Abstract
Background
Since its approval for use in recurrent glioblastoma (GBM), the survival benefit of bevacizumab (Bev) remains to be demonstrated. To address this issue, we retrospectively examined survival from first recurrence in patients treated with Bev, lomustine (CCNU), or Bev/CCNU.
Methods
We identified 168 primary GBM patients diagnosed at UCLA and Kaiser Permanente LA who received upfront radio-chemotherapy, followed by Bev and/or CCNU at first recurrence. Three patient groups, contemporaneously diagnosed from 2009 through 2015, were identified: (1) patients treated with Bev alone (n = 49), (2) CCNU alone (CCNU 09-15) (n = 36), and (3) Bev/CCNU (n = 53). Another CCNU control group (n = 30) diagnosed from 2001 through 2004 (CCNU 01-04) was also derived. We measured tumor size at first recurrence treatment initiation, using bidimensional (2D) and volumetric (3D) techniques, and analyzed overall survival (OS) from first recurrence.
Results
Among the entire cohort, larger tumor size at first recurrence was associated with poorer survival. The CCNU 01-04 group had similar tumor size as the Bev arms and low Bev crossover (7%). Treatment with Bev was associated with improved survival in patients with large tumor 2D measurements: Median OS for Bev and Bev/CCNU groups were 6.71 mo (n = 27) and 6.97 mo (n = 36) vs 4.03 mo (n = 10) in CCNU 01-04. Analysis by 3D measurement yielded similar results. Interestingly, the CCNU 09-15 group showed the highest survival, likely due to smaller tumor size and crossover to Bev (69%).
Conclusion
Survival advantage from Bev treatment was observed only among patients with large tumor burden as determined by either 2D or 3D measurement.
Keywords: bevacizumab, first recurrence, glioblastoma, overall survival, tumor size
Glioblastoma (GBM) is the most common type of malignant primary brain tumor and remains incurable for the vast majority of patients. Despite current standard upfront treatment including maximal tumor resection, followed by concurrent radiation (XRT) with temozolomide (TMZ) and maintenance TMZ,1 the median survival from diagnosis for GBM patients remains at 16-21 months,2 with nearly all patients developing recurrent disease. Although there is no standard treatment for recurrent GBM, bevacizumab (Bev) and lomustine (CCNU) represent the most common systemic recurrent treatments.
Bevacizumab, a humanized monoclonal antibody, exerts anti-angiogenic and anti-edema effects by targeting circulating vascular endothelial growth factor (VEGF). Bev received conditional accelerated approval from the United States Food and Drug Administration in 2009 and full approval in 2017 for recurrent GBM. A phase II trial (BELOB trial) conducted in the Netherlands provided evidence that Bev/CCNU was superior at first recurrence comparing to Bev alone or CCNU alone.3 Informed by this trial result, a phase III study (EORTC 26101) was conducted to compare the efficacy of concurrent Bev/CCNU vs CCNU for GBM at first recurrence.4 With the possible caveat that there was substantial crossover to Bev in the CCNU arm, the trial did not demonstrate any difference in overall survival (OS) between these arms, and there was no observed OS benefit in any subgroup.
Despite the lack of proven OS benefit at recurrence, Bev remains a common choice as salvage therapy for recurrent GBM. It remains to be determined whether particular clinical or molecular subsets of recurrent GBM patient can derive survival benefits from Bev. One possibility is that Bev might be preferentially beneficial in tumors with a higher tumor burden. However, the effect of tumor volume on outcome of recurrent GBM has not been well studied in the era of Bev availability. Tumor volume (3-dimensional (3D) measurement) of recurrent GBM has been reported to be associated with better survival,5,6 while tumor area obtained by bidimensional (2D) measurement has been shown to be nonprognostic7 with no correlation to 3D measurements.5
This retrospective study aimed to evaluate the impact of tumor burden on outcomes among patients receiving concurrent Bev/CCNU, Bev alone vs CCNU alone at first recurrence using retrospective data from primary GBM patients treated at University of California—Los Angeles (UCLA) and Kaiser Permanente—Los Angeles (KPLA). In addition, we performed parallel analysis with tumor burden assessed by 2D and 3D measurements.
Methods
Study Population
Based on a retrospective electronic database query of adult primary GBM (gliosarcoma was excluded) treated at UCLA and KPLA in 2009 through 2015, we identified a cohort of 138 patients, who received upfront XRT and TMZ, and either Bev, Bev/CCNU, or CCNU at first recurrence, including: 49 patients treated with Bev alone, 36 patients with CCNU alone (CCNU 09-15), and 53 patients with concurrent Bev/CCNU. We identified another group of 30 patients diagnosed from 2001 through 2004 who received CCNU alone (CCNU 01-04) to minimize crossing over to Bev at later recurrences.
