainen T, et al. . P16INK4a is required for hSNF5 chromatin remodeler-induced cellular senescence in malignant rhabdoid tumor cells. J Biol Chem. 2004;279(5):3807–3816. [DOI] [PubMed] [Google Scholar]
- 54. Versteege I, Medjkane S, Rouillard D, Delattre O. A key role of the hSNF5/INI1 tumour suppressor in the control of the G1-S transition of the cell cycle. Oncogene. 2002;21(42):6403–6412. [DOI] [PubMed] [Google Scholar]
- 55. Banine F, Bartlett C, Gunawardena R, et al. . SWI/SNF chromatin-remodeling factors induce changes in DNA methylation to promote transcriptional activation. Cancer Res. 2005;65(9):3542–3547. [DOI] [PubMed] [Google Scholar]
- 56. Tolstorukov MY, Sansam CG, Lu P, et al. . Swi/Snf chromatin remodeling/tumor suppressor complex establishes nucleosome occupancy at target promoters. Proc Natl Acad Sci U S A. 2013;110(25):10165–10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee S, Cimica V, Ramachandra N, Zagzag D, Kalpana GV. Aurora A is a repressed effector target of the chromatin remodeling protein INI1/hSNF5 required for rhabdoid tumor cell survival. Cancer Res. 2011;71(9):3225–3235. [DOI] [PubMed] [Google Scholar]
- 58. Strobeck MW, Fribourg AF, Puga A, Knudsen ES. Restoration of retinoblastoma mediated signaling to Cdk2 results in cell cycle arrest. Oncogene. 2000;19(15):1857–1867. [DOI] [PubMed] [Google Scholar]
- 59. Zhang HS, Gavin M, Dahiya A, et al. . Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell. 2000;101(1):79–89. [DOI] [PubMed] [Google Scholar]
- 60. Lee D, Kim JW, Seo T, Hwang SG, Choi EJ Choe J. SWI/SNF complex interacts with tumor suppressor p53 and is necessary for the activation of p53-mediated transcription. J Biol Chem. 2002;277(25):22330–22337. [DOI] [PubMed] [Google Scholar]
- 61. DelBove J, Kuwahara Y, Mora-Blanco EL, et al. . Inactivation of SNF5 cooperates with p53 loss to accelerate tumor formation in Snf5(+/–);p53(+/–) mice. Mol Carcinog. 2009;48(12):1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caramel J, Quignon F, Delattre O. RhoA-dependent regulation of cell migration by the tumor suppressor hSNF5/INI1. Cancer Res. 2008;68(15):6154–6161. [DOI] [PubMed] [Google Scholar]
- 63. Medjkane S, Novikov E, Versteege I, Delattre O.. The tumor suppressor hSNF5/INI1 modulates cell growth and actin cytoskeleton organization. Cancer Res. 2004;64(10):3406–3413. [DOI] [PubMed] [Google Scholar]
- 64. Jagani Z, Mora-Blanco EL, Sansam CG, et al. . Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog-Gli pathway. Nat Med. 2010;16(12):1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 2001;20(17):4935–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mora-Blanco EL, Mishina Y, Tillman EJ, et al. . Activation of β-catenin/TCF targets following loss of the tumor suppressor SNF5. Oncogene. 2014;33(7):933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cheng SW, Davies KP, Yung E, Beltran RJ, Yu J, Kalpana GV. c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function. Nat Genet. 1999;22(1):102–105. [DOI] [PubMed] [Google Scholar]
- 68. Park J, Wood MA, Cole MD. BAF53 forms distinct nuclear complexes and functions as a critical c-Myc-interacting nuclear cofactor for oncogenic transformation. Mol Cell Biol. 2002;22(5):1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chai B, Huang J, Cairns BR, Laurent BC. Distinct roles for the RSC and Swi/Snf ATP-dependent chromatin remodelers in DNA double-strand break repair. Genes Dev. 2005;19(14):1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hara R, Sancar A. The SWI/SNF chromatin-remodeling factor stimulates repair by human excision nuclease in the mononucleosome core particle. Mol Cell Biol. 2002;22(19):6779–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Klochendler-Yeivin A, Picarsky E, Yaniv M. Increased DNA damage sensitivity and apoptosis in cells lacking the Snf5/Ini1 subunit of the SWI/SNF chromatin remodeling