Abstract
Primary CNS lymphoma (PCNSL) is a rare diffuse large B-cell lymphoma originating within the central nervous system. The overall incidence of PCNSL is rising, particularly in the elderly population. Immunosuppression is a strong risk factor, but most patients with this tumor are apparently immunocompetent. Diagnosis of PCNSL can be challenging. Non-invasive or minimally invasive tests such as ophthalmological evaluation and spinal fluid analysis may be useful, but the majority of patients require tumor biopsy for definitive diagnosis. Our knowledge concerning optimum treatment of PCNSL is fragmentary due to paucity of adequately sized trials. Most patients are now initially treated with high-dose-methotrexate-based chemotherapy alone, as the addition of whole-brain radiotherapy at standard doses has not been shown to increase survival and does increase the risk of neurological toxicity. Ongoing trials are addressing issues such as the roles of reduced-dose radiotherapy, the addition of the CD20 antibody rituximab to chemotherapy, high-dose chemotherapy followed by autologous stem cell transplantation, and maintenance therapy in the primary management of PCNSL.
Keywords: high-dose methotrexate, neurotoxicity, primary CNS lymphoma, whole-brain radiotherapy
Clinical Case Presentation
A 75-year-old woman presented with subacute progressive confusion and unusual behavior. Imaging was obtained and corticosteroid therapy was initiated for symptomatic management. Figure 1 displays the initial imaging findings.
Fig. 1.
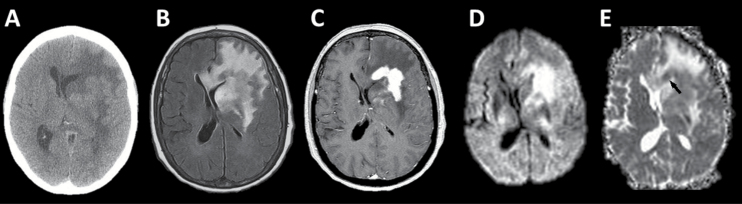
Initial CT and MR imaging. (A) Noncontrast head CT depicts a deep left-frontal mass lesion isointense to gray matter with surrounding vasogenic edema and subfalcine herniation. (B) T2-weighted fluid-attenuated inversion recovery (FLAIR) MR imaging depicts the extent of edema to better advantage. (C) Postgadolinium T1-weighted MR imaging shows intense, homogeneous enhancement of the deep left-frontal tumor. (D) Diffusion-weighted imaging (DWI) shows increased signal in this region. (E) Apparent diffusion coefficient (ADC) image demonstrates foci of reduced signal (one indicated by black arrow) within the region of increased DWI signal indicating true restricted diffusion.
Initial Supportive Care
Patients with PCNSL often demonstrate rapidly evolving symptoms, reflecting aggressive tumor behavior. Symptoms at presentation are typically nonfocal and are often cognitive and/or behavioral. Focal symptoms, seizures, and symptomatic intracranial hypertension are less common. When intraocular involvement is present (choroid, vitreous, retina, and optic nerve) patients may experience blurred vision and floaters.1
Corticosteroid Therapy
Corticosteroid therapy may rapidly ameliorate symptoms caused by PCNSL. However, prior to histopathologic diagnosis, they should be avoided because of their lymphocytotoxic effect, which may complicate neuropathologic diagnosis.2,3 At the authors’ institutions, steroids are reserved until after pathologic diagnosis confirmation when possible, then tapered and stopped as soon as clinically possible. If a significant lymphocytotoxic effect occurs prior to biopsy, it is reasonable to taper and discontinue steroids and begin serial imaging, performing biopsy at the time of tumor regrowth.4
Anticoagulation
Patients with PCNSL are at significant risk of venous thromboembolism. In a retrospective analysis of 42 patients with CNS lymphoma, 25 patients (59.5%) had venous thromboembolism, which was fatal in 3 (7%).5 The authors of this study suggested that the benefit of anticoagulant prophylaxis with low-molecular-weight heparin probably outweighs the risk in PCNSL, but this approach is not widely employed.
Clinical case relevance
The patient was treated with corticosteroid therapy prior to neurosurgical evaluation. Within a day she was essentially asymptomatic.
Initial Diagnostics
Initial Imaging
PCNSL lesions in the immunocompetent typically present as solitary, uniformly enhancing masses, although multiple lesions are seen in 20% to 40% of patients.6–9 Their most common location is in the cerebral hemispheres (particularly the frontal lobes), followed by the basal ganglia, thalami, and corpus callosum.6,8,9 Nearly all lesions abut either the ependyma or the pia,6,9,10 and periventricular white matter involvement is common.11 Leptomeningeal spread constitutes two-thirds of secondary CNS lymphoma,12,13 but is uncommon in PCNSL.8,9
Imaging characteristics of PCNSL reflect its hypercellular nature, high nuclear-to-cytoplasmic ratio, and typical disruption of the blood-brain barrier. Lesions are typically hyper- or isoattenuating on noncontrast CT,6,8 hypo- or isointense on T1-weighted MRI, and are variable on T2-weighted imaging.6–8 Calcification and gross hemorrhage within PCNSL lesions are rare prior to treatment,6–8 though pretreatment petechial hemorrhage can sometimes be detected with T2*-weighted and susceptibility weighted imaging.14,15 Nearly all PCNSL lesions enhance following intravenous gadolinium administration. Although enhancement is classically homogeneous, it can be heterogeneous.6,8–10,16 Necrosis and peripheral, ring-like enhancement are uncommon in the immunocompetent, occurring in 3% to 8% of this patient population.8,17 Intratumoral vessels or flow voids are not expected.11 Perilesional edema is variable but often moderate.6
The highly cellular nature of PCNSL contributes to the restricted diffusion of water on diffusion-weighted imaging in approximately 90% of cases.18–20 PCNSL is also typically associated with elevated lipid and choline levels on MR spectroscopy.18,21 PCNSL has only mildly increased relative cerebral blood volume on perfusion imaging and a characteristic perfusion time-intensity curve that may overshoot the baseline because of gadolinium leakage with T1 effects.19,21 Positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro- D-glucose (18F-FDG PET) readily shows the expected hypermetabolism of PCNSL before and after therapy.22–24 FDG PET can also be used to evaluate treatment response at a very early stage and to diagnose tumor recurrence.22
A wider range of imaging findings is encountered in patients with compromised immune systems. Multifocal lesions are more common in this population, as are lack of enhancement and ring enhancement.7,25,26 Hemorrhage is also more commonly seen in the immunocompromised (up to 35% of patients).26
While the previously discussed imaging findings are characteristic of PCNSL, there are no pathognomonic imaging findings for PCNSL. Intraparenchymal CNS lymphoma arising in the setting of systemic lymphoma (ie, secondary CNS lymphoma) may appear identical to PCNSL on imaging, as may other neoplastic, inflammatory, and infectious neurological conditions. Advanced imaging techniques may be helpful in some cases. Lymphoma generally has more restricted water diffusion (ie, lower apparent diffusion coefficient) than high-grade gliomas and metastases, perhaps related to its more predictably high cellularity.19,20,27,28 Intratumoral microhemorrhages visible on susceptibility weighted imaging sequences are significantly more common and extensive in glioblastoma than in PCNSL.15,29–31
In immunocompromised patients, such as those with AIDS, differentiating PCNSL from cerebral toxoplasmosis has been a notorious challenge because standard anatomic imaging findings often overlap between the 2 entities. 201Thallium SPECT shows increased thallium uptake in lymphoma but not in toxoplasmosis in lesions of sufficient size to be evaluated well with SPECT.32–34
Cerebrospinal Fluid Diagnostics
Cerebrospinal fluid (CSF) diagnostic testing is commonly performed in patients with suspected PCNSL. Though the diagnostic yield is low, CSF analysis carries little risk and may spare a patient the need for brain biopsy. CSF diagnostic testing typically includes conventional cytomorphology, flow cytometry,35 and, at some institutions, monoclonality assessment by polymerase chain reaction (PCR).36 In a large prospective study with data on meningeal involvement available for 415 patients, lymphoma was detected in the CSF by cytomorphology in 12.2%, by PCR in 10.5%, and by MRI in 4.1%. There was a significant correlation between meningeal involvement and CSF pleocytosis, but no correlation with protein elevation.37
Several additional potential diagnostic markers for PCNSL in the CSF have been described. One study found that a particular micro RNA pattern in the CSF discriminated PCNSL patients from controls with neuroinflammatory disease and other miscellaneous neurological disorders with >95% sensitivity and specificity.38 CXC chemokine ligand 13 (CXCL-13), a mediator of B-cell migration; interleukin 10 (Il-10), an anti-inflammatoty cytokine; and neopterin,39 a nonspecific marker of CNS inflammation, have been found in CSF of PCNSL patients at significantly higher concentrations than in patients with other CNS malignancies and nonmalignant CNS diseases.40,41 All of these markers require validation in independent patient populations before their use in routine practice can be recommended.
Ophthalmologic Evaluation
Ocular involvement can occur prior to brain manifestations (primary intraocular lymphoma), simultaneously with brain lesions (10% to 20% of patients), and at relapse, which might be exclusively intraocular. Many patients remain asymptomatic. In patients with ocular involvement, examination typically reveals vitreous cellular infiltration on slit lamp exam and/or subretinal tumor cell infiltrates on fundoscopy. A vitrectomy or chorioretinal biopsy is often needed for definitive diagnosis. Pathologic diagnosis is often difficult due to the predominance of inflammatory cells and relative paucity of lymphoma cells. A high ratio of interleukin 10 to interleukin 6 (>1) in the vitreous is regarded to be indicative of lymphoma.42,43 In patients with seemingly isolated vitroretinal lymphoma, potential CNS involvement should be evaluated by cranial MRI and CSF examination.44 In patients with histopathologic proof of CNS lymphoma and ocular findings highly suggestive of ocular involvement, pathologic confirmation of ocular lymphoma is generally not mandatory.