All participants signed the informed consent form in a UCLA/KPLA institutional review board-approved database study to collect clinical, pathological, and imaging information to be used for future retrospective studies. The tumor pathology was confirmed either by UCLA neuropathologists (n = 101) or outside hospital pathologists for patients with initial resection performed at another institution (n = 67). After tumor resection, included patients could have received other concurrent treatments along with upfront XRT/TMZ except for Bev or other anti-angiogenics. All patients had recurrent supra-tentorial tumor after first-line treatment with XRT/ TMZ, and were treated with at least one cycle of either Bev or CCNU or a combination of both at first recurrence. To control for possible pseudoprogression, any patient who had a first recurrence less than 12 weeks (84 days) after completion of XRT was excluded from the study (Response Assessment in Neuro-Oncology criteria).8 Patients’ demographic and clinical characteristics at initial diagnosis and first recurrence were collected for analysis. In addition, mutation status of the isocitrate dehydrogenase 1 and 2 genes (IDH1/2) was available for 71 patients, including 2 mutant IDH1 (1 patient in Bev only and 1 patient in the CCNU 09-15 group). Promoter methylation status of the O6-methylguanine-DNA-methyltransferase (MGMT) was available for 58 cases, including 18 methylated patients and 40 unmethylated patients. Further analysis did not include MGMT methylation status as only a small subset of patients (58/168) had available methylation status.
Measurement of Tumor Size at First Recurrence
Using 2D and 3D techniques, we measured tumor size at the time of first recurrent treatment initiation on axial postcontrast T1-weighted MRI obtained prior to first recurrence treatment initiation. The tumor 2D measurement was obtained from the product of the largest diameter of the contrast-enhancing region on MRI postcontrast T1-weighted imaging with its perpendicular axis on the same slide. The tumor 3D measurement was obtained using AFNI (http://afni.nimh.nih.gov/afni) to contour the contrast enhancing region of the tumor. Initially, the program semiautomatically subtracts the precontrast T1-weighted signal from the postcontrast T1-weighted image to better amplify the contrast-enhancing region. A signal threshold for the contrast-enhancing region, which was identified using the normal brain on the contralateral side as reference, was used to outline the contrast enhancing region of the tumor. This region was then manually contoured across all the slices using the guideline obtained from the setup threshold. Multifocal masses were measured independently and summed up as a total mass measurement for the patient. All tumor measurements were completed twice by independent investigators to account for interrater variability. The average 2D or 3D measurement was reported for each patient. Median tumor size from the entire cohort was later used for patient stratification.
Survival Intervals and Statistical Analysis
The primary objective of this study was to assess patient OS, which was determined from the date of first recurrent chemotherapy treatment (Bev or CCNU or Bev/CCNU) initiation to the date of death or censor. Dates of first progression were determined at the time of imaging by the treating clinicians, considering both contrast-enhancing and noncontrast-enhancing tumors using modified Levin criteria, as previously used.9,10 Patients who were lost to follow-up with unobtainable date of death or had the last follow-up before the freeze date on November 3, 2016, were censored on their last known clinical visit or imaging study. Patients with the last follow-up after the freeze date were censored on November 3, 2016.
Patient survival was evaluated using Kaplan-Meier analysis and Cox proportional hazard regression model in the R package. Patient characteristics were summarized using descriptive statistics, and differences between two groups of comparison were examined using chi-square and analysis of variance (ANOVA) tests. Because this was an exploratory study, alpha level was not adjusted for multiple comparisons, and significance level was assigned at P < .05.
Results
Patient Characteristics
As described in Methods, we derived 4 groups for comparison for a total cohort size of 168 primary GBM patients who recurred after first-line treatment. The first 3 groups of patients were contemporaneously treated between 2009 and 2015: 49 patients treated with Bev alone at first recurrence (Bev), 36 patients treated with CCNU alone (CCNU 09-15), and 53 patients treated with Bev concurrently with CCNU (Bev/CCNU). A fourth group of patients that received CCNU alone (CCNU 01-04) was derived from patients treated between 2001 and 2004 to minimize Bev crossover at later recurrences because Bev was not yet available at this time. None of the patients were exposed to Bev or other anti-angiogenics during first-line intervention. Patient characteristics are summarized in Table 1. The 4 groups of patients differed in age at the time of their initial diagnosis (ANOVA test, P = .004). Bev patients were the oldest at the time of diagnosis with a median age of 63 years, and patients with CCNU alone from 2001 through 2004 were the youngest with a median age of 53 years. Both Bev/CCNU and CCNU 09-15 groups had a median age of diagnosis of around 57 years. The 4 groups were similar in gender and performance status at first recurrent treatment. As expected, the CCNU 01-04 group had fewer patients cross over to Bev (7%) compared to the more recent CCNU 09-15 group (69%), chi-square test, P < .001.
