Abstract
Background
Currently, literature is scarce on differences across all possible tumor sites in malignant peripheral nerve sheath tumors (MPNSTs). To determine differences in treatment and survival across tumor sites and assess possible predictors for survival, we used the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
MPNST cases were obtained from the SEER database. Tumor sites were recoded into: intracranial, spinal, head and neck (H&N), limbs, core (thorax/abdomen/pelvis), and unknown site of origin. Patient and tumor characteristics, treatment modalities, and survival were extracted. Overall survival (OS) was assessed using univariable and multivariable Cox regression hazard models. Kaplan-Meier survival curves were constructed per tumor site for OS and disease-specific survival (DSS).
Results
A total of 3267 MPNST patients were registered from 1973 to 2013; 167 intracranial (5.1%), 119 spinal (3.6%), 449 H&N (13.7%), 1022 limb (31.3%), 1307 core (40.0%), and 203 unknown (6.2%). The largest tumors were found in core sites (80.0 mm, interquartile range [IQR]: 60.0-115.0 mm) and the smallest were intracranial (37.4 mm, IQR: 17.3-43.5 mm). Intracranial tumors were least frequently resected (58.1%), whereas spinal tumors were most often resected (83.0%). Radiation was administered in 35.5% to 41.8%. Independent factors associated with decreased survival were: older age, male sex, black race, no surgery, partial resection, large tumor size, high tumor grade, H&N site, and core site (all P < .05). Intracranial and pediatric tumors show superior survival (both P < .05). Intracranial tumors show superior OS and DSS curves, whereas core tumors have the worst (P < .001).
Conclusion
Superior survival is seen in intracranial and pediatric MPNSTs. Core and H&N tumors have a worse prognosis.
Keywords: malignant peripheral nerve sheath tumor, MPNST, SEER, survival, tumor site
Malignant peripheral nerve sheath tumors (MPNSTs) are rare sarcomas, encompassing only 2% to 4% of all soft tissue sarcomas.1,2 The incidence of these tumors is 1:100000 in the general population.3 In patients with neurofibromatosis type 1 (NF1), however, the incidence may be as high as 3% to 13%,4–6 and 23% to 51% of all MPNSTs are related to NF1.4,7–12 A slight predominance in males has been reported.7,9,10,13 The peak incidence of these tumors differs between NF1-related tumors and sporadic tumors. NF1 patients have an incidence peak in the third and fourth decades, while sporadic tumors are usually diagnosed in the sixth decade.12 Although some suggest a worse prognosis for NF1 patients, its influence on survival has recently been subject to debate.9–12,14,15
Currently, no standardized treatment for MPNSTs exists.3,10,16 Gross total removal of the tumor with wide margins is still considered the best prognostic factor for overall survival (OS), which is reflected in the European Society for Medical Oncology Guidelines.17 The ability for complete resection largely depends on the location of the tumor and its adjacent structures.4,18–22 The efficacy and indications of radiotherapy remain a subject of debate.23 Additionally, the role of chemotherapy in the treatment of MPNSTs is currently still under investigation,24 with recent evidence indicating an added value of neoadjuvant epirubicin and ifosfamide in high-grade, large, and deep MPNSTs.25
Differences in survival per tumor site have repeatedly been reported.10,11,14,26 However, variation in outcomes has not been assessed across all anatomical sites, mainly because of the rare nature of MPNSTs. The Surveillance, Epidemiology, and End Results (SEER) program is a cancer registry that collects data from 18 geographic areas across the United States, encompassing approximately 28% of its population. As such, the SEER database provides a means of assessing possible predictive factors of survival and treatment strategies for rare tumors such as MPNSTs at different anatomical sites. This study appraises the differences in patient characteristics, treatment, and survival for MPNSTs arising from different sites in the SEER database.
Materials and Methods
Data Source
Data were obtained from the SEER database from 1973 to 2013. The International Classification of Disease for Oncology (ICD-O-3) histology codes were used to identify cases. MPNSTs (ICD-O-3: 9540/3, 9560/3, 9561/3) from any site were selected. Our institutional review board has exempted the SEER program from review.
Covariates
Covariates extracted for analysis were: sex, age (≤18, 19–59, and ≥60 years), race (White, Black, Asian and other), tumor site, SEER tumor grade (I to IV), tumor size, extent of resection, administration of radiotherapy, timing of radiotherapy to surgery (prior to, after, during, prior to, and after surgery), and survival. Tumor sites were recoded using ICD-O-3 site codes into: intracranial, spinal, head and neck, limbs, core (including chest, abdomen, and pelvis), and NOS (not otherwise specified or unknown, Supplemental Table 1). In the SEER database, tumor size is determined from pathological reports, or from radiologic reports in case of preoperative treatment, unclear pathological reports, or in case no surgery was performed. Surgical procedures were coded differently in the SEER before and after 1998 and extent of resection can be interpreted from them. A single variable was constructed using codes prior to 1998 and after 1998 to evaluate extent of surgical resection from all time periods. These were recoded into the following subgroups: no surgery, biopsy, partial resection, gross total resection (GTR), surgery not otherwise specified, and unknown status of surgery (Supplemental Table 2).
Statistical Analysis
Data were stratified per tumor site and descriptive statistics were performed on demographics. Only primary tumors were used for survival analyses. Univariable and multivariable Cox proportional hazard analyses were performed for each tumor site to evaluate possible factors of influence on OS. Subsequently, a univariable and 3 multivariable Cox proportional hazard models were constructed for all primary MPNSTs combined with tumor site as a separate variable. These 3 models were separated to appraise influences of different therapy regimens on OS and avoid correlation among variables included. P values < .05 were considered statistically significant. Bonferroni correction was applied to correct for multiple testing. Kaplan-Meier survival curves for OS and disease-specific survival (DSS) were constructed for MPNSTs per site. Statistical analyses were conducted using IBM® (Armonk, NY) Statistical Package for the Social Sciences (SPSS)® version 24 (IBM Inc, 2016) and Kaplan-Meier curves were created using R version 3.3.3 (R Core Team, 2017).