Additional Diagnostic Work-up
A thorough patient history should be obtained, with specific emphasis on immunocompromising conditions. Blood testing should include an HIV test, serum LDH as a prognostic factor,45 and testing for hepatitis, since this may be reactivated under chemotherapy or immunotherapy.
It is important to differentiate PCNSL from systemic lymphoma with CNS involvement, as the treatments are different. Physical examination of all peripheral lymph node regions and the testes should be performed. All patients without contraindications should undergo a CT of the neck, chest, abdomen, and pelvis with contrast. Additionally, according to current guidelines, bone marrow biopsy should be performed in all patients and testicular ultrasound in men.44 With conventional staging, systemic disease is found in 4% to 12% of patients.46 Not surprisingly, systemic manifestations are diagnosed more frequently when patients are screened with FDG-PET. In a study of 42 patients, suspect systemic disease was found in 8 (19%) patients (only in 2 of the patients were these findings seen on CT). In 6 patients biopsy was performed, revealing lymphoma in 3 patients.47
Clinical case relevance
Initial imaging (Figure 1) was concerning for PCNSL. The patient had no systemic symptoms, lymphadenopathy, or history of immunosuppression. Body CT was unremarkable. She had no visual symptoms, and slit-lamp examination was negative. Lumbar puncture was contraindicated by her large intracranial mass with midline shift, so she proceeded on to surgery.
Surgery
Though patients often present with a single visible lesion, PCNSL is considered a whole-brain disease.48 Thus, surgical resection has generally not been regarded as part of therapy, and has even been deemed harmful given potential neurological deficits after surgery.49 Instead, the role of surgery is typically limited to biopsy for pathologic diagnosis. However, in a secondary analysis of a randomized phase 3 trial,50 in the subset of patients with a single lesion, a significant benefit for both progression-free survival (PFS) and overall survival (OS) was found for patients with subtotal resection as compared to biopsy only.49 The authors concluded that resection can be considered for single lesions in low-risk locations, though this approach remains controversial and is not yet part of the accepted standard of care. Such an approach, if technically possible, is indicated for patients with a single large lesion and rapid progressive deterioration of neurologic status51 and in cases with the risk of imminent brain herniation.52
Clinical case relevance
By the time of surgery, the patient had experienced symptomatic relief from corticosteroid therapy, and there was not felt to be a role for surgical debulking. She underwent a stereotactic needle biopsy, which was tolerated without complication.
Pathology
PCNSL morphologically and phenotypically corresponds to diffuse large B-cell lymphoma. Rarely, low-grade B-cell lymphoma, Burkitt lymphoma, high-grade T-cell lymphoma, and NK/T-cell lymphoma may involve the CNS in isolation.53
Microscopically, PCNSL is a highly cellular neoplasm composed of large lymphoid cells. Geographic necrosis is not typical of PCNSL and suggests immunosuppression. Characteristic angiocentric arrangement of tumor cells is frequently apparent in less cellular areas. Vascular walls with lymphoid cell infiltration show a salient finding of concentric lamellae of reticulin fibers, nearly diagnostic of PCNSL.54 Isolated tumor cells diffusely infiltrate brain parenchyma and are associated with reactive astrogliosis and microglial infiltration. Neoplastic cells are larger than macrophages and possess atypical, round nuclei with vesicular chromatin and a prominent single or multiple nucleoli, resembling centroblasts or immunoblasts. Mitotic figures and apoptotic bodies are frequently seen. Immunohistochemically, neoplastic cells express B-cell markers, such as CD20, CD79a, CD19, CD22, and PXA5.55,56 Other markers frequently expressed in diffuse large B-cell lymphoma are BCL6 (60% to 80%), MUM1/IRF4 (90%), CD10 (10% to 20%), and BCL2. Cell surface immunoglobulins are IgM and IgD with light chain restriction.
Recent advances in molecular genetic analyses have improved understanding of the molecular mechanism of diffuse large B-cell lymphoma.53,57–59 It is now commonly divided into 2 primary subtypes: germinal center B-cell–like (GCB) and activated B-cell–like (ABC), also referred to as non-GCB. In contrast to diffuse large B-cell lymphoma more generally, PCNSL neoplastic B cells almost exclusively demonstrate an ABC immunophenotype and postgerminal center origin.53 Translocation of the BCL6 gene with promoter substitution, observed in about one-third of PCNSL, induces constitutive BCL6 activity, which has oncogenic properties and contributes to keeping lymphoma cells in the germinal center stage. The toll-like receptor pathway is activated by mutations of MYD88 (mostly MYD88 L265P), which has been demonstrated in one-third of PCNSLs.60–62 MYD88 plays a key role as an adaptor molecule to transducing signals from toll-like receptor. B-cell receptor signaling cascade is also altered by mutations of CD79B and CARD11.63–66 The activation of these signaling pathways, alone or in synergy, produces constitutive activation of the nuclear factor (NF)-κB pathway and then contributes to proliferation and survival of lymphoma cells.57
The term sentinel lesion is used to indicate a peculiar CNS lesion that is antecedent to the onset of PCNSL.67 Contrast-enhancing CNS lesions, clinically suspected to be PCNSL, sometimes exhibit only inflammatory changes and myelin loss mimicking primary demyelination in the biopsy specimen, and may regress spontaneously.6 Within several months to 2 years, however, malignant lymphoma will emerge, and the preceding lesion is retrospectively designated as a sentinel lesion. In recognition of this phenomenon, patients older than 50 who present with contrast-enhancing CNS lesions and undergo biopsy with results suggestive of demyelinating disease should be closely followed.67
Clinical case relevance
The first biopsy at an outside institution demonstrated a dense inflammatory infiltrate composed predominantly of small reactive T-lymphocytes (Figure 2). Apoptosis and macrophage infiltration were noted, but no large B cells. Serial imaging demonstrated significant improvement in the short period between presentation and biopsy, with ongoing improvement for months despite the discontinuation of corticosteroid therapy (Figure 3). A second stereotactic biopsy was performed shortly thereafter and was also nondiagnostic. The patient then presented to a tertiary care center for a second opinion. She was followed radiographically for nearly two years, until a new region of enhancement developed around the temporal horn of the right lateral ventricle (Figure 4). Biopsy of this lesion revealed diffuse large B-cell lymphoma (Figure 2).
Fig. 2.
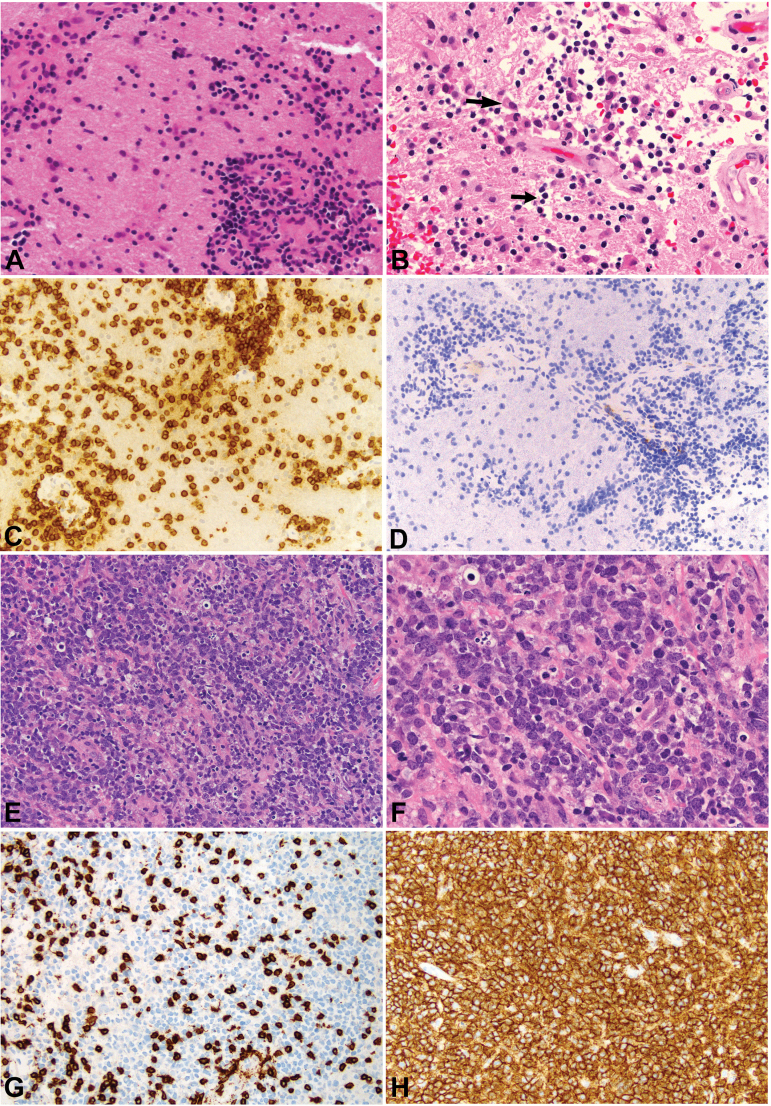
Initial biopsy after corticosteroid therapy and subsequent diagnostic biopsy at tumor recurrence. Nondiagnostic biopsy after corticosteroid therapy (A-D). White matter shows a marked lymphocytic infiltrate (A, x200) composed of small lymphocytes with dark blue nuclei, parenchymal and perivascular. Small dark apoptotic nuclei (one indicated by the short black arrow) and macrophages (one indicated by the long black arrow) are present in areas of the biopsy (B, x400). The small lymphocytes are nearly exclusively CD3-positive, consistent with reactive T-lymphocytes (C, x200). There are no CD20-positive cells in the infiltrate (D, x200). Diagnostic biopsy at recurrence (E-H). Primary CNS lymphoma cells diffusely infiltrate the parenchyma (E, x200) with monomorphous, large blastic cells largely replacing the tissue (F, x400). While a moderate number of small infiltrating CD3-positive reactive T-lymphocytes is present (G, x200), similar to the first biopsy (C), most cells express the B-cell marker CD20 (H, x200). The morphologic findings in conjunction with the immunophenotype confirm the diagnosis of diffuse large B-cell lymphoma.
