Abstract
Oral and fecal microbial biomarkers have previously been associated with cardiometabolic (CM) risk, however, no comprehensive attempt has been made to explore this association in minority populations or across different geographic regions. We characterized gut- and oral-associated microbiota and CM risk in 655 participants of African-origin, aged 25–45, from Ghana, South Africa, Jamaica, and the United States (US). CM risk was classified using the CM risk cut-points for elevated waist circumference, elevated blood pressure and elevated fasted blood glucose, low high-density lipoprotein (HDL), and elevated triglycerides. Gut-associated bacterial alpha diversity negatively correlated with elevated blood pressure and elevated fasted blood glucose. Similarly, gut bacterial beta diversity was also significantly differentiated by waist circumference, blood pressure, triglyceridemia and HDL-cholesterolemia. Notably, differences in inter- and intra-personal gut microbial diversity were geographic-region specific. Participants meeting the cut-points for 3 out of the 5 CM risk factors were significantly more enriched with Lachnospiraceae, and were significantly depleted of Clostridiaceae, Peptostreptococcaceae, and Prevotella. The predicted relative proportions of the genes involved in the pathways for lipopolysaccharides (LPS) and butyrate synthesis were also significantly differentiated by the CM risk phenotype, whereby genes involved in the butyrate synthesis via lysine, glutarate and 4-aminobutyrate/succinate pathways and LPS synthesis pathway were enriched in participants with greater CM risk. Furthermore, inter-individual oral microbiota diversity was also significantly associated with the CM risk factors, and oral-associated Streptococcus, Prevotella, and Veillonella were enriched in participants with 3 out of the 5 CM risk factors. We demonstrate that in a diverse cohort of African-origin adults, CM risk is significantly associated with reduced microbial diversity, and the enrichment of specific bacterial taxa and predicted functional traits in both gut and oral environments. As well as providing new insights into the associations between the gut and oral microbiota and CM risk, this study also highlights the potential for novel therapeutic discoveries which target the oral and gut microbiota in CM risk.
Introduction
Metabolic syndrome and cardiometabolic (CM) risk are associated with increased morbidity and mortality[1–3], and includes five risk factors: visceral obesity, elevated fasted blood glucose and elevated blood pressure, decreased high density lipoprotein (HDL) cholesterol, hypertriglyceridemia [4]. In the US, as many as 35% of all US adults present with CM risk [5], when defined using the “Adult Treatment Panel criteria”[6]. Between 1988 and 2012, the greatest increase in the prevalence of CM disease was seen among black men, estimated to be currently around 55%, and rose by 41% among black women [5]. There is increasing evidence that the composition and metabolic function of the gut microbiota correlate with the progression and pathogenesis of CM disease, although the causality remains unclear [7].
One of the hallmarks of CM-related disease is the concomitant presence of low-grade systemic inflammation [8]. Likewise, reduced intestinal bacterial diversity has also been reported in conditions with chronic inflammation and CM risk, with effects on the immune system [9, 10]. The relative abundance of specific bacterial taxa has been shown to differ between individuals with CM risk to healthy participants. For instance, individuals with obesity, diabetes and cardiovascular disease, often have a significant reduction in fecal-associated short-chain fatty acid (SCFA)-synthesizing bacteria such as Bifidobacteria, Roseburia, Faecalibacterium prausnitzii and Akkermansia muciniphilia [11–13]. All of these microorganisms have been shown to exhibit anti-inflammatory effects [13–16]. Potential opportunistic pathogens, such as Staphylococcus aureus and those from the family Enterobacteriaceae, are often enriched in the stools of individuals with CM risk [17, 18]. It has been suggested that these pro-inflammatory taxa contributes to the evolution of CM risk [17, 18].
There are several metabolic mechanisms through which the gut microbiota may contribute to the CM risk. Lipopolysaccharide (LPS) is a known precursor for the development of obesity and insulin resistance[19, 20] and an increase in the relative proportion of the bacteria that produce LPS in the gut has been associated with elevated systemic LPS concentration, likely resulting in high inflammation. Strong evidence for the role of LPS and TLR4 signaling in the development of atherosclerotic lesions has been shown, with the gut microbiota considered drivers of these atherosclerotic lesion formations [21, 22]. in atherogenesis Trimethylamine N-oxide (TMAO), which derived from microbial trimethylamine metabolism, is also increased in the stool of individuals with elevated CM risk [23, 24], although some find the inverse to be true [25] and one study found an inverse association with cardiac death in African Americans [26]. Similarly, SCFAs such as propionate, acetate and butyrate produced by bacterial fermentation of indigestible fibers, are known to regulate host energy intake, expenditure and storage, decreases their concentrations have been associated with elevated CM risk [27, 28]. Finally, specific gut bacteria have also been associated with altered bile acid composition, which also seem to play an important role in diabetes, obesity, non-alcoholic fatty liver disease and other metabolic diseases via the farnesoid X receptor (FXR) and G protein-coupled bile acid receptor (GPCR) signaling pathway [29–31]. These metabolic pathways represent mechanisms through which the gut microbiota may influence CM risk, and by which they might also serve as potential therapeutic targets for treating elevated CM risk.
Emerging data suggests that the oral microbiota may also be linked to elevated CM risk [32–34]. Oral bacteria, especially potential pro-inflammatory pathogens like Pseudomonas and Enterobacter, have been detected in human atherosclerotic plaques, associated with CM risk [33, 35]. Hence, systemic inflammation triggered by oral pathogens may be an important component in the pathogenesis of systemic disease [32, 36]. However, to date, it is unclear whether there is an association between the oral and gut microbiota and elevated CM risk, and whether the oral microbiota exhibits similar associations as gut microbiota does with CM risk. If this is the case, then, the oral microbiota, which is substantially more convenient to collect, may be a proxy to determine gut microbiota-derived associations that may have a more specific direct mechanistic association with elevated CM risk. However, a lack of large-scale cohort studies limits our interpretation of CM risk-microbiota associations, particularly across diverse human populations.
In this study, we leveraged African-origin participants enrolled in the “Modeling the Epidemiologic Transition Study” (METS) [37] cohort to determine the association between the gut (stool-derived) and oral (saliva-derived) microbiota and elevated CM risk. We characterized the gut- and oral-associated microbiota and indices of CM risk in 655 participants of African-origin, aged 25–45, from Ghana, South Africa, Jamaica, and the United States (US). The central hypothesis is that the oral microbial composition is associated with the gut microbial composition, and that both are associated with elevated CM risk in blacks.
Methods
Study participants
Participant selection
Previously, 2,506 African-origin adults (25-45yrs) were enrolled in METS between January 2010 and December 2011 and followed on a yearly basis. A detailed description of the METS protocol has previously been published [37]. For the current study, fecal samples from 655 men and women from Ghana (N = 196), South Africa (N = 176), Jamaica (N = 92) and the US (N = 191) were collected in 2014. In addition, 620 of them also supplied saliva samples. Participants were excluded from the original study if they self-reported an infectious disease, including HIV-positive individuals (South Africa), pregnant or lactating women, and unable to participate in normal physical activities [37]. METS was approved by the Institutional Review Board of Loyola University Chicago, IL, US; the Committee on Human Research Publication and Ethics of Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; the Research Ethics Committee of the University of Cape Town, South Africa; the Board for Ethics and Clinical Research of the University of Lausanne, Switzerland; and the Ethics Committee of the University of the West Indies, Kingston, Jamaica. All study procedures were explained to participants in their native languages, and participants provided written informed consent after being given the opportunity to ask any questions.
Lifestyle and biochemical measurements
All measurements were made at research clinics located in the respective communities. Weight and height were measured with participants wearing light clothing, and no shoes. Participants were asked to provide an early morning fecal sample, using a standard collection kit (EasySampler stool collection kit, Alpco, NH) at their home. Fecal samples were immediately brought to the site clinics and stored at -80°C. The same fecal sample collection procedure was carried out at all the research sites. Participants were requested to fast from 8 pm in the evening prior to the clinic examination, during which fasting capillary glucose concentrations were determined using finger stick (Accu-check Aviva, Roche).
Cardiometabolic risk
We defined CM risk using the National Cholesterol Education Program’s Adult Treatment Panel III NCEP/ ATP III criteria for cardiometabolic disease [6]. These 5 risk factors include: 1) waist circumference >102 cm in males and >88 cm in females; 2) elevated blood pressure (≥130/85 mm Hg), or receiving treatment; 3) hypertriglyceridemia (≥ 150 mg/dL), or receiving lipid-lowering treatment; 4) low high-density lipoprotein (HDL) cholesterol (<40 mg/dL in males and <50 mg/dL in females), or receiving lipid-lowering treatment; and 5) elevated fasting plasma glucose (≥ 110 mg/dL) or receiving glucose-lowering treatment.
DNA isolation and 16S ribosomal RNA (rRNA) gene sequencing
Microbial genomic DNA was extracted from the human stool samples using the DNeasy PowerSoil DNA Isolation Kit (Qiagen). The V4 region of 16S rRNA gene was amplified and sequenced using the Illumina MiSeq platform [38]. The primers used for amplification (515F-806R) contain adapters for MiSeq sequencing and single-end barcodes allowing pooling and direct sequencing of PCR products [39].