Table 1.
Demographic Summary of 168 Primary Glioblastoma Patients
| Bev N = 49 |
Bev/CCNU N = 53 |
CCNU 09-15 N = 36 |
CCNU 01-04 N = 30 |
P Value | |
|---|---|---|---|---|---|
| Alive/Censored | 6 (12%) | 10 (19%) | 13 (36%) | 2 (7%) | 0.0093a |
| Death | 43 (88%) | 43 (81%) | 23 (64%) | 28 (93%) | |
| Age at Diagnosis | |||||
| Median | 63.44 | 56.87 | 57.4 | 53.36 | 0.0043b |
| Range | 39.95-80.4 | 40.27-75.54 | 25.99-83.4 | 20.78-78.18 | |
| Gender | |||||
| Female | 25 (51%) | 17 (32%) | 17 (47%) | 12 (40%) | 0.2387a |
| Male | 24 (49%) | 36 (68%) | 19 (53%) | 18 (60%) | |
| KPS at First Recurrent Treatment | |||||
| ≥70 | 39 (80%) | 50 (94%) | 32 (89%) | 24 (80%) | 0.1127a |
| <70 | 10 (20%) | 3 (6%) | 4 (11%) | 6 (20%) | |
| Tumor 2D (mm2) | |||||
| Nonmissing N | 47 | 51 | 36 | 24 | <0.0001b |
| Median | 1196.06 | 1545.05 | 504.88 | 743.24 | |
| Range | 0-6315.35 | 0-4219.72 | 0-2818.09 | 0-4285.3 | |
| Tumor 3D (mm3) | |||||
| Nonmissing N | 46 | 50 | 36 | 22 | 0.0002b |
| Median | 12372.87 | 20991.92 | 3350.91 | 14859.23 | |
| Range | 944.48-86992.79 | 0-115250.95 | 0-44140.48 | 0-170873.55 | |
| Crossover to Bev | |||||
| No | NA | NA | 11 (31%) | 28 (93%) | <0.0001a |
| Yes | NA | NA | 25 (69%) | 2 (7%) | |
Abbreviations: Bev, bevacizumab; CCNU, lomustine; KPS, Karnofsky Performance Status; NA, not available.
achi-square test.
bAnalysis of variance test.
Larger Tumor Size (3D Measurement or 2D Measurement) at First Recurrence Was Associated with Poorer Survival
To determine whether tumor size at first recurrence can influence outcome, we determined the effect of tumor size at first recurrence prior to initiation of treatment on survival in the entire cohort. We found that the median tumor 2D and 3D measurements of the entire cohort were 1091 mm2 (n = 158) and 11698 mm3 (n = 154), respectively. In addition, the 2D and 3D measurements of the entire cohort were highly correlated (Pearson correlation and Spearman correlation tests, P < .001), suggesting that the simpler 2D measurement is adequate for clinically relevant determination of tumor size.
We performed a Kaplan-Meier analysis between large and small tumor size dichotomized by the median and found that low tumor size by either 2D or 3D measurement was associated with improved outcome. Patients with large tumor size demonstrated a median OS of 6.69 months (n = 79, 2D measurement) and 6.05 months (n = 77, 3D measurement) vs median OS of 8.81 months (n = 79, 2D measurement) and 9.67 months (n = 77, 3D measurement) in patients with small tumor size, P = .003 and P < .001, respectively (Supplementary Figure 1A, B). Multivariate Cox regression analysis including tumor size as a dichotomized variable confirmed these results (Supplementary Table 1). Patients with small tumor size showed an OS hazard ratio of 0.570 (P = .002) for 2D measurement and 0.551 (P = .001) for 3D measurement. This result indicated that tumor size independently predicts patient OS from first recurrence as measured by either 2D or 3D methodology.
