Widespread adoption of population-based screening has been associated with marked decreases in cervical cancer incidence and mortality in the United States over the last few decades. Despite these gains, an estimated 13 240 US women were diagnosed with cervical cancer in 2018, and 4170 died from the disease.1
A large body of consistent evidence implicates infection with high-risk types of human papillomavirus (hrHPV) as the causative agent in cervical cancer. These infections are common, occurring in the majority of sexually active women over their lifetime.2 While most infections resolve without clinical consequence over a period of several years, persistent infections can lead to high-grade precancerous cervical lesions (such as cervical intraepithelial neoplasia [CIN] grades 2 and 3) that can progress to cervical cancer. Approximately 30% of CIN grade 3 lesions progress to invasive cancer over a 30-year period.2 This slow progression allows many opportunities for these lesions to be detected and treated, thereby disrupting the trajectory to cancer.
In 2018, the US Preventive Services Task Force (USPSTF) updated its screening guidelines.3 In addition to continuing to recommend triennial cytology (Papanicolaou tests) for women ages 21 to 29 years followed by either continued triennial cytology or adding a test for high-risk types of HPV every 5 years from ages 30 to 65 years, the task force endorsed a strategy of hrHPV testing alone every 5 years for women ages 30 to 65 years. The USPSTF stated that referring all women with abnormal test results directly to colposcopy would lead to a much greater number of colposcopies, but it did not recommend any particular triage strategy for women with a positive test result for hrHPV; the Society of Gynecologic Oncology recommends triaging these women with HPV genotyping (tests for HPV types 16 or 18).4
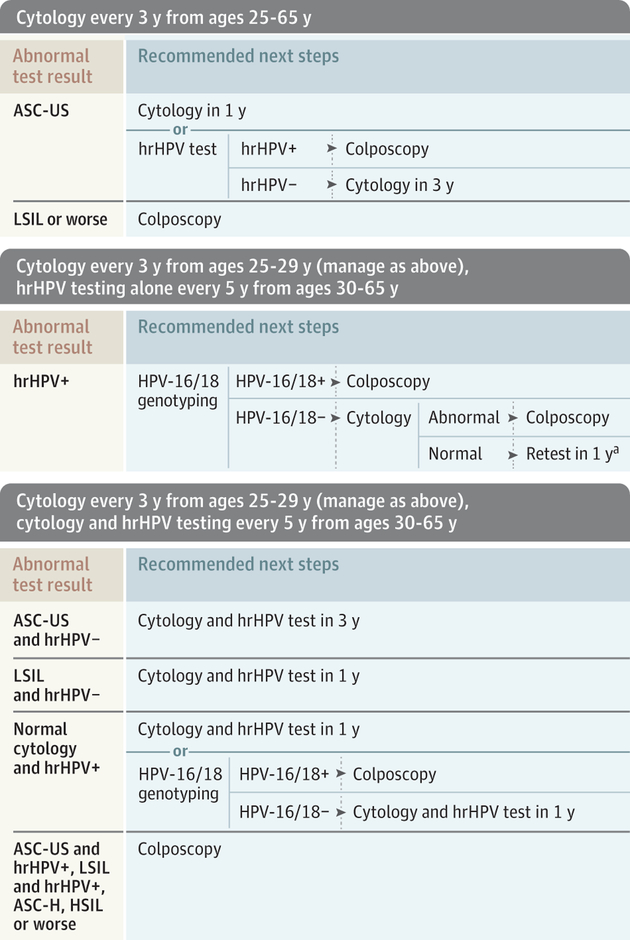
Current screening guidelines from the USPSTF, American College of Obstetricians and Gynecologists (ACOG),5 and American Cancer Society (ACS) and its partners6 are described in the eTable in the Supplement. These guidelines apply to women at average risk (no prior diagnosis of a high-grade precancerous lesion or cervical cancer, women who are not immunocompromised, and women with no in utero exposure to diethylstilbestrol). Expanded screening recommendations for women with higher risk are also included.5,7 All of these groups recommend discontinuing screening in women at average risk who have had a hysterectomy with removal of the cervix. Clinical actions following common abnormal screening test results, as recommended by professional societies,4,5,8 are displayed in the Figure.
Figure. Clinical Response to Common Abnormal Screening Test Results for Women at Average Risk of Cervical Cancer, Aged 25 to 65 Years.
Clinical responses are based on recommendations by the American College of Obstetricians and Gynecologists,5 the American Society of Colposcopy and Cervical Pathology,8 and the Society of Gynecologic Oncology.4 Average risk indicates women with no prior diagnosis of cervical intraepithelial neoplasia grade 2 or grade 3, adenocarcinoma in situ (AIS) or cervical cancer, women who are not immunocompromised, and women with no in utero exposure to diethylstilbestrol. For women aged 21 to 24 years, colposcopy is recommended for cytology interpreted as high-grade squamous intraepithelial lesion (HSIL) or worse (indicates atypical glandular cells, AIS, carcinoma, or HSIL) or atypical squamous cells, cannot exclude HSIL (ASC-H). For those with atypical squamous cells of undetermined significance (ASC-US) or low-grade squamous intraepithelial lesion (LSIL), repeat cytology at 12 and 24 months is recommended and colposcopy performed if either is ASC-H, HSIL or worse, or if cytology is persistently abnormal at 24 months. LSIL or worse indicates HSIL or worse in addition to LSIL. HPV indicates human papillomavirus; hrHPV, high-risk human papillomavirus.
a Precise test(s) not specified.