Results
Patient Population
A total of 3267 patients with MPNSTs were identified in the SEER database: 167 intracranial (5.1%), 119 spinal (3.6%), 449 head and neck (13.7%), 1022 limb (31.3%), 1307 core (40.0%), and 203 NOS and unknown (6.2%, Table 1). The mean age was 47.6 years (SD: 21.0). The majority of patients were male (54.1%) and white (78.9%). Most patients were treated only surgically (46.8%), with a combination of surgery and radiation being the second most common treatment strategy (32.8%). A total of 53.8% were of unknown tumor grade. Most often tumors were classified as grade IV (16.8%) and the median size of all tumors was 67 mm (interquartile range [IQR]: 37-100 mm). The largest tumors were found in core (median 80 mm, IQR: 60-115 mm) and limb sites (70 mm, IQR: 40-100 mm), whereas intracranial (37.4 mm, IQR: 17.3-43.5 mm), spinal (39.5 mm, IQR: 20-60 mm), and head and neck sites (38 mm, IQR: 20-65 mm) were relatively smaller in size.
Table 1.
Patient Demographics per Tumor Site
| Characteristic | Definition | Total (N = 3267) | Intracranial (N = 167) | Spinal (N = 119) | H&N (N = 449) | Limbs (N = 1022) | Core (N = 1307) | NOS and Unknown (N = 203) | P |
|---|---|---|---|---|---|---|---|---|---|
| Age | <18 | 282 (8.6) | 10 (6.0) | 9 (7.6) | 47 (10.5) | 90 (8.8) | 104 (8.0) | 22 (10.8) | <.001 |
| 19-59 | 1970 (60.3) | 105 (62.9) | 67 (56.3) | 228 (50.8) | 662 (64.8) | 791 (60.5) | 117 (57.6) | ||
| 60+ | 1015 (31.1) | 52 (31.1) | 43 (36.1) | 174 (38.8) | 270 (26.4) | 412 (31.5) | 64 (31.5) | ||
| Mean (SD) | 47.6 (21.0) | 50.9 (20.1) | 49.1 (21.7) | 51.1 (22.5) | 45.5 (20.8) | 47.5 (20.3) | 47.3 (21.9) | ||
| Sex | Female | 1501 (45.9) | 83 (49.7) | 51 (42.9) | 180 (40.1) | 478 (46.8) | 619 (47.4) | 90 (44.3) | .103 |
| Male | 1766 (54.1) | 84 (50.3) | 68 (57.1) | 269 (59.9) | 544 (53.2) | 688 (52.6) | 113 (55.7) | ||
| Race | White | 2579 (78.9) | 144 (86.2) | 93 (78.2) | 365 (81.3) | 786 (76.9) | 1020 (78.0) | 171 (84.2) | .001 |
| Black | 417 (12.8) | 16 (9.6) | 12 (10.1) | 45 (10.0) | 163 (15.9) | 163 (12.5) | 18 (8.9) | ||
| Asian and other | 253 (7.7) | 5 (3.0) | 13 (10.9) | 38 (8.5) | 64 (6.3) | 119 (9.1) | 14 (6.9) | ||
| Unknown | 18 (0.6) | 2 (1.2) | 1 (0.8) | 1 (0.2) | 9 (0.9) | 5 (0.4) | 0 (0.0) | ||
| Treatment | Sx only | 1528 (46.8) | 64 (38.3) | 61 (51.3) | 204 (45.4) | 501 (49.0) | 620 (47.4) | 78 (38.4) | <.001 |
| Rx only | 144 (4.4) | 24 (14.4) | 5 (4.2) | 15 (3.3) | 19 (1.9) | 73 (5.6) | 8 (3.9) | ||
| Sx and Rx | 1070 (32.8) | 30 (18.0) | 39 (32.8) | 165 (36.7) | 405 (39.6) | 388 (29.7) | 43 (21.2) | ||
| None | 300 (9.2) | 30 (18.0) | 9 (7.6) | 39 (8.7) | 43 (4.2) | 133 (10.2) | 46 (22.7) | ||
| Unknown | 225 (6.9) | 19 (11.4) | 5 (4.2) | 26 (5.8) | 54 (5.3) | 93 (7.1) | 28 (13.8) | ||
| Tumor Grade | I | 157 (4.8) | 0 (0.0) | 2 (1.7) | 25 (5.6) | 60 (5.9) | 64 (4.9) | 6 (3.0) | <.001 |
| II | 391 (12.0) | 5 (3.0) | 4 (3.4) | 71 (15.8) | 154 (15.1) | 141 (10.8) | 16 (7.9) | ||
| III | 411 (12.6) | 4 (2.4) | 4 (3.4) | 42 (9.4) | 172 (16.8) | 167 (12.8) | 22 (10.8) | ||
| IV | 549 (16.8) | 8 (4.8) | 9 (7.6) | 62 (13.8) | 216 (21.1) | 240 (18.4) | 14 (6.9) | ||
| Unknown | 1759 (53.8) | 150 (89.8) | 100 (84.0) | 249 (55.5) | 420 (41.1) | 695 (53.2) | 145 (71.4) | ||
| Tumor Size | ≤50 mm | 798 (24.4) | 61 (36.5) | 32 (26.9) | 171 (38.1) | 265 (25.9) | 254 (19.4) | 15 (7.4) | <.001 |
| >50 mm | 1283 (39.3) | 15 (9.0) | 14 (11.8) | 91 (20.3) | 482 (47.2) | 625 (47.8) | 56 (27.6) | ||
| Unknown | 1186 (36.3) | 91 (54.5) | 73 (61.3) | 187 (41.6) | 275 (26.9) | 428 (32.7) | 132 (65.0) | ||
| Median (mm) | 67.0 | 37.4 | 39.5 | 38.0 | 70.0 | 80.0 | 88.0 | ||
| IQR (mm) | 37.0-100.0 | 17.3-43.5 | 20.0-60.0 | 20.0-65.0 | 40.0-100.0 | 50.0-115.0 | 60.0-130.0 |
Abbreviations: H&N, head and neck; IQR, interquartile range; NOS, not otherwise specified; Rx, radiotherapy; Sx, surgery.