Tumor Size at First Recurrence Treatment Initiation Is Smallest in CCNU 09-15 Group
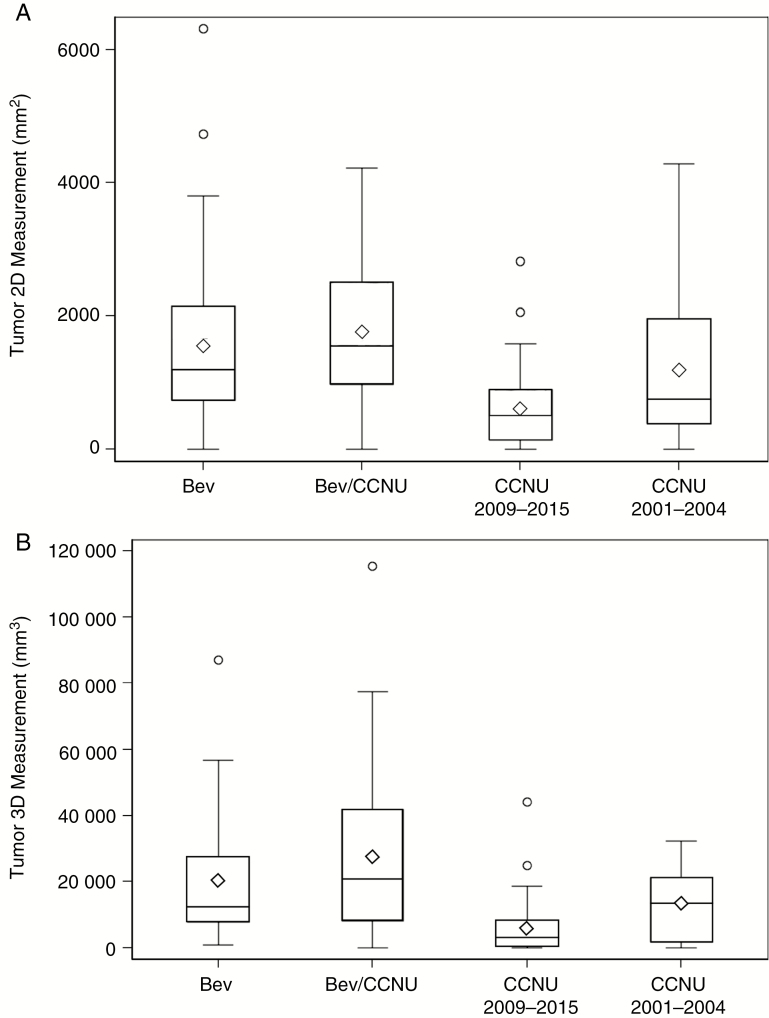
Next, we compared tumor burden at first recurrence in the 4 groups. We measured the tumor size prior to first recurrent treatment using 2D and 3D techniques and found that the 4 groups were statistically different in median tumor size (ANOVA test, P < .001 and P = .002, respectively). The 2D and 3D tumor sizes of the CCNU 09-15 group were significantly different from the tumor sizes of the Bev groups, while the tumor sizes of the CCNU 01-04 group were not. From the T test using 2D tumor sizes, CCNU 09-15 vs BEV or BEV/CCNU showed a P value < .001, while CCNU 01-04 vs BEV or BEV/CCNU showed P values = 0.121 and 0.057, respectively (similar results obtained from 3D tumor sizes). Median 2D tumor sizes for the Bev and Bev/CCNU groups were 1196 mm2 and 1545 mm2, and median 3D tumor sizes for the Bev and Bev/CCNU groups were 12373 mm2 and 20992 mm3. The CCNU 09-15 group of patients had the smallest tumor size at the time of first recurrence initiation: Median tumor 2D and 3D measurements were 505 mm2 and 3351 mm3, respectively (Fig. 1). This revealed likely selection bias in which patients with lower tumor burden were more likely to receive CCNU only vs BEV or BEV/CCNU in the contemporaneously treated 2009-2015 groups. In contrast to the smaller size of the CCNU 09-15 group, the CCNU 01-04 group was more similar to the Bev groups in both median tumor 2D and 3D assessments, with CCNU 01-04 group tumor sizes at 743 mm2 and 14859 mm3, respectively. Thus, based on comparable tumor size at first recurrence treatment as well as low Bev crossover at later recurrence, we used the CCNU 01-04 patient group as a more informative reference group than the CCNU 09-15 group.
Fig. 1.
Distribution of tumor size among four groups of patients using A, bi-dimensional (2D) measurement and B, volumetric (3D) measurement. One outlier measurement in the CCNU 2001-2004 was excluded from the graph of the 3D measurement group to prevent compression of the graph scale. In both 2D and 3D measurement analyses, the average tumor sizes of the CCNU 2009-2015 group were below the average tumor sizes of the entire cohort. CCNU indicates lomustine; 3D, 3-dimensional.
Bevacizumab treatment is associated with better OS from first recurrence when controlling for tumor size with benefits seen in larger tumors but not smaller ones.