complex. Mol Cell Biol. 2006;26(7):2661–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Park JH, Park EJ, Lee HS, et al. . Mammalian SWI/SNF complexes facilitate DNA double-strand break repair by promoting gamma-H2AX induction. EMBO J. 2006;25(17):3986–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKenna ES, Sansam CG, Cho YJ, et al. . Loss of the epigenetic tumor suppressor SNF5 leads to cancer without genomic instability. Mol Cell Biol. 2008;28(20):6223–6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guidi CJ, Sands AT, Zambrowicz BP, et al. . Disruption of Ini1 leads to peri-implantation lethality and tumorigenesis in mice. Mol Cell Biol. 2001;21(10):3598–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Klochendler-Yeivin A, Fiette L, Barra J, Muchardt C, Babinet C, Yaniv M. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO Rep. 2000;1(6):500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Roberts CW, Galusha SA, McMenamin ME, Fletcher CD, Orkin SH. Haploinsufficiency of Snf5 (integrase interactor 1) predisposes to malignant rhabdoid tumors in mice. Proc Natl Acad Sci U S A. 2000;97(25):13796–13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moreno N, Schmidt C, Ahlfeld J, et al. . Loss of Smarc proteins impairs cerebellar development. J Neurosci. 2014;34(40):13486–13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Han ZY, Richer W, Fréneaux P, et al. . The occurrence of intracranial rhabdoid tumours in mice depends on temporal control of Smarcb1 inactivation. Nat Commun. 2016;7:10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boyd C, Smith MJ, Kluwe L, Balogh A, Maccollin M, Plotkin SR. Alterations in the SMARCB1 (INI1) tumor suppressor gene in familial schwannomatosis. Clin Genet. 2008;74(4):358–366. [DOI] [PubMed] [Google Scholar]
- 80. Hadfield KD, Newman WG, Bowers NL, et al. . Molecular characterisation of SMARCB1 and NF2 in familial and sporadic schwannomatosis. J Med Genet. 2008;45(6):332–339. [DOI] [PubMed] [Google Scholar]
- 81. Hulsebos TJ, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet. 2007;80(4):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vitte J, Gao F, Coppola G, Judkins AR, Giovannini M. Timing of Smarcb1 and Nf2 inactivation determines schwannoma versus rhabdoid tumor development. Nat Commun. 2017;8(1):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hashizume R, Gupta N, Berger MS, et al. . Morphologic and molecular characterization of ATRT xenografts adapted for orthotopic therapeutic testing. Neuro Oncol. 2010;12(4):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kaur H, Hütt-Cabezas M, Weingart MF, et al. . The chromatin-modifying protein HMGA2 promotes atypical teratoid/rhabdoid cell tumorigenicity. J Neuropathol Exp Neurol. 2015;74(2):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Girard E, Ditzler S, Lee D, et al. . Efficacy of cabazitaxel in mouse models of pediatric brain tumors. Neuro Oncol. 2015;17(1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hashizume R, Zhang A, Mueller S, et al. . Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol. 2016;18(11):1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alimova I, Pierce AM, Harris P, et al. . Targeting Polo-like kinase 1 in SMARCB1 deleted atypical teratoid rhabdoid tumor. Oncotarget. 2017;8(57):97290–97303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Singh A, Lun X, Jayanthan A, et al. . Profiling pathway-specific novel therapeutics in preclinical assessment for central nervous system atypical teratoid rhabdoid tumors (CNS ATRT): favorable activity of targeting EGFR- ErbB2 signaling with lapatinib. Mol Oncol. 2013;7(3):497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Weingart MF, Roth JJ, Hutt-Cabezas M, et al. . Disrupting LIN28 in atypical teratoid rhabdoid tumors reveals the importance of the mitogen activated protein kinase pathway as a therapeutic target. Oncotarget. 