The endorsement of 3 strategies expands screening options and accommodates a variety of clinical settings. The 2018 USPSTF recommendation3 states that women aged 30 to 65 years should discuss with their health care professional which testing strategy is best for them, suggesting that their preferences may be important considerations when choosing a particular strategy. The USPSTF summarizes the clinical implications of choosing hrHPV testing alone or cotesting over the cytology strategy as follows: over a lifetime of screening, hrHPV-based strategies would avert approximately 1 additional cancer case per 1000 women screened compared with cytology, representing a “very small” improvement in life-years gained; hrHPV-based strategies, however, would subject women to more tests and procedures compared with cytology alone.
Shared decision making is easy to invoke but can be challenging to implement.9 Explaining the trade-offs between longer screening intervals and a higher likelihood of more testing due to surveillance can be complex and time consuming but is necessary if women’s informed preferences are to be integrated into clinical practice. Many women, however, may prefer not to engage in such a detailed and nuanced discussion and would seek the advice of their clinician regarding which strategy best balances benefits and harms. While the USPSTF does not consider costs in its deliberations, it states that cytology or hrH PV testing alone are preferred to cotesting, based on its assessment of this balance. Recent cost-effectiveness analyses suggest that cytology every 3 years for women ages 21 to 29 years with either continued triennial cytology or switching to a low-cost hrHPV test every 5 years from ages 30 to 65 years confers a reasonable balance of benefits, harms, and costs from both a societal and health care sector perspective.10
From a health systems vantage point, the hrHPV primary screening option requires availability of at least 1 of the tests that have been approved by the US Food and Drug Administration for that indication. The logistics of implementing the algorithms for follow-up of abnormal test results is another factor that may determine which strategies are most feasible for office and system workflow efficiencies. The Figure shows different clinical actions for women with abnormal test results; the complexity of the algorithms has the potential to challenge systems charged with coordinating follow-up visits and ensuring high-quality services if all 3 screening strategies are used.
In addition to screening, clinicians can further the goal of cervical cancer prevention by recommending appropriate HPV vaccination. A 2-dose schedule is recommended for girls and boys initiating vaccination at ages 9 to 14 years; 3 doses are recommended for those initiating the series at ages 15 to 26 years and for those who are immunocompromised. Until additional evidence emerges regarding the population effect of vaccination, all guideline groups recommend that vaccinated women be screened the same as unvaccinated women.
The field of cervical cancer prevention is highly dynamic, and clinicians should expect additional changes to clinical guidelines as newer evidence emerges. Amid enthusiasm for new strategies for women who participate in screening programs, however, clinicians should be aware that the most profound effect of screening on cervical cancer incidence and mortality can be achieved by providing unscreened and underscreened women with easy access to low-cost screening, diagnostic testing, and treatment. The appearance of a new approved strategy of hrHPV testing alone may enable the possibility of self-sampling, a strategy that may prove to be effective and more acceptable to some women than a clinic-collected sample. Until guideline groups recommend this screening approach, clinicians can further the goals of cervical cancer prevention by identifying and screening at-risk women in their practices and supporting outreach programs for women who are not receiving regular care.
Supplementary Material
Conflict of Interest Disclosures:
Dr Sawaya reported receipt of grants from the National Cancer Institute (NCI) during the conduct of the study. Dr Smith-McCune reported receipt of grants from the National Institutes of Health (NIH) during the conduct of the study. Dr Kuppermann reported grants from NIH/NCI during the conduct of the study.
Footnotes
Submissions: The Women’s Health editors welcome proposals for features in the section. Submit yours to ccrandall@mednet.ucla.edu or edward.livingston@jamanetwork.org.
REFERENCES
- 1.American Cancer Society. Cancer facts & figures 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed March 20, 2019.
- 2.Agency for Healthcare Research and Quality. Evidence synthesis No. 158: screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Home/GetFileBylD/3279. Accessed April 13, 2019. [PubMed]
- 3.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018:320(7): 674–686. doi: 10.1001/jama.2018.10897 [DOI] [PubMed] [Google Scholar]
- 4.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337. doi: 10.1097/A0G.0000000000000669 [DOI] [PubMed] [Google Scholar]
- 5.Practice bulletin No. 168 summary: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):923–925.doi: 10.1097/A0G.0000000000001699 [DOI] [PubMed] [Google Scholar]
- 6.Saslow D, Solomon D, Lawson HW, et al. ; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed March 20, 2019.
- 8.Massad LS, Einstein MH, Huh WK, et al. ; 2012 ASCCP Consensus Guidelines Conference. 2012 Updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4): 829–846. doi: 10.1097/A0G.0b013e3182883a34 [DOI] [PubMed] [Google Scholar]
- 9.Kuppermann M, Sawaya GF. Shared decision-making: easy to evoke, challenging to implement. JAMA Intern Med. 2015;175(2):167–168. doi: 10.1001/jamainternmed.2014.4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawaya GF, Sansted E, Alarid-Escudero F, et al. Estimated quality of life and economic outcomes associated with 12 cervical cancer screening strategies: a cost-effectiveness analysis. JAMA Intern Med. doi: 10.1001/jamainternmed.2019.0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.