Treatment Modalities
Most patients were treated with surgery (46.8%), which was followed by radiotherapy in 32.8% of patients. Intracranial tumors were less frequently resected (58.1%), whereas spinal tumors were treated surgically in 83.0% of cases. GTR was achieved in only 28.0% of cases, and 30.0% of surgeries resulted in a subtotal resection (Table 2). GTR was most often achieved in spinal tumors (42.6%) and least frequently in core tumors (24.9%). Overall, 38.9% of patients underwent a form of radiation, and percentages varied slightly from 35.5% of intracranial cases to 41.8% of cases in extremities. Radiotherapy was given in a neoadjuvant setting in 4.2% and adjuvant in 28.0% of all cases. Preoperative radiation was most often used in limb sites (6.8%). Intraoperative radiation was administered in only 0.6% of cases. A combination of both pre- and postoperative radiotherapy was given only in 0.8% of all cases.
Table 2.
Treatment Modalities Across Sites for Primary Tumors
| Definition | Total (N = 2732) | Intracranial (N = 141) | Spinal (N = 94) | H&N (N = 344) | Limbs (N = 878) | Core (N = 1106) | NOS and Unknown (N = 169) | P | |
|---|---|---|---|---|---|---|---|---|---|
| Extent of Resection | No surgery | 306 (11.2) | 43 (30.5) | 9 (9.6) | 29 (8.4) | 49 (5.6) | 132 (11.9) | 44 (26.0) | <.001 |
| Biopsy | 62 (2.3) | 3 (2.1) | 0 (0.0) | 11 (3.2) | 6 (0.7) | 38 (3.4) | 4 (2.4) | ||
| Partial resection | 820 (30.0) | 4 (2.8) | 9 (9.6) | 129 (37.5) | 317 (36.1) | 334 (30.2) | 27 (16.0) | ||
| GTR | 765 (28.0) | 38 (27.0) | 40 (42.6) | 89 (25.9) | 298 (33.9) | 275 (24.9) | 25 (14.8) | ||
| Surgery NOS | 658 (24.1) | 37 (26.2) | 34 (36.2) | 73 (21.2) | 195 (22.2) | 270 (24.4) | 49 (29.0) | ||
| Unknown | 121 (4.4) | 16 (11.3) | 2 (2.1) | 13 (3.8) | 13 (1.5) | 57 (5.2) | 20 (11.8) | ||
| Rx | No radiation | 1591 (58.2) | 88 (62.4) | 59 (62.8) | 195 (56.7) | 477 (54.3) | 660 (59.7) | 112 (66.3) | .048 |
| Any form radiation | 1064 (38.9) | 50 (35.5) | 34 (36.2) | 141 (41.0) | 367 (41.8) | 421 (38.1) | 51 (30.2) | ||
| Unknown | 77 (2.8) | 3 (2.1) | 1 (1.1) | 8 (2.3) | 34 (3.9) | 25 (2.3) | 6 (3.6) | ||
| Rx Sequence | No Rx or no Sx | 1802 (66.0) | 112 (79.4) | 64 (68.1) | 216 (62.8) | 523 (59.6) | 758 (68.5) | 129 (76.3) | <.001 |
| Rx after Sx | 766 (28.0) | 27 (19.1) | 28 (29.8) | 114 (33.1) | 274 (31.2) | 288 (26.0) | 35 (20.7) | ||
| Rx before Sx | 116 (4.2) | 0 (0.0) | 1 (1.1) | 6 (1.7) | 60 (6.8) | 45 (4.1) | 4 (2.4) | ||
| Rx before and after Sx | 23 (0.8) | 1 (0.7) | 1 (1.1) | 4 (1.2) | 12 (1.4) | 5 (0.5) | 0 (0.0) | ||
| Intraoperative Rx | 16 (0.6) | 1 (0.7) | 0 (0.0) | 3 (0.9) | 6 (0.7) | 6 (0.5) | 0 (0.0) | ||
| Unknown sequence | 9 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 3 (0.3) | 4 (0.4) | 1 (0.6) | ||
Abbreviations: GTR, gross total resection; H&N, head and neck; NOS, not otherwise specified; Rx, radiotherapy; Sx, surgery.
Univariable and Multivariable Analyses
Univariable analysis for intracranial MPNSTs showed that older age (>60 years), surgical procedure in the form of a biopsy, and larger size were associated with decreased survival (all P < .05, Supplemental Table 3). In multivariable analyses, older age and larger size were significantly associated with decreased survival even after correction for multiple testing. In univariable analysis for spinal tumors, treatment strategies that included radiation and larger size are associated with worse survival (P < .05 for both). Larger size lost significance in multivariable analyses. Treatment with radiotherapy only was significantly associated with worse survival even after Bonferroni correction. Older age, higher tumor grade (grade ≥3), and large size are associated with higher mortality in head and neck tumors (all P < .05) in univariable analysis. These factors were still associated with poorer survival in multivariable analyses and with correction for multiple testing. Older age, expectant management or radiation solely, large size, and higher grade were associated with higher mortality in limb tumors (all P < .05, Supplemental Table 4) in univariable analysis and multivariable analyses with Bonferroni correction. Similar characteristics were associated with decreased survival in core MPNSTs. In the latter, patients who received radiation after surgery seemed to have a better OS in univariable analysis. In multivariable analyses older age, high tumor grade, large size, and treatment modalities without surgery were all still significantly associated with worse OS, even after Bonferroni correction. Pediatric cases and those that received radiotherapy after surgery had an increased survival in multivariable analyses, but this was no longer significant after correction for multiple testing.