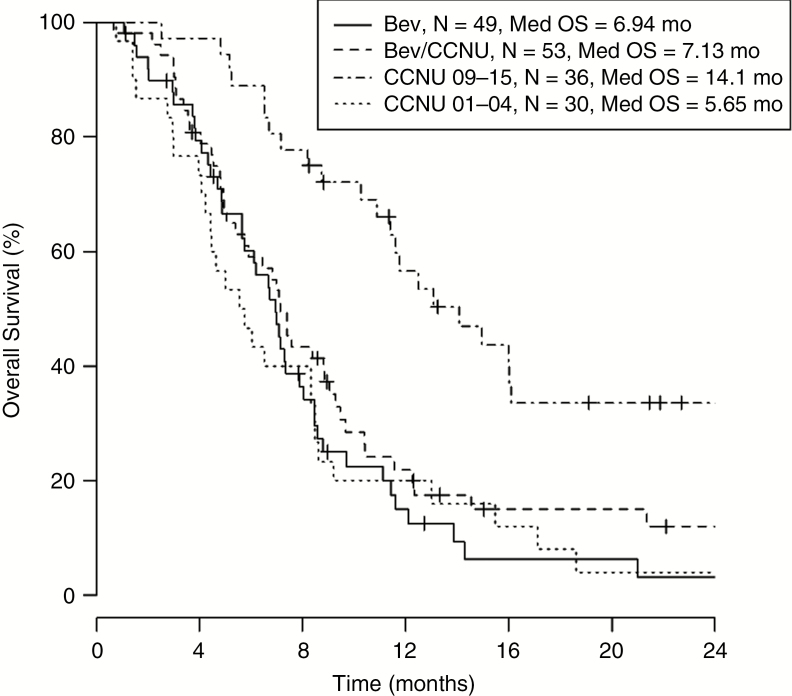
To evaluate the OS of all patient groups from first recurrence, we performed a Kaplan-Meier analysis, which showed similar survival between Bev, Bev/CCNU, and CCNU 01-04 patient groups, and substantially longer OS in CCNU 09-15 patient groups. The median overall survivals of Bev, Bev/CCNU and CCNU 01-04 patients were 6.94 months (n = 49), 7.13 months (n = 53), and 5.65 months (n = 30), respectively, while median OS of CCNU 09-15 patients was 14.1 months (n = 36) (Fig. 2). Multivariate Cox regression analysis, in which age at treatment initiation and KPS at first recurrent treatment were included in the model in addition to treatment at first recurrence, yielded similar result to the Kaplan-Meier analysis (Table 2). Using CCNU 2001-2004 survival as reference, Bev and Bev/CCNU patient groups showed insignificant hazard ratios of 0.803 (P = .387) and 0.748 (P = .238), respectively, while CCNU 09-15 showed a hazard ratio of 0.305 (P < .001).
Fig. 2 .
Kaplan-Meier analysis evaluating overall survival (OS) of patients by treatment group. Survival appeared to be similar among bevacizumab (Bev)-, Bev/lomustine (CCNU)-, and CCNU 01-04–treated patients, while patients treated with CCNU in 09-15 survived longer than the other groups. Median OS for patients receiving Bev alone at first recurrence was 6.94 months (n = 49), for patients with Bev/CCNU was 7.13 months (n = 53), for patients with CCNU 09-15 was 14.1 months (n = 36), and for patients with CCNU 01-04 was 5.65 months (n = 30).
Table 2 .
Cox Regression Analysis of Overall Survival
| OS Without Controlling for Tumor Size | OS Controlling for Tumor Size | |||
|---|---|---|---|---|
| Overall Survival, Tumor 2D | Overall Survival, Tumor 3D | |||
| Hazard Ratio (95% CI), P Value | Hazard Ratio (95% CI), P Value | Hazard Ratio (95% CI), P Value | ||
| Age at Treatment Initiation | 1.022 (1.006-1.039), P = .007 | 1.031 (1.013-1.049), P < .001 | 1.024 (1.005-1.043), P = .012 | |
| KPS at First Recurrent Treatment | <70 | Reference | Reference | Reference |
| ≥70 | 0.552 (0.341-0.893), P = .016 | 0.487 (0.289-0.820), P = .007 | 0.550 (0.319-0.949), P = .032 | |
| Treatment Group | CCNU 01-04 | Reference | Reference | Reference |
| Bev | 0.803 (0.489-1.320), P = .387 | 0.482 (0.269-0.864), P = .014 | 0.584 (0.336-1.016), P = .057 | |
| Bev/CCNU | 0.748 (0.462-1.212), P = .238 | 0.478 (0.273-0.837), P = .010 | 0.538 (0.316-0.916), P = .023 | |
| CCNU 09-15 | 0.305 (0.174-0.535), P < .001 | 0.235 (0.129-0.431), P < .001 | 0.253 (0.136-0.471), P < .001 | |
| Tumor Measurement | High | Reference | Reference | |
| Low | 0.623 (0.419-0.924), P = .019 | 0.711 (0.484-1.046), P = .083 | ||
Abbreviations: 2D, 2-dimensional; 3D, 3-dimensional; Bev, bevacizumab; CI, confidence interval; CCNU, lomustine; OS, overall survival; KPS, Karnofsky Performance Status.
