Abstract
Cardiovascular diseases are the leading cause of death around the world. Despite the larger number of genes and loci identified, the precise mechanisms by which these genes influence risk of cardiovascular disease is not well understood. Recent advances in the development and optimization of high-throughput technologies for the generation of “omics data” have provided a deeper understanding of the processes and dynamic interactions involved in human diseases. However, the integrative analysis of “omics” data is not straightforward and represents several logistic and computational challenges. In spite of these difficulties, several studies have successfully applied integrative genomics approaches for the investigation of novel mechanisms and plasma biomarkers involved in cardiovascular diseases. In this review, we summarized recent studies aimed to understand the molecular framework of these diseases using multi-omics data from mice and humans. We discuss examples of omics studies for cardiovascular diseases focused on the integration of genomics, epigenomics, transcriptomics, and proteomics. This review also describes current gaps in the study of complex diseases using systems genetics approaches as well as potential limitations and future directions of this emerging field.
Keywords: multi-omics, cardiovascular disease, heart disease, systems biology, data integration
Introduction
Coronary artery disease (CAD) is the most common cause of cardiovascular death (1). Studies conducted in twins (2, 3) and in the general population have estimated a heritability of CAD at ~40–50% (4). In addition, genome-wide association studies (GWAS) have identified more than 150 genetic loci associated with CAD risk (5–18). Although GWAS studies have been successful on identifying common DNA variation implicated in cardiovascular diseases, they provide little or no molecular evidence of gene causality. In this context, the premise that rare genetic variation could have stronger functional effects on disease manifestation still is arguable (19). This realization has motivated researchers to integrate genetics studies with additional high-throughput data designed to interrogate the transcriptome, epigenome, proteome, metabolome, etc. Recent studies have implemented the integration of multi-omics data to accelerate the identification of novel mechanisms for complex diseases and understand the dynamics of disease manifestation (20–23). The relevance of integrating multi-omics data and the current statistical tools available for data integration have been reviewed in detail elsewhere (24–34). In this review, we summarize the state-of-the-art of multi-omics studies conducted in mice and humans to understand the molecular mechanisms underlying cardiovascular diseases including CAD (35–47), stroke (42, 48), heart failure (13, 49, 50), cardiac hypertrophy (13, 51), aortic valve disease (52, 53), and heart regeneration (54). We also discuss the gaps of multi-omics studies including the utility of generating multi-omics data in animal models, the importance of sex stratification on gene discovery, the inclusion of diverse populations and the integration of metabolomics and metagenomics with other omics platforms. Finally, we discuss future directions of multi-omics approaches for cardiovascular diseases and their importance in the era of precision health.
Multi-Omics Studies for the Investigation of Cardiovascular Disease
The simultaneous integration of multi-omics approaches including but not limited to genomics, epigenomics, transcriptomics, proteomics, and metabolomics (Figure 1), represents a powerful approach for understanding the mechanisms connecting identified genetic variation to cardiovascular diseases with gene causality, where many sources of variability are integrated into statistical models to identify key drivers and pathways that have the largest contribution to the disease (25). Importantly, most of the risk variants associated with CAD or other cardiovascular diseases (5, 7, 14, 17, 18, 37, 55, 56) identified by GWAS are located in noncoding regions of the genome (intronic or intergenic), suggesting that these variants are likely to affect cis or trans regulatory elements that bind transcription factors, enhancers or promoters (57). Previous multi-omic studies for CAD were mainly focused on the integration of GWAS data with global transcriptomics using eQTL analysis. In recent years, high-throughput technology have further facilitated the integration of omics data for the identification of causal genes and molecular mechanisms involved in the development of cardiovascular events in mice (13, 37, 39, 41, 58) and humans (36–39, 48) (Table 1).
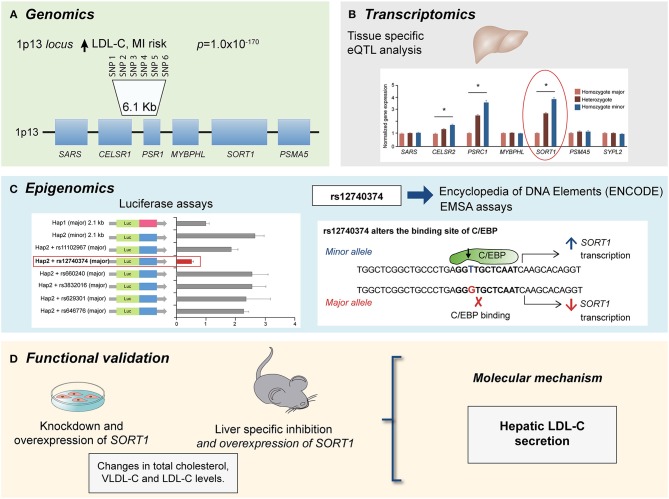
Figure 1.
Multi-omics approach to identify the causal gene associated with LDL-C levels and CAD risk at the 1p13 locus. (A) GWAs meta-analysis showed several SNPs at the 1p13 locus strongly associated with LDL-C levels (p = 1.0 × 10−170) and CAD risk. The 1p13 locus contains several genes (squares). The most significantly associated haplotype for LDL-C comprise six SNPs in high linkage disequilibrium (LD) and is located between CELSR1 and PSR1 genes. (B) Liver eQTL analysis showed the minor haplotype significantly associated with higher expression of CELSR1, PSR1, and SORT1 genes with SORT1 gene showed the largest difference modified from Musunuru et al. (74). (C) By using luciferase assays and ENCODE database it was identified a common polymorphism at the 1p13 locus, rs12740374 that alters the expression of the SORT1 gene in liver with the minor allele (T) creating a C/EBP (CCAAT/enhancer binding protein) transcription factor binding site and the major allele (G) disrupting it. The C/EBP transcriptional factor regulates the expression of hepatic genes involved in metabolism. (D) Functional approaches for SORT1 using small interfering RNA (siRNA) knockdown and viral overexpression in mouse liver showed that SORT1 results in significant changes in plasma LDL-C and very low-density lipoprotein (VLDL) particle levels by modulating hepatic VLDL secretion.
Table 1.
Studies using Multi-omics approaches for the investigation of cardiovascular diseases.