2015;6(5):3165–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Alimova I, Birks DK, Harris PS, et al. . Inhibition of EZH2 suppresses self-renewal and induces radiation sensitivity in atypical rhabdoid teratoid tumor cells. Neuro Oncol. 2013;15(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Knipstein JA, Birks DK, Donson AM, Alimova I,Foreman NK, Vibhakar R. Histone deacetylase inhibition decreases proliferation and potentiates the effect of ionizing radiation in atypical teratoid/rhabdoid tumor cells. Neuro Oncol. 2012;14(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McGovern SL, Okcu MF, Munsell MF, et al. . Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int J Radiat Oncol Biol Phys. 2014;90(5):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lun X, Ruan Y, Jayanthan A, et al. . Double-deleted vaccinia virus in virotherapy for refractory and metastatic pediatric solid tumors. Mol Oncol. 2013;7(5):944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Studebaker AW, Hutzen B, Pierson CR, Shaffer TA, Raffel C, Jackson EM. Oncolytic measles virus efficacy in murine xenograft models of atypical teratoid rhabdoid tumors. Neuro Oncol. 2015;17(12):1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Studebaker AW, Hutzen BJ, Pierson CR, et al. . Oncolytic herpes virus rRp450 shows efficacy in orthotopic xenograft group 3/4 medulloblastomas and atypical teratoid/rhabdoid tumors. Mol Ther Oncolytics. 2017;6:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wu Y, Lun X, Zhou H, et al. . Oncolytic efficacy of recombinant vesicular stomatitis virus and myxoma virus in experimental models of rhabdoid tumors. Clin Cancer Res. 2008;14(4):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ardon H, De Vleeschouwer S, Van Calenbergh F, et al. . Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer. 2010;54(4):519–525. [DOI] [PubMed] [Google Scholar]
- 98. van Gool SW, Holm S, Rachor J, et al. . Immunotherapy in atypical teratoid-rhabdoid tumors: Data from a survey of the HGG-Immuno Group. Cytotherapy. 2016;18(9):1178–1186. [DOI] [PubMed] [Google Scholar]
- 99. Gholamin S, Mitra SS, Feroze AH, et al. . Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med. 2017;9(381). [DOI] [PubMed] [Google Scholar]
- 100. Fischer-Valuck BW, Chen I, Srivastava AJ, et al. . Assessment of the treatment approach and survival outcomes in a modern cohort of patients with atypical teratoid rhabdoid tumors using the National Cancer Database. Cancer. 2017;123(4):682–687. [DOI] [PubMed] [Google Scholar]
- 101. Schrey D, Carceller Lechón F, Malietzis G, et al. . Multimodal therapy in children and adolescents with newly diagnosed atypical teratoid rhabdoid tumor: individual pooled data analysis and review of the literature. J Neurooncol. 2016;126(1):81–90. [DOI] [PubMed] [Google Scholar]
- 102. Buscariollo DL, Park HS, Roberts KB, Yu JB. Survival outcomes in atypical teratoid rhabdoid tumor for patients undergoing radiotherapy in a Surveillance, Epidemiology, and End Results analysis. Cancer. 2012;118(17):4212–4219. [DOI] [PubMed] [Google Scholar]
- 103. Chen YW, Wong TT, Ho DM, et al. . Impact of radiotherapy for pediatric CNS atypical teratoid/rhabdoid tumor (single institute experience). Int J Radiat Oncol Biol Phys. 2006;64(4):1038–1043. [DOI] [PubMed] [Google Scholar]
- 104. Fossey M, Li H, Afzal S, et al. . Atypical teratoid rhabdoid tumor in the first year of life: the Canadian ATRT registry experience and review of the literature. J Neurooncol. 2017;132(1):155–162. [DOI] [PubMed] [Google Scholar]
- 105. Lafay-Cousin L, Hawkins C, Carret AS, et al. . Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012;48(3):353–359. [DOI] [PubMed] [Google Scholar]
- 106. Lee J, Kim DS, Han JW, Suh CO. Atypical teratoid/rhabdoid tumors in children treated with multimodal therapies: the necessity of upfront radiotherapy after surgery. Pediatr Blood Cancer. 2017;64(12). [DOI] [PubMed] [Google Scholar]