In contrast with multivariate models that did not include tumor size, when size was added to the Cox regression model, both Bev patient groups had improved OS compared to the CCNU 01-04 patient groups (Table 2). Bev and Bev/CCNU patients showed hazard ratios of 0.482 (P = .014) and 0.478 (P = .010), respectively, for 2D analysis, and 0.584 (P = .057) and 0.538 (P = .023), respectively, for 3D analysis. The CCNU 09-15 patient groups continued to show better survival compared to the reference group in both 2D and 3D analysis: Hazard ratios are 0.235 (P < .001) and 0.253 (P < .001), respectively, indicating possible effects of Bev “crossover” as well as other unclear selection biases.
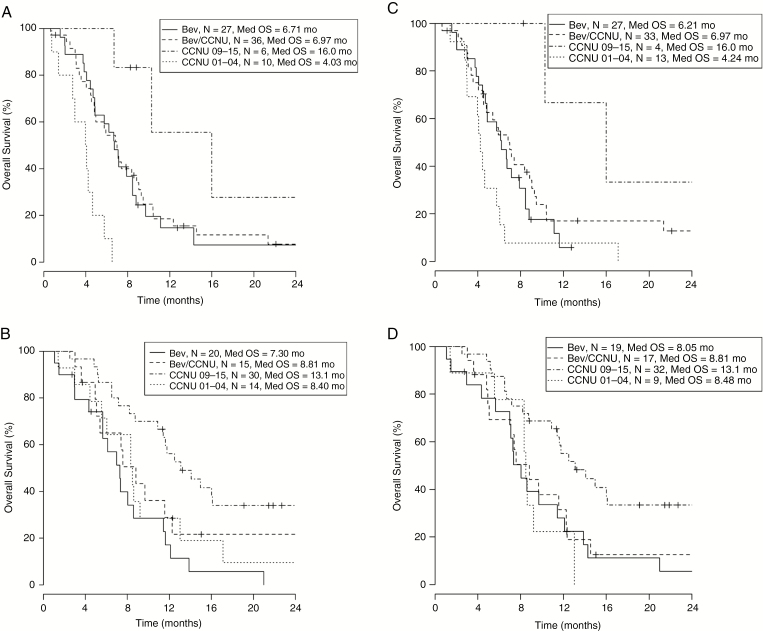
To better understand and illustrate the effect of tumor size on survival at first recurrence, we substratified each group based on tumor size and analyzed OS by treatment for large and small tumor size separately. Median 2D and 3D tumor measurements (1091 mm2 and 11698 mm3, respectively) were used as the threshold for stratification. Kaplan-Meier analyses showed improved OS between the Bev groups (Bev or Bev/CCNU) and CCNU 01-04 only among patients with large tumor size. In 2D analysis, Bev and Bev/CCNU patients with large tumor size showed median OS of 6.71 months (n = 27) and 6.97 months (n = 36) vs 4.03 months (n = 10) in CCNU 01-04 patients (Fig. 3A); while Bev and Bev/CCNU patients with small tumor size showed median OS of 7.30 months (n = 20) and 8.81 months (n = 15) vs 8.40 months in CCNU 01-04 patients (n = 14) (Fig. 3B). The 3D analysis yielded similar results (Fig. 3C, D). The CCNU 09-15 patients continued to perform substantially better than the other 3 groups in all 4 patient subgroups substratified by tumor sizes.
Fig. 3.
Kaplan-Meier analysis evaluating patient overall survival (OS) with prestratification by tumor measurements (2D and 3D) and substratification by treatment group. There is significant separation in OS between the Bev groups (Bev or Bev/CCNU) and CCNU 01-04 among all patients with large tumor size, 2D and 3D measurement, respectively (Figure 3A and 3C). Such separation was not observed among patients with small tumor sizes, 2D and 3D measurement, respectively (Figure 3B and 3D). 2D indicates 2-dimensional; 3D, 3-dimensional; Bev, bevacizumab; CCNU, lomustine.
Confirming the Kaplan-Meier results, Cox regression analyses showed a survival advantage in Bev and Bev/CCNU patients compared to the CCNU 01-04 group only among patients with large tumor size, both in 2D and 3D measurements (Table 3). Bev and Bev/CCNU patients with large tumor size showed a hazard ratio of 0.218 (P < .001) and 0.234 (P < .001), respectively for 2D analysis. In 3D analysis, Bev patients with large tumors tended to have better survival with a hazard ratio of 0.507 (P = .061), and Bev/CCNU patients with large tumor showed significantly better survival with a hazard ratio of 0.441 (P = .020). No significant difference was observed among Bev and Bev/CCNU patients compared to the CCNU 01-04 reference patient group among patients with small tumors in both analyses. The CCNU 09-15 patient group also demonstrated significant better survival compared to all 3 of the other patient groups in all subanalyses. Our results indicated that a survival benefit from Bev at first recurrence relative to CCNU 01-04 was observed only in patients with large tumors.