| References | Phenotypes | Population of study | Omic strategy | Tissue | Analysis strategy | Main findings | Genes involved | Functional confirmation |
|---|---|---|---|---|---|---|---|---|
| Santolini et al. (13) | Isoproterenol-induced cardiac hypertrophy and heart failure | Mice (HMDP) 100 genetically diverse strains of mice |
Genomics (genomic diversity) Transcriptomics (microarray platform Illumina) |
H | Correlation-based method | Identification of 36 genes associated with severity of cardiac hypertrophy | Rffl, Wdr1, Nppb, Atp6v0a1, Ankrd1, Eif4a1, Dtr (HB-EGF), Kcnip2, Pcdhgc4, Hes1, 4930504E06Rik, Akap9, 2310022B05Rik, Bclaf1, Ttc13, Nipsnap3b, Gss, Klhl23, Tspan17, Tnni2, Cab39l, Ptrf (Cavin-1), Dedd, 9430041O17Rik, Fgf16, Ehd2, Ppp1r9a, Kremen, Scara5, Zfp523, Nfatc1, Corin, Prnpip1, Lrrc1, AW549877, and Mkrn3 | Knockdown of Hes1 reduces hypertrophy by 80–90% in neonatal rat ventricular myocytes |
| Foroughi Asl et al. (36) | CAD | CAD patients from the Stockholm Atherosclerosis Gene Expression (STAGE) study |
Genomics [microarray platform, Affymetrix] Transcriptomics (microarray platform, Affymetrix) |
B, AAW, MAM, LIV, SKLM, SF, VAF | Cis- and trans-gene regulation by GWAS risk loci across tissues and CAD phenotypes | Identification of 3 master regulators of CAD across 7 tissues | FLYWCH1, PSORSIC3 and G3BP1 | Knockdown of FLYWCH1, PSORSIC3, G3BP1 genes affect cholesterol-ester accumulation in foam cells |
| Braenne et al. (37) | CAD | STAGE study Mice (HMDP) |
Genomics (microarray platform, Illumina) Transcriptomics (microarray platform, Affymetrix) |
LIV, SF, and M | GWAS and eQTL analysis | The majority of the GWAS loci for CAD affect gene expression (41%) | LIPA, TOM1L2, GALNT4, SERPINH1, VAMP8, VAMP5, GGCX, PSCR1, CELSR2, SORT1, DRG2, C17orf39, MYO15A, TOM1L2, SREBF1, mir-224, hsa-miR-130a-5p, hsa-miR-4722-5p, hsa-miR-3198, hsa-miR-5197-3p, miR-378a-5p | NA |
| Zhao et al. (48) | Carotid plaque, Stroke | Gene-expression profiles of 11 publically gene expression datasets of carotid plaque (n = 1,546). GWA studies of ischemic stroke from the International Stroke Genetics Consortium |
Genomics (microarray platform, Illumina) Transcriptomics (microarray platform, Affymetrix) |
H | Marker Set Enrichment Analysis (co-expression modules) | Seventeen co-expression modules were enriched for stroke. Enriched modules for stroke we associated with toll-like receptor pathway, homocysteine metabolism and phagosome formation and maturation | F2, APOH, and AMBP | NA |
| Lempiainen et al. (46) | CAD | GWAS studies and exome array studies for CAD.eQTL STAGE study |
Genomics (microarray platform, Illumina) Transcriptomics (microarray platform, Affymetrix) |
B, AAW, SKLM, SF, VAF | Construction of network modules for tissue-specific gene–protein interactions affected by genetic variance in CAD risk loci | Identification of modules with tissue-specific activity associated with CAD. Most of the modules were druggable. The top modules were implicated in extracellular matrix organization and disassembly, blood coagulation, or platelet degranulation/activation process | LDLR, APOE, SCARB1, NOS3, CSNK2A1, HTRA1, LRP1, COL4A1, FN1, RELA, TNF, SHC1, LRP1, LYN, SYK, IGF1R, SHC1, IL6R, CXCR4, LCAT, VLDLR, PLTP, APP, SCH1, RELA, FN1, TNF, FN1, PCSK9, TRIB3, CXCR4, and CCR1. | NA |
| Franzen et al. (38) | CAD | Patients with CAD from the STARNET studyRoad Epigenomics Consortium |
Genomics (microarray platform, Illumina) Transcriptomics (mRNA sequencing, Illumina) Epigenomics (microarray platform, Illumina) |
B, MAM, AOR, SF, VAF, SKLM, LIV | Cis- and trans-gene regulation across different tissues and CAD phenotypes | Tissue-specific gene-regulatory effects of CAD-associated SNPs identified by GWAS. Identification of 26 key drivers regulated in cis-trans by CAD SNPs | FAM117B, LIPA, SARS, ATP5G1, GGCX, CARF, ICA1L, SH2B3, AC023271.1, RPL7P14, MAT2A, EDNRA, LINC00310, SLC22A5, NT5C2, FES, USP39, ADAMTS7, FURIN, PSMA5, ABCG5, CNNM2, SLC5A3, CACFD1, ZNF76, TCF21, PSRC1, and PDGFD | NA |
| Liu et al. (59) | CAD | HCASMCs from 52 unrelated donors. |
Genomics
Transcriptomics Epigenomics (ATAC-seq) |
HCASMCs | Jointly eQTL modeling and GWAS analyses | Identification of 5 genes that modulate CAD risk via HCASMCs. | SIPA1, TCF21, SMAD3,FES,PDGFRA | NA |
| Haitjema et al. (42) | CAD, Stroke | GWAS of METASTROKE and CARDIoGRAMplusC4D |
Genomics (microarray platform, Illumina) Transcriptomics (mRNA sequencing, Illumina) Chromatin Organization (4C sequencing, Illumina) |
M, CEC | Association of eQTLs with chromatin interaction | Integrative analysis of gene expression and chromatin conformation to elucidate mechanisms involved in atherosclerosis | MIA3, PSRC1, SORT1, GGCX, VAMP5, VAMP8, NBEAL1, WDR12, MRAS, PHACTR1, TRIB1, CDKN2A, CDKN2B, KIAA1462, LIPA, COL4A1, COL4A2, PEMT, RASD1, SMG6, UBE2Z, LDLR | NA |
| Lee et al. (60) | H | |||||||
| Meder et al. (49) | Heart failure | 135 patients with dilated cardiomyopathy 31 control subjects |
Transcriptomics (mRNA sequencing, Illumina) Epigenomics (microarray platform, Illumina) |
H, B | Methylation-expression quantitative trait locus analysis | Integration of methylation and gene expression data identified enrichment of cell adhesion, cardiac development, and muscle function in HF | PLXNA2, RGS3, NPPA, NPPB, B9D1, doublecortin-like kinase 2 and neurotrimin | NA |
| Rask-Andersen et al. (61) | Hypertension MI Stroke Thrombosis Arrhythmia |
729 subjects from the Northern Sweden Population Health Study |
Epigenomics
Illumina Infinium 450 BeadChip |
B | Integration of EWAS and ChIA-PET data | Identification of 196 genes associated with cardiac-related traits | ESRRG, ST6GALNAC5, RYR2, NMNAT2, EPHA2, TGFB2, ABCG5, FMNL2, DYSF, MEIS1, MECOM, WNT7A, SOX2, HAND2, F2RL1, KCNN2, ME1* |
NA |
| Dekkers K et al. (62) | Blood lipids | 3,296 subjects from the Biobank Based Integrative Omics Study |
Transcriptomics
Epigenomics |
B | Integration of EWAS and gene expression | Identification of CpGs associated with the expression of lipids |
CPT1A and SREBF1 (TGs)
DHCR24 (LDL-C) ABCG1 (HDL-C) |
NA |
| Howson JMM, et al. (43) | CAD | 88,192 CAD cases 162,544 controls including CARDIoGRAMplusC4D database |
Genomics (microarray platform, Illumina, Affymetrix) Transcriptomics (microarray platform, Illumina) Epigenomics (microarray platform, Illumina) Proteomics (multiplexed aptamer based affinity proteomics platform, SomaLogic) |
30 cells/tissues including P, B, LIV, SF, VAF, H, and DT |
Genomic meta-analysis, eQTL, pQTL. Enrichment analysis (Ingenuity Pathway Analysis software) | Integrative analysis showed enrichment of genes involved in biological processes active in the arterial wall as cellular adhesion, leucocyte migration, vascular smooth muscle cell differentiation, coagulation, inflammation, and atherosclerosis | ATP1B1, NME7, CAMSAP2, DDX59, LMOD1, TNS1, TBXAS1, SERPINH1,SCARB1, TRIP4 HP, PECAM1, PROCR | NA |
| Yao C, et al. (44) | CAD | 6,861 subjects from the Framingham Heart Study and CARDIoGRAMplusC4D |
Genomics (microarray platform, Illumina, Affymetrix) Transcriptomics (microarray platform, Affymetrix) Proteomics (multiplexed aptamer based affinity proteomics platform, Luminex) |
P | Multi-stage strategy of proteomic analysis | pQTL analysis identified six causal proteins for CHD | LPA, BCHE, PON1, MCAM, MPO, Cystatin C | NA |
| Chen G, et al. (45) | CAD, MI | 7,242 participants from the Framingham Heart Study |
