Abstract
Ependymomas are rare primary central nervous system (CNS) tumors in adults. They occur most commonly in the spinal cord, and have classically been graded histologically into World Health Organization (WHO) grades I, II, or III based on the level of anaplasia. Recent data are showing that genetic heterogeneity occurs within the same histological subgroup and that ependymomas arising from different CNS locations have different molecular signatures. This has renewed interest in developing targeting therapies based on molecular profiles especially given the variable outcomes with radiation and the poor results with cytotoxic agents. In this paper, we present the case of a 46-year-old woman with a classic presentation of spinal cord ependymoma and discuss the current histopathological and molecular classification for ependymomas as well as current guidelines for patient management.
Keywords: adults, chemotherapy, ependymoma, radiation, surgery
Clinical Case Presentation
A 46-year-old woman presented with a 6-week history of neck pain radiating down both upper extremities, associated with numbness involving the medial aspect of both hands. The patient denied any history of trauma, any sphincter disturbances or weakness, or any other neurological symptoms. On examination she had evidence of mild right upper extremity hyperreflexia with spread to her right biceps reflex. Otherwise her neurological examination was normal. Magnetic resonance imaging (MRI) of the cervical spine was obtained (Fig. 1).
Fig. 1.
Intramedullary Spinal Cord Ependymoma.
A, Axial and C, sagittal T1-weighted postcontrast magnetic resonance images (MRIs) demonstrate a well-demarcated intramedullary enhancing lesion centered at the level of C6. B, Axial and D, sagittal T2-weighted MRIs show the mass to be hyperintense, with adjacent edema and a subtle T2 hyperintense cap along the inferior tumor margin (arrow).
Etiology and Presentation
Ependymomas are rare tumors constituting only 1.8% of central nervous system (CNS) tumors. Ependymomas develop in all age groups but occur more frequently in children, being the most common spinal cord tumor in children and adolescents ages 0–19 years with an incidence of 23.3% of all cord, spinal meninges, and cauda equina tumors according to the latest 2017 report published by the Central Brain Tumor Registry of the United States (CBTRUS).1 In adults (ages 20+), they constitute 19.1% of tumors arising in these locations, with tumors of the meninges and nerve sheet tumors being more common in this age group (38.9% and 26.5%, respectively). There also appears to be a racial disparity, with an incidence ratio in whites vs African Americans of 1.73 when including cranial and spinal ependymomas.
In a large retrospective series of adults with ependymomas, pain was the most common presenting symptom for both brain and spinal cord ependymomas (70% vs 85%, respectively).2 For spinal cord tumors, other common presenting symptoms were sensory symptoms (70%), weakness (30%), bladder (25%), and bowel (11%) dysfunction. Patients with spinal cord ependymomas had symptoms for an average of 8 months prior to diagnosis compared to 3.5 months for patients with brain ependymomas.
Initial Workup
Imaging
Intramedullary spinal cord ependymoma
MRI is the imaging modality of choice for evaluating spinal cord tumors, often revealing features strongly suggestive of ependymoma. The prototypical spinal cord ependymoma is a well-circumscribed intensely enhancing mass located centrally within the spinal cord, most commonly involving the cervical spine, and spanning multiple vertebral segments.3 Nontumoral “polar cysts” at the superior and inferior margins of the tumor due to accumulation of fluid in the central canal are seen in the majority of cases, while true tumoral cysts are less common. Syringohydromyelia occurs in up to 50% of cases. The solid portions of intramedullary spinal cord ependymomas are isointense or hypointense on T1-weighted sequences, and homogenously or heterogeneously enhancing on gadolinium-enhanced images. On T2-weighted sequences, the tumors are usually hyperintense and frequently demonstrate hypointense caps of hemosiderin at the cranial and/or caudal margins due to previous hemorrhage.
When neck or back pain is the predominant presenting symptom, patients may initially be imaged with radiographs or computer tomography (CT). As ependymomas generally demonstrate indolent growth, they may cause vertebral remodeling with segmental widening of the spinal canal and vertebral body scalloping.3 On CT, the tumors themselves may be visualized as expansion of the cord, with central enhancement present if contrast is given.
The radiographic differential diagnosis for spinal ependymoma includes both neoplastic and nonneoplastic entities. When significant cord expansion is present, primary differential considerations include tumors such as hemangioblastoma and astrocytoma, as well as less common spinal tumors including lymphoma, subependymoma, and metastasis. Spinal cord expansion can also be seen with inflammatory and demyelinating conditions such as neurosarcoidosis and neuromyelitis optica and other causes of transverse myelitis. Spinal cord compressive myelopathy could also be considered for a focal enhancing and edematous spinal cord lesion centered at a level with spinal canal stenosis.
Myxopapillary ependymoma
Myxopapillary ependymoma (MPE) is considered an intraaxial extramedullary spinal cord tumor and differs significantly from the previously discussed ependymoma subtypes, including with respect to imaging. MPE arises from ependymal cells of the filum terminale, though it may be centered at the conus medullaris, filum terminale, or cauda equina. Tumors too small to fill the spinal canal are often discovered incidentally, while larger tumors that entirely fill the canal may present with low back pain or symptoms of nerve root compression.
Using MRI, an MPE most often appears as a well-circumscribed ovoid or sausage-shaped mass that displaces the traversing nerve roots. On T1-weighted images, the tumors are isointense to the spinal cord, with avid enhancement on contrast-enhanced sequences. On T2-weighted images, MPEs are hyperintense, with frequent hypointense margins reflecting regions of hemosiderin deposition.3 A characteristic example of an MPE MRI is shown in Fig. 2. As is the case with intramedullary spinal cord ependymomas, bony remodeling and expansion of the spinal canal may provide indirect evidence of lumbar spine MPE on radiographs and CT images. CT can also demonstrate the presence of an isodense intradural mass. Primary differential diagnostic considerations include nerve sheath tumors arising from cauda equina nerve roots, spinal meningiomas, metastatic disease, paragangliomas, or very rarely a non-myxopapillary ependymoma.
Fig. 2.
Myxopapillary Ependymoma.