Fig. 3.
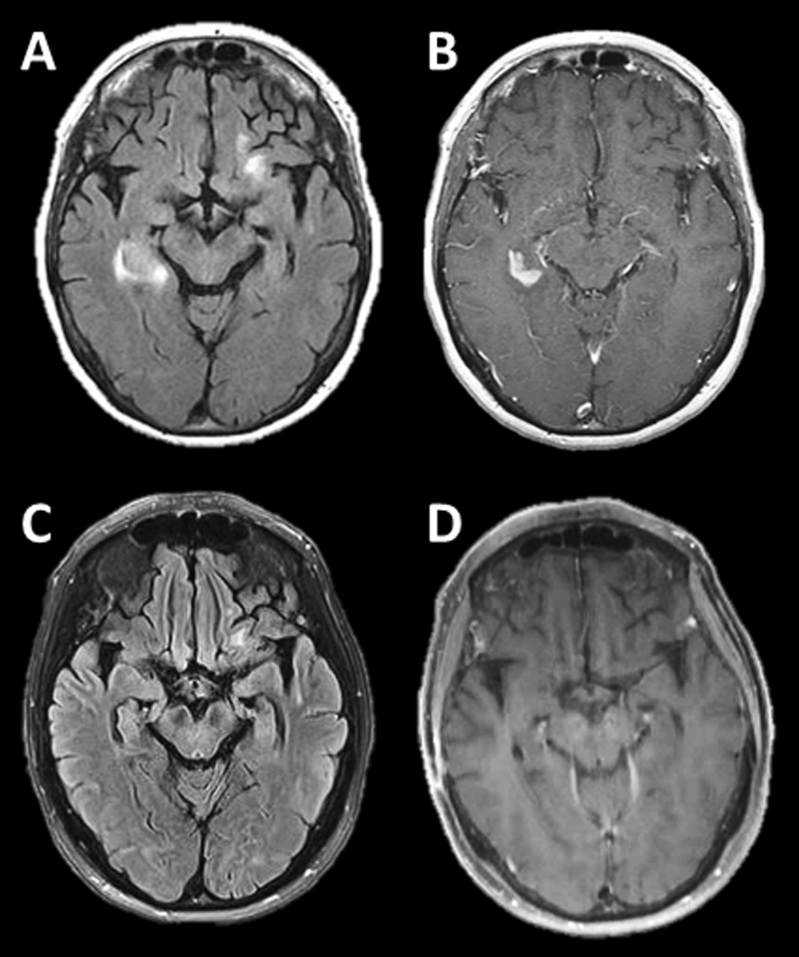
Serial MR imaging after corticosteroid therapy. Serial gadolinium-enhanced T1-weighted MR images (A) prior to corticosteroid therapy, (B) 3 days after the initiation of corticosteroids, (C) 5 days after the initiation of corticosteroids; note interval biopsy defect within the left frontal region of enhancement, (D) 3 months after biopsy, and (E) 8 months after biopsy.
Fig. 4.
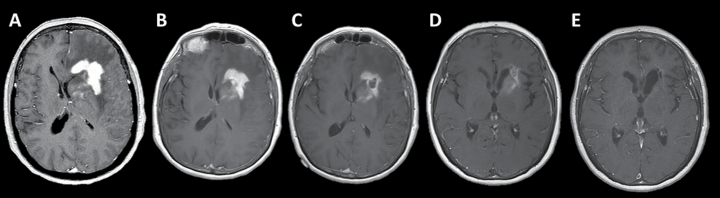
MR imaging at recurrence and after high-dose methotrexate (HDMTX)-based chemotherapy. (A) T2-weighted fluid-attenuated inversion recovery (FLAIR) and (B) T1-weighted post-gadolinium images at time of radiographic progression. (C) T2-weighted FLAIR and (D) T1-weighted post-gadolinium images following the completion of HDMTX-based chemotherapy demonstrate a complete response.
Epidemiology
PCNSL accounts for 2% to 4% of intracranial neoplasms and 4% to 6% of extranodal lymphomas.68 Median age at diagnosis is 65 years, and this tumor is extremely rare in children and adolescents.69 Immunosuppression is the only risk factor identified thus far. The incidence of PCNSL in the United States increased in the 1980s and 1990s, peaking in 1995 with 10.2 new cases per one million persons per years.70 This increase was driven largely by AIDS-related disease. More recently, the incidence of PCNSL in young patients with immunosuppression has decreased, but it is becoming more common in elderly patients without other clear risk factors.71
Standard-of-Care Treatment
As PCNSL is a diffuse brain disease, systemic chemotherapy is an integral part of effective treatment. The ability to cross the intact blood-brain barrier is a prerequisite for systemic chemotherapy to be effective in PCNSL. When a CHOP-like protocol (cyclophosphamide, doxorubicine, vincristine, and dexamethasone instead of prednisolone)—standard treatment for systemic lymphomas—was given before whole-brain radiotherapy (WBRT) in PCNSL patients, survival was comparable to WBRT alone.72
Systemic Chemotherapy
The paucity of randomized trials to support therapeutic decisions is a critical problem in the management of PCNSL. While there is no universally accepted treatment, consensus exists that high-dose methotrexate (HDMTX) is the most important drug in the treatment of PCNSL. However, there is great variability in HDMTX dose and schedule in practice. It is generally accepted that doses ≥ 3 g/m2 and infusion times of approximately 4 hours should be used to reliably achieve cytotoxic levels in the CSF.73,74
A wide spectrum of complete response rates with HDMTX monochemotherapy, ranging between 15% (despite a very high dose of 8 g/m2 and combination with rituximab) and 52%, has been reported.75,76 HDMTX-based polychemotherapy is generally regarded to be more effective than HDMTX alone. Several noncomparative trials using different HDMTX-based combinations have been published. With an intensive chemotherapy regimen, including HDMTX (5 g/m2), high-dose cytarabine (HDAraC) (3 g/m2), vincristine, alkylating agents, and dexamethasone combined with intensive intraventricular chemotherapy via Ommaya reservoir, a median event-free survival of 21 months and a median OS of 50 months was reached.77 However, this regimen has not found broad acceptance because of the risk of Ommaya reservoir infection.
In a small (n = 79), randomized, phase 2 study, HDMTX alone was compared with HDMTX in combination with HDAraC, both arms followed by WBRT.78 The complete response rate was 46% with the combination vs 18% with HDMTX alone (P = .006), and 3-year OS was 46% vs 32% (P = .07), respectively. However, this difference was achieved with undertreatment in the “standard” arm, since HDMTX alone (3.5 g/m2) was given every 3 weeks only, thus resulting in exceptionally low complete response rate. A more recent trial compared HDMTX and HDAraC (group A) with the same combination plus rituximab (group B) and plus both thiotepa and rituximab (group C, MATRix), followed by a second randomization to either WBRT or high-dose chemotherapy (carmustine and thiotepa) and autologous stem cell transplantation (HD-ASCT).79 Results of the first randomization have been reported and demonstrated a significant improvement of the complete response rate (primary endpoint) with MATRix (49%; 95% CI, 38–60%) as compared with both comparator arms. This trial, however, was not prospectively designed to test the benefit of addition of rituximab or thiotepa to HDMTX and HDAraC.
Consolidation of HDMTX-based primary chemotherapy by other non-cross-resistant chemotherapy is the current concept in PCNSL treatment. In the CALGB 50202 study, 44 patients with complete response to 4 courses of rituximab/ HDMTX/ temozolomide (R-MT) were consolidated with etoposide/HDAraC. The complete response rate to R-MT was 66%, the 2-year PFS rate was 57%, and median time to progression was 4.0 years.80 In a German study of patients <65 years of age, induction chemotherapy with rituximab/HDMTX was followed by rituximab/HDAraC/thiotepa and HD-ASCT (carmustine/thiotepa). The 3-year PFS was 63% and 3-year OS was 78%.81 In an American study, 32 patients ≤ 67 years received 5 courses of rituximab/HDMTX/procarbacine/vincristine (R-MPV), which was in responders consolidated by high-dose regimen with thiotepa/cyclophosphamide/busulfan followed by autologous stem cell transplant (ASCT). The complete response rate to R-MPV was 66% and the 3-year PFS estimate was 79%; however, there was a relatively high toxic death rate of 10%.82 The final results of the IELSG-32 study mentioned above comparing different consolidations in the second randomization are pending. In the preliminary report it is stated that the PFS at 2 years—the primary endpoint of the second randomization—of 61% was significantly better in the MATRix group, and very poor 2-year PFS of 36% and 46% in groups A and B, respectively.86 However, an intrinsic limitation of the 2-stage randomization design is the potential for interaction between the randomization arm for induction chemotherapy and the randomization arm for consolidation.