Genomics (microarray platform, Illumina, Affymetrix) Targeted proteomics (bead-based multiplex immunoassays, Luminex) |
P | Cis- and trans-protein regulation by GWAS CAD risk loci | Identification of 210 pQTLs for 12 proteins associated with CAD and MI | CELSR2/SORT1 locus (granulin) | NA |
| Fernandes, M, et al. (47) | CAD | Public databases of human samples |
Genomics (microarray platform, Illumina, Affymetrix) Transcriptomics (microarray platform, Illumina) Epigenomics (microarray platform, Illumina) Proteomics (LC-MS/MS, MALDI-TOF/TOF, Thermo) Metabolomics (LC-MS/MS, HPLC-MS, Thermo) |
ART, B, H, and LIV | Supervised development of a multi-omics integrative molecular model | Integrative analysis of omics studies showed enrichment of lipid metabolism, extracellular matrix remodeling, inflammation, and cardiac hypertrophy pathways | LCAT, FABP1, FASN, APOA1, FASN, mir-1305 (PPARA and APOA1), mir-1303 (FASN) | NA |
| Lau E, et al. (51) | Cardiac hypertrophy | Mice (inbred from six diverse genetic backgrounds) |
Transcriptomics (microarray platform, Illumina) Proteomics (LC-MS/MS platform, Thermo) Proteome dynamics |
H | Clustering of co-expression | Modules associated with heart hypertrophy across the mouse strains were involved in biological processes including cell adhesion, glycolytic process, actin filament organization, translation, and sodium ion transport | ANXA2, ANXA5, COL4A2, LDHA, and PGAM1 | NA |
| Schlotter F, et al. (52) | Calcific aortic valve disease | 25 human stenotic aortic valves |
Transcriptomics (mRNA sequencing, Illumina) Proteomics (unlabeled and label-based tandem-mass–tagged, Thermo) |
AV | Correlation of gene and protein expression differentiated between calcification stage. Protein-protein interaction |
Identification of novel regulatory networks for CAVD | SOD3. MGP, SERPINA1, VWF, C8A, C8B, SLPI, ELANE, HLA-DRA, and CD14 | NA |
| Matic LP, et al. (53) | Carotid atheroma | Patients from the Karolinska Biobank |
Transcriptomics (microarray platform, Illumina, Affymetrix) Proteomics (LC-MS/MS platform, Thermo) |
CP, P | Systems biology | Identification of enriched pathways for carotid atheroma including cell proliferation, nitric oxide signaling, lipoprotein, and apoptotic particle clearance, immune cell activation, chemokine secretion, blood coagulation, and extracellular matrix disassembly were dominant in plaques by transcriptomics. Extracellular matrix, heme-binding, and platelet-derived growth factor binding were the most enriched functional categories by plaque proteomics. Integrative analysis showed BLVRB as the only significant candidate enriched both in plaques and plasma | BLVRB- HMOX1 | In THP-1 macrophages iron stimulated an induction of BLVRB and HMOX1 was observed. |
| Lalowski MM, et al. (54) | Heart regeneration | Mice |
Transcriptomics (mRNA sequencing, Illumina) Proteomics (LC/MS platform, Waters) Metabolomics (UPLC-MS/MS platform, Metabolon) |
H | Systems biology | The decrease of the heart regeneration capacity was associated with a transition from fructose-induced glycolysis under hypoxic conditions to oxidative phosphorylation, with an increase in oxidative stress, suggesting a switch from hyperplasia to hypertrophy growth. Furthermore, they found enrichment of the glycolytic pathway, mTOR, plasmalogen metabolism, methionine and histidine metabolism, lipid peroxidation, and sphingolipid signaling as novel pathways involved in heart regeneration | Cpt I and II, Acaa2, Acsl1, Ecl1, Hadha, Hadhb, and Hsd17b10 | NA |
| Suhre K, et al. (35) | CAD | KORA and TwinsUK cohorts.CARDIoGRAM. |
Genomics (microarray platform, Illumina, Affymetrix) Metabolomics (HPLC/MS platform, Metabolon) |
B, P. | Genotype-dependent metabolic phenotypes | Some genetic loci that regulate blood metabolite concentrations were also associated with CAD risk (NAT2, ABO, CPS1, NAT8, ALPL, KLKB1). The biochemical function of the associated metabolic traits identified may support a possible role in heart disease. | NAT2 (1-methylxanthine/ 4-acetamidobutanoate); ABO (ADpSGEGDFXAEGGGVR/ADSGEGDFXAEGGGVR); CPS1 (Glycine); NAT8 (N-acetylornithine); ALPL (ADpSGEGDFXAEGGGVR/ DSGEGDFXAEGGGVR); KLKB1 bradykinin des-arg(9). | NA |
| Feng Q, et al. (40) | CAD | 59 CAD patients and 43 healthy controls |
Metabolomics (HPLC/MS platform, Thermo) Metagenomics (DNA sequencing, Illumina) |
P | Association of metabolites with microbiome data | Some metabolites were significantly associated with gut microbiota and CAD risk (GlcNAc-6-P, mannitol, and 15 plasma cholines). Moreover, these identified metabolites show correlations with species of intestinal microbiota (Clostridium sp. and Streptococcus sp.). | LPCs, glycerophosphocholines, L-Arginine, GlcNAc-6-P, and paraxanthine | NA |
| Cui X, et al. (50) | Chronic heart failure | 53 CHF patients and 41 controls |
Metabolomics (LC/MS platform, Thermo) Metagenomics (DNA sequencing, Illumina) |
P | Correlation between changes in metabolites and gut microbiome associated with CHF | Enriched bacteria in CHF such as Veillonella were inversely correlated with cardiovascular protective metabolites such as niacin, cinnamic acid, and orotic acid. Furthermore, they found a positive correlation between the high sphingosine 1-phosphate levels and several CHF-enriched bacteria such as Veillonella, Coprobacillus, and Streptococcus. |
Veionella- niacin, cinnamic acid, and orotic acid Veillonella, Coprobacillus, and Streptococcus- sphingosine 1-phosphate |
NA |
| Talukdar H, et al. (39) | CAD | GWAS of CARDIoGRAMplusC4D and DIAGRAM studies. Mice (HMDP) |
Genomics (microarray platform, Illumina, Affymetrix) Transcriptomics (microarray platform, Affymetrix) |
AAW, SF, VAF, LIV | Marker Set Enrichment Analysis (co-expression modules). Cross-species validation using the HMDP | Identification of 30 CAD-causal regulatory gene networks interconnected in vascular and metabolic tissues | POLR21, PQBP1, AIP, DRAP1, MRPL28, PCBD1, ZNF91 | Validation of key divers in a THP-1 foam cells |
| Shu L, et al. (41) | CAD T2D |
GWAS data of five multi-ethnic studies including AA, EA, and HA. GWAS of CARDIoGRAMplusC4D and DIAGRAM studies. Mice (HMDP) |
Genomics (microarray platform, Illumina, Affymetrix) Transcriptomics (microarray platform and mRNA sequencing, Affymetrix, Illumina) PheWAS |
16 tissues including B, SF, ADR, ART, DT, IS, HY, LIV, LY, SKLM, TG, VE | Marker Set Enrichment Analysis (co-expression modules). Cross-species validation using cardiometabolic traits in the HMDP | Co-expression modules between CAD and T2D showed enrichment of pathways that regulate the metabolism of lipids, glucose, branched-chain amino acids, oxidation, extracellular matrix, immune response, and neuronal system. Identification of 15 key drivers associated with both CAD and T2D | ACAT2, ACLY, CAV1, COL6A2, COX7A2, DBI, HMGCR, IDI1, IGF1, MCAM, MEST, MSMO1, PCOLCE, SPARC, and ZFP36 | SiRNA knockout and in vivo knockout of CAV1 resulted in metabolic perturbations |
CAD, Cardiovascular Artery Disease; P, plasma; H, heart; B, blood; LIV, liver; AW, atherosclerotic arterial wall; MAM, atherosclerotic-lesion-free internal mammary artery; AOR, atherosclerotic aortic root; SF, subcutaneous fat; VAF, visceral abdominal fat; SKLM, skeletal muscle; ADR, Adrenal gland; HCASMCs, Human coronary artery smooth muscle cells; ART, Artery; DT, Digestive tract; IS, Islet; HY, Hypothalamus LY, Lymphocyte; TG, Thyiroid gland; VE, Vascular endothelium; AV, Aortic valve; M, monocytes; CEC, Coronary endothelial cells; CP, Carotid plaque.