A, Sagittal T2-weighted magnetic resonance image (MRI) demonstrates a sausage-shaped hyperintense mass centered at the L2-3 interspace that displaces traversing cauda equina nerve roots. B, Postcontrast T1-weighted sagittal MRI demonstrates avid homogenous tumoral enhancement. C, An axial T2-weighted MRI shows the tumor filling the canal with lateral displacement and compression of nerve roots (arrow).
Intracranial ependymoma
Posterior Fossa Ependymoma.
Posterior fossa ependymomas in adults share many imaging features with pediatric tumors. The typical CT appearance is a mass filling the fourth ventricle, which is isodense or hypodense, and often results in obstructive hydrocephalus.4 Macroscopic calcification is seen in approximately one-half of patients, which is more frequent than in other posterior fossa masses such as medulloblastoma, pilocytic astrocytoma, and metastasis from systemic cancers.5
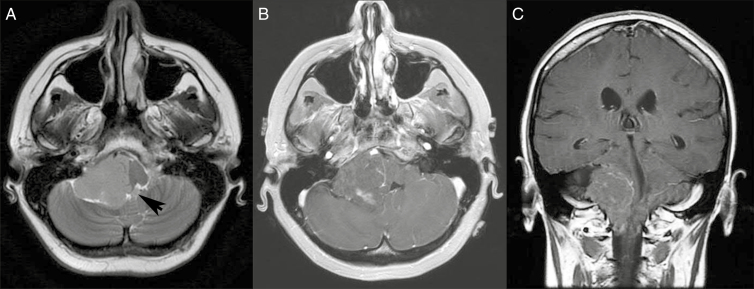
On MRI, posterior fossa ependymomas often extend in a “plastic” way through the foramina of Luschka and/or Magendie. As these tumors often arise from the floor of the fourth ventricle, there is frequently a cerebrospinal fluid (CSF) cleft along the roof of the ventricle, a pattern opposite that seen with many medulloblastomas. The solid portions of the tumor are typically isointense or hypointense relative to the brain on T1-weighted images and hyperintense on T2-weighted images. Heterogeneous enhancement is seen on post-gadolinium images. Hemorrhage and/or calcification may be apparent on T2* imaging sequences such as susceptibility-weighted sequences (SWI) or gradient echo (GRE). Restricted diffusion may be present in highly cellular solid regions of tumor, though typically apparent diffusion coefficients in ependymoma are not as low as in medulloblastoma.4,6 An example of a posterior fossa ependymoma in an adult is shown in Figure 3.
Fig. 3.
Posterior Fossa Ependymoma.
A, Axial T2-weighted magnetic resonance image (MRI) demonstrates a mass centered in the right cerebellopontine angle with extension through the foramen of Luschka into the fourth ventricle (arrow). B and C, Axial and coronal T1-weighed postcontrast MRIs demonstrate heterogeneous enhancement within the mass.
Supratentorial Ependymoma.
CT imaging of supratentorial ependymomas typically shows a heterogeneous solid tumor, often isodense or hypodense to white matter, and frequently containing coarse calcifications, cysts, or both. MRI reveals that the solid portions of tumor are typically isointense or hypointense relative to the brain on T1-weighted images and hyperintense on T2-weighted images, and heterogeneous enhancement is the rule. Most ependymomas will demonstrate evidence of previous hemorrhage or calcification on SWI or GRE.7 In one large case series of patients with intraparenchymal supratentorial ependymoma, frontal lobe localization was most common (21/37; 57%), followed by parietal localization (14/37; 38%).8 More than one-half of supratentorial ependymomas arise in the brain parenchyma without ventricular involvement, presumably from fetal ependymal cell remnants migrating from periventricular areas.9 The third ventricle is the next most common site, followed by the lateral ventricles. Figure 4 provides an example of the imaging appearance of a supratentorial ependymoma.
Fig. 4.
Supratentorial Ependymoma.
A, Axial computed tomography image demonstrates a heterogeneous, primarily hyperdense, right frontal mass. B, T1-weighted postcontrast magnetic resonance axial and D, coronal images (MRIs) show heterogeneous tumor enhancement. C, Axial T2-weighted MRI confirms the presence of a cystic space within the tumor, as well as peri-tumoral edema along the posteromedial aspect of the tumor (arrow). E, Coronal fluid-attenuated inversion recovery image suggests purely intraparenchymal tumor location, without extension to the ventricle.
Unlike spinal cord and posterior fossa ependymomas, which can often be identified with reasonable confidence by imaging, supratentorial ependymomas in adults are only one type of tumor in the differential diagnosis and tissue confirmation is necessary. Differential considerations for tumors arising within or adjacent to the ventricles include central neurocytoma, subependymoma, and choroid plexus papilloma. Preoperative identification of tumors that do not involve the ventricles is especially difficult, as they may appear similar to high-grade infiltrating gliomas such as glioblastoma or to metastases, both of which are far more common than intraparenchymal ependymoma.
Clinical Case Relevance
Our patient presented with pain and sensory symptoms, which are the most common presenting symptoms for a spinal cord ependymoma. MRI of the cervical spine showed a well-demarcated intramedullary enhancing mass (Fig. 1). Based on this information, the patient was advised to proceed with surgery
Surgical Management
Gross total resection (GTR) of ependymomas of all grades is the most consistent factor associated with improved survival in multiple studies.10 Surgery offers several benefits including the removal of mass effect, procurement of tissue for pathologic diagnosis and grading, and most important, oncologic benefit. As a result, surgical resection remains the cornerstone of management for these tumors irrespective of their location in the CNS.