16S rRNA gene pyrosequencing data preprocessing and analysis
Raw sequences were pre-processed, quality filtered and analyzed using the next-generation microbiome bioinformatics platform (QIIME2 version 2018.6 pipeline) according to the developer’s suggestion [40, 41]. We used the DADA2 algorithm [42], a software package wrapped in QIIME2, to identify exact sequence variants (ESVs). Quality control, filtering low quality regions of the sequences, identification and removal of chimera sequences, merging paired-end reads, which yielded the ESV feature table (ESV table). Alpha and beta-diversity analyses were performed in R using the phyloseq package [43]. Alpha diversity was calculated by Shannon’s diversity index, observed OTUs, and Chao1 diversity [44]. Results were adjusted for BMI, age, sex and country. Principal coordinate analysis (PCoA) was performed based on weighted and unweighted UniFrac distances, a method for computing differences between microbial communities based on phylogenetic information [45]. Weighted UniFranc considered both ESVs presence and absence and abundance distances, and unweighted UniFrac only considered ESVs presence. Permutational multivariate analysis of variance (PERMANOVA, R function adonis (vegan, 999 permutations)) was used to analyze statistical differences in beta diversity[46]. Benjamini–Hochberg false discovery rate (fdr) correction was used to correct for multiple hypothesis testing [47].
The contribution of CM risk factors and environmental factors (sex, age, BMI, sleep, smoke and alcohol consumption) to the overall weighted and unweighted UniFrac dissimilarities in gut and oral microbiota composition was also assessed using PERMANOVA (R function adonis (vegan), 999 permutations), which decomposes the dissimilarity matrix into ‘variance’ explained by each covariate. The obtained R2 gives the proportion of variability observed in the entire dissimilarity matrix that can be independently attributed to the studied variables.
For taxonomic comparisons, relative abundances based on all obtained reads were used. We used the QIIME2 plugin “q2-feature-classifier” and the Naïve Bayes classifier [48] that was trained on the metagenome annotation package Greengenes13.8 [49] 99% operational taxonomic units (OTUs) full-length sequences to obtain the taxonomy for each ESV. Significantly differential ESVs were determined using the statistical framework called analysis of composition of microbiomes (ANCOM) [42] for two group comparisons. FDR correction was used to correct for multiple hypothesis testing. Results were adjusted for BMI, age, sex and country.
A two-side Mantel test using Spearman correlation coefficients (999 permutations) was applied to identify the correlation between the beta diversity of the oral and the gut microbiota, with both unweighted and weighted UniFrac distance matrices in software R with the function “mantel.test” [50].
To test for correlations between oral microbiota and the Shannon index or ESVs, which their relative abundances was greater than 1% and are also significantly correlated with CM risk in gut microbiota, random forest regression and generalized linear models (GLM) were performed. Random forest regression was done with 1,000 regression trees based on 10-fold cross-validation and performed with QIIME2 plugin “qiime sample-classifier regress-samples” and the Random Forest regressor in the R programming environment. A randomly drawn 80% of samples were used for model training and the remaining 20% were used for validation.
Metagenome functional predictions of the microbial pathways
We used Piphillin an algorithm to predict the functional profiles of the microbiome [51]. Briefly, this algorithm uses direct nearest-neighbor matching between 16S rRNA gene sequencing datasets and microbial genomic databases to infer the metagenomic content of the samples. Gene prediction was performed on ESVs table using online Piphillin (http://secondgenome.com/Piphillin), with KEGG database version 2017 and 97% identity cut-off. Predicted gene content and gene copy numbers within each genome were retrieved and classified in terms of the ezymes code by each gene as KEGG orthology (KOs) [52]. Results were adjusted for BMI, age, sex and country. Statistical analyses were performed in R. Student's t-test (normally distributed) or Mann-Whitney U test (not normally distributed) was used for to detect differentially abundant KOs between two groups. FDR correction was used to correct for multiple hypothesis testing.
Results
Participant characteristics
Previously 2,506 adults from Ghana, South Africa, Jamaica, The Seychelles, and US were recruited in 2009, and prospectively followed on an annual basis in METS [37]. The current study included a subsample of 655 participants, of which 196 were Ghanaian, 176 were South African, 92 were Jamaican, and 191 were from the US (Table 1). Approximately 60% of the participants were women. The average age among all the participants was 34.9 ± 6.4 years, and participants from South Africa and Jamaica were significantly younger than US participants (p <0.001 and p = 0.016, respectively). Men and women from Ghana, South Africa and Jamaica weighed significantly less (p <0.001) than their US counterparts (63.2 ± 12.0, 76.2 ± 20.5, and 80.0 ± 21.1 kg, respectively), and there were significantly more US men and women that were overweight and obese (81.3%, p <0.001 for all) compared with the other cohorts (i.e., Ghana, 33.2%; South Africa, 55.7%; Jamaica 65.2%) (Table 1). Americans also slept the least number of nightly hours (6.7 ± 1.4 hrs) compared to Ghanaians (7.9 ± 1.4 hrs, p<0.001), Jamaicans (7.3 ± 2.1 hrs, p<0.033), and South Africans (10.5 ± 1.7 hrs, p<0.001), and reported significantly higher prevalences of cigarette smoking (37.7%, p<0.001), and alcohol consumption (84.3%, p<0.001) compared to the other 3 sites, after adjusting for age, sex and BMI (Table 1).
Table 1. Participant characteristics by site.
The US is the reference site.
| Ghana | South Africa | Jamaica | US | Overall | |
|---|---|---|---|---|---|
| N | N = 196 | N = 176 | N = 92 | N = 191 | N = 655 |
| Age (y) | 35.8 ± 6.6 | 33.3 ± 5.9* | 33.9 ± 6.2* | 36.0 ± 6.3 | 34.9 ± 6.4 |
| Weight (kg) | 63.2 ± 12.0** | 76.2 ± 20.5** | 80.0 ± 21.1** | 94.2 ± 25.0 | 78.1 ± 23.3 |
| Height (cm) | 161.7± 7.6** | 163.7 ± 7.7** | 166.8 ± 10.9** | 169.6 ± 8.4 | 165.2 ±9.0 |
| BMI (kg/m2) | 24.3 ± 5.0** | 28.7 ± 8.2** | 29.1 ± 8.9** | 32.8 ± 8.8 | 28.6 ±8.4 |
| Normal weight (BMI<25 kg/m2), N, % | 131, 66.8%** | 78, 44.3%** | 32, 34.8%** | 36, 18.9% | 277, 42.3% |
| Overweight (BMI≥25 kg/m2-<30 kg/m2) | 41, 20.9% | 28, 15.9%** | 28, 30.4% | 52, 27.2% | 149, 22.8% |
| Obese (BMI≥30 kg/m2) | 24, 12.2%** | 70, 39.8%** | 32, 34.8%** | 103, 53.9% | 229, 35.0% |
| Fat-free mass (kg) | 44.3 ± 7.4** | 44.6 ± 7.2** | - | 55.1 ± 11.2 | 48.1 ± 10.2 |
| Fat mass (kg) | 19.0 ± 9.2** | 31.7 ± 16.2** | - | 39.3 ± 18.4 | 29.9 ± 17.3 |
| % Body fat | 29.2 ± 9.84** | 39.3 ± 11.0 | - | 39.9 ± 10.8 | 36.0 ± 11.6 |
| Sleep hours (hrs/night) ¥ | 7.9 ± 1.4** | 10.5 ± 1.7** | 7.3± 2.1* | 6.7 ± 1.4 | 8.2 ± 2.2 |
| Smokers¥ | 4, 2.0%** | 48, 27.3%** | 10, 10.9%** | 72, 37.7% | 134, 20.5% |
| Drinkers¥ | 59, 30.6%** | 75, 42.9%** | - | 161, 84.3% | 295, 52.8% |
*p<0.05
**p<0.01 compared to US.
¥Adjusted for age, sex and BMI
Cardiometabolic risk
We used the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP/ ATP III) criteria for metabolic syndrome [6] to indicate CM risk, and as follows; waist circumference >102 cm in men and >88 cm in females; elevated blood pressure (≥130/85 mm Hg), or receiving treatment for hypertension; hypertriglyceridemia (≥150 mg/dL) or receiving treatment; low high-density lipoprotein (HDL) cholesterol (<40 mg/dL in males and <50 mg/dL in females), or receiving treatment; and elevated fasting plasma glucose (≥110 mg/dL) or receiving treatment for type 2 diabetes. Individuals with a BMI ≥25 kg/m2 were classified as overweight, and ≥30 kg/m2 were categorized as obese [53]. For the purpose of our analysis, we dichotomized CM risk creating a CM risk phenotype, whereby those with elevated CM risk had at least 3 of the five CM risk factors, compared to those with 2 or less. Because the Jamaican participants were missing HDL and triglyceride concentration measures in blood, they were excluded from the overall CM risk analysis. This resulted in a total of 563 participants with complete CM risk data. Overall, 46 of these 563 participants (8.2%) presented with elevated CM risk. Compared to the US participants (14.7%), the prevalence of elevated CM risk was significantly lower among the Ghanaians (1.5%, p<0.001), and trended lower among the South Africans (8.5%, p = 0.09) (Table 2).
Table 2. Cardiometabolic risk by site, and overall.