For complete list of genes see reference.
Success Stories of Multi-Omics Studies in Cardiovascular Diseases
Although there have been few studies integrating multi-omics profiles for the investigation of mechanisms associated with cardiovascular diseases, this approach has revealed the potential function of previously identified GWAS loci and respective mechanisms involved in these common diseases. In this section, we summarize recent studies using multi-omics approaches focusing on the integration of genomics, epigenomics, transcriptomics, and proteomics.
Genomics, Transcriptomics, and Epigenomics
There is a large body of literature linking genetic variation with gene expression and/or epigenetic marks to understand the potential mechanisms of identified DNA variants in disease manifestation. One example on the integration of genomics with transcriptomics is a study conducted to investigate the role of the 9p21 locus (63), which was identified as one of the most significant loci for CAD in previous GWAs (64, 65). The association of CAD with this locus have been consistently replicated in multiple studies (56, 66), although the causal link of this locus remained unclear. This locus contains several genes including CDKN2A (encoding cyclin p14, p16), CDKN2B (encoding cyclin p15), MTAP (encoding methylthioadenosine phosphorylase), and the long non-coding RNA ANRIL. Integration of genetic and transcriptomic data led to the identification of ANRIL as the top candidate causal gene for CAD at the 9p21 region (63). Functional studies in cell lines showed possible mechanisms that could explain the role of 9p21 in CAD (67, 68). For instance, a previous study showed that alleles at the 9p21 locus were associated with different isoforms of ANRIL (linear or circular isoforms), where linear transcripts were associated with atherosclerosis and circular transcripts were protective against atherosclerosis. This process is mediated through the expression of multiple genes regulated in both, cis and trans (69, 70). Moreover, a recent study showed that ANRIL (DQ485454) is involved in endothelial cells functions important to the development of CAD including monocyte adhesion to endothelial cells, trans-endothelial monocyte migration, and endothelial cell migration (71).
Another example is the investigation of the region of the gene cluster CELSR2-PSRC1-MYBPHL-SORT at the 1p13.3 locus associated with low-density lipoprotein cholesterol (LDL-C) levels and cardiovascular risk (55, 72, 73). Incorporation of eQTL analysis also showed that SNPs associated with a lower risk of CAD in the 1p13.3 locus were associated with an increased gene expression of SORT1, PSRC1, and CELSR2, with SORT1 displaying the largest expression change in the liver (73, 74). This finding allowed the construction of new hypothesis to elucidate the molecular mechanism of the 1p13.3 locus on CAD development. Studies of SORT1 and PSRC1 overexpression in mouse models of hyperlipidemia showed that, while PSCR1 overexpression had no metabolic effects, SORT1 overexpression led to a significant reduction in plasma LDL-C and very low-density lipoprotein (VLDL) particle levels by modulating hepatic VLDL secretion, suggesting an important role of SORT1 in CAD (74). Finally, a similar omics approach was applied to identify genes associated with isoproterenol-induced hypertrophy and heart failure in the Hybrid Mouse Diversity Panel (HMDP) (13, 22, 23, 41, 75–83). The integration of genomic information and cardiac transcriptome enabled the identification of several candidate causal genes that determined the degree of cardiac hypertrophy. Specifically, Hes1 was predicted to be involved in the progression of heart damage in cardiac hypertrophy (13). This study showed that knocking down Hes1 in ventricular myocytes resulted in a reduction of up to 90% hypertrophy, confirming the role of Hes1 in cardiac hypertrophy (13). More recently, several studies have demonstrated that epigenetic modifications are associated with CAD risk (38, 42, 43, 47, 49, 59, 61, 62, 84, 85), and other CVD related risk factors (61, 62, 84). Epigenetic changes that have been investigated in the context of CVD include DNA methylation (38, 43, 49), chromatin organization (42), and microRNAs (47). In recent years, efforts have been conducted to identify interactions between functional non-coding active elements of the genome and enhancers, defined as cis-acting DNA sequences that can increase the transcription of genes (60, 61, 86). Several methods have been developed for the identification of these interactions including, chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-seq), chromatin conformation capture (3C, HiC), and most recently, chromatin interaction paired-end tagging (ChIA-PET). These technologies offer the advantage to identify genome-wide protein-DNA interactions.
Adding Another Layer: Proteomics
The incorporation of protein expression profiles into the multi-omics studies for CAD has been less explored compared with multi-omics studies incorporating mRNA expression (43–45, 47, 51–54). This may be due to the costs and the highly specialized expertise required for instrument operation, data acquisition, and analysis of quantitative proteomics (87). Recently, Emilsson et al. showed that co-expression protein modules associated with complex diseases are highly regulated by cis and trans acting genetic variants (88). Therefore, the integration of proteomic data can add valuable information about the molecular processes involved in the development of CAD. One of the more interesting studies incorporating proteomic data in mice was conducted by Lau et al. which in addition to genomic and proteomic data, integrated protein dynamics (51). This study showed modules involved in cell adhesion, glycolytic process, actin filament organization, translation, and sodium ion transport associated with heart hypertrophy (51). In another multi-omics study conducted by Schlotter et al. for the identification of mechanisms involved in calcified aortic valve disease (CAVD) (52), the authors performed global transcriptomics and proteomics of human stenotic valves to identified novel regulatory networks in CAVD. Novel potential molecular drivers of CAVD development and progression were identified including alkaline phosphatase, apolipoprotein B, matrix metalloproteinase activation, and mitogen-activated protein kinase. Moreover, this approach also identified inflammation pathways as a significant contributor to CVD (52). This study emphasizes the relevance of extensive phenotypic characterization for multi-omics approaches to define markers associated with disease subgroups and to design more specific therapeutic strategies. In summary, these studies showed that the knowledge generated from the integration of genomics, epigenomics, transcriptomics, and proteomics could provide initial insights into the identification of mechanisms for cardiovascular diseases.