Intramedullary Spinal Cord Ependymoma
Operative management of spinal ependymomas can be technically challenging. Accordingly, the oncologic benefit of resection must be weighed against the potential for iatrogenic neurologic complications. Regardless of grade, en bloc GTR results in the best overall oncologic outcome, most strikingly in MPE, for which it is curative.11
GTR has been shown in multiple studies to improve progression free survival (PFS) and overall survival (OS).10,12,13 For example, Oh et al demonstrated that patients with GTR of either grade II or III ependymomas had a 98.8% 5-year survival rate, while those with subtotal resection (STR), with or without radiation therapy (RT), had a 79.3% and 73.7% 5-year survival rate, respectively.14 The ability to achieve GTR for intramedullary grade II ependymomas can be as high as 93% while being safely performed in most patients.15–17 GTR is facilitated in characteristically well-defined and encapsulated World Health Organization (WHO) Grade I tumors; however, this is less likely in infiltrative grade II and the more aggressive grade III ependymomas.18 Whenever possible, intramedullary ependymomas should be removed en bloc, as piecemeal removal may facilitate CSF dissemination.19
Although surgical excision of spinal ependymomas is the most important factor determining PFS and OS, it is not without risk. Postoperative deficits are common after resection of spinal cord neoplasms with up to 67% of patients experiencing neurologic decline. Fortunately, many of these deficits will improve within several months postoperatively.17,20 Generally, the more profoundly affected the patient is prior to surgical excision, the more potential exists for postoperative neurological deficits. The most important predictor of postoperative outcome is preoperative neurological status with those patients exhibiting mild symptoms the most likely to return to a functional neurologic status. Those with profound deficits preoperatively are at much higher risk of postoperative decline and less likely to recover.20,21
Myxopapillary Ependymoma
GTR of suspected MPE should be attempted in all cases. It is associated with better long-term control, OS, and PFS when compared to STR.22,23 If possible, en bloc resection is preferable to piecemeal resection, as tumor capsule violation has been reported to increase the risk of CSF seeding and metastasis, while GTR without tumor capsule violation may be curative.19,24
Despite its extramedullary location and benign grade, MPE carries a long-term prognosis similar to that of WHO grade II ependymoma, perhaps because of a lower overall GTR rate.25 Oh et al reported in their meta-analysis no difference in OS or PFS between MPE and grade II ependymoma.15 Weber et al demonstrated a treatment failure rate of 33% in their series.26 Surgically, this may be explained by the complexity of these lesions, which can be densely adherent, even appearing to be infiltrating the conus medullaris and/or cauda equina, making GTR in conjunction with neurologic preservation technically challenging.
Intracranial Ependymomas
Surgery is the mainstay of therapy for intracranial grade II and III ependymomas. Multivariate analysis of the Collaborative Ependymoma Research Network Foundation (CERN) and Surveillance, Epidemiology, and End Results (SEER) databases demonstrated GTR to be superior to STR in terms of PFS.12 In cases in which GTR is not achieved, a “second-look” surgery is indicated if residual tumor is seen on postoperative MRI, and there is a low or otherwise acceptable risk of neurologic deficit with pursuing an additional resection.27 In addition to extent of resection, it appears that location plays an important role in PFS with infratentorial vs supratentorial tumors having a PFS of 12.3 vs 3.9 years, respectively. Tumor grade is also prognostic, with anaplastic (grade III) ependymomas associated with a shorter median time to progression as compared to grade II ependymomas (2.3 and 15.8 years, respectively).28 Consensus on the management of recurrent ependymoma is less well defined. Maximal surgical resection may be beneficial when weighed against risks and baseline patient condition.29
Clinical Case Relevance
The patient underwent a GTR of the tumor. Postoperatively the patient developed sensory loss from the waist down with no weakness. Symptoms improved gradually over several months. These symptoms were thought to be secondary to surgical manipulation of the cord, since imaging postoperatively showed no radiographic etiology for this presentation.
Pathology
Ependymomas are usually circumscribed neoplasms (Fig. 5A), arising near or in the ventricular system, but they can also arise in the cerebral hemispheres and rarely have an infiltrative pattern. On gross inspection (Fig. 5A) they are usually tan, soft tumors sometimes gritty on palpation because of the deposition of calcium. Based on the most current WHO Classification of Tumors of the CNS, ependymomas are primarily classified based on histomorphology as classic/WHO grade II (Fig. 5B) or anaplastic/WHO grade III (Fig. 5C and D).30 Histopathologic examination reveals that classic ependymomas are composed of small, monomorphic cells with round to oval nuclei forming perivascular, anucleate zones or pseudorosettes, which are generally seen in all tumors. Ependymomas can also rarely display true rosettes, which form when the ependymal cells arrange themselves in a small circle preserving the anucleate zones, but creating a central, empty lumen; it is believed that this structure results in an attempt to recapitulate the ependymal canal. Ependymomas can have low mitotic counts or few foci of necrosis with pseudopalisading and/or foci of microvascular proliferation. Anaplastic ependymoma is characterized by increased cellular density, cellular atypia and pleomorphism, increased mitotic activity, numerous foci of microvascular proliferation, and/or necrosis with pseudopalisading (Fig. 5C and D). Papillary, clear cell, and tanycytic ependymoma are morphological variants without clinical significance beyond that of classical (morphological) WHO grading.30
Fig. 5.
A, Gross features of ependymal tumors: Well-circumscribed, tan tumor, relatively homogenous on cross-section is here represented by a myxopapillary ependymoma attached to a nerve fascicle from cauda equina (specimen is from a different patient). B, For the patient presented here, resection of intramedullary tumor showed a World Health Organization (WHO) grade II ependymoma composed of a monomorphic neoplastic proliferation with perivascular anucleate zones (pseudorosettes) (arrows) (100×, scale = 100 μm). The tumor cells were bland, with round to oval nuclei. Detail, 400×, scale = 20 μm) showed glial fibrillary acidic protein positivity with strong pseudorosette accentuation (left inset, 100×, scale = 100 μm) and perinuclear, “dot-like” epithelial membrane antigen reactivity (right inset, 400×, scale = 20 μm). C, Anaplastic ependymoma, WHO grade III: Hypercellular ependymoma with pseudorosettes, foci of microvascular proliferation (yellow long arrows) and necrosis (bounded by short black arrows in C) (100×, scale = 100 μm). D, Anaplastic ependymoma, higher power: Hypercellularity, microvascular proliferation (red circles), and numerous mitotic figures (long yellow arrows) (200×, scale = 50 μm) (specimens showed in C and D are from different patients).