Since there is no proof of better efficacy of higher HDMTX doses, it is our practice to treat newly diagnosed PCNSL patients with creatinine clearance of ≥50 ml/min with a minimum of 4 cycles of HDMTX at a dose of 3-4 g/m2 as a 4-hour infusion and to combine it with an alkylating drug (eg, ifosfamide, temozolomide) or HDAraC. At many centers, chemotherapy is combined with the CD20 antibody rituximab, as will be discussed below.
Role of Rituximab
As a large protein, penetration of the CD20 antibody rituximab into the CNS is poor, with less than 1% of systemic concentration reaching the leptomeningeal compartment,83 which provides an indirect measure of intraparenchymal concentration. Adding rituximab to HDMTX-based chemotherapy has proved feasible and active in small studies.84–86 Retrospective analyses of the role or addition of rituximab to HDMTX yielded conflicting results.87–89
Recently, the first study evaluating the role of rituximab in a randomized fashion was published (IELSG-32). The complete response rate was 23% for HDMTX+HDAraC (group A), 30% when rituximab was added (group B), and 49% when both rituximab and thiotepa were added (group C). Multivariate logistic regression analysis including treatment group and the IELSG risk score revealed a significant response advantage for group C vs A and B but no significant difference between A and B.87
Role of Radiotherapy
Although never proven in a randomized trial, it is concluded from a retrospective analysis,90 and generally accepted, that radiotherapy alone is inferior to its combination with HDMTX-based chemotherapy. With WBRT alone, a high response rate, but usually no significant long-term control, can be achieved.
The role of WBRT given in addition to HDMTX-based primary chemotherapy has been defined by the G-PCNSL-SG1 trial. Patients randomized to radiation received HDMTX-based chemotherapy followed by immediate WBRT. Patients randomized to chemotherapy alone received no further therapy if they had achieved complete response, and second-line chemotherapy with HDAraC if they had not. No significant difference in OS was found, with a median OS in the intent-to-treat population (n = 411) of 32.1 months with WBRT and 34.4 months without WBRT. Patients treated with WBRT had prolonged PFS: 15.5 vs 9.9 months (P = .04).50 These results were confirmed in a long-term analysis of this study with a median follow-up of 82 months.91 This trial has been a source of controversy, with some experts pointing out that the unmet primary endpoint for noninferiority and the high rate of protocol violations prevent definitive conclusions, and others noting that the results contribute to the accumulating scientific literature,90,92 which suggests that omission of WBRT from first-line treatment does not compromise OS and may prevent neurological toxicity.
The role of focal radiotherapy and reduced-dose WBRT, with the aim to reduce neurotoxicity, is unclear due to the lack of comparative trials. A small prospective study of 52 patients suggested that consolidating dose-reduced WBRT (23.4 Gy) given to patients with complete response after HDMTX-based chemotherapy (R-MPV) and before HDAraC was associated with good tumor control: the 2-year PFS of the 31 patients with complete response after R-MPV was 77% and median PFS was 7.7 years.85,86
In general, WBRT is not routinely utilized for primary treatment of PCNSL and is currently offered primarily to patients for whom systemic chemotherapy is contraindicated.
Side Effect Management
Due to a relatively high risk of complications, HDMTX therapy should be administered in experienced centers. The presence of more than 500 ml of “third space” fluid such as pleural effusion or ascites may lead to a prolonged effective MTX half-life, and represents a relative contraindication to HDMTX.
HDMTX therapy would be unacceptably toxic without the use of folinic acid (leucovorin), often dosed at 30 mg every 6 hours starting 24 hours after the initiation of HDMTX. Monitoring of MTX elimination is mandatory to ensure normal clearance. In case of disturbed MTX elimination, an intensified leucovorin rescue is necessary. In severe cases, application of carboxypeptidase-G2—an enzyme rapidly cleaving MTX—should be considered since hemodialysis has only modest efficacy in removing MTX.
Renal insufficiency is a serious acute side effect of HDMTX therapy. At many centers, MTX dose is adapted to the creatinine clearance.93 To reduce the risk of renal insufficiency, all potentially nephrotoxic agents should be stopped, and intensive intravenous hydration and urine alkalization (goal urine pH ≥ 7) is necessary. Acute HDMTX-induced lung injury (pneumonitis) with fever, dry cough, and dyspnea is relatively rare and usually managed with steroids. The differentiation from pneumonia caused by infectious agents can be difficult. Nausea and vomiting are only rarely seen with antiemetic prophylaxis based on 5-HT3-antagonists. Stomatitis is most frequent with 24-hour infusions and rarely seen with a 4-hour infusion. Acute CNS toxicity with somnolence, confusion, fatigue, disorientation, and seizures may occur during or within hours after HDMTX infusion. Subacute neurotoxicity may occur within days to weeks and is characterized by focal symptoms, seizures, and somnolence. Both are transient and reversible without special treatment in most cases.94
Delayed treatment-related neurotoxicity represents a major long-term toxicity in patients with PCNSL, particularly after combination of HDMTX-based chemotherapy with WBRT.95,96 The risk of neurotoxicity clearly increases with age.95 It is appreciated that WBRT rather than chemotherapy is the primary mediator of neurotoxicity in PCNSL.96–98 In fact, in a retrospective analysis of 185 patients, WBRT was the only factor associated with late neurotoxicity (evaluated by clinical examination only) in the multivariate setting.99 In a recent series, PCNSL patients treated with WBRT had significant impairments across most cognitive domains, interfering with quality of life, while those treated with chemotherapy alone had significantly higher scores in neuropsychometric testing.100
Treatment of Elderly Patients
Older age is a major negative prognostic factor in PCNSL, being associated both with reduced survival and an increased risk of treatment-relate toxicities. 45,50,101,102
Due to very high risk of delayed neurotoxicity, reaching 100% after 4 years in patients 60 years and older,95 WBRT has largely been abandoned as a primary therapy in this population. Several trials designed for elderly patients using HDMTX-based chemotherapy alone have been published.103–107 These trials have demonstrated response rates of approximately 50% and median PFS of 5 to 10 months, but with substantial acute toxicity and high rate of treatment discontinuation in some trials. In a recent randomized, phase 2 study of patients >60 years, the MPV-A (HDMTX, procarbazine, vincristine, HDAraC) regimen was compared to MT (HDMTX with temozolomide). The complete response rate was 62% and 45%, respectively; the objective response rate was 82% vs 71%; and median OS was 31 vs 14 months, with comparable levels of acute toxicity.108 While all of these efficacy endpoints favor the MPV-A regimen, the trial was not powered for a direct comparison and the differences did not reach statistical significance. In another recent phase 2 study, all 66 patients were treated with a dose-adjusted HDMTX-baseed and HDAraC-based protocol combined with liposomal AraC intrathecally; those between 66 and 75 years (n = 27) additionally received a maintenance therapy with temozolomide for 1 year or until progression.109 The outcome of elderly patients was comparable with that of the younger patients, probably reflecting the benefit of maintenance therapy.
In summary, HDMTX is feasible in elderly PCNSL patients without serious comorbidities and most likely produces a better outcome than WBRT alone, which should be reserved for patients not able to receive chemotherapy. To minimize the risk of late neurotoxicity, use of WBRT after HDMTX should be avoided.
Primary Intraocular Lymphoma
The median survival of patients with isolated primary intraocular lymphoma and no CNS involvement is approximately 60 months.110 Local eye-dedicated therapy such as ocular radiation or intravitreal chemotherapy with MTX or immunotherapy with rituximab can be given to patients with primary intraocular lymphoma. These approaches have not been directly compared, but do not seem to differ substantially with respect to local tumor control or treatment-related visual compromise.111
Since CNS relapse is universal in patients with primary intraocular lymphoma who undergo local treatment alone, chemotherapy regimens analogous to those used in PCNSL are employed for isolated primary intraocular lymphoma at some institutions, while others reserve systemic chemotherapy until CNS relapse.110 The efficacy of systemic chemotherapy depends on ocular penetration. In one study of patients treated with HDMTX (8 g/m2), anterior chamber concentrations of <10% and vitreous concentrations <1% of the corresponding plasma level were found, nonetheless achieving micromolar concentrations and producing responses in 7 of 9 patients.112 With ifosfamide therapy, anterior chamber concentrations are approximately 19% to 54% of the corresponding plasma concentration.113 In a small prospective study of ifosfamide or its oral analogue as monotherapy for primary intraocular lymphoma, all 10 patients responded with a median PFS of 18 months and median OS of 32 months.114
A systemic HDMTX-based chemotherapy, as used in PCNSL, is the treatment of choice in patients with simultaneous ocular and CNS involvement. The role of additional local ocular therapy in this setting remains unclear. In a retrospective analysis of 221 patients with concomitant PCNSL and intraocular lymphoma, the addition of local ocular therapy in addition to PCNSL treatment was found to improve disease control but not survival.43
Treatment of Immunosuppressed Patients
Patients with chronic drug-induced immunosuppression for treatment of autoimmune disorders or following organ transplantation are at risk for the development of PCNSL. The post-transplantation lymphoproliferative disorders are B-cell lymphomas developed on the basis of proliferation stimulated by Epstein-Barr virus infection. Immunosuppressive agents associated with this condition include cyclosporine, tacrolimus, and mycophenolate mofetil.
