Abstract
Objective:
Visfatin, a novel adipokine with diabetogenic and immunomodulatory properties, has been implicated in the pathophysiology of insulin resistance, as well as in various acute and chronic inflammatory disorders. We have previously reported that amniotic fluid concentrations of visfatin are higher in patients with preterm labor and intra-amniotic infection than in patients with preterm labor without inflammation. The aim of this study was to determine whether spontaneous preterm labor (PTL) with intact membranes and intra-amniotic infection/inflammation (IAI) is associated with changes in maternal plasma circulating visfatin concentrations.
Study design:
This cross-sectional study included patients in the following groups: 1) normal pregnant women (n=123); 2) patients with an episode of preterm labor and intact membranes without IAI who delivered at term (n=57); 3) preterm labor without IAI who delivered preterm (n=47); and 4) preterm labor with IAI who delivered preterm (n=57). Plasma visfatin concentrations were determined by ELISA. Non parametric statistics were used for analysis.
Results:
1) Preterm labor with IAI leading to preterm delivery was associated with a higher median maternal plasma concentration of visfatin than normal pregnancy; 2) among patients with preterm labor, those with IAI had the highest median maternal concentration of visfatin; 3) the changes in maternal plasma visfatin remained significant after adjusting for maternal age, body mass index, gestational age at sampling, and birth weight.
Conclusion:
1) Preterm labor with IAI is characterized by high maternal circulating visfatin concentrations; 2) these findings suggest that visfatin plays a role in the regulation of the metabolic adaptations to insults resulting in preterm labor in the context of IAI.
Keywords: Visfatin, Adipokines, Pregnancy, Preterm labor, Intra-amniotic infection, Inflammation, Chorioamnionitis, Preterm delivery, Energy Requirements, Energy Expenditure, Preterm Birth, Metabolism
Introduction
Infection and/or inflammation has been implicated as a mechanism of disease responsible for preterm parturition, as well as fetal injury.1–14 Moreover, infection/inflammation is the only pathologic processes for which a solid causal link with preterm parturition has been established.10;15 Indeed, there is experimental, epidemiologic and clinical evidence linking intra-amniotic infection and/or inflammation and preterm parturition.2–5;8;10;11;15–58 In addition, a large body of evidence supports the same cause and effect relationship between systemic infection/inflammation and preterm parturition: 1) systemic administration of microbial products to pregnant mice can result in spontaneous preterm labor and preterm birth;33;59;60 2) preterm labor and preterm birth can be induced by the administration of IL-1 to pregnant mice61 and exposure of these mice to IL-1 receptor antagonist abrogates parturition;62 3) systemic infection (e.g. malaria,63–65 pyelonephritis,66–70 pneumonia71–73 and periodontal disease74–83) have been associated with preterm birth; and 4) disorders characterized by a chronic inflammatory state such as systemic lupus erythematosus,84;85 inflammatory bowel diseases,86;87 asthma,88;89 and morbid obesity90–92 have been associated with preterm parturition.
Adipose tissue has emerged as a highly active endocrine organ93–96 that can orchestrate a metabolic response to insults, but also an inflammatory response via the production of soluble factors known as adipocytokines. Indeed, adipocytokines have been implicated in the pathophysiology of inflammatory disorders including asthma,97;98 ulcerative colitis,99;100 Crohn’s Disease,99 rheumatoid arthritis,101;102 multiple sclerosis103;104 and obesity.105–114 Moreover, these highly active peptide and proteins have immunoregulatory effects on the innate (e.g. resistin,58;115;116 visfatin57;117;118) adaptive or both limbs of the immune response (e.g. leptin,119–122 adiponectin123–126). Importantly, adipokines have also been implicated in the adaptation to gestation and complications of pregnancy.57;108;110;111;113;114;127–134
Visfatin, a 52 kDa newly discovered adipokine, has already been identified more than a decade ago as a growth factor for early B cell, termed pre-B cell colony-enhancing factor (PBEF).117;118;135–137 The re-discovery of visfatin as an adipokine that is preferentially produced by visceral adipose tissue,138–140 has facilitated the investigation of its metabolic and immunoregulatory effects. Indeed, in addition to its insulin-mimicking effects,139;141 visfatin plays a role as a regulator of the innate immune response117;137;142 as well as in inflammation associated with infection.143 Recently, we have reported that intra-amniotic infection/inflammation (IAI) is characterized by elevated amniotic fluid concentrations of adipocytokines.57;58 Specifically, amniotic fluid concentrations of visfatin were higher in patients with preterm labor and infection than those with preterm labor without infection.