In multivariable analysis of all primary MPNST cases, pediatric cases and intracranial tumors were independently associated with superior survival (both P < .05, Tables 3 and 4). MPNSTs originating from the head and neck or core sites showed significantly poorer survival (both P < .05). Also, older age, male sex, black race, higher tumor grade (grade III and IV), and large tumor size were independently prognostic for worse survival (all P < .05). Patients who did not receive surgical treatment or only a biopsy were significantly associated with worse survival (Table 4). The sequence of radiotherapy did not have any influence on patient survival (Table 4), nor did any addition of radiotherapy to surgery (all P > .05, Table 3). After applying a Bonferroni correction to all 3 models, only large tumor size, high tumor grades, core site, and treatment modalities without surgery significantly reduced OS (all P < .002).
Table 3.
Cox Univariable and Multivariable Analysis of Overall Survival in All Primary Malignant Peripheral Nerve Sheath Tumors: Therapy Regimen
| Characteristic | Definition | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | 19-59 | Ref. | Ref. | ||||
| <18 | 0.84 | 0.70-1.02 | .071 | 0.80 | 0.66-0.97 | .022 | |
| 60+ | 1.65 | 1.49-1.84 | <.001 | 1.79 | 1.60-1.99 | <.001 | |
| Sex | Female | Ref. | Ref. | ||||
| Male | 1.18 | 1.07-1.30 | .001 | 1.15 | 1.04-1.27 | .006 | |
| Race | White | Ref. | Ref. | ||||
| Black | 1.19 | 1.03-1.37 | .018 | 1.22 | 1.05-1.41 | .008 | |
| Asian and other | 0.94 | 0.78-1.14 | .528 | 0.93 | 0.77-1.12 | .435 | |
| Unknown | 0.32 | 0.10-0.99 | .047 | 0.42 | 0.13-1.30 | .132 | |
| Therapy | Sx only | Ref. | Ref. | ||||
| Rx only | 2.50 | 2.01-3.11 | <.001 | 2.07 | 1.65-2.59 | <.001 | |
| Sx and Rx | 1.17 | 1.05-1.31 | .006 | 1.09 | 0.97-1.23 | .153 | |
| None | 1.63 | 1.35-1.96 | <.001 | 1.58 | 1.31-1.91 | <.001 | |
| Unknown | 1.71 | 1.42-2.06 | <.001 | 1.54 | 1.27-1.87 | <.001 | |
| Surgery | GTR | Ref. | |||||
| PR | 1.00 | 0.87-1.15 | .989 | ||||
| Biopsy | 1.55 | 1.13-2.13 | .006 | ||||
| No Sx | 1.88 | 1.58-2.25 | <.001 | ||||
| Sx NOS | 1.09 | 0.95-1.25 | .238 | ||||
| Unknown | 1.93 | 1.54-2.43 | <.001 | ||||
| Radiation Sequence | No Sx or no Rx | Ref. | |||||
| Rx after Sx | 1.01 | 0.91-1.13 | .821 | ||||
| Rx before Sx | 1.01 | 0.79-1.28 | .949 | ||||
| Rx b/a Sx | 0.71 | 0.38-1.32 | .277 | ||||
| Rx intraop | 0.60 | 0.27-1.36 | .210 | ||||
| Unknown | 1.20 | 0.54-2.68 | .658 | ||||
| Tumor Location | Limbs | Ref. | Ref. | ||||
| Intracranial | 0.80 | 0.61-1.07 | .128 | 0.74 | 0.55-0.99 | .045 | |
| Spinal | 1.27 | 0.94-1.70 | .115 | 1.28 | 0.94-1.72 | .113 | |
| H&N | 1.18 | 1.00-1.40 | .052 | 1.27 | 1.07-1.52 | .007 | |
| Core | 1.68 | 1.49-1.89 | <.001 | 1.58 | 1.40-1.78 | <.001 | |
| NOS and Unknown | 2.07 | 1.69-2.54 | <.001 | 1.80 | 1.45-2.23 | <.001 | |
| Tumor Grade | I | Ref. | Ref. | ||||
| II | 1.34 | 0.94-1.89 | .102 | 1.33 | 0.94-1.89 | .106 | |
| III | 2.91 | 2.08-4.06 | <.001 | 2.74 | 1.96-3.84 | <.001 | |
| IV | 3.69 | 2.67-5.10 | <.001 | 3.24 | 2.33-4.49 | <.001 | |
| Unknown | 2.53 | 1.86-3.45 | <.001 | 2.34 | 1.71-3.19 | <.001 | |
| Tumor Size | ≤50 mm | Ref. | Ref. | ||||
| >50 mm | 2.43 | 2.09-2.82 | <.001 | 2.26 | 1.93-2.64 | <.001 | |
| Unknown | 2.03 | 1.75-2.36 | <.001 | 1.91 | 1.63-2.22 | <.001 | |
Abbreviations: b/a, before and after; CI, confidence interval; GTR, gross total resection; H&N, head and neck; HR, hazard ratio; intraop, intraoperatively, NOS, not otherwise specified; PR, partial resection; Ref., reference; Rx, radiotherapy; Sx, surgery.
Table 4.