In patients on long-term immunosuppressive drugs diagnosed with PCNSL, immunosuppression is reduced when possible. In HIV-positive patients with PCNSL, immunocompetence can be achieved with antiretroviral therapy, which has been shown to prolong survival.115 In the largest retrospective analysis of 84 patients with post-transplant lymphoproliferative disorder (PTLD)–PCNSL, immunosuppressive medications were reduced in 93% of patients. Additional primary therapies in this patient group included HDMTX in 48%, HDAraC in 33%, WBRT in 24%, and/or rituximab in 44%. The objective response rate was 60%, but with a treatment-related mortality of 13%; 3-year PFS was 32%; and 3-year OS was 43%.116 These findings suggest that patients with immunosuppression-caused PCNSL can be treated similarly to immunocompetent patients, but with higher risk of serious infectious complications.
Important Ongoing Clinical Trials
Though the number of completed prospective trials for PCNSL remains small, numerous planned and ongoing trials promise to provide new insight into therapy. Some of these trials seek to optimize the use of agents already employed in the treatment of PCNSL. Others expand upon recent progress in systemic lymphoma and focus on treatments suggested to be promising in the ABC subtype of diffuse large B-cell lymphoma,117 which represents the vast majority of PCNSL.64
The phase 3 HOVON 105 PCNSL/ALLG NHL 24 collaborative study (EudraCT # 2009-014722-42) will enroll 200 patients randomized to the combination of HDMTX (3 g/m2), teniposide, carmustine, and prednisone, with or without rituximab. Responders will also receive HDAraC consolidation, and patients<60 years who maintain their response status after cytarabine will then receive WBRT. Two studies, one phase 3 (NCT02531841) and one randomized phase 2 (NCT01511562), will evaluate the roll of consolidation therapy with HD-ASCT by randomizing patients to HD-ASCT or consolidating conventional chemotherapy following the completion of induction chemotherapy. An additional trial aimed at maximizing the efficacy of HDMTX therapy while limiting toxicity is the randomized phase 2 trial of reduced-dose WBRT after initial therapy composed of rituximab, HDMTX, vincristine, procarbazine, and HDAraC (NCT01399372). Obinotuzumab, a novel glycoengineered type-II CD20 monoclonal antibody that proved superior to rituximab in inducing cell death is being tested in a randomized phase 2 study as maintenance therapy in patients who previously achieved complete response with primary therapy (NCT02498951). As previously discussed, the poor blood-brain barrier penetration of most chemotherapy regimens represents a major obstacle to treatment. Though this has typically been overcome by using drugs that can cross the blood-brain barrier, therapy to disrupt the blood-brain barrier combined with rituximab, carboplatin, and MTX is being tested in an ongoing phase 1/2 study (NCT00293475).
Several ongoing trials are evaluating targeted therapy. Dysregulation of various cellular pathways (such as NF- κB, toll-like receptor, or B-cell receptor signaling pathways) has been reported in PCNSL, and these pathways constitute potential therapeutic targets. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, is currently being evaluated in combination with chemotherapy in newly diagnosed PCNSL patients (NCT02203526) and in 2 studies with relapsed/refractory patients (NCT02315326, NCT02542514). A novel dual PI3K/mTOR inhibitor, PQR309, and buparlisib (BKM120), a PIK3-inhibitor, are being tested in relapsed or refractory patients (NCT02669511 and NCT02301364). Immunomodulation is another promising therapeutic strategy in PCNSL. The thalidomide derivative pomalidomide is currently being evaluated in intraocular lymphoma and relapsed or refractory PCNSL (NCT01722305) and lenalidomide is being tested in the same indication in combination with rituximab (NCT01542918). The checkpoint blocker pembrolizumab is being evaluated in relapsed PCNSL (NCT02779101).
Salvage Therapy
PCNSL shows a continuing tendency to recur with longer follow-up even after intensive initial therapy.91,118,119 Patients generally benefit from salvage therapy after failure of primary treatment if not in a severely compromised condition.120 Choice of salvage treatment should be driven by a patient’s age, performance status, prior therapy, and duration of previous response.
WBRT is a very effective salvage treatment, with response rates of 60% to 79% among refractory and relapsed patients, and median OS of 10.9 to 16 months after progression or relapse.121,122 However, WBRT exposes patients to higher risk of late neurotoxicity than second-line chemotherapy. Thus, salvage chemotherapy is generally preferred, particularly in patients with good performance status and response to previous chemotherapy.
Numerous chemotherapy and immunotherapy approaches to recurrent PNCSL have been evaluated, utilizing agents such as topotecan,123,124 rituximab,83 temozolomide with or without concurrent rituximab,125–127 bendamustine,128 HDAraC,129 ifosfamide-based and/or etoposide-based polychemotherapy,81,130,131 and yttrium-90–labeled ibritumomab tiuxetan.132 The median PFS figures were uniformly short at 2 to 5 months. Lenalidomide, an immunomodulatory agent, most probably acting by modifying the tumor microenvironment and activating cytotoxic T-cells and natural killer cells, induced a complete response in 2 of 6 patients with relapsed PCNSL.133 A phase 2 study with the mTOR inhibitor temsirolimus is the first completed prospective trial of a targeted agent in PCNSL.134 Complete response was seen in 5 of the 37 patients (13.5%), “complete response unconfirmed” in 3 (8%), and partial response in 12 (32.4%), for an overall response rate (primary endpoint) of 54%. However, median PFS was only 2.6 months. Rechallenge with HDMTX is a reasonable option for patients who experienced a long-term remission after primary HDMTX therapy.135,136
For younger patients who are able to tolerate intensive therapy, HD-ASCT can be considered. In a prospective trial, 43 patients with a median age of 52 years received HDAraC and etoposide (CYVE) followed by thiotepa, busulfan, and cyclophosphamide and ASCT. The median PFS was 11.6 months and the 2-year OS 45%; however, 3 patients died on CYVE and 5 patients (all <60 years) developed clinically manifest late neurotoxicity.137,138
Relapse or progression outside the CNS is very rare in PCNSL. Patients with isolated extra-CNS relapses are usually managed with anthracycline-based chemotherapy with or without HDC-ASCT.4
Clinical case relevance
First-line treatment with HDMTX, temozolomide, and rituximab was initiated. Her first post-treatment MRI demonstrated excellent response to therapy (Figure 4).
Follow-up
The International Primary CNS Lymphoma Collaborative Group recommends that patients in clinical studies are reassessed every 3 months in the first 2 years after therapy completion, then every 6 months for 3 years, and then annually for at least 5 years for a total of 10 years of follow-up.44 This schedule is also reasonable for patients not treated on clinical studies. However, very late relapses after more than 10 years have been reported in patients with PCNSL.
Routine follow-up reassessment should include history, physical examination, and gadolinium-enhanced MRI. Patients with initial CSF or eye involvement should undergo a re-evaluation of these compartments. Additional examinations should be performed at clinical suspicion; a routine search for systemic manifestations is not recommended since the vast majority of relapses are localized in the CNS. For remission status estimation, IPCG criteria44 rather than Macdonald criteria should be used. These criteria include a response category of “complete remission unconfirmed,” which allows for the presence of a small amount of contrast enhancement at the site of the initial tumor or surgery.
At relapse, pathologic confirmation of brain lesions with a typical radiomorphology is usually not performed. In rare cases, contrast enhancement may be absent, complicating the distinction between recurrent tumor and small infarctions or toxic leukoencephalopathy.139 In these patients, a brain biopsy should be considered for definitive diagnosis.
Ideally, monitoring of late neurotoxicity should be performed. At many institutions the Mini-Mental State Examination is being used due to its simplicity and practicability. However, because its sensitivity is low, detailed psychometric testing is preferred, at least in clinical trials.4
Prognosis and Survivorship
Overall Survival
The prognosis of PCNSL remains poor, despite recent advances. In the G-PCNSL-SG1 study, the median OS was 35.3 months in the per-protocol population (n = 311), but only 21.5 months for the 526 patients who started treatment.50 Moreover, while current therapeutic approaches have reduced neurotoxicity by deferring WBRT, it is not clear that survival has improved.140
Older age and reduced performance status are uniformly accepted as negative prognostic factors in PCNSL, as well as in many other tumor types. The Memorial Sloan Kettering Cancer Center prognosis score based on these 2 factors divides patients into 3 risk classes with different outcomes: class 1 (age <50 years, median OS 8.5 years, median event-free survival 2 years), class 2 (age ≥50 and KPS ≥70, median OS 3.2 years, median event-free survival 1.8 years), and class 3 (age ≥50 and KPS <70, median OS 1.1 years, median event-free survival 0.6 months).101 The International Extranodal Lymphoma Study Group (IELSG) identified 5 variables independently associated with worse survival: age more than 60 years, ECOG performance status more than 1, elevated LDH serum level, high CSF protein concentration, and involvement of deep regions of the brain (periventricular regions, basal ganglia, brainstem, and/or cerebellum). These 5 variables were used to design a prognostic score: the 2-year OS was 80%, 48%, and 15% (P = .00001) for patients with 0 to 1, 2 to 3, and 4 to 5 unfavorable features, respectively.45
The prognostic impact of biomarkers in PCNSL remains to be fully defined. Outcome prediction based upon the Hans algorithm as reported for systemic diffuse large B-cell lymphoma141 was not reproducible in a post hoc analysis of 119 patients from the G-PCNSL-SG1.142 The prognostic determination based upon individual markers from this algorithm in PCNSL is either indeterminate or of variable significance; eg, BCL6 was associated with favorable prognosis in some studies143,144 but with inferior prognosis in others.80,142,145 MYC protein expression detected by immunohistochemistry, found in 43% of 42 patients with CNS diffuse large B-cell lymphoma, identified a patient subset with worse prognosis.146 The results of all these analyses, however, have to be interpreted with caution since the vast majority of them were retrospective in nature, evaluated small numbers of patients, and involved different therapeutic regimens.