- 107. Pai Panandiker AS, Merchant TE, Beltran C, et al. . Sequencing of local therapy affects the pattern of treatment failure and survival in children with atypical teratoid rhabdoid tumors of the central nervous system. Int J Radiat Oncol Biol Phys. 2012;82(5):1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chi SN, Zimmerman MA, Yao X, et al. . Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27(3):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. von Hoff K, Hinkes B, Dannenmann-Stern E, et al. . Frequency, risk-factors and survival of children with atypical teratoid rhabdoid tumors (AT/RT) of the CNS diagnosed between 1988 and 2004, and registered to the German HIT database. Pediatr Blood Cancer. 2011;57(6):978–985. [DOI] [PubMed] [Google Scholar]
- 110. Bartelheim K, Nemes K, Seeringer A, et al. . Improved 6-year overall survival in AT/RT—results of the registry study Rhabdoid 2007. Cancer Med. 2016;5(8):1765–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dufour C, Beaugrand A, Le Deley MC, et al. . Clinicopathologic prognostic factors in childhood atypical teratoid and rhabdoid tumor of the central nervous system: a multicenter study. Cancer. 2012;118(15):3812–3821. [DOI] [PubMed] [Google Scholar]
- 112. Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008;51(2):235–240. [DOI] [PubMed] [Google Scholar]
- 113. Slavc I, Chocholous M, Leiss U, et al. . Atypical teratoid rhabdoid tumor: improved long-term survival with an intensive multimodal therapy and delayed radiotherapy. The Medical University of Vienna Experience 1992-2012. Cancer Med. 2014;3(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zaky W, Dhall G, Ji L, et al. . Intensive induction chemotherapy followed by myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for young children newly-diagnosed with central nervous system atypical teratoid/rhabdoid tumors: the Head Start III experience. Pediatr Blood Cancer. 2014;61(1):95–101. [DOI] [PubMed] [Google Scholar]
- 115. Benesch M, Bartelheim K, Fleischhack G, et al. . High-dose chemotherapy (HDCT) with auto-SCT in children with atypical teratoid/rhabdoid tumors (AT/RT): a report from the European Rhabdoid Registry (EU-RHAB). Bone Marrow Transplant. 2014;49(3):370–375. [DOI] [PubMed] [Google Scholar]
- 116. Reddy A, Strother D, Judkins A, et al. . Treatment of atypical teratoid rhabdoid tumors (ATRT) of the central nervous system with surgery, intensive chemotherapy, and 3-D conformal radiation (ACNS0333). A report from the Children’s Oncology Group. Paper presented at: 17th International Symposium on Pediatric Neuro-Oncology; June 13, 2016; Liverpool, United Kingdom. [Google Scholar]
- 117. Wetmore C, Boyett J, Li S, et al. . Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol. 2015;17(6):882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Geoerger B, Bourdeaut F, DuBois SG, et al. . A phase I study of the CDK4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumors. Clin Cancer Res. 2017;23(10):2433–2441. [DOI] [PubMed] [Google Scholar]
- 119. Forest F, David A, Arrufat S, et al. . Conventional chondrosarcoma in a survivor of rhabdoid tumor: enlarging the spectrum of tumors associated with SMARCB1 germline mutations. Am J Surg Pathol. 2012;36(12):1892–1896. [DOI] [PubMed] [Google Scholar]
- 120. Bendel A, Toledano H, Cohen I, Chi S, Biegel J. Late development of metachronous atypical teratoid/rhabdoid tumors (AT/RTs) or malignant rhabdoid tumor (MRT) in three patients with germline SMARCB1 mutations. Neuro Oncol. 2018;20(Suppl 2):i27–i28. [Google Scholar]
- 121. Birks DK, Donson AM, Patel PR, et al. . High expression of BMP pathway genes distinguishes a subset of atypical teratoid/rhabdoid tumors associated with shorter survival. Neuro Oncol. 2011;13(12):1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]