Cox Multivariable Analysis of Overall Survival in All Primary Malignant Peripheral Nerve Sheath Tumors: Radiation Sequence and Extent of Surgery
| Characteristic | Definition | Multivariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | 19-59 | Ref. | Ref. | ||||
| <18 | 0.79 | 0.66-0.97 | .020 | 0.80 | 0.66-0.97 | .023 | |
| 60+ | 1.79 | 1.60-1.99 | <.001 | 1.78 | 1.59-1.98 | <.001 | |
| Sex | Female | Ref. | Ref. | ||||
| Male | 1.15 | 1.04-1.27 | .008 | 1.16 | 1.05-1.28 | .005 | |
| Race | White | Ref. | Ref. | ||||
| Black | 1.27 | 1.09-1.46 | .002 | 1.23 | 1.06-1.42 | .006 | |
| Asian and other | 0.93 | 0.77-1.12 | .432 | 0.93 | 0.77-1.12 | .431 | |
| Unknown | 0.42 | 0.14-1.32 | .137 | 0.40 | 0.13-1.26 | .117 | |
| Therapy | Sx only | ||||||
| Rx only | |||||||
| Sx and Rx | |||||||
| None | |||||||
| Unknown | |||||||
| Surgery | GTR | Ref. | |||||
| PR | 1.10 | 0.96-1.27 | .174 | ||||
| Biopsy | 1.56 | 1.13-2.14 | .007 | ||||
| No Sx | 1.93 | 1.60-2.32 | <.001 | ||||
| Sx NOS | 1.16 | 1.00-1.35 | .055 | ||||
| Unknown | 1.97 | 1.53-2.52 | <.001 | ||||
| Radiation Sequence | No Sx or no Rx | Ref. | |||||
| Rx after Sx | 0.97 | 0.86-1.09 | .569 | ||||
| Rx before Sx | 0.85 | 0.67-1.09 | .209 | ||||
| Rx b/a Sx | 0.74 | 0.40-1.39 | .355 | ||||
| Rx intraop | 0.72 | 0.32-1.60 | .418 | ||||
| Unknown | 0.98 | 0.44-2.19 | .958 | ||||
| Tumor Location | Limbs | Ref. | Ref. | ||||
| Intracranial | 0.88 | 0.66-1.17 | .380 | 0.74 | 0.55-1.00 | .046 | |
| Spinal | 1.23 | 0.91-1.66 | .177 | 1.31 | 0.97-1.77 | .084 | |
| H&N | 1.32 | 1.11-1.58 | .002 | 1.28 | 1.08-1.53 | .005 | |
| Core | 1.62 | 1.44-1.83 | <.001 | 1.57 | 1.39-1.77 | <.001 | |
| NOS and Unknown | 1.99 | 1.61-2.45 | <.001 | 1.73 | 1.40-2.15 | <.001 | |
| Tumor Grade | I | Ref. | Ref. | ||||
| II | 1.33 | 0.94-1.88 | .113 | 1.35 | 0.96-1.92 | .101 | |
| III | 2.83 | 2.02-3.96 | <.001 | 2.82 | 2.02-3.95 | <.001 | |
| IV | 3.40 | 2.45-4.71 | <.001 | 3.40 | 2.45-4.71 | <.001 | |
| Unknown | 2.44 | 1.79-3.34 | <.001 | 2.32 | 2.32-1.69 | <.001 | |
| Tumor Size | ≤50 mm | Ref. | Ref. | ||||
| >50 mm | 2.33 | 2.00-2.72 | <.001 | 2.33 | 1.99-2.72 | <.001 | |
| Unknown | 1.93 | 1.65-2.25 | <.001 | 1.82 | 1.55-2.13 | <.001 | |
Abbreviations: b/a, before and after; CI, confidence interval; GTR, gross total resection; H&N, head and neck; HR, hazard ratio; intraop, intraoperatively; NOS, not otherwise specified; PR, partial resection; Ref., reference; Rx, radiotherapy; Sx, surgery.
Overall and Disease-Specific Survival
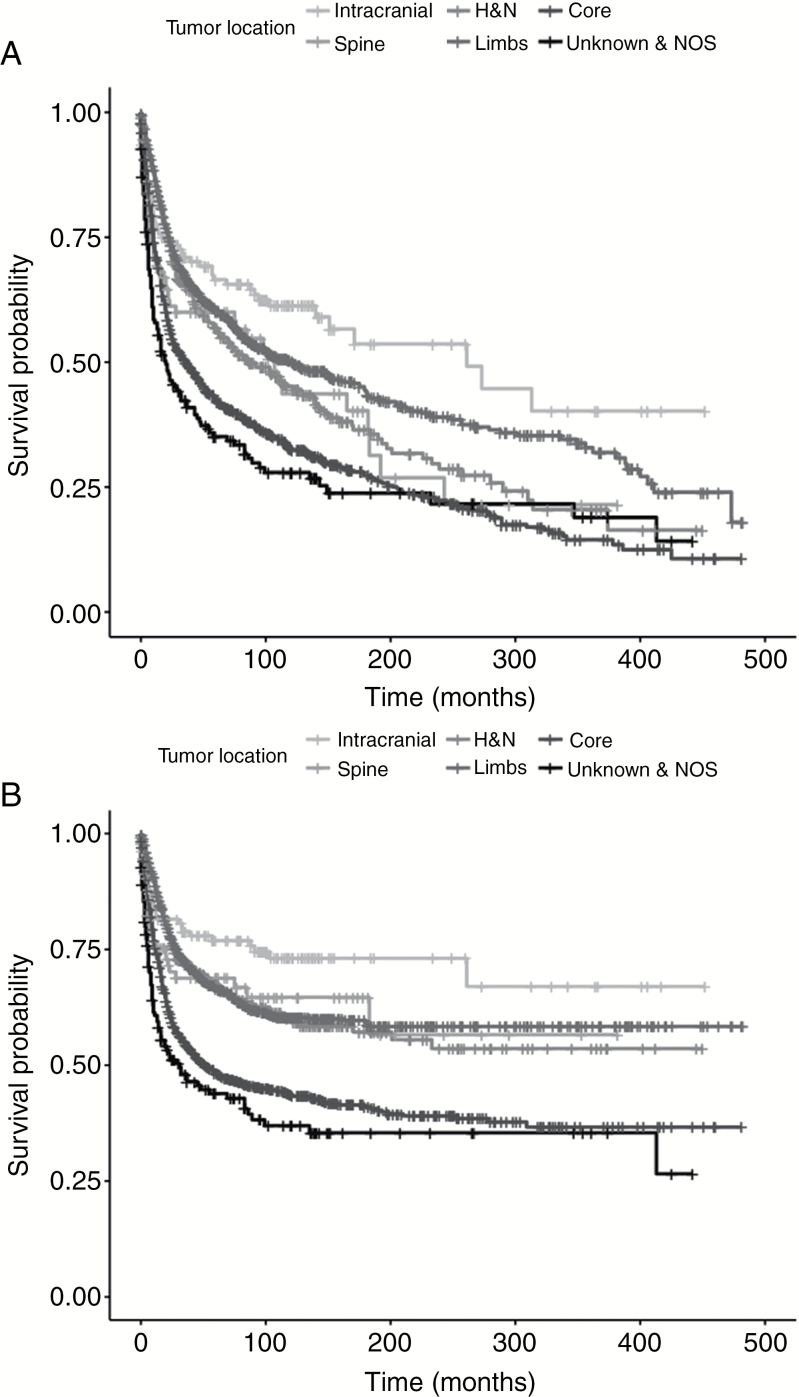
Patients with intracranial tumors showed superior OS followed by limbs, head and neck, and spine. Tumors arising from the core had the worst OS (overall difference between curves P < .001, Fig. 1A). Differences across sites in DSS seem to be similar to OS, but disparities among limbs, head and neck, and spine were less obvious (overall difference between curves P < .001, Fig. 1B).