The US is the reference site, and comparisons are adjusted for age, sex and BMI.
| Ghana | South Africa | Jamaica | United States | Overall | |
|---|---|---|---|---|---|
| N = 196 | N = 176 | N = 92 | N = 191 | N = 655 | |
| Fasted plasma glucose (mg/dL) | 100.7 ± 12.2 | 83.3 ± 18.1** | 99.6 ± 15.5 | 106.2 ± 40.4 | 97.5 ± 26.8 |
| Elevated fasted plasma glucose, N, % | 2, 1.0%** | 8, 4.6%** | 18, 19.6% | 45, 23.9% | 73, 11.2% |
| Systolic blood pressure (mmHg) | 112.9 ± 13.5** | 124.4 ± 20.5* | 112.7 ± 12.5** | 123.3 ± 17.6 | 119.0 ± 17.5 |
| Diastolic blood pressure (mmHg) | 66.6 ± 11.0** | 79.4 ± 12.8 | 72.0 ± 34.0** | 80.5 ± 13.1 | 74.9 ± 18.1 |
| Elevated blood pressure, N, % | 6, 3.1%** | 26, 14.8% | 1, 1.1%* | 25, 13.1% | 58, 8.9 |
| Triglycerides (mg/dL) | 82.1 ± 36.5 | 88.2 ± 55.1 | - | 98.5 ± 62.1 | 89.5 ± 52.5 |
| Hypertriglyceridemia, N, % (N = 563) | 12, 6.2% | 16, 9.1% | - | 29, 15.7% | 57, 10.3% |
| HDL-cholesterol (mg/dL) | 46.1 ± 13.6** | 49.9 ± 15.0* | - | 52.1 ± 15.1 | 49.3 ± 14.7 |
| Low HDL-cholesterol, N, % N = 563 | 104, 53.6%** | 79, 45.4%* | - | 64, 34.8% | 247, 44.8% |
| LDL-cholesterol (mg/dL) | 100.7 ± 29.1 | 92.9 ± 32.6** | - | 110.1 ± 35.5 | 101.4 ± 33.1 |
| Total cholesterol (mg/dL) | 163.3 ± 34.3** | 160.1 ± 36.1** | - | 183.0 ± 38.6 | 168.9 ± 37.7 |
| High waist circumference N, % N = 563 | 37, 18.9%** | 78, 44.3%** | 37, 40.2%** | 116, 60.7 | 268, 40.9% |
| Cardiometabolic disease N, % N = 563 | 3, 1.5%* | 15, 8.5% | - | 28, 14.7 | 46, 8.2% |
Cardiometabolic risk is defined by three out of five risk factors: waist circumference >102 cm in males and >88 cm in females; elevated blood pressure (≥ 130/85 mm Hg), or receiving treatment; hypertriglyceridemia (≥ 150 mg/dL), or receiving treatment; low HDL (<40 mg/dL in males and <50 mg/dL in females), or receiving treatment; and elevated fasting plasma glucose (≥ 110 mg/dL) or receiving treatment.
*p<0.05
**p<0.01 compared to US.
Gut-derived bacterial diversity associates with country of origin and CM risk factors
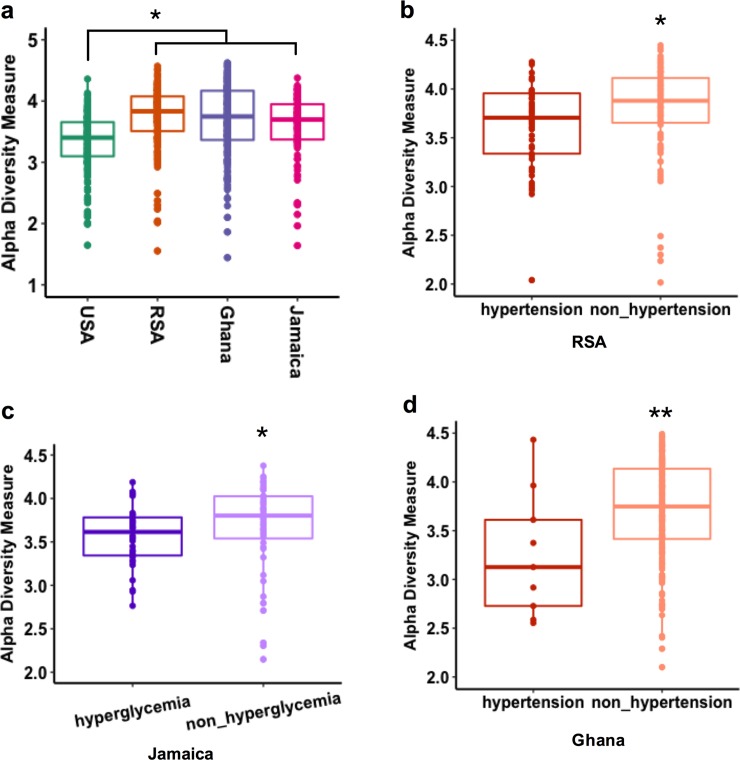
The associations between the gut-derived microbial diversity measured by 16S rRNA amplicon sequences using exact sequence variants (ESVs) and indices of elevated CM risk were calculated across the four cohorts. All analyses were adjusted for age, sex and BMI. Gut bacterial alpha diversity (intrapersonal gut diversity, measured by Shannon and Chao1 indexes)[44] was significantly greater among the Ghanaians and South Africans, compared to the US participants (p <0.001; Fig 1, S1 Fig). The Shannon index, which incorporates both the richness and evenness of the community, was lower among participants with elevated blood pressure from South Africa (p <0.05) and Ghana (p <0.01) but showed no significant relationship among the US participants or the Jamaicans (Fig 1, S2 Fig). Elevated fasted blood glucose was significantly associated with lower alpha diversity, but only among the Jamaicans (p <0.05; Fig 1, S2 Fig). Individuals with elevated waist circumference, triglyceride concentration and HDL levels showed no significant associations for gut microbial alpha diversity (adjusted for age, sex and BMI, p >0.05, S2 Fig). These results suggest that decreased gut microbial alpha diversity is associated some of the CM risk factors (i.e., elevated blood pressure and elevated fasting blood glucose), however, these associations are country specific.
Fig 1. Gut bacterial alpha diversity associations with cardiometabolic risk factors (adjusted for age, sex and BMI, only significant associated were shown here).
a) Alpha diversity (Shannon index) in the four study sites (USA, RSA, Ghana and Jamaica); b) association with hypertension in RSA; c); association with elevated fasting blood glucose in Jamaica; d); association with hypertension in Ghana. * p <0.05; ** p <0.01.
Gut bacterial beta diversity was significantly different between countries [false discovery rate (fdr)-corrected p <0.01; S3 Fig]. Therefore, as with alpha diversity, we derived associations of beta diversity against CM risk within each country independently (S4 and S5 Figs). Weighted and unweighted UniFrac distances, which compute differences between microbial communities based on phylogenetic information[45], were significantly different between high and low waist circumference, but only among the South Africans (fdr-corrected p<0.05) and Ghanaians (fdr-corrected p<0.01). The same was true for elevated blood pressure, whereby weighted UniFrac was significantly different among the South Africans (fdr-corrected p <0.05) and Ghanaians (fdr-corrected p <0.05), but interestingly, unweighted UniFrac was only significantly different in the South Africa cohort (fdr-corrected p <0.05). This might suggest that the differences seen in the South Africans may be in part due to differences in the abundance of rare bacterial taxa, while in Ghana the differences may be due to more abundant bacterial taxa in the fecal samples. Similarly, for triglyceride concentrations, only weighted UniFrac was significantly different and only among the South Africans (fdr-corrected p <0.05), while for HDL concentrations only weighted UniFrac was significantly different among US participants (fdr-corrected p <0.05). Hyperglycemia was not significantly correlated with beta diversity in any of the cohorts. These results suggest that gut microbial structure is significantly associated with individual CM risk factors, and that these associations are geographically dependent, given that the contribution of the abundant versus rare bacterial taxa varied with country of origin. Taken together, contributions of CM risk as well as environmental factors, including country of origin, result in inter-individual dissimilarities in the gut microbial composition (S1 Table).
Gut microbial structure and function correlate with CM risk phenotype
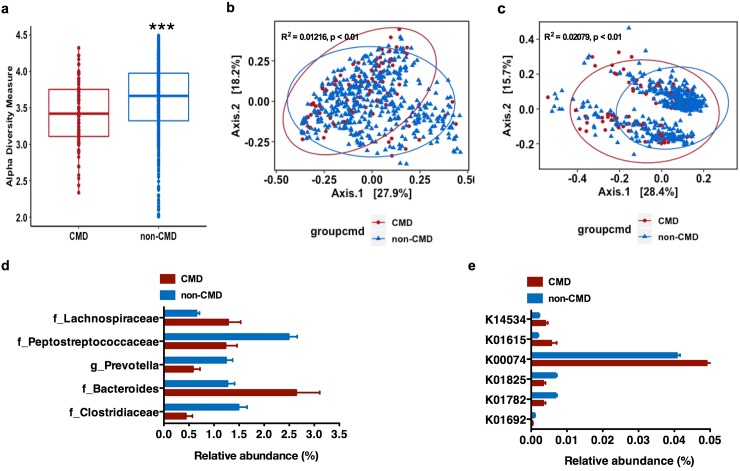
We next investigated whether the gut microbiota correlated with an elevated CM risk phenotype in the participants from USA, Ghana and South Africa. Jamaican’s were excluded from this analysis due to missing lipid data, and analyses were adjusted for age, sex and BMI. Participants with elevated CM risk phenotype (at least 3 risk factors) had significantly lower fecal alpha diversity (measured by Shannon index), compared to those without elevated CM risk (p <0.001; Fig 2A). Furthermore, weighted and unweighted UniFrac distances were significantly different between the two CM risk phenotypes (fdr-corrected p < 0.05; Fig 2B and 2C). We were able to identify the fecal bacterial ESVs which had significantly different abundance as a function of elevated CM risk. For these ESVs with a relative abundance ≥1% in at least one group (adjusted for country, sex, age and BMI), participants with elevated CM risk were significantly enriched with 2 bacterial ESVs annotated to family Lachnospiraceae and genus Bacteroides, while participants without elevated CM risk had were enriched in 3 bacterial ESVs annotated to family Clostridiaceae, Peptostreptococcaceae, and genus Prevotella (fdr-corrected p <0.05; Fig 2D).