Metabolomics and Metagenomic Studies for the Study of CAD
Metabolomics and metagenomics represent additional layers of complexity because they integrate the influences of the intake, utilization and flux of nutrients. Moreover, these omics data have proven to be useful tools for the identification of biomarkers with potential clinical applicability (89). However, studies integrating metabolomics, lipidomics, or metagenomics data in the context of CAD are limited (Table 1). In a GWAS study for metabolite levels conducted by Suhre et al. (35), the authors found several loci including ABO, NAT2, CPS1, NAT8, ALPL, KLKB1 genes associated with both metabolites and a high risk of CAD (35). Interestingly, KLKB1 was associated with bradykinin concentrations and with a higher CAD risk. It is known that bradykinin is a potent endothelium-dependent vasodilator that contributes to vasodilation and hypotension (90). These findings suggest that the integration of metabolomic data with other omic data can help to identify novel biomarkers for CVD diagnosis. Regarding studies integrating metagenomic data, there are only two studies for CVD so far that integrate metabolomics and metagenomics data (40, 50) (Table 1). These studies have shown species of bacteria associated with risk of CAD and plasma metabolites. For example, the bacteria Veillonella was associated with chronic heart failure and was also inversely correlated with known cardiovascular protective metabolites such as niacin, cinnamic acid and orotic acid (50). Nevertheless, it should be noted that these studies are only based on correlations and do not make an integrative analysis of the data, which reflects the complexity and the opportunity to develop novel statistical approaches.
Integration of Multi-Omics, Multi-Ethnic, and Multi-Species Models of Disease
It has been suggested that comparison of “omics” data between human and animal models can provide an important contribution to the understanding of the molecular mechanism implicated in CAD (24). While studies in humans have greater translational potential, studies using animal models can help validate their biological relevance and to recapitulate the findings in humans under different environmental stimulus (22, 24, 78). This has been demonstrated in recent studies integrating multi-omics approaches for the study of CAD in both humans and animal models (39, 41). An example of a large-scale integrative multi-omic approach is the study conducted by Shu and colleagues that involved CAD and T2D GWAS data of five multi-ethnic studies (41). In this study, genetic and transcriptomic data of 16 relevant tissues for CAD were included to construct co-regulation networks for CVD and T2D (41). This network modeling allowed the identification of pathways involved in lipid metabolism, glucose, and branched-chain amino acids, along with process involved in oxidation, extracellular matrix, immune response, and neuronal system in CAD and T2D (41). Moreover, this strategy helped to dissect the molecular mechanism of HMGCR, identified as a top key driver for both CAD and T2D. Interestingly, the authors showed that HMGCR was associated with CVD and T2D in opposite directions, while genetic variants in HMGCR decrease CVD risk, they increase T2D risk. These findings could have important implications in the pharmacological treatment of both diseases. The integration of existing omics-data from mice and humans deposited in the cardiovascular disease database (C/VDdb), including, microRNA, genomics, proteomics and metabolomics, has recently been analyzed to identified novel drivers for CVD. In an exercise to demonstrate the utility of the C/VD database, integrative analysis of this “omics” studies showed enrichment of lipid metabolism, extracellular matrix remodeling, inflammation, and cardiac hypertrophy pathways. In addition, regulatory mechanisms mediated through miRNAs associated with the development of CAD were reported (47). Altogether, these studies illustrate that high-level integration approaches are powerful tools to extract robust biological signals across molecular layers, phenotypes, tissue types, and even species and to prioritize new therapeutic avenues for cardiometabolic diseases. Of note, there is a limited overlap in the metabolic regulators, co-expression modules and key driver gene identified across different multi-omics studies for CVD, except for markers involved in lipid metabolism which seem to be consistent among different studies. This highlights the importance of lipid metabolism in the development of cardiovascular disorders (91–93). Discrepancies of these findings could be explained by differences in the statistical tools, phenotypic characterization, ethnic origin, sex, and pathophysiological conditions (13, 23–25, 79, 94).
Data Integration Using Freely Available Public Databases
The access to big biologic public databases allows the integration of genomic data with other “omics” including transcriptomics, proteomics and metabolomics datasets through freely available public databases such as GTEx (95) Encode (Encode project c, Roadmap (Roadmap Epigenomics Consortium, 2015), Snyderome (96) and bioRxiv, to mention a few. One of the main advantages of these databases is that allow simultaneous analysis of regulatory mechanism in different tissues, which are usually difficult to obtain in genetic studies conducted in humans. In this regard, the Genotype-Tissue Expression (GTEx) project is one of the most complete gene expression datasets currently available. This database was generated as a repository for identifying genetic variants associated with changes in gene expression (expression quantitative trait loci, eQTLs) and contains a broad tissue collection obtained from deceased donors. The last release v7, provides 11,688 transcriptomes from 714 individuals and 53 tissues. In addition GTEx also includes pathology and histology data as well as other characteristics as ethnicity, age, and sex (95). Moreover, in order to increase information about potential molecular mechanisms, the Enhancing GTEx (eGTEx) project extends the GTEx project to combine gene expression with DNase I hypersensitivity, ChIP–seq, DNA and RNA methylation, ASE, protein expression, somatic mutation, and telomere length assays (97). The Encyclopedia of DNA Elements (ENCODE) project has identified and annotated a significant amount of functional elements in the human and mice genome through diverse approaches as DNA hypersensitivity, DNA methylation, and immunoprecipitation (IP) assays of proteins that interact with DNA and RNA. The last version includes over 35 high-throughput experimental methods in > 250 different cell and tissue types, resulting in over 4,000 experiments. As GTEx database, ENCODE also includes relevant information about ethnicity, sex and age (98). Additional databases such as Roadmap (99), which has an extensive collection of DNA methylation, histone modifications, chromatin accessibility, and small RNA transcripts. The utility of these databases has been demonstrated in several studies for CAD, where their integration with genetic data facilitated the identification of regulatory mechanisms, potential targets and allows the functional validation. One example, is the prediction of the disruption of C/EBP binding site by the G allele of rs12740374 SNP using ENCODE data, functional studies showed that this variant results in a lower transcription of the SORT1 gene in liver and a higher VLDL-secretion, explaining the association of the variant with LDL-C levels in genetic studies (Figure 1) (74). Therefore, the integration of various data frameworks could be highly successfully to understand the mechanisms implicated in disease manifestation.
Future Directions
The identification of causal genes is a critical step toward the translation of genetic loci into biologic processes. The integration of “omic” strategies will accelerate the identification, in a more precise way, of novel molecular mechanisms implicated in CVD. This may eventually result in the characterization of novel pathways and drug targets. Although multi-omics approaches have been successfully applied for the investigation of cardiovascular diseases, the number of studies using this approach is still limited. These studies have been primarily focused on the integration of genomics, transcriptomics, epigenomics, and proteomics. Given the potential of metabolomics, metatranscriptomics, and metagenomics as tools for the identification of biomarkers with potential clinical applicability, the integration of such data will increase the understanding of cardiovascular diseases and accelerate the identification of new diagnostics or therapeutic targets (100). Finally, research efforts should be directed to the application of multi-omics and the generation of big data in more diverse populations and into the investigation of sex-specific mechanisms.