Fig. 1.
A, Kaplan-Meier curves of overall survival per tumor site. B, Kaplan-Meier curves of disease-specific survival per tumor site. H&N indicates head and neck; NOS, not otherwise specified.
Discussion
Using the SEER database we identified that the site of origin is an independent prognostic factor for survival in MPNSTs. Intracranial tumors tend to have a better survival than those arising in extremities. Tumors arising from core sites are associated with the poorest survival; head and neck tumors were also associated with worse survival compared to limb sites. Pediatric cases were significantly associated with better survival compared to adult cases independent from tumor site, size, and treatment modality. Other factors associated with worse survival were older age, male gender, black race, higher tumor grade, and large tumors. Treatment modalities appear to vary slightly across site of origin.
Intracranial Malignant Peripheral Nerve Sheath Tumors
Literature on intracranial MPNSTs is scarce, consisting only of multiple case reports, small case series, and some systematic reviews. This analysis presents the largest group of intracranial MPNSTs reported in the literature to date. Patients with intracranial MPNSTs are believed to have a short survival.27–30 One-year survival has been reported to be as little as 33%, while others found a 3-year overall survival of 64.0%.27,28,31 The survival of the 141 primary intracranial MPNSTs presented in this paper seems to be better than currently suggested in the literature. This difference could be the result of different grades of tumors included, treatment modalities used, and extent of resection achieved. On the other hand, lymphatic metastases have not been reported in intracranial MPNSTs, which may be associated with improved prognosis for this site of origin.29,30 It is assumed that metastases from intracranial MPNSTs mainly occur as a consequence of cerebrospinal fluid dissemination that results in drop metastases.29
Head and Neck Malignant Peripheral Nerve Sheath Tumors
MPNSTs arising from extracranial head and neck sites have previously been associated with a worse prognosis, but this rarely reached statistical significance, mainly because of small population sizes.10,11,14,32 This is in line with findings of this study suggesting that they have worse survival compared to limb and intracranial sites. Five-year survival rates have been reported to vary from 20% to 47%.14,32–34 Unlike intracranial MPNSTs, these tumors have been reported to metastasize to the lymph nodes, but also to the lungs.34,35
Spinal Malignant Peripheral Nerve Sheath Tumors
Reports about spinal MPNSTs are as rare as those about intracranial tumors. Small case series have shown that survival in spinal tumors is generally unfavorable.20,36–38 Reported 5-year survival rates vary from 16% to 44%.20,37,38 Generally, MPNSTs of spinal origin are considered difficult to resect completely because of close vital structures adjacent to the tumor site.20,37,38 Although radiotherapy is recommended for local control in spinal MPNSTs, it has not been shown to have an effect on survival.36,37 Likewise, this study did not find an additional benefit for radiotherapy in spinal tumors. Radiotherapy as a monotherapy was significantly associated with worse OS independent of tumor and patient-specific characteristics. Because large amounts of radiation may induce myelopathy,37,39 tumor control using radiotherapy must be executed only in cases in which tumor invasiveness causes symptoms.
Core Malignant Peripheral Nerve Sheath Tumors
Core tumors are among the most frequent MPNSTs; prevalence reported in large series varies from 34% to 55%.4,9–11,14,32,40 This is consistent with the SEER data, which show a prevalence of 40%. This location is more frequently affected in NF1 patients compared to sporadic MPNSTs.4,40 Although generally seen as tumors with a less favorable outcome, only 3 large institutional studies have previously shown this difference to be significant.11,14,32 This study supports their findings that core site tumors tend to have a worse prognosis.
Extremity Malignant Peripheral Nerve Sheath Tumors
Extremities are also a common tumor site for MPNSTs with a prevalence in large series varying between 35% and 57%.4,9–11,14,15,32,40 MPNSTs arising from extremities tend to be more easily completely resected compared to other tumor locations.4,9,11,14,32 Therefore, most authors believe that survival is better in these patients. All but intracranial tumors had a worse overall survival. In the literature, 5-year OS ranged from 39% to 72%.19,32 Although limb salvage treatment is possible, amputations are still not uncommon for large and deep tumors.1
Pediatric Malignant Peripheral Nerve Sheath Tumors
MPNSTs in pediatric patients have been described previously.32,41–45 Five-year OS in children varies between 34.6% and 51%.32,41,42,44,45 No institutional study has yet been able to find a difference in survival between pediatric and adult tumors. However, in 2 studies including only pediatric cases, a prolonged survival was seen in younger children compared to adults.32,44 The SEER data suggest that pediatric patients tend to have a better survival in general, possibly by controlling for many risk factors previously shown to influence survival.