Fig 2. Gut bacterial structure and function correlates with cardiometabolic (CM) risk phenotype.
(a), Alpha diversity analysis (Shannon Index) from 16S rRNA gene sequence data of gut microbiota against CM risk; (b-c), Principal coordinate analyses (PCoA) of weighted (a) and unweighted (b) UniFrac distance of gut microbiota composition against CM risk (fdr-corrected p <0.01); (d), Specific gut bacterial taxa are differentially abundant (in relative terms) between study sites with and without elevated CM risk (only significantly differential exact sequence variants (ESVs) with relative abundance ≥ 1% in at least one group shown. Data shown are means ± S.E.M.; p(fdr-corrected) <0.05); (e), Relative abundance of the genes involved in the four different pathways for butyrate synthesis against CM risk of gut microbiota across all study sites. (Data shown are means of percentages ± S.E.M. p(fdr-corrected) < 0.05). CMD means CM risk defined as at least 3 CM risk factors from five: waist circumference, elevated blood pressure, elevated blood fasting glucose, elevated triglyceride and low HDL concentration in USA, RSA, and Ghana *** p < 0.001. false discovery rate.
Specific gut bacterial taxa were also associated with individual CM risk across the entire cohort (adjusted for country, age, sex and BMI, S6 Fig and S2 Table) or when stratified by sex (adjusted for country, age and BMI, S6 Fig and S2 Table). We found that the gut microbial taxa level was profoundly altered in participants for each of the CM risk factors. For example, among participants with an elevated waist circumference, hyperglyceridemia, elevated blood pressure or low HDL concentration, there were a significant enrichment of bacterial ESVs annotated to genera belonging to family Lachnospiraceae, Enterobacteriaceae and Clostridiaceae; genera Streptococcus, Coprococcus and Blautia (fdr-corrected p <0.05). Conversely, bacterial ESVs annotated to genus Prevotella (family Prevotellaceae), and family Enterobacteriaceae, Clostridiaceae, and Peptostreptococcaceae were also significantly enriched among participants without CM risk (fdr-corrected p <0.05; S1 File). As expected, there were also several gut bacterial taxa that were differentially abundant between each country and each CM risk factor (S7 Fig and S2 Table).
The 16S rRNA amplicon data was used to predict gene abundance using Piphillin[51] to establish differences in the gut microbial metabolic potential in several well-known pathways associated with elevated CM risk, such as LPS biosynthesis, SCFA metabolism, TMA N-oxide (TMAO) biosynthesis, and genes associated with secondary bile acid biosynthesis. No statistically significant differences in the proportion of genes encoding components of TMA N-oxide (TMAO) biosynthesis, LPS biosynthesis, and secondary bile acid biosynthesis were observed among participants who had an elevated CM risk phenotype (at least 3 risk factors), compared to those without CM risk (adjusted for country, sex, BMI and age, p >0.05). However, the relative abundance of the genes involved in the four different pathways for butyrate synthesis showed that several genes that encode for enoyl-CoA hydratase enzymes (K01692, K01782, K01825) in the acetyl-CoA pathway were enriched in participants without elevated CM risk; while, crotonase (K01715) and β-hydroxybutyryl-CoA dehydrogenase (K00074) in the acetyl-CoA pathway were enriched in participants with elevated CM risk. Notably, the abundance of predictive genes that encode for glutaconyl-CoA decarboxylase (α, β subunits) (K01615) in the glutarate pathway and the 4-hydroxybutyryl-CoA dehydratase (K14534) in the 4-aminobytyrate/succinate pathway were enriched in participants with elevated CM risk (adjusted for country, sex, age and BMI, fdr-corrected p < 0.05; Fig 2D).
We also explored whether gut bacterial functions were associated with each of the 5 individual CM risk factor within each of the countries. Gut microbial predicted genes involved in LPS and SCFA biosynthesis pathways showed that genes involved in the butyrate synthesis via lysine, glutarate and 4-aminobutyrate/succinate pathways and LPS synthesis pathway were differentially enriched among the cohort with an elevated individual CM risk factor (S8–S11 Figs). Specifically, predicted genes involved in LPS biosynthesis pathways were enriched among participants with an elevated waist circumference in Ghana and US; participants with an elevated blood pressure in Ghana and Jamaica; participants with an elevated fasted blood glucose in Jamaica and the US, and those with low HDL in South Africa and the US. As the butyrate synthesis via lysine, glutarate and 4-aminobutyrate/succinate pathways, predicted genes involved in the glutarate pathway were significantly enriched in participants with a high waist circumference in South Africa and Ghana, and also participants with low HDL concentrations in the US and South Africa; predicted genes involved in the lysine pathway were enriched in participants with elevated blood pressure in Ghana; and predicted genes involved in the 4-aminobytyrate/ succinate pathway in South Africans were significantly associated with low HDL concentrations (see detail in S1 File; S8–S11 Figs).
Generalized linear models (GLM) were applied to explore the associations between alpha diversity, bacterial taxa and predicted functional genes and total CM risk (captured as the sum of CM risk factors in each participant). For instance, there was a significant positive correlation between the proportion of KEGG ID K01615 in the glutarate pathway and K14534 in the 4-aminobytyrate/succinate pathway and total CM risk score (i.e. a z-score transformation to generate continuous discrete measurements, controlling for country, age, sex, and BMI, p <0.05, see methods for more details). However, there were no significant associations between diversity or differential bacterial taxa and total CM risk (p >0.05). To explore whether the gut microbiota predicted elevated CM risk (three out of the five risk factors), random forest regression was used to determine the gut bacterial ESVs against the total CM risk (z-score transformed) pooling the 3 sites with CM risk data. Elevated CM risk could be predicted by gut-associated ESVs and explained almost 15% of the elevated CM risk variance among those with CM risk data.
Oral-associated bacterial diversity associates with country of origin and CM risk factors
On the other hand, oral microbiota diversity was associated with the country of origin, but not with the individual CM risk factors. Shannon diversity was significantly greater among the Ghanaians compared to the US participants (adjusted for age, sex and BMI, p <0.05; S12 Fig). As oral microbial alpha diversity was significantly different between countries, we correlated CM risk against alpha diversity for each country independently and found that none of the CM risk factors were significantly associated with oral microbial alpha diversity (p >0.05, S13 Fig). However, oral microbial beta diversity was significantly different between participants from different countries (fdr-corrected p <0.01, S14 Fig), and therefore, tests of association were performed separately for each country. In the US sample, weighted UniFrac distance was significantly different between individuals with either high and normal waist circumference (fdr-corrected p <0.01) and similarly, between participants with either elevated or normal glucose concentrations (fdr-corrected p <0.05, S15 Fig). Unweighted UniFrac distances were significantly different between individuals with either elevated and normal waist circumference in the US (fdr-corrected p ≤ 0.01), Ghana (fdr-corrected p <0.05), and South Africa (fdr-corrected p <0.05) (S16 Fig). In the US alone, unweighted UniFrac was significantly different by HDL risk (fdr-corrected p <0.05) (S16 Fig). Therefore, oral beta diversity, and hence microbial structure, was significantly associated with several CM risk factors, but similarly differed by country, suggesting that environmental factors are critical for inter-individual dissimilarities in oral microbial composition (S3 Table).