Conclusion
Despite substantial progress in management, cure is still questionable in PCNSL patients and exceptional in the elderly. Multidisciplinary care including neuroradiologists, neuropathologists, neurosurgeons, neurologists, medical oncologists, radiation oncologists, and psychologists is necessary for optimal patient management.4 Risk-tailored treatment choice, adequate monitoring of response and side effects, and careful follow-up in experienced centers substantially improve outcome. Initial treatment is usually based on HDMTX without WBRT, and WBRT should be deferred as long as possible to minimize the risk of delayed neurotoxicity.
Ongoing clinical trials will hopefully elucidate optimal induction and consolidation chemotherapy as well as the role of new agents. Incorporation of neuropsychologic testing into studies is to be mandated. Better understanding of tumor pathogenesis is probably essential to establish optimal therapy of this disease.
Funding
None.
Conflict of interest statement. No conflicts of interest are reported for any author.
References
- 1. Korfel A, Schlegel U. Diagnosis and treatment of primary CNS lymphoma. Nat Rev Neurol. 2013;9(6):317–327. [DOI] [PubMed] [Google Scholar]
- 2. Porter AB, Giannini C, Kaufmann T, et al. Primary central nervous system lymphoma can be histologically diagnosed after previous corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma. Ann Neurol. 2008;63(5):662–667. [DOI] [PubMed] [Google Scholar]
- 3. Haldorsen IS, Espeland A, Larsen JL, et al. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol. 2005;44(7):728–734. [DOI] [PubMed] [Google Scholar]
- 4. Hoang-Xuan K, Bessell E, Bromberg J, et al. ; European Association for Neuro-Oncology Task Force on Primary CNS Lymphoma. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015;16(7):e322–e332. [DOI] [PubMed] [Google Scholar]
- 5. Goldschmidt N, Linetsky E, Shalom E, et al. High incidence of thromboembolism in patients with central nervous system lymphoma. Cancer. 2003;98(6):1239–1242. [DOI] [PubMed] [Google Scholar]
- 6. Coulon A, Lafitte F, Hoang-Xuan K, et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol. 2002;12(2):329–340. [DOI] [PubMed] [Google Scholar]
- 7. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32(6):984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haldorsen IS, Krakenes J, Krossnes BK, et al. CT and MR imaging features of primary central nervous system lymphoma in Norway, 1989–2003. AJNR Am J Neuroradiol. 2009;30(4):744–751. doi:10.3174/ajnr.A1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Küker W, Nägele T, Korfel A, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72(2):169–177. [DOI] [PubMed] [Google Scholar]
- 10. Yap KK, Sutherland T, Liew E, et al. Magnetic resonance features of primary central nervous system lymphoma in the immunocompetent patient: a pictorial essay. J Med Imaging Radiat Oncol. 2012;56(2):179–186. [DOI] [PubMed] [Google Scholar]
- 11. Keraliya AR, Krajewski KM, Giardino AA, et al. Imaging of nervous system involvement in hematologic malignancies: what radiologists need to know. AJR Am J Roentgenol. 2015;205(3):604–617. [DOI] [PubMed] [Google Scholar]
- 12. Senocak E, Oguz KK, Ozgen B, et al. Parenchymal lymphoma of the brain on initial MR imaging: a comparative study between primary and secondary brain lymphoma. Eur J Radiol. 2011;79(2):288–294. [DOI] [PubMed] [Google Scholar]
- 13. Hill QA, Owen RG. CNS prophylaxis in lymphoma: who to target and what therapy to use. Blood Rev. 2006;20(6):319–332. [DOI] [PubMed] [Google Scholar]
- 14. Kickingereder P, Wiestler B, Sahm F, et al. Primary central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion-, perfusion-, and susceptibility-weighted MR imaging. Radiology. 2014;272(3):843–850. [DOI] [PubMed] [Google Scholar]
- 15. Radbruch A, Wiestler B, Kramp L, et al. Differentiation of glioblastoma and primary CNS lymphomas using susceptibility weighted imaging. Eur J Radiol. 2013;82(3):552–556. [DOI] [PubMed] [Google Scholar]
- 16. Tang YZ, Booth TC, Bhogal P, et al. Imaging of primary central nervous system lymphoma. Clin Radiol. 2011;66(8):768–777. [DOI] [PubMed] [Google Scholar]
- 17. Johnson BA, Fram EK, Johnson PC, et al. The variable MR appearance of primary lymphoma of the central nervous system: comparison with histopathologic features. AJNR Am J Neuroradiol. 1997;18(3):563–572. [PMC free article] [PubMed] [Google Scholar]
- 18. Zacharia TT, Law M, Naidich TP, et al. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18(4):411–417. [DOI] [PubMed] [Google Scholar]
- 19. Calli C, Kitis O, Yunten N, et al. Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur J Radiol. 2006;58(3):394–403. [DOI] [PubMed] [Google Scholar]
- 20. Toh CH, Castillo M, Wong AM, et al. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29(3):471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harting I, Hartmann M, Jost G, et al. Differentiating primary central nervous system lymphoma from glioma in humans using localised proton magnetic resonance spectroscopy. Neurosci Lett. 2003;342(3):163–166. [DOI] [PubMed] [Google Scholar]
- 22. Kawai N, Miyake K, Yamamoto Y, et al. 18F-FDG PET in the diagnosis and treatment of primary central nervous system lymphoma. Biomed Res Int. 2013;2013:247152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palmedo H, Urbach H, Bender H, et al. FDG-PET in immunocompetent patients with primary central nervous system lymphoma: correlation with MRI and clinical follow-up. Eur J Nucl Med Mol Imaging. 2006;33(2):164–168. [DOI] [PubMed] [Google Scholar]
- 24. Ogawa T, Kanno I, Hatazawa J, et al. Methionine PET for follow-up of radiation therapy of primary lymphoma of the brain. Radiographics. 1994;14(1):101–110. [DOI] [PubMed] [Google Scholar]
- 25. Haldorsen IS, Krakenes J, Goplen AK, et al. AIDS-related primary central nervous system lymphoma: a Norwegian national survey 1989–2003. BMC Cancer. 2008;8:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thurnher MM, Rieger A, Kleibl-Popov C, et al. Primary central nervous system lymphoma in AIDS: a wider spectrum of CT and MRI findings. Neuroradiology. 2001;43(1):29–35. [DOI] [PubMed] [Google Scholar]
- 27. Doskaliyev A, Yamasaki F, Ohtaki M, et al. Lymphomas and glioblastomas: differences in the apparent diffusion coefficient evaluated with high b-value diffusion-weighted magnetic resonance imaging at 3T. Eur J Radiol. 2012;81(2):339–344. [DOI] [PubMed] [Google Scholar]
- 28. Horger M, Fenchel M, Nägele T, et al. Water diffusivity: comparison of primary CNS lymphoma and astrocytic tumor infiltrating the corpus callosum. AJR Am J Roentgenol. 2009;193(5):1384–1387. [DOI] [PubMed] [Google Scholar]
- 29. Kim HS, Jahng GH, Ryu CW, et al. Added value and diagnostic performance of intratumoral susceptibility signals in the differential diagnosis of solitary enhancing brain lesions: preliminary study. AJNR Am J Neuroradiol. 2009;30(8):1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Furtner J, Schöpf V, Preusser M, et al. Non-invasive assessment of intratumoral vascularity using arterial spin labeling: a comparison to susceptibility-weighted imaging for the differentiation of primary cerebral lymphoma and glioblastoma. Eur J Radiol. 2014;83(5):806–810. [DOI] [PubMed] [Google Scholar]
- 31. Peters S, Knöß N, Wodarg F, et al. Glioblastomas vs. lymphomas: more diagnostic certainty by using susceptibility-weighted imaging (SWI). Rofo. 2012;184(8):713–718. [DOI] [PubMed] [Google Scholar]
- 32. Ruiz A, Ganz WI, Post MJ, et al. Use of thallium-201 brain SPECT to differentiate cerebral lymphoma from toxoplasma encephalitis in AIDS patients. AJNR Am J Neuroradiol. 1994;15(10):1885–1894. [PMC free article] [PubMed] [Google Scholar]
- 33. Pomper MG, Constantinides CD, Barker PB, et al. Quantitative MR spectroscopic imaging of brain lesions in patients with AIDS: correlation with [11C-methyl]thymidine PET and thallium-201 SPECT. Acad Radiol. 2002;9(4):398–409. [DOI] [PubMed] [Google Scholar]
- 34. Young RJ, Ghesani MV, Kagetsu NJ, et al. Lesion size determines accuracy of thallium-201 brain single-photon emission tomography in differentiating between intracranial malignancy and infection in AIDS patients. AJNR Am J Neuroradiol. 2005;26(8):1973–1979. [PMC free article] [PubMed] [Google Scholar]
- 35. Schroers R, Baraniskin A, Heute C, et al. Diagnosis of leptomeningeal disease in diffuse large B-cell lymphomas of the central nervous system by flow cytometry and cytopathology. Eur J Haematol. 2010;85(6):520–528. [DOI] [PubMed] [Google Scholar]
- 36. Gleissner B, Siehl J, Korfel A, et al. CSF evaluation in primary CNS lymphoma patients by PCR of the CDR III IgH genes. Neurology. 2002;58(3):390–396. [DOI] [PubMed] [Google Scholar]
- 37. Korfel A, Weller M, Martus P, et al. Prognostic impact of meningeal dissemination in primary CNS lymphoma (PCNSL): experience from the G-PCNSL-SG1 trial. Ann Oncol. 2012;23(9):2374–2380. [DOI] [PubMed] [Google Scholar]
- 38. Baraniskin A, Kuhnhenn J, Schlegel U, et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117(11):3140–3146. [DOI] [PubMed] [Google Scholar]