Ependymomas express the glial fibrillary acidic protein (GFAP) and S100, but usually not OLIG2, which is consistently expressed in other glial tumors (eg, diffuse gliomas). GFAP will show a strong perivascular accentuation in the pseudorosetted areas (Fig. 5B). The epithelial membrane antigen (EMA) often shows a perinuclear, “dot-like” or “ring-like” cytoplasmic pattern (Fig. 5B).30 A very promising biomarker has been recently described in ependymoma. NHERF1/EBP50, a protein required for epithelial morphogenesis, appears to be more specific than EMA for labeling ependymomas.31 In our experience NHERF1 is superior to EMA and extremely helpful in diagnosing difficult cases.
In addition to classic and anaplastic ependymoma, a genetically defined ependymoma variant, RELA-fusion positive ependymoma (RFPE), is incorporated in the new WHO classification as a standalone diagnostic entity.30 RFPE is characterized by a RELA fusion, most commonly the C11orf95-RELA (~70%), by supratentorial location, usually affecting children, and less commonly, adults.32,33 According to a single study, RFPEs have a worse prognosis compared to other supratentorial molecular groups (see below).34 This entity is morphologically diverse and can be either classic or anaplastic (WHO grade II or III). RELA fusions result from chromosome 11q13.1 chromotripsis and drive nuclear factor (NF)-κB pathway signaling. L1CAM immunoexpression is associated with the presence of the RELA fusion and with NF-κB signaling.32 RFPE associates with loss of chromosome 9, homozygous deletion of CDKN2A (p16), previously described as poor prognostic markers.35
Investigations have identified additional ependymoma subtypes. To date, 9 large molecular subgroups of ependymal tumors have been defined based on large-scale genomic profiling.34,36–39 They span the supratentorial, infratentorial (posterior fossa), and spinal cord anatomical compartments. Each anatomical compartment has 3 molecular subgroups (Fig. 6). The supratentorial ependymoma compartment includes the favorable prognostic category of ependymomas with YAP1 fusions.32,34 By using an antibody against the trimethylated Histone H3 at Lysine 27 (H3K27me3), a recent study was able to distinguish between groups A and B ependymomas of posterior fossa with increased accuracy. All PF-EPN-A (72/72, 100%) showed loss of H3K27me3 immunoexpression, while most PF-EPN-B (39/40, 97.5%) retained H3K27me3 immunoexpression.40 This is exciting as immunohistochemistry is cost effective compared to methylation arrays allowing for faster and easier clinical utilization.
Fig. 6.
Molecular Classification of Ependymal Tumors.
EPN indicates ependymoma; MPE, myxopapillary ependymoma; PF, posterior fossa; SC, spinal cord; SE, subependymoma; ST, supratentorial.
Additionally, an individual molecular alteration spanning several large molecular subgroups has been identified in ependymoma. Gain of 1q, associated with poor prognosis, was described in PF-EPN-A, PF-EPN-B, and ST-EPN-RELA in approximately 20% to 25% of cases.34,35,41–43 Gain of 1q could be individually tested by fluorescent in situ hybridization or chromosomal microarray.
Given the new molecular advances, a consensus meeting among multispecialty ependymoma experts recently concluded that supratentorial and infratentorial ependymomas are distinct biological entities. Therefore, as different diseases, treatment decisions for patients with ependymomas outside of clinical trials should not be based on WHO grading (II vs III).44
Clinical Case Relevance
Histopathological examination of the resected tumor showed a monomorphic neoplastic proliferation composed of bland glial cells with round to oval nuclei and with perivascular anucleate zones (pseudorosettes). Mitotic figures were inconspicuous. Areas of necrosis and/or microvascular proliferation were not present. GFAP immunohistochemistry confirmed the glial nature of the neoplastic cells and showed strong perivascular accentuation. EMA immunohistochemistry showed perinuclear, “dot-like” reactivity (Fig. 5B). The histological and immunophenotypical features were diagnostic of ependymoma, WHO grade II. Microarray-based chromosome analysis (single nucleotide polymorphism array) molecular analysis of the tumor showed a single clone comprising 95% of the cells analyzed with hyperdiploid genotype with gain of chromosomes 9, 12, 15, and X, and with loss of chromosome 22 compatible with spinal ependymoma.30,45
Postoperative Assessment
Given the prognostic significance of GTR, postoperative MRI to assess for residual disease should take place within 48 hours of surgery and ideally within 24 hours. After this period, postoperative changes along the margin of the surgical site may be indistinguishable from residual disease. At a minimum, this MR study should include a pregadolinium T1-weighted sequence to identify blood products, a postgadolinium T1-weighted sequence to demonstrate enhancing residual disease, and a diffusion-weighted sequence to identify areas of infarction that may demonstrate enhancement on follow-up images.
As ependymoma can spread through the CSF, MRI of the brain and entire spine is recommended for staging in patients with pathologically proven disease. If this is not completed prior to surgery, MRI should be delayed for 2 to 3 weeks following surgery to avoid false-positive results due to transient postoperative changes. The National Comprehensive Cancer Network (NCCN) recommendations state that complete neuraxis imaging should be performed for all grades of ependymoma, including MPE.46
Similarly, if no CSF analysis has been performed preoperatively, the recommendations are to wait at least 2 weeks postoperatively to avoid false-positive cytology.46
Clinical Case Relevance
The patient had an MRI of the cervical spine 24 hours postoperatively and MRI brain, thoracic and lumbar spine 3 weeks later. This was then followed by a spinal tap. There was no evidence of dissemination of the ependymoma along the neuroaxis either by imaging or CSF analysis.
Postoperative Treatment
Radiation Therapy
Intramedullary spinal cord ependymoma
GTR has been shown to be significantly associated with reduced recurrence and improved OS in multiple series.23,47,48 RT is recommended after STR, but there is no clear evidence for RT after GTR.49
Myxopapillary ependymoma
MPEs are uncommon low-grade spinal tumors. RT is recommended after STR to reduce the risk of recurrence, but recent evidence suggests that RT may also reduce local recurrence in patients with GTR whose tumors could not be removed en bloc.49
Treatment Technique for Spinal Ependymoma
Historically, RT was given to the level of disease plus 2 vertebral bodies above and below the cephalad and caudal extent of the tumor to doses of 45-54 Gy.49 Currently, doses of 45-54 Gy are given with consideration to a boost to 59.4 Gy if below the level of the spinal cord. If there is disseminated disease then craniospinal RT to 36 Gy would be given. If there are drop metastases below (and not above) the level of the tumor, then consideration could be given to treating the level of the tumor and below to 36 Gy with a boost to the tumor bed and any gross disease to limit the late toxicity of cranial RT.