Author Contributions
PL-M, JW, and AH-V drafted and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. AH-V is funded by the NIH U54 DK120342 grant and NIH/CTSI UL1 TR00188. JW is funded by the NIH K08 HL133491 and NIH R01 HL129639.
References
- 1.Writing Group M. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: a report from the american heart association. Circulation. (2016) 133:e38–360. 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2.Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, De Faire U. Heritability of death from coronary heart disease: a 36-year follow-up of 20 966 Swedish twins. J Intern Med. (2002) 252:247–54. 10.1046/j.1365-2796.2002.01029.x [DOI] [PubMed] [Google Scholar]
- 3.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. (1994) 330:1041–6. 10.1056/NEJM199404143301503 [DOI] [PubMed] [Google Scholar]
- 4.Won HH, Natarajan P, Dobbyn A, Jordan DM, Roussos P, Lage K, et al. Disproportionate contributions of select genomic compartments and cell types to genetic risk for coronary artery disease. PLoS Genet. (2015) 11:e1005622. 10.1371/journal.pgen.1005622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consortium CAD, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. (2013) 45:25–33. 10.1038/ng.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coram MA, Duan Q, Hoffmann TJ, Thornton T, Knowles JW, Johnson NA, et al. Genome-wide characterization of shared and distinct genetic components that influence blood lipid levels in ethnically diverse human populations. Am J Hum Genet. (2013) 92:904–16. 10.1016/j.ajhg.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coronary Artery Disease Genetics C A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. (2011) 43:339–44. 10.1038/ng.782 [DOI] [PubMed] [Google Scholar]
- 8.Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, et al. Genetics of blood lipids among ~300,000 multi-ethnic participants of the Million Veteran Program. Nat Genet. (2018) 50:1514–23. 10.1038/s41588-018-0222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myocardial Infarction G, Investigators CAEC. Stitziel NO, Stirrups KE, Masca NG, Erdmann J, et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. (2016) 374:1134–44. 10.1056/NEJMoa1507652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myocardial Infarction Genetics C. Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. (2009) 41:334–41. 10.1038/ng.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson CP, Goel A, Butterworth AS, Kanoni S, Webb TR, Marouli E, et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet. (2017) 49:1385–91. 10.1038/ng.3913 [DOI] [PubMed] [Google Scholar]
- 12.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. (2015) 47:1121–30. 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santolini M, Romay MC, Yukhtman CL, Rau CD, Ren S, Saucerman JJ, et al. A personalized, multiomics approach identifies genes involved in cardiac hypertrophy and heart failure. NPJ Syst Biol Appl. (2018) 4:12. 10.1038/s41540-018-0046-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. (2011) 43:333–8. 10.1038/ng.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabb KL, Hellwege JN, Palmer ND, Dimitrov L, Sajuthi S, Taylor KD, et al. Analysis of whole exome sequencing with cardiometabolic traits using family-based linkage and association in the IRAS family study. Ann Hum Genet. (2017) 81:49–58. 10.1111/ahg.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. (2010) 466:707–13. 10.1038/nature09270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Harst, Verweij NP. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. (2018) 122:433–43. 10.1161/CIRCRESAHA.117.312086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verweij N, Eppinga RN, Hagemeijer Y, van der Harst P. Identification of 15 novel risk loci for coronary artery disease and genetic risk of recurrent events, atrial fibrillation, and heart failure. Sci Rep. (2017) 7:2761. 10.1038/s41598-017-03062-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wray NR, Purcell SM, Visscher PM. Synthetic associations created by rare variants do not explain most GWAS results. PLoS Biol. (2011) 9:e1000579 10.1371/journal.pbio.1000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramazzotti D, Lal A, Wang B, Batzoglou S, Sidow A. Multi-omic tumor data reveal diversity of molecular mechanisms that correlate with survival. Nat Commun. (2018) 9:4453. 10.1038/s41467-018-06921-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao H, Bartoszek K, Lio P. Multi-omic analysis of signalling factors in inflammatory comorbidities. BMC Bioinformat. (2018) 19(Suppl. 15):439. 10.1186/s12859-018-2413-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chella Krishnan K, Kurt Z, Barrere-Cain R, Sabir S, Das A, Floyd R, et al. Integration of multi-omics data from mouse diversity panel highlights mitochondrial dysfunction in non-alcoholic fatty liver disease. Cell Syst. (2018) 6:103–15 e7. 10.1016/j.cels.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurt Z, Barrere-Cain R, LaGuardia J, Mehrabian M, Pan C, Hui ST, et al. Tissue-specific pathways and networks underlying sexual dimorphism in non-alcoholic fatty liver disease. Biol Sex Differ. (2018) 9:46. 10.1186/s13293-018-0205-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. (2017) 18:83. 10.1186/s13059-017-1215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arneson D, Shu L, Tsai B, Barrere-Cain R, Sun C, Yang X. Multidimensional integrative genomics approaches to dissecting cardiovascular disease. Front Cardiovasc Med. (2017) 4:8. 10.3389/fcvm.2017.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilne B, Schunkert H. Integrating genes affecting coronary artery disease in functional networks by multi-OMICs approach. Front Cardiovasc Med. (2018) 5:89. 10.3389/fcvm.2018.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnett DK. Genetics of CVD in 2015: using genomic approaches to identify CVD-causing variants. Nat Rev Cardiol. (2016) 13:72–4. 10.1038/nrcardio.2015.202 [DOI] [PubMed] [Google Scholar]
- 28.Raghow R. An 'omics' perspective on cardiomyopathies and heart failure. Trends Mol Med. (2016) 22:813–27. 10.1016/j.molmed.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 29.Misra BB, Langefeld CD, Olivier M, Cox LA. Integrated omics: tools, advances, and future approaches. J Mol Endocrinol. (2018) 62:R21–45. 10.1530/JME-18-0055 [DOI] [PubMed] [Google Scholar]
- 30.Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet. (2014) 15:34–48. 10.1038/nrg3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLellan WR, Wang Y, Lusis AJ. Systems-based approaches to cardiovascular disease. Nat Rev Cardiol. (2012) 9:172–84. 10.1038/nrcardio.2011.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S, Lusis AJ, Drake TA. A systems-based framework for understanding complex metabolic and cardiovascular disorders. J Lipid Res. (2009) 50 Suppl:S358–63. 10.1194/jlr.R800067-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lusis AJ, Weiss JN. Cardiovascular networks: systems-based approaches to cardiovascular disease. Circulation. (2010) 121:157–70. 10.1161/CIRCULATIONAHA.108.847699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lusis AJ. A thematic review series: systems biology approaches to metabolic and cardiovascular disorders. J Lipid Res. (2006) 47:1887–90. 10.1194/jlr.E600004-JLR200 [DOI] [PubMed] [Google Scholar]