Oral-associated bacterial community structure correlates with the elevated CM risk phenotype
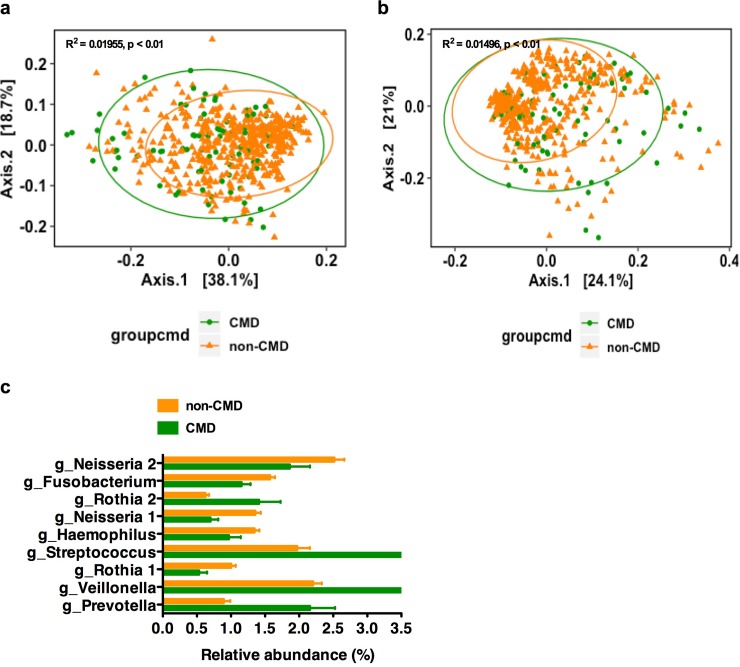
We next investigated whether the oral microbiota correlated with the elevated CM risk phenotype (3 out of five risk factors) and CM risk score in the US, Ghanaian and South African participants. Alpha diversity was not significantly different between low and elevated CM risk (adjusted for age, sex and BMI, p(fdr-corrected) <0.05, S17 Fig). However, both weighted and unweighted UniFrac distance were significantly differentiated by CM risk (fdr-corrected p <0.01, Fig 3A and 3B). The differential taxa analysis (with relative abundance ≥1% in at least one group) showed that participants with elevated CM risk had a significant enrichment of 4 oral-bacterial ESVs annotated to the genera Prevotella, Veillonella, and Streptococcus; while participants without elevated CM risk had a significant enrichment of 5 bacterial ESVs annotated to genus Haemophilus, Neisseria, Fusobacterium (adjusted for country, sex, BMI and age, p(fdr-corrected) <0.05; Fig 3C). Several oral-bacterial ESVs annotated to the genus Rothia presented opposite behaviors (i.e., some were enriched, and some were depleted in participants with elevated CM risk compared with low CM risk)
Fig 3. Oral-associated bacterial structure correlates with CM risk phenotype.
(a-b), Principal coordinate analyses (PCoA) of weighted (a) and unweighted (b) UniFrac distance of oral microbiota composition against CM risk (fdr-corrected p <0.01). (c), Specific oral bacterial taxa are differentially abundant (in relative terms) between study sites with and without CM risk (only significantly differential exact sequence variants (ESVs) with relative abundance ≥ 1% in at least one group shown. Data shown are mean± S.E.M. p(fdr-corrected) < 0.05). CMD means CM risk, including at least 3 CM risk factors of waist circumference, elevated blood pressure, elevated blood fasting glucose, high triglyceridemia and low HDL-cholesterolemia in USA, RSA, and Ghana. fdr, false discovery rate.
At a more granular level, specific oral bacterial taxa were also associated with each CM risk factor across the entire cohort (adjusted for country, age, sex and BMI; S18 Fig and S4 Table) or when stratified by sex (adjusted for country, BMI and age; S18 Fig and S4 Table), separately. The results indicate that the oral microbiota of participants who have greater CM risk, e.g. a higher waist circumference, elevated blood pressure or low HDL concentration, are significantly enriched for potentially pro-inflammatory taxa, including Streptococcus, Prevotella, and Veillonella (fdr-corrected p <0.05; S1 File). There were also specific oral bacteria that were differentially abundant between each country by each CM risk factor (fdr-corrected p <0.05; S1 File; S19 Fig and S2 Table).
To determine whether the taxa could identify participants with elevated CM risk, we once again used GLM to determine the association between the differential proportional oral bacterial taxa and total CM risk (sum of the number of CM risk factors for each participant). There was a significantly positive association between the proportion of a Streptococcus ESV and total CM risk (z-score transformation), controlling for country, age, and sex p <0.05). To test whether the oral microbiota can identify participants with the elevated CM risk phenotype, random forest regression was used to examine the association of oral bacterial ESVs against CM risk (z-score transformed). Among participants with elevated CM risk, oral bacterial ESVs, accounted for almost 8% of the variance.
Correlation of beta diversity between gut and oral microbiota
Finally, to explore whether the beta diversity trends seen in the oral microbiota correlated with those in the gut microbiota, we applied a Mantel test using both unweighted and weighted UniFrac distance matrices. Pooling all countries, there was a significant correlation with both unweighted (r = 0.094, p = 0.001) and weighted (r = 0.082, p = 0.001) UniFrac distance between the gut and oral microbiota (S20 and S21 Figs). However, within each country, only the weighted UniFrac metric had a significant correlation between oral and gut microbial beta diversity, and only among the US and South African participants (p = 0.034, r = 0.073 and p = 0.021, r = 0.09 respectively) (S20 and S21 Figs).
Discussion
In our study of African-origin adults from Ghana, South Africa, Jamaica and the US, we performed a comprehensive analysis exploring the relationship between the gut and oral microbiota and elevated CM risk, representing 4 geographically diverse countries spanning an epidemiologic transition. Overall, our results provide evidence that the gut and oral microbiota may potentially be both predictive as well as a therapeutic target for elevated CM risk, and is in line with previous evidence that the human microbiota are associated with CM risk [54, 55].
Consistent with previous studies [9, 56], we found that the gut microbial alpha diversity was significantly lower in participants with elevated CM risk. However, these associations are mostly geographical and dependent on the type of CM risk factor, e.g. for elevated blood pressure, the association was only found among South Africans and Ghanaians, while only Jamaicans had differences for elevated fasted blood glucose. Similarly, gut bacterial beta diversity was also significantly different between participants with an elevated waist circumference, elevated blood pressure, hypertriglyceridemia or low HDL concentration, compared with their healthy counterparts. Although again, the associations differed by country, e.g. for an elevated waist circumference, the associations were only found among the South African and Ghanaian participants, and for low HDL concentration, only among the US participants.
Previous studies have reported conflicting results as to which specific bacterial taxa associate with CM risk factors [11–13, 17, 18]. In our study, gut bacterial ESVs annotated to family Lachnospiraceae were significantly enriched in participants with the elevated CM risk phenotype, along with individual risk factors, including elevated waist circumference and hypertriglyceridemia. Previously, a number of studies have found that an enrichment of Lachnospiraceae was associated with the development of obesity, insulin resistance and other metabolic disorders [57–61]. Notably, participants with an elevated waist circumference also showed an enrichment for the genus Streptococcus, which has previously been found to be enriched in some CM diseases [17]. In our study, participants with healthy waist circumferences, and blood pressure and a normal fasting blood glucose exhibited a significant enrichment of the Ruminococcaceae family (S1 File), which includes several kinds of beneficial bacteria known to be negatively correlated to metabolic disease and associated with lower CM risk, e.g. genus Faecalibacterium [62, 63] and genus Oscillospira [64]. Similarly, gut bacterial ESVs annotated to the Clostridiaceae and Peptostreptococcaceae families and Prevotella genus were enriched in participants with a lower total CM risk as well as individual CM risk factors, e.g. healthy waist circumferences and normal fasting blood glucose levels, consistent with previous research, reporting a negative association with obesity or other CM risk factors [18, 65, 66]. Although it should also be noted that there are a handful of studies that suggest that Clostridiaceae and Prevotella are significantly enriched in patients with metabolic diseases [66–68].
Our predicted metagenomic functional analysis showed that participants with elevated CM risk harbored a more pronounced inflammatory phenotype. Indeed, predicted genes involved in LPS biosynthesis pathways were significantly enriched in participants presenting with individual CM risk factors across diverse geographic settings. Similar findings have previously been reported and suggest that a capacity for an increased LPS synthesis potential in the gut microbiota of people with obesity, diabetes, symptomatic atherosclerosis, arterial thrombosis, and other related metabolic diseases [19–22, 69]. Of note, the predicted relative proportion of genes that encode for enzymes involved in butyrate synthesis pathway suggested that the lysine pathway, glutarate pathway, and 4-aminobytyrate/succinate pathway were significantly enriched in individuals with elevated CM risk and also individual risk factor. Butyrate can be synthesized via different substrates, driven by enzymes that are produced and secreted by different bacteria [70]. While the most common pathway for butyrate synthesis is via pyruvate and acetyl-coenzyme A, other less-dominant pathways include amino-acids (lysine, glutarate and 4-aminobytyrate/succinate) as substrates via the 4-aminobutyrate pathway, which can produce pro-inflammatory byproducts [70] and are related with obesity. Together, these results suggest that the gut microbial metabolic functional potential of participants with elevated CM risk had a marked inflammation-driving capacity, which may influence host systemic inflammatory levels and may ultimately lead to the CM disease consequence.
While the impact of ethnicity on the core of gut microbiota has been demonstrated by several studies [71, 72], exploring the impact of ethnicity and the associations between gut microbiota and CM risk has not been performed to date. Some studies find that there is a decrease in the gut microbial alpha-diversity that is associated with CM risk from different ethnicities, e.g. Danish obese individuals when compared to non-obese individuals9 and obese compared with lean in a mid-western US female adolescent twin cohort study [56]. Similarly, previous studies find that there is a shared alteration in the gut microbial composition of the individuals with elevated CM risk and adiposity derived from different ethnicities. For example there was an adiposity-related enrichment of Lachnospiraceae in the gut microbiota of participants enrolled in the Twins UK cohort study[61], as well as among British obese individuals [60], and obese, Colombian subjects [73] with increased cardiometabolic risk, and finally among individuals in the midst of Westernization [74] when compared to the lean individuals, respectively. Interestingly, the association between TMAO and cardiovascular disease has been found to be significantly greater in whites compared to blacks [26]. In our study of African-origin adults, there was no significant association between predicted genes involved in TMAO biosynthetic pathway and total CM risk or individual CM risk factors, consistent with previous research, finding a negative association between TMAO concentration and cardiac death in black participants [26].