Proton RT may also be of benefit in spinal ependymomas. In particular, proton RT would reduce the dose to the heart in thoracic ependymomas. One small series has shown favorable toxicity outcomes in pediatric patients treated with proton RT for spinal ependymoma with reduced dose to anterior structures compared to photon-based plans.50
Intracranial ependymomas
The most significant factor affecting PFS and OS of patients with intracranial ependymoma is the ability to attain a GTR.51 However, GTR is often not achievable because of the adherence or invasion of critical structures such as the brainstem. Historically, outcomes for ependymoma were poor, and RT was seen to reduce recurrence and improve survival in several series.52,53 Even with localized disease, RT was often given to the craniospinal axis or the whole brain.54 These large fields resulted in significant late neurocognitive toxicity.55,56 However, as imaging and treatment techniques improved, it was found that the majority of recurrences occurred in the local tumor bed and conformal RT to the tumor bed resulted in high rates of control.57,58
Posterior Fossa Ependymoma.
Multiple retrospective series and population-based data support the role of adjuvant RT in the management of infratentorial ependymoma.59–61 Two analyses from the SEER program have shown improved overall survival with adjuvant RT in infratentorial ependymoma.59,62 A large hospital-based registry series, however, did not find a benefit to RT in adults.63 More recently, 2 distinct molecular subgroups of posterior fossa ependymomas (excluding subependymomas) have been characterized, EPN_PFA and EPN_PFB.34,37 EPN_PFA tumors have a poor prognosis and occur commonly in infants and young children, and EPN_PFB have a better prognosis and occur more often in older children and adults. A recent series of 820 patients with infratentorial ependymomas from 4 institutions (83% were EPN_PFA and 17% EPN_PFB) demonstrated that GTR improved PFS and OS for both subgroups.64 Few patients with EPN_PFA did not receive RT, and RT continues to be recommended because of the poor prognosis. Patients with EPN_PFA tumors and STR fared particularly poorly despite RT, and further research is needed to improve prognosis in this group. Patients with EPN_PFB had a favorable prognosis with GTR alone and may not benefit from RT; however, further research should be performed to confirm these findings because of the small number of patients with EPN_PFB.
Supratentorial Ependymoma.
Observation has been commonly recommended after GTR for WHO grade II supratentorial ependymomas as multiple series have shown favorable outcomes.65,66 RT has been recommended for WHO grade III tumors and patients with STR. However, supratentorial WHO grade II/III ependymomas have been classified into two distinct molecular subgroups, EPN-YAP1 and EPN-RELA.34 EPN-RELA tumors occur in adults and pediatric patients, and EPN-YAP1 tumors occur in pediatric patients only. Current consensus guidelines recommend against using tumor grading in treatment decisions because of poor reproducibility and incorporating molecular subgrouping in clinical trials, although this remains controversial.44,67 Thus, RT should be given for patients with residual tumor after surgery, while clinical trials should evaluate the benefit of RT in the setting of GTR.
Disseminated Ependymoma.
Disseminated ependymoma at diagnosis is uncommon and represents less than 7% of cases.68 Craniospinal RT to 36 Gy is standard in these cases with a boost to 54-59.4 Gy to the primary tumor bed and a boost to 54 Gy to gross disease.69
Treatment Technique and Late Toxicity
For patients with localized intracranial ependymoma, the tumor bed is commonly treated to 59.4 Gy, with an anatomically constrained 3-5 mm geometric expansion about the tumor bed treated to 54 Gy. A prospective series of 153 patients found that 7-year local control was 87.3% using conformal RT with a 1 cm clinical target volume (CTV) expansion.57 Current prospective trials (ACNS 0831) use a 5 mm CTV expansion. Intensity modulated radiotherapy or volumetric modulated arc therapy are commonly used to give a conformal dose to the planning target volume while reducing the dose to normal structures such as the cochlea, brainstem, temporal lobes, hypothalamus, and hippocampus.70
Proton RT may reduce late toxicity, particularly in children, by reducing the dose to normal tissues through elimination of the exit dose and reduction in the entrance dose of radiation. Protons have been shown to result in lower doses to normal tissues (cochlea, hypothalamus, temporal lobes, hippocampus) in studies comparing proton vs photon plans.71,72 Initial studies have shown that protons have comparable local control to photons; however, further information on comparing late toxicities has not been reported.73 Nonetheless, recent series evaluating neurocognitive data in pediatric patients with localized ependymoma treated with photon RT have shown significantly improved outcomes with minimal impairment.74,75 Proton RT may also be of benefit in patients receiving craniospinal RT in reducing the dose to structures in front of the spine such as the heart, lungs, bowel, breasts, and thyroid.76
Chemotherapy
Surgery and radiation are the main therapeutic modalities in the management of ependymomas. Chemotherapy is typically left as a salvage therapy in recurrent cases but therapeutic activity using cytotoxic agents is modest at best.77 A wide variety of chemotherapy agents have been used with anecdotal and retrospective reports available for most regimens tested. With new data elucidating the molecular profile of these tumors, targeted therapies are being investigated as potential therapy. For example, the receptor tyrosine kinases ERBB2, ERBB4, and EGFR are overexpressed in ependymomas and this overexpression is associated with cellular proliferation, tumor growth, and recurrence.78 Hence the rationale in using drugs targeting tyrosine kinase receptors like lapatinib. A clinical trial in recurrent, adult ependymomas combining lapatinib and dose-intense temozolomide has been completed, and the preliminary results indicate that this regimen has clinical activity and is well tolerated.79
As described above and shown in Fig. 6, gene expression profiling has now categorized ependymomas into 9 specific subgroups. Each subgroup has its own prognosis as well as distinctive cytogenetic and epigenetic changes, elucidating new prospective therapeutic targets. At the moment the most obvious potential targets are RELA fusion, unique to supratentorial ependymomas, and YAP1, also found in supratentorial ependymomas. Additionally, a recent study through profiling of transcriptional enhancers, also identified FGFR1 and WEE1 as possible therapeutic targets in ependymoma.80 Owing to the rarity of these tumors, only significant collaborative efforts between clinicians and researchers will lead to further unravelling of the molecular profile and improved clinical outcomes for these patients.