Table 3 .
Cox Regression Analysis of Overall Survival with Substratification by Tumor Size
| High Tumor 2D Measurement | Low Tumor 2D Measurement | High Tumor 3D Measurement | Low Tumor 3D Measurement | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI), P Value | Hazard Ratio (95% CI), P Value | Hazard Ratio (95% CI), P Value | Hazard Ratio (95% CI), P Value | ||
| Age at Treatment Initiation | 1.020 (0.994-1.047), P = .138 | 1.034 (1.007-1.062), P = .012 | 1.016 (0.986-1.046), P = .298 | 1.026 (1.001-1.052), P = .046 | |
| KPS at First Recurrent Treatment | <70 | Reference | Reference | Reference | Reference |
| ≥70 | 0.678 (0.303-1.516), P = .344 | 0.421 (0.197-0.898), P = .025 | 0.833 (0.404-1.718), P = .620 | 0.246 (0.105-0.577), P = .001 | |
| Treatment Group | CCNU 01-04 | Reference | Reference | Reference | Reference |
| Bev | 0.218 (0.095-0.497), P < .001 | 0.847 (0.379-1.895), P = .687 | 0.507 (0.249-1.032), P = .061 | 0.631 (0.260-1.531), P = .309 | |
| Bev/CCNU | 0.234 (0.104-0.523), P < .001 | 0.606 (0.263-1.398), P = .241 | 0.441 (0.221-0.879), P = .020 | 0.532 (0.214-1.319), P = .173 | |
| CCNU 09-15 | 0.076 (0.020-0.295), P < .001 | 0.350 (0.166-0.740), P = .006 | 0.133 (0.029-0.604), P = .009 | 0.292 (0.123-0.690), P = .005 | |
Abbreviations: 2D, 2-dimensional; 3D, 3-dimensional; Bev, bevacizumab; CCNU, lomustine; CI, confidence interval; KPS, Karnofsky Performance Status; OS, overall survival.
Discussion
Bev remains a cornerstone of GBM salvage therapy since its conditional FDA approval in 2009. The EORTC 26101 study recently enabled Bev to gain full FDA approval in 2017 for adult GBM patients who progressed after prior therapy. To further assess Bev’s potential as a salvage therapy, we performed a retrospective study to evaluate our institution’s experience on the efficacy of Bev at recurrence. Our study involved 4 groups of patients, consisting of 3 groups contemporaneously diagnosed and treated between 2009 and 2015 that received (1) Bev alone, (2) CCNU alone or (3) concurrent Bev/CCNU at first recurrence, and 1 group diagnosed from 2001 through 2004 that received CCNU alone as a historical control group from the era during which Bev was not available for salvage therapy. Without controlling for tumor size, patients receiving Bev or Bev/CCNU showed similar OS to the CCNU 01-04–alone patient group.
Interestingly, the CCNU 09-15 group displayed the best OS; we hypothesized that this effect could result from “selection bias” in which Bev usage at first recurrence is reserved for patients with poor function, generally due to significant tumor and edema burden. In our blinded 2D and 3D tumor size analyses, our tumor size data showed that the 75th percentile of the tumor size in the CCNU 09-15 group was below the median tumor measurement of the entire cohort (892 mm2 vs 1091 mm2 for 2D measurement and 8124 mm3 vs 11698 mm3 for 3D measurement), indicating a much lower tumor burden seen in patients in this group compared to the other 3 groups. This clearly reflects the current practice at UCLA and KPLA, in which Bev tends to be offered to patients with significantly larger tumor burden or as the last treatment option after multiple chemotherapy failures. Further, large tumor size is associated with poorer outcome in our entire cohort. Even after controlling for tumor size, CCNU 09-15 continued to show increased OS compared to other groups, indicating that other factors may be contributing. One of these factors could be that 69% of the patients in the CCNU 09-15 group crossed over to Bev at later recurrence. Thus, both lower tumor burden and the high rate of Bev crossover as well as other unknown factors might have contributed to the significant improved survival in the CCNU 09-15 group.