Numerous reports have implicated a close linkage between oral infections, particularly periodontitis, and several systemic diseases, e.g. atherosclerosis, type 2 diabetes mellitus, atherosclerotic cardiovascular disease [32–34]. In our study, we found that the oral microbial structure (measured by beta diversity) was associated with indices of elevated CM risk, e.g. high waist circumference, elevated fasted blood glucose, and low HDL concentration. However, as with gut microbiota, these associations varied according to the geographic region. There were also several ESVs significantly associated with total elevated CM risk as well as the individual CM risk factors. For example, participants with total elevated CM risk or individual CM risk factors, e.g. elevated waist circumference, low HDL concentration, elevated blood pressure or hypertriglyceridemia, exhibited enriched oral bacterial ESVs annotated to genus Streptococcus, Prevotella, or Veillonella, which were previously reported to be associated with obesity and other cardiometabolic disease [75–78]. Systemic inflammation is an important component of the role of oral bacteria in the pathogenesis of systemic diseases [32, 36]. Some strains in genera Streptococcus, Prevotella, or Veillonella are opportunistic pathogens and have been previously reported to be associated with inflammatory diseases [79–81], even though, we also found that ESVs in genus Neisseria were higher in participants without individual CM risk, e.g. elevated waist circumference, elevated blood pressure or hypertriglyceridemia, inconsistent with previous reports as described above. Importantly, we found a number of instances whereby different ESVs of the same genus were associated with differential CM risk. For example, different ESVs belonging to the genus Rothia were significantly enriched in the oral microbiota of participants both with and without elevated CM risk. While it is possible that these are spurious relationships, it is also possible that these two unclassified Rothia strains have different genetic makeup in non-genera conserved regions or different transcriptional and/or translational profiles in genus conserved regions associated with CM risk (e.g., distinct immunological and/or metabolic properties) that could result in different host-associated impact. Indeed, this may explain the conflicting results with certain studies [82]. The oral microbiota may significantly impact the CM risk through triggering of inflammatory processes. Consequently, an analysis of the oral microbiota can be imagined to be used as a pre-screening test for CM risk, despite differences between the gut and oral bacterial diversity and taxa associated with elevated CM risk. Notably, in this study, we also found a weak but significant association between the oral and gut microbiota, within the structure and also the functional taxa. Thus, it is conceivable that the oral microbiota might be used as a proxy to determine gut-derived associations with CM risk.
Conclusions
In conclusion, our findings extend our insights into the relationship between the human microbiota and elevated CM risk at the structural and functional level, pointing to possible future therapeutic modalities for CM risk targeting the gut and oral microbiota. Our findings identify previously unknown links between gut and oral microbiota alterations, and CM risk, suggesting that gut and oral microbial composition, structure and predicted functional potential may serve as predictive biomarkers for identifying CM risk, as well as therapeutic targets. The features of association between human microbiome and CM risk is diverse in different populations and indicates that different interventions and potential individualized treatment methods targeting the microbiome need to be developed to control the development of CM risk across the world. Indeed, we are continuing our exploration of these relationships in our large international cohort of African-origin adults spanning the epidemiologic transition [37].
Supporting information
(DOCX)
(a-c) Alpha diversity analysis (Shannon Index (a), observed OTU (b) and Chao1 (c)) from 16S rRNA gene sequences of stool microbiota shows the differences among population from USA, RSA, Ghana and Jamaica. USA, the United States of America; RSA, South Africa. * p < 0.05.
(TIF)
Alpha diversity analysis (Shannon Index) from 16S rRNA gene sequences of stool microbiota against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa. * p < 0.05, ** p < 0.01.
(TIF)
Principal coordinate analyses (PCoA) of weighted (a) and unweighted (b) UniFrac distances of stool microbiota composition in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
(a-r), Principal coordinate analyses (PCoA) of weighted UniFrac distance of stool microbiota composition against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
(a-r), Principal coordinate analyses (PCoA) of unweighted UniFrac distance of stool microbiota composition against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
(a-c), Waist circumference across all, male and female participants; (d-f), elevated blood pressure across all, male and female participants; (g-h), elevated fasted blood glucose across all and female participants; (i-j), hypertriglyceridemia across all and female participants; (k), HDL concentration across all and female participants. Only significantly differential ESAs with relative abundance ≥ 1% in at least one group are shown. Data shown are mean± S.E.M. p(fdr-corrected) <0.05. USA, the United States of America; RSA, South Africa.
(TIF)
(a), Waist circumference in Ghanaian and RSA population; (b), elevated fasting plasma glucose in Jamaican population; (c), elevated fasting plasma glucose in RSA population; (d), elevated fasting plasma glucose in RSA population. Only significantly differential ESAs with relative abundance ≥ 1% in at least one group are shown. Data shown are mean± S.E.M. p(fdr-corrected) < 0.05. USA, the United States of America; RSA, South Africa.
(TIF)
(a), Waist circumference in Ghanaian and USA population; (b), elevated blood pressure in Ghanaian and Jamaican population; (c), elevated fasting plasma glucose in USA and Jamaican population; (d), elevated fasting plasma glucose in USA and RSA population. USA, the United States of America; RSA, South Africa. Only significantly differential KOs are shown. Data shown are mean± S.E.M. p(fdr-corrected) < 0.05.
(TIF)
Only significantly differential KOs are shown. Data shown are mean± S.E.M. * p(fdr-corrected) <0.05.
(TIF)
(a), Waist circumference in Ghanaian and RSA population; (b), elevated blood pressure in RSA population. ko00620, pyruvate metabolism, ko00640, propanoate metabolism, and ko00650, butanoate metabolism. USA, the United States of America; RSA, South Africa. Only significantly differential KOs are shown. Data shown are mean± S.E.M. p(fdr-corrected) < 0.05.
(TIF)
USA, the United States of America; RSA, South Africa. Only significantly differential KOs are shown. Data shown are mean± S.E.M. * p(fdr-corrected) < 0.05.
(TIF)
(a-c) Alpha diversity analysis (Shannon Index (a), observed OTU (b) and Chao1 (c)) from 16S rRNA gene sequences from oral microbiota shows the difference among population from USA, RSA, Ghana and Jamaica. USA, the United States of America; RSA, South Africa. No shared letters above the boxplot means p < 0.05 between two groups.
(TIF)
Alpha diversity analysis (Shannon Index) from 16S rRNA gene sequence data of oral microbiota against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
Principal coordinate analyses (PCoA) of weighted (a) and unweighted (b) UniFrac distances of oral microbiota composition in USA, RSA, Ghanaian and Jamaican population. USA, the United States of America; RSA, South Africa.
(TIF)
(a-r), Principal coordinate analyses (PCoA) of weighted UniFrac distance of oral microbiota composition against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
(a-r), Principal coordinate analyses (PCoA) of unweighted UniFrac distance of oral microbiota composition against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
Alpha diversity analysis (Shannon Index) from 16S rRNA gene sequence data of oral microbiota against elevated CM risk, defined as at least of 3 CM risk factors of waist circumference, elevated blood pressure, elevated blood fasting glucose, triglyceride and HDL concentration in USA, RSA, and Ghanaian populations. CMD, elevated CM risk.
(TIF)
(a-c), Waist circumference across all, male and female participants; (d-f), elevated blood pressure across all, male and female participants; (g), hypertriglyceridemia across female participants; (h) HDL concentration across male participants. Only significantly differential ESAs with relative abundance ≥ 1% in at least one group are shown. Data shown are mean± S.E.M. p(fdr-corrected) <0.05. USA, the United States of America; RSA, South Africa.
(TIF)
(a), Waist circumference in USA, Ghanaian and RSA population; (b), elevated fasting plasma glucose in USA population; (c), hypertriglyceridemia in Ghanaian population. Only significantly differential ESAs with relative abundance ≥ 1% in at least one group are shown. Data shown are mean± S.E.M. p(fdr-corrected) < 0.05. USA, the United States of America; RSA, South Africa.
(TIF)
(a) Scatter plot of beta diversity in the gut microbiota versus the oral microbiome independently of the country of origin; (b-e) and for each country (b) USA, (c) RSA, (d), Ghana (e) and Jamaica. USA, the United States of America; RSA, South Africa.
(TIF)
(a) Scatter plot of beta diversity in the gut microbiota versus the oral microbiome independently of the country of origin; (b-e) and for each country (b) USA, (c) RSA, (d), Ghana (e) and Jamaica. USA, the United States of America; RSA, South Africa.
(TIF)
USA, the United States of America; RSA, South Africa.
(DOCX)
USA, the United States of America; RSA, South Africa. ESV, Exact Sequence Variant. Data shown are mean± S.E.M.
(DOCX)
USA, the United States of America; RSA, South Africa.
(DOCX)
USA, the United States of America; RSA, South Africa. ESV, Exact Sequence Variant. Data shown are mean± S.E.M.
(DOCX)
Acknowledgments
The authors would like to acknowledge the original METS study participants and site-specific clinic staff members. The current work is funded in part by the National Institutes of Health R01DK80763, R01DK090360 and R01DK111848. BTL is supported by the National Institutes of Health under award number, R01DK104927-01A1 and Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, VA merit (Grant no. 1I01BX003382-01-A1). BPB is supported by the Arnold O. Beckman Postdoctoral Award.