Surveillance Imaging
Following definitive treatment of ependymoma, the NCCN recommends MRI of any site involved by tumor prior to treatment (ie, brain and/or spine, depending on presentation) every 3 to 4 months for a year, then every 4 to 6 months for a year, then every 6 to 12 months thereafter, with complete restaging of the brain and entire spine if recurrent disease is seen anywhere.46 In clinical practice, however, follow-up is variable, particularly in patients with spinal MPE in whom surgery may be curative.
Clinical Case Relevance
Because our patient had a GTR of her spinal ependymoma, it was decided to monitor her closely clinically and by serial imaging. Three years out from her surgery, she is clinically stable, and on recent MRI there is no evidence of tumor recurrence.
Treatment of Recurrent Disease
Management of patients with recurrent disease may include re-resection, often followed by re-irradiation. Re-irradiation with fractionated RT after attempted GTR is an effective treatment and can produce long-term survivors. If there is disseminated disease, then 36 Gy craniospinal RT is given with a boost to the tumor bed to 50.4-54 Gy. If the recurrence is local only then fractionated RT (50.4-54) Gy, hypofractionated RT (2.5-6 Gy/fx), or stereotactic radiosurgery (SRS) may be given to the tumor bed, with multiple series showing high rates of local control and acceptable toxicity.81,82
Conclusion
In summary, ependymomas are a heterogeneous group of neoplasms with varied clinical characteristics, pathologic appearance, survival outcomes, cytogenetic, gene expression, and epigenetic profiles. The rarity of the disease makes it difficult to perform large-scale or randomized clinical trials. This is compounded by the added complexity of the recent findings that although histologically similar, tumor biology may vary based on molecular subtype, thereby mandating either separate studies or stratification into subgroups based on their distinct genomic and epigenomic features. The variable outcomes with adjuvant RT and the meager success of cytotoxic chemotherapies point to a need to develop personalized, targeted approaches.
Funding
None.
Conflict of interest statement. None declared.
References
- 1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(Suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armstrong TS, Vera-Bolanos E, Bekele BN, Aldape K, Gilbert MR. Adult ependymal tumors: prognosis and the M.D. Anderson Cancer Center experience. Neuro Oncol. 2010;12(8):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koeller KK, Rosenblum RS, Morrison AL. Neoplasms of the spinal cord and filum terminale: radiologic-pathologic correlation. Radiographics. 2000;20(6):1721–1749. [DOI] [PubMed] [Google Scholar]
- 4. Yuh EL, Barkovich AJ, Gupta N. Imaging of ependymomas: MRI and CT. Childs Nerv Syst. 2009;25(10):1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandão LA, Young Poussaint T. Posterior fossa tumors. Neuroimaging Clin N Am. 2017;27(1):1–37. [DOI] [PubMed] [Google Scholar]
- 6. Pierce T, Kranz PG, Roth C, Leong D, Wei P, Provenzale JM. Use of apparent diffusion coefficient values for diagnosis of pediatric posterior fossa tumors. Neuroradiol J. 2014;27(2):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furie DM, Provenzale JM. Supratentorial ependymomas and subependymomas: CT and MR appearance. J Comput Assist Tomogr. 1995;19(4):518–526. [DOI] [PubMed] [Google Scholar]
- 8. Mangalore S, Aryan S, Prasad C, Santosh V. Imaging characteristics of supratentorial ependymomas: study on a large single institutional cohort with histopathological correlation. Asian J Neurosurg. 2015;10(4):276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centeno RS, Lee AA, Winter J, Barba D. Supratentorial ependymomas. Neuroimaging and clinicopathological correlation. J Neurosurg. 1986;64(2):209–215. [DOI] [PubMed] [Google Scholar]
- 10. Gilbert MR, Ruda R, Soffietti R. Ependymomas in adults. Curr Neurol Neurosci Rep. 2010;10(3):240–247. [DOI] [PubMed] [Google Scholar]
- 11. Hanbali F, Fourney DR, Marmor E, et al. Spinal cord ependymoma: radical surgical resection and outcome. Neurosurgery. 2002;51(5):1162–1172; discussion 1172–1174. [DOI] [PubMed] [Google Scholar]
- 12. Amirian ES, Armstrong TS, Aldape KD, Gilbert MR, Scheurer ME. Predictors of survival among pediatric and adult ependymoma cases: a study using Surveillance, Epidemiology, and End Results data from 1973-2007. Neuroepidemiology. 2012;39(2):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guidetti B, Mercuri S, Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981;54(3):323–330. [DOI] [PubMed] [Google Scholar]
- 14. Oh MC, Tarapore PE, Kim JM, et al. Spinal ependymomas: benefits of extent of resection for different histological grades. J Clin Neurosci. 2013;20(10):1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sweeney KJ, Reynolds M, Farrell M, Bolger C. Gross total resection rates of grade II/III intramedullary ependymomas using the surgical strategy of en-bloc resection without intra-operative neurophysiological monitoring. Br J Neurosurg. 2017;31(3):364–368. [DOI] [PubMed] [Google Scholar]
- 16. Yang SS, Seet RC, Lim EC. A pain in the neck. Diagnosis: ependymoma. J Clin Neurosci. 2009;16(5):683, 730. [PubMed] [Google Scholar]
- 17. Rashad S, Elwany A, Farhoud A. Surgery for spinal intramedullary tumors: technique, outcome and factors affecting resectability. Neurosurg Rev. 2018;41(2):503–511. [DOI] [PubMed] [Google Scholar]
- 18. Celano E, Salehani A, Malcolm JG, Reinertsen E, Hadjipanayis CG. Spinal cord ependymoma: a review of the literature and case series of ten patients. J Neurooncol. 2016;128(3):377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakamura M, Ishii K, Watanabe K, et al. Long-term surgical outcomes for myxopapillary ependymomas of the cauda equina. Spine (Phila Pa 1976). 2009;34(21):E756–E760. [DOI] [PubMed] [Google Scholar]