Strengths and Limitations
This study has several registry-associated limitations. Many data of interest were missing, for instance, data about tumor grade, tumor size, extent of resection, and site of origin. All missing groups were examined as separate entities and were associated with significantly worse outcomes. This may have resulted in over- or underrepresentation of certain variables. Also, it was not possible to conduct separate analyses for patients with NF1. While many studies found that NF1 patients show poorer survival,4,10,22,26 more recent studies did not find this difference.9,11,14 In a meta-analysis by Kolberg et al, NF1 negatively affected survival in studies published before 2000, but significance was lost in data after 2000.15 Not only better surveillance may have had an impact on this difference; NF1 patients tend to present with larger tumors more frequently originating from trunk sites, both factors associated with worse survival.10 A total of 11.2% of all patients did not receive cancer-directed surgery, which mainly includes patients who were diagnosed at autopsy, but possibly a small heterogeneous group including clinical diagnoses as well. The latter may impede the interpretation of this group of patients. The SEER tumor grading system is also not completely comparable to World Health Organization grading, which may make comparisons to other studies more difficult. Unfortunately, the registry does not contain any information on recurrence or progression-free survival; mode and dosage of radiotherapy are not registered either, nor is the indication of its use. This makes the interpretation of the impact that radiotherapy has, adjacent to surgery, difficult. It is possible that most patients receiving radiotherapy had positive margins, another variable that is not available in the SEER registry, which could skew data on survival. Furthermore, the use and regimen of chemotherapy cannot be extracted either. Nevertheless, the effect of chemotherapy is still the subject of debate.9,14,22,32,46,47
Despite these limitations, the SEER database allows for investigation of small subpopulations of rare tumors such as MPNSTs, including pediatric populations and rare tumor sites. Tumors that arise in different sites may be etiologically different from one another as location seems to be of great influence. Thoroughly examining clinical differences between different sites of origin may, therefore, lead to a better understanding of these rare tumors. In the future, large databases with prospective registration could be set up to track all outcomes relevant to MPNSTs through multicenter interdisciplinary efforts. Exact clinical-pathological differences between tumor sites and between pediatric and adult tumors should be investigated. This could help formulate specific treatment strategies to improve outcomes for these patients.
Conclusion
This study of the SEER database shows that intracranial and pediatric MPNSTs are associated with better OS, independent from treatment and other tumor-specific factors. Worse prognosis is seen in core sites and tumors arising in the head and neck. Treatment modalities and extent of resection also vary slightly among tumor sites. Apart from tumor origin, older age, male gender, black race, higher tumor grade, and large tumors may be associated with decreased survival.
Funding
This study was not supported by funding from any extramural resources.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. Vauthey JN, Woodruff JM, Brennan MF. Extremity malignant peripheral nerve sheath tumors (neurogenic sarcomas): a 10-year experience. Ann Surg Oncol. 1995;2(2):126–131. [DOI] [PubMed] [Google Scholar]
- 2. Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33(6):2597–2604. [PubMed] [Google Scholar]
- 3. Staedtke V, Bai RY, Blakeley JO. Cancer of the peripheral nerve in neurofibromatosis type 1. Neurotherapeutics. 2017;14(2):298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57(10):2006–2021. [DOI] [PubMed] [Google Scholar]
- 5. Evans DG, Baser ME, McGaughran J, Sharif F, Howard E Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasmant E, Sabbagh A, Spurlock G, et al. ; members of the NF France Network NF1 microdeletions in neurofibromatosis type 1: from genotype to phenotype. Hum Mutat. 2010;31(6):E1506–E1518. [DOI] [PubMed] [Google Scholar]
- 7. Doorn PF, Molenaar WM, Buter J, Hoekstra HJ. Malignant peripheral nerve sheath tumors in patients with and without neurofibromatosis. Eur J Surg Oncol. 1995;21(1):78–82. [DOI] [PubMed] [Google Scholar]
- 8. Cashen DV, Parisien RC, Raskin K, Hornicek FJ, Gebhardt MC, Mankin HJ. Survival data for patients with malignant schwannoma. Clin Orthop Relat Res. 2004;(426):69–73. [DOI] [PubMed] [Google Scholar]
- 9. Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249(6):1014–1022. [DOI] [PubMed] [Google Scholar]
- 10. Valentin T, Le Cesne A, Ray-Coquard I, et al. Management and prognosis of malignant peripheral nerve sheath tumors: the experience of the French Sarcoma Group (GSF-GETO). Eur J Cancer. 2016;56:77–84. [DOI] [PubMed] [Google Scholar]
- 11. Stucky CC, Johnson KN, Gray RJ, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19(3):878–885. [DOI] [PubMed] [Google Scholar]
- 12. Porter DE, Prasad V, Foster L, Dall GF, Birch R,Grimer RJ. Survival in malignant peripheral nerve sheath tumours: a comparison between sporadic and neurofibromatosis type 1-associated tumours. Sarcoma. 2009;2009:756395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ariel IM. Tumors of the peripheral nervous system. Semin Surg Oncol. 1988;4(1):7–12. [DOI] [PubMed] [Google Scholar]
- 14. Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107(5):1065–1074. [DOI] [PubMed] [Google Scholar]
- 15. Kolberg M, Høland M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15(2):135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brekke HR, Kolberg M, Høland M, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. 2013;15(2):135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii102–iii112. [DOI] [PubMed] [Google Scholar]
- 18. Baehring JM, Betensky RA, Batchelor TT. Malignant peripheral nerve sheath tumor: the clinical spectrum and outcome of treatment. Neurology. 2003;61(5):696–698. [DOI] [PubMed] [Google Scholar]