On the other hand, the historical control CCNU 01-04 patient group has more clinical and demographic characteristics comparable to the Bev groups than the CCNU 09-15 patient group. This group had only 2 patients (7%) cross over to Bev at later recurrences, and the median tumor measurement was more similar to the Bev groups in both tumor 2D and 3D assessments. From this observation, the CCNU 01-04 patient group served as the most relevant reference group for all survival analyses.
Tumor burden measurement in the recurrent setting has been shown to have prognostic value, though it was not correlated to tumor area measurement, which could be assessed readily in the clinic setting.5 Only 70 patients were included in previous studies, which recruited patients from institutions in France, Sweden, and the United Kingdom between 1994 and 1996. The cohort also did not have standardized pathological diagnoses. Our study included only confirmed recurrent GBM patients who were uniformly treated by physicians at only 2 institutions (UCLA and KPLA) throughout 2001 and 2009, which was an era with more advanced radiological imaging. Our results indicated a strong correlation between tumor burdens measured by 3D technique with a more clinically applicable 2D measurement technique. Both measurements indicated a negative prognosis associated with larger tumors in a first recurrence setting. These data support the use of conventional 2D measurement to assess patients’ prognoses with recurrent GBM.
Results from phase II and III trials (BELOB and EORTC)3,4 did not reach the same consensus on the efficacy of Bev in the recurrent setting. Partly, these studies have not looked at parameters such as patient tumor size and controlled for Bev crossover at a later recurrence. While our results showed that receiving Bev-containing treatments was not associated with OS calculated from first recurrent treatment initiation for all patients, Bev was associated with improved OS for patients with larger 2D tumor size at the time of treatment initiation: Median OS for patients with Bev alone is 6.71 months (n = 27) and Bev/CCNU is 6.97 months (n = 36) compared to median OS for patients with CCNU 01-04 of 4.03 months (n = 10). In addition, the 2D and 3D measurements in our study were strongly correlated (P < .001). Cox proportional regression models containing each type of measurement separately gave similar results (data not shown), suggesting the adequacy of use of conventional 2D tumor measurement to better understand the benefit of Bev based on tumor size.
While we tried to obtain a homogenous patient cohort of primary GBM patients who received the same first-line treatment and first recurrence treatment modalities of interest, we acknowledge that this is a retrospective study and could include selection biases or treatment variation that may affect the results. Our 2D and 3D measurements were completed twice by two independent investigators to minimize user error and interrater variability; however, as tumor size was measured based on contrast-enhancing regions, a small fraction of GBMs with a noncontrast-enhancing mass might not have been captured in the analysis. In addition, some patients might have had multiple recurrences after first recurrence treatment and received further treatments other than the chemotherapy of interest, which might potentially have influenced the patient outcome. Given the patient diversity and no standard treatment protocol established at later recurrence, we did not further report or stratify patients based on treatment received at later recurrence. Because the historical control cohort of the CCNU 01-04 group was derived from a pre-Stupp era, a small number (30%) of patients received XRT followed by TMZ instead of a concurrent regimen. Lastly, our results may be influenced by the arbitrary intervals between MRI scans that could influence tumor size measurements at first recurrence.
In summary, our results showed that Bev treatment at first recurrence appears to be associated with an improvement of OS in GBM patients with large tumor sizes. Tumor 2D and 3D measurements showed a strong correlation, suggesting the adequacy of using conventional 2D measurement to assess patients’ tumor sizes and response to Bev in the recurrent setting. Further studies, preferably prospective, are needed to corroborate our findings.
Funding
This work was supported by Genentech, which provided research funding to UCLA for the data abstraction, analysis, and reporting of this research.
Conflicts of interest: Arliene Ravelo and Nicolas Sommer are employees of Roche/Genentech. All other authors have nothing to declare.
Supplementary Material
References
- 1. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Taillibert S, Kanner AA et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 3. Taal W, Oosterkamp HM, Walenkamp AM et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 4. Wick W, Gorlia T, Bendszus M et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 5. Dempsey MF, Condon BR, Hadley DM. Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D?AJNR Am J Neuroradiol. 2005;26(4):770–776. [PMC free article] [PubMed] [Google Scholar]
- 6. Chow KL, Gobin YP, Cloughesy T, Sayre JW, Villablanca JP, Viñuela F.. Prognostic factors in recurrent glioblastoma multiforme and anaplastic astrocytoma treated with selective intra-arterial chemotherapy. AJNR Am J Neuroradiol. 2000;21(3):471–478. [PMC free article] [PubMed] [Google Scholar]
- 7. Reeves GI, Marks JE. Prognostic significance of lesion size for glioblastoma multiforme. Radiology. 1979;132(2):469–471. [DOI] [PubMed] [Google Scholar]
- 8. Wen PY, Macdonald DR, Reardon DA et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 9. Lai A, Tran A, Nghiemphu PL et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47(3):329–335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.