- 20. Halvorsen CM, Kolstad F, Hald J, et al. Long-term outcome after resection of intraspinal ependymomas: report of 86 consecutive cases. Neurosurgery. 2010;67(6):1622–1631; discussion 1631. [DOI] [PubMed] [Google Scholar]
- 21. Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery. 2005;56(5):972–981; discussion 972–981. [PubMed] [Google Scholar]
- 22. Lee SH, Chung CK, Kim CH, et al. Long-term outcomes of surgical resection with or without adjuvant radiation therapy for treatment of spinal ependymoma: a retrospective multicenter study by the Korea Spinal Oncology Research Group. Neuro Oncol. 2013;15(7):921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin Y, Smith ZA, Wong AP, Melkonian S, Harris DA, Lam S. Predictors of survival in patients with spinal ependymoma. Neurol Res. 2015;37(7):650–655. [DOI] [PubMed] [Google Scholar]
- 24. Abdulaziz M, Mallory GW, Bydon M, et al. Outcomes following myxopapillary ependymoma resection: the importance of capsule integrity. Neurosurg Focus. 2015;39(2):E8. [DOI] [PubMed] [Google Scholar]
- 25. Boström A, von Lehe M, Hartmann W, et al. Surgery for spinal cord ependymomas: outcome and prognostic factors. Neurosurgery. 2011;68(2):302–308; discussion 309. [DOI] [PubMed] [Google Scholar]
- 26. Weber DC, Wang Y, Miller R, et al. Long-term outcome of patients with spinal myxopapillary ependymoma: treatment results from the MD Anderson Cancer Center and institutions from the Rare Cancer Network. Neuro Oncol. 2015;17(4):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawabata Y, Takahashi JA, Arakawa Y, Hashimoto N. Long-term outcome in patients harboring intracranial ependymoma. J Neurosurg. 2005;103(1):31–37. [DOI] [PubMed] [Google Scholar]
- 28. Vera-Bolanos E, Aldape K, Yuan Y, et al. ; CERN Foundation Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015;17(3):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iqbal MS, Lewis J. An overview of the management of adult ependymomas with emphasis on relapsed disease. Clin Oncol (R Coll Radiol). 2013;25(12):726–733. [DOI] [PubMed] [Google Scholar]
- 30. Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. 4th revised ed Lyon, France: IARC; 2016. [Google Scholar]
- 31. Georgescu MM, Yell P, Mobley BC, et al. NHERF1/EBP50 is an organizer of polarity structures and a diagnostic marker in ependymoma. Acta Neuropathol Commun. 2015;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parker M, Mohankumar KM, Punchihewa C, et al. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature. 2014;506(7489):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pietsch T, Wohlers I, Goschzik T, et al. Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-κB signaling pathway. Acta Neuropathol. 2014;127(4):609–611. [DOI] [PubMed] [Google Scholar]
- 34. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korshunov A, Witt H, Hielscher T, et al. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol. 2010;28(19):3182–3190. [DOI] [PubMed] [Google Scholar]
- 36. Wani K, Armstrong TS, Vera-Bolanos E, et al. ; Collaborative Ependymoma Research Network A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol. 2012;123(5):727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Witt H, Mack SC, Ryzhova M, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20(2):143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoffman LM, Donson AM, Nakachi I, et al. Molecular sub-group-specific immunophenotypic changes are associated with outcome in recurrent posterior fossa ependymoma. Acta Neuropathol. 2014;127(5):731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mack SC, Witt H, Piro RM, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506(7489):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Panwalkar P, Clark J, Ramaswamy V, et al. Immunohistochemical analysis of H3K27me3 demonstrates global reduction in group-A childhood posterior fossa ependymoma and is a powerful predictor of outcome. Acta Neuropathol. 2017;134(5):705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kilday JP, Mitra B, Domerg C, et al. Copy number gain of 1q25 predicts poor progression-free survival for pediatric intracranial ependymomas and enables patient risk stratification: a prospective European clinical trial cohort analysis on behalf of the Children’s Cancer Leukaemia Group (CCLG), Societe Francaise d’Oncologie Pediatrique (SFOP), and International Society for Pediatric Oncology (SIOP). Clin Cancer Res. 2012;18(7):2001–2011. [DOI] [PubMed] [Google Scholar]
- 42. Mendrzyk F, Korshunov A, Benner A, et al. Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res. 2006;12(7 Pt 1):2070–2079. [DOI] [PubMed] [Google Scholar]
- 43. Carter M, Nicholson J, Ross F, et al. Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br J Cancer. 2002;86(6):929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yao Y, Mack SC, Taylor MD. Molecular genetics of ependymoma. Chin J Cancer. 2011;30(10):669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nabors LB, Portnow J, Ammirati M, et al. Central nervous system cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13(10):1191–1202. [DOI] [PubMed] [Google Scholar]
- 47. Lin YH, Huang CI, Wong TT, et al. Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol. 2005;71(2):205–210. [DOI] [PubMed] [Google Scholar]
- 48. Volpp PB, Han K, Kagan AR, Tome M. Outcomes in treatment for intradural spinal cord ependymomas. Int J Radiat Oncol Biol Phys. 2007;69(4):1199–1204. [DOI] [PubMed] [Google Scholar]
- 49. Figueiredo N, Brooks N, Resnick DK. Evidence-based review and guidelines for the management of myxopapillary and intramedullary ependymoma. J Neurosurg Sci. 2013;57(4):327–341. [PubMed] [Google Scholar]