- 39. Viaccoz A, Ducray F, Tholance Y, et al. CSF neopterin level as a diagnostic marker in primary central nervous system lymphoma. Neuro Oncol. 2015;17(11):1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fischer L, Korfel A, Pfeiffer S, et al. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res. 2009;15(19):5968–5973. [DOI] [PubMed] [Google Scholar]
- 41. Rubenstein JL, Wong VS, Kadoch C, et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood. 2013;121(23):4740–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chan CC, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16(11):1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grimm SA, McCannel CA, Omuro AM, et al. Primary CNS lymphoma with intraocular involvement: International PCNSL Collaborative Group Report. Neurology. 2008;71(17):1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abrey LE, Batchelor TT, Ferreri AJ, et al. ; International Primary CNS Lymphoma Collaborative Group. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. [DOI] [PubMed] [Google Scholar]
- 45. Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the international extranodal lymphoma study group experience. J Clin Oncol. 2003;21(2):266–272. [DOI] [PubMed] [Google Scholar]
- 46. Schiff D, Suman VJ, Yang P, et al. Risk factors for primary central nervous system lymphoma: a case-control study. Cancer. 1998;82(5):975–982. [PubMed] [Google Scholar]
- 47. Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol. 2008;10(2):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology. 2002;59(10):1557–1562. [DOI] [PubMed] [Google Scholar]
- 49. Weller M, Martus P, Roth P, et al. ; German PCNSL Study Group. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol. 2012;14(12):1481–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–1047. [DOI] [PubMed] [Google Scholar]
- 51. Bellinzona M, Roser F, Ostertag H, et al. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. Eur J Surg Oncol. 2005;31(1):100–105. [DOI] [PubMed] [Google Scholar]
- 52. Elder JB, Chen TC. Surgical interventions for primary central nervous system lymphoma. Neurosurg Focus. 2006;21(5):E13. [DOI] [PubMed] [Google Scholar]
- 53. Deckert M, Montesinos-Rongen M, Brunn A, et al. Systems biology of primary CNS lymphoma: from genetic aberrations to modeling in mice. Acta Neuropathol. 2014;127(2):175–188. [DOI] [PubMed] [Google Scholar]
- 54. Burger PC, Scheithauer BW. Tumors of the central nervous system. AFIP Atlas of Tumor Pathology, Series 4. Fascicle 7. Washington DC: American Registry of Pathology; 2007. [Google Scholar]
- 55. Kluin PM, Deckert M, Ferry JA. Primary diffuse large B-cell lymphoma of the CNS. In: Swerdlow SH, Campo E, Harris NLet al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008:240–241. [Google Scholar]
- 56. Deckert M, Paulus W. Malignant lymphomas. In: Louis DN, Ohgaki H, Wiestler OD, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC; 2007:188–192. [Google Scholar]
- 57. Akhter A, Masir N, Elyamany G, et al. Differential expression of Toll-like receptor (TLR) and B cell receptor (BCR) signaling molecules in primary diffuse large B-cell lymphoma of the central nervous system. J Neurooncol. 2015;121(2):289–296. [DOI] [PubMed] [Google Scholar]
- 58. Kraan W, van Keimpema M, Horlings HM, et al. High prevalence of oncogenic MYD88 and CD79B mutations in primary testicular diffuse large B-cell lymphoma. Leukemia. 2014;28(3):719–720. [DOI] [PubMed] [Google Scholar]
- 59. Wang JQ, Jeelall YS, Horikawa K. Emerging targets in human lymphoma: targeting the MYD88 mutation. Blood Lymphat Cancer. 2013;3:53–61. [Google Scholar]
- 60. Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319(5870):1676–1679. [DOI] [PubMed] [Google Scholar]
- 62. Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Braggio E, Van Wier S, Ojha J, et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res. 2015;21(17):3986–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bruno A, Boisselier B, Labreche K, et al. Mutational analysis of primary central nervous system lymphoma. Oncotarget. 2014;5(13):5065–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gonzalez-Aguilar A, Idbaih A, Boisselier B, et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res. 2012;18(19):5203–5211. [DOI] [PubMed] [Google Scholar]
- 66. Vater I, Montesinos-Rongen M, Schlesner M, et al. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia. 2015;29(3):677–685. [DOI] [PubMed] [Google Scholar]
- 67. Alderson L, Fetell MR, Sisti M, et al. Sentinel lesions of primary CNS lymphoma. J Neurol Neurosurg Psychiatry. 1996;60(1):102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14Suppl 5:v1–v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17Suppl 4:iv1–iv62. doi:10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kadan-Lottick NS, Skluzacek MC, et al. Decreasing incidence rates of primary central nervous system lymphoma. Cancer. 2002;95(1):193–202. [DOI] [PubMed] [Google Scholar]
- 71. O’Neill BP, Decker PA, Tieu C, et al. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin’s lymphoma. Am J Hematol. 2013;88(12):997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schultz C, Scott C, Sherman W, et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol. 1996;14(2):556–564. [DOI] [PubMed] [Google Scholar]
- 73. Borsi JD, Moe PJ. A comparative study on the pharmacokinetics of methotrexate in a dose range of 0.5 g to 33.6 g/m2 in children with acute lymphoblastic leukemia. Cancer. 1987;60(1):5–13. [DOI] [PubMed] [Google Scholar]
- 74. Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med. 1975;293(4):161–166. [DOI] [PubMed] [Google Scholar]
- 75. Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol. 2003;21(6):1044–1049. [DOI] [PubMed] [Google Scholar]
- 76. Herrlinger U, Schabet M, Brugger W, et al. German Cancer Society Neuro-Oncology Working Group NOA-03 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma. Ann Neurol. 2002;51(2):247–252. [DOI] [PubMed] [Google Scholar]
- 77. Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21(24):4489–4495. [DOI] [PubMed] [Google Scholar]
- 78. Ferreri AJ, Reni M, Foppoli M, et al. ; International Extranodal Lymphoma Study Group (IELSG). High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512–1520. [DOI] [PubMed] [Google Scholar]
- 79. Ferreri AJ, Cwynarski K, Pulczynski E, et al. ; International Extranodal Lymphoma Study Group (IELSG). Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217–e227. [DOI] [PubMed] [Google Scholar]
- 80. Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061–3068. doi:10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mappa S, Marturano E, Licata G, et al. Salvage chemoimmunotherapy with rituximab, ifosfamide and etoposide (R-IE regimen) in patients with primary CNS lymphoma relapsed or refractory to high-dose methotrexate-based chemotherapy. Hematol Oncol. 2013;31(3):143–150. [DOI] [PubMed] [Google Scholar]
- 82. Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466–468. [DOI] [PubMed] [Google Scholar]
- 84. Chamberlain MC, Johnston SK. High-dose methotrexate and rituximab with deferred radiotherapy for newly diagnosed primary B-cell CNS lymphoma. Neuro Oncol. 2010;12(7):736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25(30):4730–4735. [DOI] [PubMed] [Google Scholar]
- 87. Kansara R, Shenkier TN, Connors JM, et al. Rituximab with high-dose methotrexate in primary central nervous system lymphoma. Am J Hematol. 2015;90(12):1149–1154. [DOI] [PubMed] [Google Scholar]
- 88. Holdhoff M, Ambady P, Abdelaziz A, et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology. 2014;83(3):235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gregory G, Arumugaswamy A, Leung T, et al. Rituximab is associated with improved survival for aggressive B cell CNS lymphoma. Neuro Oncol. 2013;15(8):1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ferreri AJ, Reni M, Pasini F, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58(10):1513–1520. [DOI] [PubMed] [Google Scholar]
- 91. Korfel A, Thiel E, Martus P, et al. Randomized phase III study of whole-brain radiotherapy for primary CNS lymphoma. Neurology. 2015;84(12):1242–1248. [DOI] [PubMed] [Google Scholar]
- 92. Ekenel M, Iwamoto FM, Ben-Porat LS, et al. Primary central nervous system lymphoma: the role of consolidation treatment after a complete response to high-dose methotrexate-based chemotherapy. Cancer. 2008;113(5):1025–1031. [DOI] [PubMed] [Google Scholar]
- 93. Jahnke K, Korfel A, Martus P, et al. ; German Primary Central Nervous System Lymphoma Study Group (G-PCNSL-SG). High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma. Ann Oncol. 2005;16(3):445–449. [DOI] [PubMed] [Google Scholar]
- 94. Vezmar S, Becker A, Bode U, et al. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy. 2003;49(1-2):92–104. [DOI] [PubMed] [Google Scholar]
- 95. Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16(3):859–863. [DOI] [PubMed] [Google Scholar]
- 96. Doolittle ND, Korfel A, Lubow MA, et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology. 2013;81(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Correa DD, DeAngelis LM, Shi W, et al. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62(4):548–555. [DOI] [PubMed] [Google Scholar]