Data Availability
All 16S rRNA sequences and sample metadata are publicly available via the QIITA platform under study ID 11888 (https://qiita.ucsd.edu/study/description/11888) and in EBI under accession no.ERP115612 (Study: PRJEB32880).
Funding Statement
The current work is funded in part by the National Institutes of Health R01DK080763, and R01DK090360 to AL, and R01DK111848 to LRD. BTL is supported by the National Institutes of Health under award number, R01DK104927-01A1 and Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, VA merit (Grant no. 1I01BX003382-01-A1). BPB is supported by the Arnold O. Beckman Postdoctoral Award.
References
- 1.Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25(6):375–84. Epub 2010/04/29. 10.1007/s10654-010-9459-z ; PubMed Central PMCID [DOI] [PubMed] [Google Scholar]
- 2.Fan J, Song Y, Chen Y, Hui R, Zhang W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;168(5):4761–8. Epub 2013/08/27. 10.1016/j.ijcard.2013.07.230 ; PubMed Central PMCID [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Yang X, Yang J, Yang Z, Wang S, Sun F, et al. The Cohort Study on Prediction of Incidence of All-Cause Mortality by Metabolic Syndrome. PLoS One. 2016;11(5):e0154990 Epub 2016/05/20. 10.1371/journal.pone.0154990 ; PubMed Central PMCID: PMC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. Epub 2005/09/15. 10.1161/CIRCULATIONAHA.105.169404 . [DOI] [PubMed] [Google Scholar]
- 5.Moore JX, Chaudhary N, Akinyemiju T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24 10.5888/pcd14.160287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. . [PubMed] [Google Scholar]
- 7.Aron-Wisnewsky J, Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12(3):169–81. 10.1038/nrneph.2015.191 . [DOI] [PubMed] [Google Scholar]
- 8.Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4(7):676–94. Epub 2012/08/02. 10.3390/nu4070676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. Epub 2013/08/30. 10.1038/nature12506 . [DOI] [PubMed] [Google Scholar]
- 10.Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS (London, England). 2015;29(18):2409–18. Epub 2015/09/12. 10.1097/qad.0000000000000869 . [DOI] [PubMed] [Google Scholar]
- 11.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71. 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui X, Ye L, Li J, Jin L, Wang W, Li S, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8(1):635 10.1038/s41598-017-18756-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105(43):16731–6. 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7(9):503–14. Epub 2010/07/29. 10.1038/nrgastro.2010.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneeberger M, Everard A, Gomez-Valades AG, Matamoros S, Ramirez S, Delzenne NM, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643 Epub 2015/11/14. 10.1038/srep16643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–83. Epub 2013/09/12. 10.1136/gutjnl-2013-304833 . [DOI] [PubMed] [Google Scholar]
- 17.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–56. 10.1136/gutjnl-2015-310861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845 10.1038/s41467-017-00900-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. Epub 2007/04/26. 10.2337/db06-1491 . [DOI] [PubMed] [Google Scholar]
- 20.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. Isme J. 2013;7(4):880–4. Epub 2012/12/14. 10.1038/ismej.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiouptsi K, Reinhardt C. Impact of the commensal microbiota in atherosclerosis and arterial thrombosis. British journal of pharmacology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proceedings of the National Academy of Sciences. 2004;101(29):10679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21(2):91–6. 10.1016/j.cardfail.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaysen GA, Johansen KL, Chertow GM, Dalrymple LS, Kornak J, Grimes B, et al. Associations of Trimethylamine N-Oxide With Nutritional and Inflammatory Biomarkers and Cardiovascular Outcomes in Patients New to Dialysis. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2015;25(4):351–6. Epub 2015/03/25. 10.1053/j.jrn.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafi T, Powe NR, Meyer TW, Hwang S, Hai X, Melamed ML, et al. Trimethylamine N-Oxide and Cardiovascular Events in Hemodialysis Patients. J Am Soc Nephrol. 2017;28(1):321–31. Epub 2016/07/21. 10.1681/ASN.2016030374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829 Epub 2013/05/09. 10.1038/ncomms2852 ncomms2852 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–17. 10.2337/db08-1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–35. 10.1016/j.cmet.2013.01.003 . [DOI] [PubMed] [Google Scholar]
- 30.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9. 10.1038/nature04330 . [DOI] [PubMed] [Google Scholar]
- 31.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77. 10.1016/j.cmet.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kholy KE, Genco RJ, Van Dyke TE. Oral infections and cardiovascular disease. Trends Endocrinol Metab. 2015;26(6):315–21. 10.1016/j.tem.2015.03.001 . [DOI] [PubMed] [Google Scholar]
- 33.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4592–8. 10.1073/pnas.1011383107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitra S, Drautz-Moses DI, Alhede M, Maw MT, Liu Y, Purbojati RW, et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome. 2015;3:38 10.1186/s40168-015-0100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serra e Silva Filho W, Casarin RC, Nicolela EL Jr., Passos HM, Sallum AW, Goncalves RB. Microbial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patients. PLoS One. 2014;9(10):e109761 10.1371/journal.pone.0109761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inaba H, Amano A. Roles of oral bacteria in cardiovascular diseases—from molecular mechanisms to clinical cases: Implication of periodontal diseases in development of systemic diseases. J Pharmacol Sci. 2010;113(2):103–9. Epub 2010/05/27. . [DOI] [PubMed] [Google Scholar]
- 37.Luke A, Bovet P, Forrester TE, Lambert EV, Plange-Rhule J, Schoeller DA, et al. Protocol for the modeling the epidemiologic transition study: a longitudinal observational study of energy balance and change in body weight, diabetes and cardiovascular disease risk. BMC Public Health. 2011;11:927 Epub 2011/12/16. 10.1186/1471-2458-11-927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walters W, Hyde ER, Berg-Lyons D, Ackermann G, Humphrey G, Parada A, et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems. 2016;1(1). Epub 2016/11/09. 10.1128/mSystems.00009-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. Isme J. 2012;6(8):1621–4. 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. Epub 2010/04/13. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall M, Beiko RG. 16S rRNA Gene Analysis with QIIME2. Methods in molecular biology (Clifton, NJ). 2018;1849:113–29. Epub 2018/10/10. 10.1007/978-1-4939-8728-3_8 . [DOI] [PubMed] [Google Scholar]
- 42.Mandal S, Van Treuren W, White RA, Eggesbo M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis. 2015;26:27663 10.3402/mehd.v26.27663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peet RK. The measurement of species diversity. Annual review of ecology and systematics. 1974;5(1):285–307. [Google Scholar]
- 45.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Applied and environmental microbiology. 2005;71(12):8228–35. 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson MJ. A new method for non‐parametric multivariate analysis of variance. Austral ecology. 2001;26(1):32–46. [Google Scholar]
- 47.Storey JD. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology). 2002;64(3):479–98. [Google Scholar]
- 48.Murphy KP. Naive bayes classifiers. University of British Columbia; 2006;18. [Google Scholar]
- 49.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and environmental microbiology. 2006;72(7):5069–72. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantel N. The detection of disease clustering and a generalized regression approach. Cancer research. 1967;27(2):209–20. Epub 1967/02/01. . [PubMed] [Google Scholar]
- 51.Iwai S, Weinmaier T, Schmidt BL, Albertson DG, Poloso NJ, Dabbagh K, et al. Piphillin: Improved Prediction of Metagenomic Content by Direct Inference from Human Microbiomes. PLoS One. 2016;11(11):e0166104 Epub 2016/11/08. 10.1371/journal.pone.0166104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic acids research. 2012;40(Database issue):D109–14. Epub 2011/11/15. 10.1093/nar/gkr988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68(4):899–917. 10.1093/ajcn/68.4.899 . [DOI] [PubMed] [Google Scholar]
- 54.Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. 2015;66:343–59. Epub 2015/01/15. 10.1146/annurev-med-060513-093205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, et al. Development of a gut microbe–targeted nonlethal therapeutic to inhibit thrombosis potential. Nature medicine. 2018;24(9):1407 10.1038/s41591-018-0128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. Epub 2008/12/02. 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meehan CJ, Beiko RG. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol. 2014;6(3):703–13. 10.1093/gbe/evu050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6. 10.1038/nature11400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci Rep. 2016;6:35405 10.1038/srep35405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salonen A, Lahti L, Salojarvi J, Holtrop G, Korpela K, Duncan SH, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. Isme J. 2014;8(11):2218–30. 10.1038/ismej.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beaumont M, Goodrich JK, Jackson MA, Yet I, Davenport ER, Vieira-Silva S, et al. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol. 2016;17(1):189 Epub 2016/09/27. 10.1186/s13059-016-1052-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balamurugan R, George G, Kabeerdoss J, Hepsiba J, Chandragunasekaran AM, Ramakrishna BS. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br J Nutr. 2010;103(3):335–8. Epub 2009/10/24. 10.1017/S0007114509992182 . [DOI] [PubMed] [Google Scholar]