- 35.Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wagele B, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. (2011) 477:54–60. 10.1038/nature10354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foroughi Asl H, Talukdar HA, Kindt AS, Jain RK, Ermel R, Ruusalepp A, et al. Expression quantitative trait Loci acting across multiple tissues are enriched in inherited risk for coronary artery disease. Circ Cardiovasc Genet. (2015) 8:305–15. 10.1161/CIRCGENETICS.114.000640 [DOI] [PubMed] [Google Scholar]
- 37.Braenne I, Civelek M, Vilne B, Di Narzo A, Johnson AD, Zhao Y, et al. Prediction of causal candidate genes in coronary artery disease loci. Arterioscler Thromb Vasc Biol. (2015) 35:2207–17. 10.1161/ATVBAHA.115.306108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franzen O, Ermel R, Cohain A, Akers NK, Di Narzo A, Talukdar HA, et al. Cardiometabolic risk loci share downstream cis- and trans-gene regulation across tissues and diseases. Science. (2016) 353:827–30. 10.1126/science.aad6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talukdar HA, Foroughi Asl H, Jain RK, Ermel R, Ruusalepp A, Franzen O, et al. Cross-tissue regulatory gene networks in coronary artery disease. Cell Syst. (2016) 2:196–208. 10.1016/j.cels.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Q, Liu Z, Zhong S, Li R, Xia H, Jie Z, et al. Integrated metabolomics and metagenomics analysis of plasma and urine identified microbial metabolites associated with coronary heart disease. Sci Rep. (2016) 6:22525. 10.1038/srep22525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shu L, Chan KHK, Zhang G, Huan T, Kurt Z, Zhao Y, et al. Shared genetic regulatory networks for cardiovascular disease and type 2 diabetes in multiple populations of diverse ethnicities in the United States. PLoS Genet. (2017) 13:e1007040. 10.1371/journal.pgen.1007040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haitjema S, Meddens CA, van der Laan SW, Kofink D, Harakalova M, Tragante V, et al. Additional candidate genes for human atherosclerotic disease identified through annotation based on chromatin organization. Circ Cardiovasc Genet. (2017) 10:e001664. 10.1161/CIRCGENETICS.116.001664 [DOI] [PubMed] [Google Scholar]
- 43.Howson JMM, Zhao W, Barnes DR, Ho WK, Young R, Paul DS, et al. Fifteen new risk loci for coronary artery disease highlight arterial-wall-specific mechanisms. Nat Genet. (2017) 49:1113–9. 10.1038/ng.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao C, Chen G, Song C, Keefe J, Mendelson M, Huan T, et al. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat Commun. (2018) 9:3268 10.1038/s41467-018-06231-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G, Yao C, Hwang SJ, Liu C, Song C, Huan T, et al. Integrated proteomic analysis of cardiovascular disease reveals novel protein quantitative trait loci. Circulation. (2016) 134:A18806. [Google Scholar]
- 46.Lempiainen H, Braenne I, Michoel T, Tragante V, Vilne B, Webb TR, et al. Network analysis of coronary artery disease risk genes elucidates disease mechanisms and druggable targets. Sci Rep. (2018) 8:3434. 10.1038/s41598-018-20721-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandes M, Patel A, Husi H. C/VDdb: a multi-omics expression profiling database for a knowledge-driven approach in cardiovascular disease. (CVD). PLoS ONE. (2018) 13:e0207371. 10.1371/journal.pone.0207371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao YQ, Kurt Z, Yang X. Multi-omics modeling of carotid atherosclerotic plaques reveals molecular networks and regulators of stroke. Circulation. (2017) 136:A20541. [Google Scholar]
- 49.Meder B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, Frese K, Lai A, et al. Epigenome-wide association study identifies cardiac gene patterning and a novel class of biomarkers for heart failure. Circulation. (2017) 136:1528–44. 10.1161/CIRCULATIONAHA.117.027355 [DOI] [PubMed] [Google Scholar]
- 50.Cui X, Ye L, Li J, Jin L, Wang W, Li S, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. (2018) 8:635. 10.1038/s41598-017-18756-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau E, Cao Q, Lam MPY, Wang J, Ng DCM, Bleakley BJ, et al. Integrated omics dissection of proteome dynamics during cardiac remodeling. Nat Commun. (2018) 9:120. 10.1038/s41467-017-02467-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlotter F, Halu A, Goto S, Blaser MC, Body SC, Lee LH, et al. Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation. (2018) 138:377–93. 10.1161/CIRCULATIONAHA.117.032291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matic LP, Jesus Iglesias M, Vesterlund M, Lengquist M, Hong MG, Saieed S, et al. Novel multiomics profiling of human carotid atherosclerotic plaques and plasma reveals biliverdin reductase B as a marker of intraplaque hemorrhage. JACC Basic Transl Sci. (2018) 3:464–80. 10.1016/j.jacbts.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lalowski MM, Bjork S, Finckenberg P, Soliymani R, Tarkia M, Calza G, et al. Characterizing the key metabolic pathways of the neonatal mouse heart using a quantitative combinatorial omics approach. Front Physiol. (2018) 9:365. 10.3389/fphys.2018.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samani NJ, Braund PS, Erdmann J, Gotz A, Tomaszewski M, Linsel-Nitschke P, et al. The novel genetic variant predisposing to coronary artery disease in the region of the PSRC1 and CELSR2 genes on chromosome 1 associates with serum cholesterol. J Mol Med. (2008) 86:1233–41. 10.1007/s00109-008-0387-2 [DOI] [PubMed] [Google Scholar]
- 56.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. (2007) 357:443–53. 10.1056/NEJMoa072366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J, et al. Genetics of gene expression and its effect on disease. Nature. (2008) 452:423–8. 10.1038/nature06758 [DOI] [PubMed] [Google Scholar]
- 58.Lau E, Wu JC. Omics, big data, and precision medicine in cardiovascular sciences. Circ Res. (2018) 122:1165–8. 10.1161/CIRCRESAHA.118.313161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu B, Pjanic M, Wang T, Nguyen T, Gloudemans M, Rao A, et al. Genetic regulatory mechanisms of smooth muscle cells map to coronary artery disease risk loci. Am J Hum Genet. (2018) 103:377–88. 10.1016/j.ajhg.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee D, Kapoor A, Safi A, Song L, Halushka MK, Crawford GE, et al. Human cardiac cis-regulatory elements, their cognate transcription factors, and regulatory DNA sequence variants. Genome Res. (2018) 28:1577–88. 10.1101/gr.234633.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rask-Andersen M, Martinsson D, Ahsan M, Enroth S, Ek WE, Gyllensten U, et al. Epigenome-wide association study reveals differential DNA methylation in individuals with a history of myocardial infarction. Hum Mol Genet. (2016) 25:4739–48. 10.1093/hmg/ddw302 [DOI] [PubMed] [Google Scholar]
- 62.Dekkers KF, van Iterson M, Slieker RC, Moed MH, Bonder MJ, van Galen M, et al. Blood lipids influence DNA methylation in circulating cells. Genome Biol. (2016) 17:138. 10.1186/s13059-016-1000-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jarinova O, Stewart AF, Roberts R, Wells G, Lau P, Naing T, et al. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler Thromb Vasc Biol. (2009) 29:1671–7. 10.1161/ATVBAHA.109.189522 [DOI] [PubMed] [Google Scholar]
- 64.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. (2007) 316:1488–91. 10.1126/science.1142447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F, Xu CQ, He Q, Cai JP, Li XC, Wang D, et al. Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat Genet. (2011) 43:345–9. 10.1038/ng.783 [DOI] [PubMed] [Google Scholar]