- 19. Hruban RH, Shiu MH, Senie RT, Woodruff JM. Malignant peripheral nerve sheath tumors of the buttock and lower extremity. A study of 43 cases. Cancer. 1990;66(6):1253–1265. [DOI] [PubMed] [Google Scholar]
- 20. Kourea HP, Bilsky MH, Leung DH, Lewis JJ, Woodruff JM.. Subdiaphragmatic and intrathoracic paraspinal malignant peripheral nerve sheath tumors: a clinicopathologic study of 25 patients and 26 tumors. Cancer. 1998;82(11):2191–2203. [DOI] [PubMed] [Google Scholar]
- 21. Wanebo JE, Malik JM, VandenBerg SR, Wanebo HJ, Driesen N, Persing JA. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer. 1993;71(4):1247–1253. [DOI] [PubMed] [Google Scholar]
- 22. Wong WW, Hirose T, Scheithauer BW, Schild SE,Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998;42(2):351–360. [DOI] [PubMed] [Google Scholar]
- 23. Kahn J, Gillespie A, Tsokos M, et al. Radiation therapy in management of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Front Oncol. 2014;4:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Widemann BC, Reinke DK, Helman LJ, et al. SARC006: Phase II trial of chemotherapy in sporadic and neurofibromatosis type 1 (NF1)-associated high-grade malignant peripheral nerve sheath tumors (MPNSTs). J Clin Oncol. 2013;31(15 Suppl):10522. [Google Scholar]
- 25. Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18(6):812–822. [DOI] [PubMed] [Google Scholar]
- 26. Sordillo PP, Helson L, Hajdu SI, et al. Malignant schwannoma—Clinical characteristics, survival, and response to therapy. Cancer. 1981;47(10):2503–2509. [DOI] [PubMed] [Google Scholar]
- 27. Gousias K, Boström J, Kovacs A, Niehusmann P, Wagner I,Kristof R. Factors of influence upon overall survival in the treatment of intracranial MPNSTs. Review of the literature and report of a case. Radiat Oncol. 2010;5:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scheithauer BW, Erdogan S, Rodriguez FJ, et al. Malignant peripheral nerve sheath tumors of cranial nerves and intracranial contents: a clinicopathologic study of 17 cases. Am J Surg Pathol. 2009;33(3):325–338. [DOI] [PubMed] [Google Scholar]
- 29. L’heureux-Lebeau B, Saliba I. Updates on the diagnosis and treatment of intracranial nerve malignant peripheral nerve sheath tumors. Onco Targets Ther. 2013;6:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen L, Mao Y, Chen H, Zhou LF. Diagnosis and management of intracranial malignant peripheral nerve sheath tumors. Neurosurgery. 2008;62(4):825–832; discussion 832. [DOI] [PubMed] [Google Scholar]
- 31. Ren X, Wang J, Hu M, Jiang H, Yang J, Jiang Z. Clinical, radiological, and pathological features of 26 intracranial and intraspinal malignant peripheral nerve sheath tumors. J Neurosurg. 2013;119(3):695–708. [DOI] [PubMed] [Google Scholar]
- 32. Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23(33):8422–8430. [DOI] [PubMed] [Google Scholar]
- 33. Vege DS, Chinoy RF, Ganesh B, Parikh DM. Malignant peripheral nerve sheath tumors of the head and neck: a clinicopathological study. J Surg Oncol. 1994;55(2):100–103. [DOI] [PubMed] [Google Scholar]
- 34. Minovi A, Basten O, Hunter B, Draf W Bockmühl U. Malignant peripheral nerve sheath tumors of the head and neck: management of 10 cases and literature review. Head Neck. 2007;29(5):439–445. [DOI] [PubMed] [Google Scholar]
- 35. Ma C, Ow A, Shan OH, et al. Malignant peripheral nerve sheath tumours in the head and neck region: retrospective analysis of clinicopathological features and treatment outcomes. Int J Oral Maxillofac Surg. 2014;43(8):924–932. [DOI] [PubMed] [Google Scholar]
- 36. Chou D, Bilsky MH, Luzzati A, et al. ; AOSpine Knowledge Forum Tumor Malignant peripheral nerve sheath tumors of the spine: results of surgical management from a multicenter study. J Neurosurg Spine. 2017;26(3):291–298. [DOI] [PubMed] [Google Scholar]
- 37. Wang T, Yin H, Han S, et al. Malignant peripheral nerve sheath tumor (MPNST) in the spine: a retrospective analysis of clinical and molecular prognostic factors. J Neurooncol. 2015;122(2):349–355. [DOI] [PubMed] [Google Scholar]
- 38. Zhu B, Liu X, Liu Z, et al. Malignant peripheral nerve sheath tumours of the spine: clinical manifestations, classification, treatment, and prognostic factors. Eur Spine J. 2012;21(5):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gibbs IC, Patil C, Gerszten PC, Adler JR Jr, Burton SA. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64(2 Suppl):A67–A72. [DOI] [PubMed] [Google Scholar]
- 40. Hwang IK, Hahn SM, Kim HS, et al. Outcomes of treatment for malignant peripheral nerve sheath tumors: different clinical features associated with neurofibromatosis type 1. Cancer Res Treat. 2017;49(3):717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. deCou JM, Rao BN, Parham DM, et al. Malignant peripheral nerve sheath tumors: the St. Jude Children’s Research Hospital experience. Ann Surg Oncol. 1995;2(6):524–529. [DOI] [PubMed] [Google Scholar]
- 42. Demir HA, Varan A, Yalçn B, Akyüz C, Kutluk T, Büyükpamukçu M. Malignant peripheral nerve sheath tumors in childhood: 13 cases from a single center. J Pediatr Hematol Oncol. 2012;34(3):204–207. [DOI] [PubMed] [Google Scholar]
- 43. Raney B, Schnaufer L, Ziegler M, Chatten J, Littman P, Jarrett P. Treatment of children with neurogenic sarcoma. Experience at the Children’s Hospital of Philadelphia, 1958-1984. Cancer. 1987;59(1):1–5. [DOI] [PubMed] [Google Scholar]
- 44. Meis JM, Enzinger FM, Martz KL, Neal JA. Malignant peripheral nerve sheath tumors (malignant schwannomas) in children. Am J Surg Pathol. 1992;16(7):694–707. [DOI] [PubMed] [Google Scholar]
- 45. Casanova M, Ferrari A, Spreafico F, et al. Malignant peripheral nerve sheath tumors in children: a single-institution twenty-year experience. J Pediatr Hematol Oncol. 1999;21(6):509–513. [PubMed] [Google Scholar]
- 46. Longhi A, Errani C, Magagnoli G, et al. High grade malignant peripheral nerve sheath tumors: outcome of 62 patients with localized disease and review of the literature. J Chemother. 2010;22(6):413–418. [DOI] [PubMed] [Google Scholar]
- 47. Moretti VM, Crawford EA, Staddon AP, Lackman RD, Ogilvie CM. Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy. Am J Clin Oncol. 2011;34(4):417–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.