- 63.Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, et al. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio. 2014;5(4). Epub 2014/08/15. 10.1128/mBio.01438-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konikoff T, Gophna U. Oscillospira: a Central, Enigmatic Component of the Human Gut Microbiota. Trends in microbiology. 2016;24(7):523–4. Epub 2016/03/22. 10.1016/j.tim.2016.02.015 . [DOI] [PubMed] [Google Scholar]
- 65.Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245 Epub 2012/12/06. 10.1038/ncomms2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, et al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One. 2011;6(6):e20944 Epub 2011/06/23. 10.1371/journal.pone.0020944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085 Epub 2010/02/09. 10.1371/journal.pone.0009085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–70. Epub 2009/01/24. 10.1073/pnas.0812600106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nature communications. 2012;3:1245 10.1038/ncomms2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5(2):e00889 Epub 2014/04/24. 10.1128/mBio.00889-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Guo Z, Xue Z, Sun Z, Zhang M, Wang L, et al. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. The ISME journal. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nature medicine. 2018;24(10):1526 10.1038/s41591-018-0160-1 [DOI] [PubMed] [Google Scholar]
- 73.De La Cuesta-Zuluaga J, Corrales-Agudelo V, Carmona J, Abad J, Escobar J. Body size phenotypes comprehensively assess cardiometabolic risk and refine the association between obesity and gut microbiota. International Journal of Obesity. 2017. [DOI] [PubMed] [Google Scholar]
- 74.de la Cuesta-Zuluaga J, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. Gut microbiota is associated with obesity and cardiometabolic disease in a population in the midst of Westernization. Scientific reports. 2018;8(1):11356 10.1038/s41598-018-29687-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casarin RC, Barbagallo A, Meulman T, Santos VR, Sallum EA, Nociti FH, et al. Subgingival biodiversity in subjects with uncontrolled type-2 diabetes and chronic periodontitis. Journal of periodontal research. 2013;48(1):30–6. Epub 2012/07/06. 10.1111/j.1600-0765.2012.01498.x . [DOI] [PubMed] [Google Scholar]
- 76.Palleja A, Kashani A, Allin KH, Nielsen T, Zhang C, Li Y, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome medicine. 2016;8(1):67 Epub 2016/06/17. 10.1186/s13073-016-0312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramos E, Gaudier FL, Hearing LR, Del Valle GO, Jenkins S, Briones D. Group B streptococcus colonization in pregnant diabetic women. Obstetrics and gynecology. 1997;89(2):257–60. Epub 1997/02/01. . [DOI] [PubMed] [Google Scholar]
- 78.Zeigler CC, Persson GR, Wondimu B, Marcus C, Sobko T, Modeer T. Microbiota in the oral subgingival biofilm is associated with obesity in adolescence. Obesity (Silver Spring). 2012;20(1):157–64. Epub 2011/10/15. 10.1038/oby.2011.305 . [DOI] [PubMed] [Google Scholar]
- 79.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. The Journal of infectious diseases. 2002;186(6):798–806. Epub 2002/08/29. 10.1086/342845 . [DOI] [PubMed] [Google Scholar]
- 80.Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151(4):363–74. Epub 2017/05/26. 10.1111/imm.12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. Journal of leukocyte biology. 2006;80(2):267–77. Epub 2006/05/30. 10.1189/jlb.1105626 . [DOI] [PubMed] [Google Scholar]
- 82.Bhat M, Pasini E, Copeland J, Angeli M, Husain S, Kumar D, et al. Impact of Immunosuppression on the Metagenomic Composition of the Intestinal Microbiome: a Systems Biology Approach to Post-Transplant Diabetes. Sci Rep. 2017;7(1):10277 Epub 2017/09/02. 10.1038/s41598-017-10471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(a-c) Alpha diversity analysis (Shannon Index (a), observed OTU (b) and Chao1 (c)) from 16S rRNA gene sequences of stool microbiota shows the differences among population from USA, RSA, Ghana and Jamaica. USA, the United States of America; RSA, South Africa. * p < 0.05.
(TIF)
Alpha diversity analysis (Shannon Index) from 16S rRNA gene sequences of stool microbiota against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa. * p < 0.05, ** p < 0.01.
(TIF)
Principal coordinate analyses (PCoA) of weighted (a) and unweighted (b) UniFrac distances of stool microbiota composition in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
(a-r), Principal coordinate analyses (PCoA) of weighted UniFrac distance of stool microbiota composition against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
(a-r), Principal coordinate analyses (PCoA) of unweighted UniFrac distance of stool microbiota composition against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
(a-c), Waist circumference across all, male and female participants; (d-f), elevated blood pressure across all, male and female participants; (g-h), elevated fasted blood glucose across all and female participants; (i-j), hypertriglyceridemia across all and female participants; (k), HDL concentration across all and female participants. Only significantly differential ESAs with relative abundance ≥ 1% in at least one group are shown. Data shown are mean± S.E.M. p(fdr-corrected) <0.05. USA, the United States of America; RSA, South Africa.
(TIF)
(a), Waist circumference in Ghanaian and RSA population; (b), elevated fasting plasma glucose in Jamaican population; (c), elevated fasting plasma glucose in RSA population; (d), elevated fasting plasma glucose in RSA population. Only significantly differential ESAs with relative abundance ≥ 1% in at least one group are shown. Data shown are mean± S.E.M. p(fdr-corrected) < 0.05. USA, the United States of America; RSA, South Africa.
(TIF)
(a), Waist circumference in Ghanaian and USA population; (b), elevated blood pressure in Ghanaian and Jamaican population; (c), elevated fasting plasma glucose in USA and Jamaican population; (d), elevated fasting plasma glucose in USA and RSA population. USA, the United States of America; RSA, South Africa. Only significantly differential KOs are shown. Data shown are mean± S.E.M. p(fdr-corrected) < 0.05.
(TIF)
Only significantly differential KOs are shown. Data shown are mean± S.E.M. * p(fdr-corrected) <0.05.
(TIF)
(a), Waist circumference in Ghanaian and RSA population; (b), elevated blood pressure in RSA population. ko00620, pyruvate metabolism, ko00640, propanoate metabolism, and ko00650, butanoate metabolism. USA, the United States of America; RSA, South Africa. Only significantly differential KOs are shown. Data shown are mean± S.E.M. p(fdr-corrected) < 0.05.
(TIF)
USA, the United States of America; RSA, South Africa. Only significantly differential KOs are shown. Data shown are mean± S.E.M. * p(fdr-corrected) < 0.05.
(TIF)
(a-c) Alpha diversity analysis (Shannon Index (a), observed OTU (b) and Chao1 (c)) from 16S rRNA gene sequences from oral microbiota shows the difference among population from USA, RSA, Ghana and Jamaica. USA, the United States of America; RSA, South Africa. No shared letters above the boxplot means p < 0.05 between two groups.
(TIF)
Alpha diversity analysis (Shannon Index) from 16S rRNA gene sequence data of oral microbiota against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
Principal coordinate analyses (PCoA) of weighted (a) and unweighted (b) UniFrac distances of oral microbiota composition in USA, RSA, Ghanaian and Jamaican population. USA, the United States of America; RSA, South Africa.
(TIF)
(a-r), Principal coordinate analyses (PCoA) of weighted UniFrac distance of oral microbiota composition against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
(a-r), Principal coordinate analyses (PCoA) of unweighted UniFrac distance of oral microbiota composition against each CM risk factors, including waist circumference, blood pressure, blood fasting glucose, triglyceride and HDL concentration in USA, RSA, Ghanaian and Jamaican populations. USA, the United States of America; RSA, South Africa.
(TIF)
Alpha diversity analysis (Shannon Index) from 16S rRNA gene sequence data of oral microbiota against elevated CM risk, defined as at least of 3 CM risk factors of waist circumference, elevated blood pressure, elevated blood fasting glucose, triglyceride and HDL concentration in USA, RSA, and Ghanaian populations. CMD, elevated CM risk.
(TIF)
(a-c), Waist circumference across all, male and female participants; (d-f), elevated blood pressure across all, male and female participants; (g), hypertriglyceridemia across female participants; (h) HDL concentration across male participants. Only significantly differential ESAs with relative abundance ≥ 1% in at least one group are shown. Data shown are mean± S.E.M. p(fdr-corrected) <0.05. USA, the United States of America; RSA, South Africa.
(TIF)
(a), Waist circumference in USA, Ghanaian and RSA population; (b), elevated fasting plasma glucose in USA population; (c), hypertriglyceridemia in Ghanaian population. Only significantly differential ESAs with relative abundance ≥ 1% in at least one group are shown. Data shown are mean± S.E.M. p(fdr-corrected) < 0.05. USA, the United States of America; RSA, South Africa.
(TIF)
(a) Scatter plot of beta diversity in the gut microbiota versus the oral microbiome independently of the country of origin; (b-e) and for each country (b) USA, (c) RSA, (d), Ghana (e) and Jamaica. USA, the United States of America; RSA, South Africa.
(TIF)
(a) Scatter plot of beta diversity in the gut microbiota versus the oral microbiome independently of the country of origin; (b-e) and for each country (b) USA, (c) RSA, (d), Ghana (e) and Jamaica. USA, the United States of America; RSA, South Africa.
(TIF)
USA, the United States of America; RSA, South Africa.
(DOCX)
USA, the United States of America; RSA, South Africa. ESV, Exact Sequence Variant. Data shown are mean± S.E.M.
(DOCX)
USA, the United States of America; RSA, South Africa.
(DOCX)
USA, the United States of America; RSA, South Africa. ESV, Exact Sequence Variant. Data shown are mean± S.E.M.
(DOCX)
Data Availability Statement
All 16S rRNA sequences and sample metadata are publicly available via the QIITA platform under study ID 11888 (https://qiita.ucsd.edu/study/description/11888) and in EBI under accession no.ERP115612 (Study: PRJEB32880).