- 66.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. (2007) 316:1491–3. 10.1126/science.1142842 [DOI] [PubMed] [Google Scholar]
- 67.Holdt LM, Teupser D. Long non-coding RNA ANRIL: Lnc-ing genetic variation at the chromosome 9p21 locus to molecular mechanisms of atherosclerosis. Front Cardiovasc Med. (2018) 5:145 10.3389/fcvm.2018.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musunuru K, Post WS, Herzog W, Shen H, O'Connell JR, McArdle PF, et al. Association of single nucleotide polymorphisms on chromosome 9p21.3 with platelet reactivity: a potential mechanism for increased vascular disease. Circ Cardiovasc Genet. (2010) 3:445–53. 10.1161/CIRCGENETICS.109.923508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. (2010) 6:e1001233. 10.1371/journal.pgen.1001233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. (2016) 7:12429. 10.1038/ncomms12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho H, Shen GQ, Wang X, Wang F, Archacki S, Li Y, et al. Long non-coding RNA ANRIL regulates endothelial cell activities associated with coronary artery disease by up-regulating CLIP1, EZR, and LYVE1 genes. J Biol Chem. (2019) 294:3881–98. 10.1074/jbc.RA118.005050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. (2008) 40:161–9. 10.1038/ng.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. (2008) 40:189–97. 10.1038/ng.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. (2010) 466:714–9. 10.1038/nature09266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lusis AJ, Seldin MM, Allayee H, Bennett BJ, Civelek M, Davis RC, et al. The hybrid mouse diversity panel: a resource for systems genetics analyses of metabolic and cardiovascular traits. J Lipid Res. (2016) 57:925–42. 10.1194/jlr.R066944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin LY, Chun Chang S, O'Hearn J, Hui ST, Seldin M, Gupta P, et al. Systems genetics approach to biomarker discovery: GPNMB and heart failure in mice and humans. G3. (2018) 8:3499–506. 10.1534/g3.118.200655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bennett BJ, Davis RC, Civelek M, Orozco L, Wu J, Qi H, et al. Genetic architecture of atherosclerosis in mice: a systems genetics analysis of common inbred strains. PLoS Genet. (2015) 11:e1005711. 10.1371/journal.pgen.1005711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hui ST, Kurt Z, Tuominen I, Norheim F, C Davis R, Pan C, et al. The genetic architecture of diet-induced hepatic fibrosis in mice. Hepatology. (2018) 68:2182–96. 10.1002/hep.30113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Norheim F, Hasin-Brumshtein Y, Vergnes L, Chella Krishnan K, Pan C, Seldin MM, et al. Gene-by-sex interactions in mitochondrial functions and cardio-metabolic traits. Cell Metab. (2019) 29:932–49.e4. 10.1016/j.cmet.2018.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park S, Ranjbarvaziri S, Lay FD, Zhao P, Miller MJ, Dhaliwal JS, et al. Genetic regulation of fibroblast activation and proliferation in cardiac fibrosis. Circulation. (2018) 138:1224–35. 10.1161/CIRCULATIONAHA.118.035420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buscher K, Ehinger E, Gupta P, Pramod AB, Wolf D, Tweet G, et al. Natural variation of macrophage activation as disease-relevant phenotype predictive of inflammation and cancer survival. Nat Commun. (2017) 8:16041. 10.1038/ncomms16041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rau CD, Civelek M, Pan C, Lusis AJ. A suite of tools for biologists that improve accessibility and visualization of large systems genetics datasets: applications to the hybrid mouse diversity panel. Methods Mol Biol. (2017) 1488:153–88. 10.1007/978-1-4939-6427-7_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rau CD, Romay MC, Tuteryan M, Wang JJ, Santolini M, Ren S, et al. Systems genetics approach identifies gene pathways and Adamts2 as drivers of isoproterenol-induced cardiac hypertrophy and cardiomyopathy in mice. Cell Syst. (2017) 4:121–8 e4. 10.1016/j.cels.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Breitling LP, Yang RX, Korn B, Burwinkel B, Brenner H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet. (2011) 88:450–7. 10.1016/j.ajhg.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernandez-Sanles A, Sayols-Baixeras S, Curcio S, Subirana I, Marrugat J, Elosua R. DNA methylation and age-independent cardiovascular risk, an epigenome-wide approach: the REGICOR study (REgistre GIroni del COR). Arterioscler Thromb Vasc Biol. (2018) 38:645–52. 10.1161/ATVBAHA.117.310340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, He L, Goggin SM, Saadat A, Wang L, Sinnott-Armstrong N, et al. High-resolution genome-wide functional dissection of transcriptional regulatory regions and nucleotides in human. Nat Commun. (2018) 9:5380. 10.1038/s41467-018-07746-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schubert OT, Rost HL, Collins BC, Rosenberger G, Aebersold R. Quantitative proteomics: challenges and opportunities in basic and applied research. Nat Protoc. (2017) 12:1289–94. 10.1038/nprot.2017.040 [DOI] [PubMed] [Google Scholar]
- 88.Emilsson V, Ilkov M, Lamb JR, Finkel N, Gudmundsson EF, Pitts R, et al. Co-regulatory networks of human serum proteins link genetics to disease. Science. (2018) 361:769–73. 10.1126/science.aaq1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang N, Zhu F, Chen L, Chen K. Proteomics, metabolomics, and metagenomics for type 2 diabetes and its complications. Life Sci. (2018) 212:194–202. 10.1016/j.lfs.2018.09.035 [DOI] [PubMed] [Google Scholar]
- 90.Cruden NL, Witherow FN, Webb DJ, Fox KA, Newby DE. Bradykinin contributes to the systemic hemodynamic effects of chronic angiotensin-converting enzyme inhibition in patients with heart failure. Arterioscler Thromb Vasc Biol. (2004) 24:1043–8. 10.1161/01.ATV.0000129331.21092.1d [DOI] [PubMed] [Google Scholar]
- 91.Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet. (2014) 384:607–17. 10.1016/S0140-6736(14)61009-6 [DOI] [PubMed] [Google Scholar]
- 92.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. (2014) 384:618–25. 10.1016/S0140-6736(14)61217-4 [DOI] [PubMed] [Google Scholar]
- 93.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. (2014) 384:626–35. 10.1016/S0140-6736(14)61177-6 [DOI] [PubMed] [Google Scholar]
- 94.Johnson KW, Shameer K, Glicksberg BS, Readhead B, Sengupta PP, Bjorkegren JLM, et al. Enabling precision cardiology through multiscale biology and systems medicine. JACC Basic Transl Sci. (2017) 2:311–27. 10.1016/j.jacbts.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Consortium GT. The genotype-tissue expression (GTEx) project. Nat Genet. (2013) 45:580–5. 10.1038/ng.2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen R, Mias GI, Li-Pook-Than J, Jiang L, Lam HY, Chen R, et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell. (2012) 148:1293–307. 10.1016/j.cell.2012.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.eGTEx Project. Enhancing GTEx by bridging the gaps between genotype, gene expression, and disease. Nat Genet. (2017) 49:1664–70. 10.1038/ng.3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. (2012) 489:57–74. 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roadmap Epigenomics C, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. Nature. (2015) 518:317–30. 10.1038/nature14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. (2016) 17:451–9. 10.1038/nrm.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]