- 50. Amsbaugh MJ, Grosshans DR, McAleer MF, et al. Proton therapy for spinal ependymomas: planning, acute toxicities, and preliminary outcomes. Int J Radiat Oncol Biol Phys. 2012;83(5):1419–1424. [DOI] [PubMed] [Google Scholar]
- 51. Godfraind C, Kaczmarska JM, Kocak M, et al. Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol. 2012;124(2):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mork SJ, Loken AC. Ependymoma: a follow-up study of 101 cases. Cancer. 1977;40(2):907–915. [DOI] [PubMed] [Google Scholar]
- 53. Salazar OM, Rubin P, Bassano D, Marcial VA. Improved survival of patients with intracranial ependymomas by irradiation: dose selection and field extension. Cancer. 1975;35(6):1563–1573. [DOI] [PubMed] [Google Scholar]
- 54. Salazar OM, Castro-Vita H, VanHoutte P, Rubin P, Aygun C. Improved survival in cases of intracranial ependymoma after radiation therapy. Late report and recommendations. J Neurosurg. 1983;59(4):652–659. [DOI] [PubMed] [Google Scholar]
- 55. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. [DOI] [PubMed] [Google Scholar]
- 56. Riva D, Giorgi C. The neurodevelopmental price of survival in children with malignant brain tumours. Childs Nerv Syst. 2000;16(10-11):751–754. [DOI] [PubMed] [Google Scholar]
- 57. Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paulino AC. The local field in infratentorial ependymoma: does the entire posterior fossa need to be treated?Int J Radiat Oncol Biol Phys. 2001;49(3):757–761. [DOI] [PubMed] [Google Scholar]
- 59. Koshy M, Rich S, Merchant TE, Mahmood U, Regine WF, Kwok Y. Post-operative radiation improves survival in children younger than 3 years with intracranial ependymoma. J Neurooncol. 2011;105(3):583–590. [DOI] [PubMed] [Google Scholar]
- 60. Liu AP, Shing MM, Yuen HL, et al. Timing of adjuvant radiotherapy and treatment outcome in childhood ependymoma. Pediatr Blood Cancer. 2014;61(4):606–611. [DOI] [PubMed] [Google Scholar]
- 61. Rogers L, Pueschel J, Spetzler R, et al. Is gross-total resection sufficient treatment for posterior fossa ependymomas?J Neurosurg. 2005;102(4):629–636. [DOI] [PubMed] [Google Scholar]
- 62. McGuire CS, Sainani KL, Fisher PG. Both location and age predict survival in ependymoma: a SEER study. Pediatr Blood Cancer. 2009;52(1):65–69. [DOI] [PubMed] [Google Scholar]
- 63. Nuño M, Yu JJ, Varshneya K, et al. Treatment and survival of supratentorial and posterior fossa ependymomas in adults. J Clin Neurosci. 2016;28:24–30. [DOI] [PubMed] [Google Scholar]
- 64. Ramaswamy V, Hielscher T, Mack SC, et al. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: a retrospective multicohort analysis. J Clin Oncol. 2016;34(21):2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hukin J, Epstein F, Lefton D, Allen J. Treatment of intracranial ependymoma by surgery alone. Pediatr Neurosurg. 1998;29(1):40–45. [DOI] [PubMed] [Google Scholar]
- 66. Little AS, Sheean T, Manoharan R, Darbar A, Teo C. The management of completely resected childhood intracranial ependymoma: the argument for observation only. Childs Nerv Syst. 2009;25(3):281–284. [DOI] [PubMed] [Google Scholar]
- 67. Ellison DW, Kocak M, Figarella-Branger D, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Perilongo G, Massimino M, Sotti G, et al. Analyses of prognostic factors in a retrospective review of 92 children with ependymoma: Italian Pediatric Neuro-oncology Group. Med Pediatr Oncol. 1997;29(2):79–85. [DOI] [PubMed] [Google Scholar]
- 69. Merchant TE, Fouladi M. Ependymoma: new therapeutic approaches including radiation and chemotherapy. J Neurooncol. 2005;75(3):287–299. [DOI] [PubMed] [Google Scholar]
- 70. Merchant TE. Three-dimensional conformal radiation therapy for ependymoma. Childs Nerv Syst. 2009;25(10):1261–1268. [DOI] [PubMed] [Google Scholar]
- 71. MacDonald SM, Safai S, Trofimov A, et al. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71(4):979–986. [DOI] [PubMed] [Google Scholar]
- 72. Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51(1):110–117. [DOI] [PubMed] [Google Scholar]
- 73. Sato M, Gunther JR, Mahajan A, et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer. 2017;123(13):2570–2578. [DOI] [PubMed] [Google Scholar]
- 74. Morris EB, Li C, Khan RB, et al. Evolution of neurological impairment in pediatric infratentorial ependymoma patients. J Neurooncol. 2009;94(3):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. A 5-year investigation of children’s adaptive functioning following conformal radiation therapy for localized ependymoma. Int J Radiat Oncol Biol Phys. 2012;84(1):217–223.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Howell RM, Giebeler A, Koontz-Raisig W, et al. Comparison of therapeutic dosimetric data from passively scattered proton and photon craniospinal irradiations for medulloblastoma. Radiat Oncol. 2012;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ruda R, Reifenberger G, Frappaz D, et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol. 2018;20(4):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gilbertson RJ, Bentley L, Hernan R, et al. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res. 2002;8(10):3054–3064. [PubMed] [Google Scholar]
- 79. Armstrong TS, Vera-Bolanos E, Gilbert M, et al. AT-07 A phase II study of lapatinib and dose-dense temozolomide (TMZ) for adults with recurrent ependymoma: patient reported outcomes (PRO) from a CERN clinical trial. Neuro Oncol. 2014;16(Suppl 5):v9. [Google Scholar]
- 80. Mack SC, Pajtler KW, Chavez L, et al. Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature. 2018;553(7686):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lobón MJ, Bautista F, Riet F, et al. Re-irradiation of recurrent pediatric ependymoma: modalities and outcomes: a twenty-year survey. Springerplus. 2016;5(1):879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Merchant TE, Boop FA, Kun LE, Sanford RA. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys. 2008;71(1):87–97. [DOI] [PubMed] [Google Scholar]