- 98. Harder H, Holtel H, Bromberg JE, et al. Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology. 2004;62(4):544–547. [DOI] [PubMed] [Google Scholar]
- 99. Omuro AM, Ben-Porat LS, Panageas KS, et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol. 2005;62(10):1595–1600. [DOI] [PubMed] [Google Scholar]
- 100. Correa DD, Shi W, Abrey LE, et al. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol. 2012;14(1):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the memorial Sloan-Kettering cancer center prognostic model. J Clin Oncol. 2006;24(36):5711–5715. [DOI] [PubMed] [Google Scholar]
- 102. Roth P, Martus P, Kiewe P, et al. Outcome of elderly patients with primary CNS lymphoma in the G-PCNSL-SG-1 trial. Neurology. 2012;79(9):890–896. [DOI] [PubMed] [Google Scholar]
- 103. Fritsch K, Kasenda B, Hader C, et al. Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann Oncol. 2011;22(9):2080–2085. [DOI] [PubMed] [Google Scholar]
- 104. Hoang-Xuan K, Taillandier L, Chinot O, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Group. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21(14):2726–2731. [DOI] [PubMed] [Google Scholar]
- 105. Illerhaus G, Marks R, Müller F, et al. High-dose methotrexate combined with procarbazine and CCNU for primary CNS lymphoma in the elderly: results of a prospective pilot and phase II study. Ann Oncol. 2009;20(2):319–325. [DOI] [PubMed] [Google Scholar]
- 106. Kurzwelly D, Glas M, Roth P, et al. Primary CNS lymphoma in the elderly: temozolomide therapy and MGMT status. J Neurooncol. 2010;97(3):389–392. [DOI] [PubMed] [Google Scholar]
- 107. Zhu JJ, Gerstner ER, Engler DA, et al. High-dose methotrexate for elderly patients with primary CNS lymphoma. Neuro Oncol. 2009;11(2):211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Omuro A, Chinot O, Taillandier L, et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251–e259. [DOI] [PubMed] [Google Scholar]
- 109. Pulczynski EJ, Kuittinen O, Erlanson M, et al. Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: results from a phase II study by the Nordic Lymphoma Group. Haematologica. 2015;100(4):534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Grimm SA, Pulido JS, Jahnke K, et al. Primary intraocular lymphoma: an International Primary Central Nervous System Lymphoma Collaborative Group Report. Ann Oncol. 2007;18(11):1851–1855. [DOI] [PubMed] [Google Scholar]
- 111. Sagoo MS, Mehta H, Swampillai AJ, et al. Primary intraocular lymphoma. Surv Ophthalmol. 2014;59(5):503–516. [DOI] [PubMed] [Google Scholar]
- 112. Batchelor TT, Kolak G, Ciordia R, et al. High-dose methotrexate for intraocular lymphoma. Clin Cancer Res. 2003;9(2):711–715. [PubMed] [Google Scholar]
- 113. Jahnke K, Wagner T, Bechrakis NE, et al. Pharmacokinetics and efficacy of ifosfamide or trofosfamide in patients with intraocular lymphoma. Ann Oncol. 2005;16(12):1974–1978. [DOI] [PubMed] [Google Scholar]
- 114. Jahnke K, Thiel E, Bechrakis NE, et al. Ifosfamide or trofosfamide in patients with intraocular lymphoma. J Neurooncol. 2009;93(2):213–217. [DOI] [PubMed] [Google Scholar]
- 115. Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS. 2003;17(12):1787–1793. [DOI] [PubMed] [Google Scholar]
- 116. Evens AM, Choquet S, Kroll-Desrosiers AR, et al. Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. Am J Transplant. 2013;13(6):1512–1522. [DOI] [PubMed] [Google Scholar]
- 117. Nowakowski GS, Czuczman MS. ABC, GCB, and double-hit diffuse large B-cell lymphoma: does subtype make a difference in therapy selection? Am Soc Clin Oncol Educ Book. 2015:e449–e457. [DOI] [PubMed] [Google Scholar]
- 118. Ferreri AJ, Ciceri F, Brandes AA, et al. MATILDE chemotherapy regimen for primary CNS lymphoma: results at a median follow-up of 12 years. Neurology. 2014;82(15):1370–1373. [DOI] [PubMed] [Google Scholar]
- 119. Harjama L, Kuitunen H, Turpeenniemi-Hujanen T, et al. Constant pattern of relapse in primary central nervous lymphoma patients treated with high-dose methotrexate combinations. A Finnish retrospective study. Acta Oncol. 2015;54(6):939–943. [DOI] [PubMed] [Google Scholar]
- 120. Reni M, Mazza E, Foppoli M, et al. Primary central nervous system lymphomas: salvage treatment after failure to high-dose methotrexate. Cancer Lett. 2007;258(2):165–170. [DOI] [PubMed] [Google Scholar]
- 121. Hottinger AF, DeAngelis LM, Yahalom J, et al. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69(11):1178–1182. [DOI] [PubMed] [Google Scholar]
- 122. Nguyen PL, Chakravarti A, Finkelstein DM, et al. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23(7):1507–1513. [DOI] [PubMed] [Google Scholar]
- 123. Fischer L, Thiel E, Klasen HA, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17(7):1141–1145. [DOI] [PubMed] [Google Scholar]
- 124. Voloschin AD, Betensky R, Wen PY, et al. Topotecan as salvage therapy for relapsed or refractory primary central nervous system lymphoma. J Neurooncol. 2008;86(2):211–215. [DOI] [PubMed] [Google Scholar]
- 125. Enting RH, Demopoulos A, DeAngelis LM, et al. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63(5):901–903. [DOI] [PubMed] [Google Scholar]
- 126. Nayak L, Abrey LE, Drappatz J, et al. ; North American Brain Tumor Consortium. Multicenter phase II study of rituximab and temozolomide in recurrent primary central nervous system lymphoma. Leuk Lymphoma. 2013;54(1):58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96(6):864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chamberlain MC. Salvage therapy with bendamustine for methotrexate refractory recurrent primary CNS lymphoma: a retrospective case series. J Neurooncol. 2014;118(1):155–162. [DOI] [PubMed] [Google Scholar]
- 129. Chamberlain MC. High-dose cytarabine salvage therapy for recurrent primary CNS lymphoma. J Neurooncol. 2016;126(3):545–550. [DOI] [PubMed] [Google Scholar]
- 130. Arellano-Rodrigo E, López-Guillermo A, Bessell EM, et al. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70(4):219–224. [DOI] [PubMed] [Google Scholar]
- 131. Tyson RM, Siegal T, Doolittle ND, et al. Current status and future of relapsed primary central nervous system lymphoma (PCNSL). Leuk Lymphoma. 2003;44(4):627–633. [DOI] [PubMed] [Google Scholar]
- 132. Maza S, Kiewe P, Munz DL, et al. First report on a prospective trial with yttrium-90-labeled ibritumomab tiuxetan (Zevalin) in primary CNS lymphoma. Neuro Oncol. 2009;11(4):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Houillier C, Choquet S, Touitou V, et al. Lenalidomide monotherapy as salvage treatment for recurrent primary CNS lymphoma. Neurology. 2015;84(3):325–326. [DOI] [PubMed] [Google Scholar]
- 134. Korfel A, Schlegel U, Herrlinger U. Phase II trial of temsirolimus for relapsed/refractory primary central nervous system lymphoma (PCNSL). J Clin Oncol. 2016;34(15):1757–1763. [DOI] [PubMed] [Google Scholar]
- 135. Pentsova E, Deangelis LM, Omuro A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol. 2014;117(1):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10(17):5643–5646. [DOI] [PubMed] [Google Scholar]
- 137. Soussain C, Choquet S, Fourme E, et al. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica. 2012;97(11):1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Soussain C, Hoang-Xuan K, Taillandier L, et al. ; Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26(15):2512–2518. [DOI] [PubMed] [Google Scholar]
- 139. Fischer L, Koch A, Schlegel U, et al. Non-enhancing relapse of a primary CNS lymphoma with multiple diffusion-restricted lesions. J Neurooncol. 2011;102(1):163–166. [DOI] [PubMed] [Google Scholar]
- 140. Shenkier TN, Voss N, Chhanabhai M, et al. The treatment of primary central nervous system lymphoma in 122 immunocompetent patients: a population-based study of successively treated cohorts from the British Colombia Cancer Agency. Cancer. 2005;103(5):1008–1017. [DOI] [PubMed] [Google Scholar]
- 141. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. [DOI] [PubMed] [Google Scholar]
- 142. Kreher S, Jöhrens K, Strehlow F, et al. Prognostic impact of B-cell lymphoma 6 in primary CNS lymphoma. Neuro Oncol. 2015;17(7):1016–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Braaten KM, Betensky RA, de Leval L, et al. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9(3):1063–1069. [PubMed] [Google Scholar]
- 144. Levy O, Deangelis LM, Filippa DA, et al. Bcl-6 predicts improved prognosis in primary central nervous system lymphoma. Cancer. 2008;112(1):151–156. [DOI] [PubMed] [Google Scholar]
- 145. Chang CC, Kampalath B, Schultz C, et al. Expression of p53, c-Myc, or Bcl-6 suggests a poor prognosis in primary central nervous system diffuse large B-cell lymphoma among immunocompetent individuals. Arch Pathol Lab Med. 2003;127(2):208–212. [DOI] [PubMed] [Google Scholar]
- 146. Tapia G, Baptista MJ, Muñoz-Marmol AM, et al. MYC protein expression is associated with poor prognosis in primary diffuse large B-cell lymphoma of the central nervous system. APMIS. 2015;123(7):596–603. [DOI] [PubMed] [Google Scholar]