Currently, there are no reports concerning maternal plasma visfatin in the presence of preterm labor or infectious disease. Thus, the aim of this study was to determine whether spontaneous preterm labor (PTL) with intact membranes and intra-amniotic infection/inflammation (IAI) is associated with changes in maternal plasma circulating visfatin concentrations.
Materials and methods
Study design and population
A cross-sectional study was designed by searching our clinical database and bank of biological samples, including 284 patients in the following groups: 1) normal pregnant women (n=123); 2) patients with an episode of preterm labor and intact membranes without IAI who delivered at term (n=57); 3) preterm labor without IAI who delivered preterm (<37 weeks gestation) (n=47); and 4) preterm labor with IAI who delivered preterm (n=57).
All women provided written informed consent prior to enrollment and the collection of blood and amniotic fluid. The collection and utilization of blood and amniotic fluid for research purposes was approved by the Institutional Review Boards of the Sotero del Rio Hospital (Chile), the Wayne State University (Detroit, Michigan, USA) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (Bethesda, Maryland, USA). Many of these samples have previously been used to study the biology of inflammation, homeostasis, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Clinical Definitions
Patients were considered to have a normal pregnancy if they had a singleton gestation, a normal oral glucose challenge test,144;145 and if they did not have any medical, obstetrical, or surgical complications, and delivered a term neonate (≥37 weeks) of appropriate birth weight for gestational age 146;147 without complications. Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical change before 37 completed weeks of gestation that required hospitalization. Microbial invasion of the amniotic cavity was defined as a positive amniotic fluid culture for micro-organisms. Intra-amniotic inflammation was diagnosed by an amniotic fluid IL-6 concentration ≥2.6 ng/mL.148
Amniotic fluid collection
Amniotic fluid samples were obtained by trans-abdominal amniocentesis performed for evaluation of microbial status of the amniotic cavity. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. An amniotic fluid white blood cell (WBC) count, glucose concentration and Gram-stain were also performed shortly after collection as previously described.149–151 The results of these tests were used for clinical management. Amniotic fluid IL-6 concentrations were used only for research purposes. Amniotic fluid not required for clinical assessment was centrifuged for 10 minutes at 4°C and the supernatant was aliquoted and stored at –70°C until analysis.
Maternal plasma sample collection and Human visfatin C-terminal immunoassay:
Maternal blood samples were collected immediately before or after the amniocentesis into vacutainer tubes. Blood was centrifuged at 1300 × g for 10 minutes at 4°C. The plasma obtained was stored at −80°C until analysis. The concentrations of visfatin in maternal plasma were determined using specific and sensitive enzyme immunoassays purchased from Phoenix Pharmaceuticals, Inc (Belmont, CA USA). Visfatin C-terminal assays were validated in our laboratory for human plasma prior to the conduction of this study. Validation included spike and recovery experiments, which produced parallel curves indicating that maternal plasma matrix constituents did not interfere with antigen- antibody binding in this assay system. Visfatin enzyme immunoassays are based on the principle of competitive binding and were conducted according to manufacturer recommendations. Briefly, assay plates are pre-coated with a secondary antibody and the non-specific binding sites have been blocked. Standards and samples were incubated in the assay plates along with primary antiserum and biotinylated peptide. The secondary antibody in the assay plates bound to the Fc fragment of the primary antibody whose Fab fragment competitively bound with both the biotinylated peptide and peptide standard or targeted peptide in the samples. Following incubation, the assay plates were repeatedly washed to remove unbound materials and incubated with a streptavidin-horseradish peroxidase (SA-HRP) solution. Following incubation, unbound enzyme conjugate was removed by repeated washing and a substrate solution was added to the wells of the assay plates and color developed in proportion to the amount of biotinylated peptide-SA-HRP complex but inversely proportional to the amount of peptide in the standard solutions or the samples. Color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA USA). Maternal plasma concentrations of visfatin C were determined by interpolation from individual standard curves composed of human visfatin peptide. The calculated inter and intra assay coefficients of variation (CVs) for visfatin C-terminal immunoassays in our laboratory were 5.3% and 2.4%, respectively. The sensitivity was calculated to be 0.04 ng/ml.
Statistical analysis
Normality of the data was tested using the Shapiro-Wilk or Kolmogorov-Smirnov tests. Since plasma visfatin concentrations were not normally distributed, non-parametric tests were used for analyses. Kruskal–Wallis tests with post-hoc analysis by Mann-Whitney U tests were used for comparisons of continuous variables. Comparison of proportions was performed by Chi-square test. Spearman rank correlation was utilized to assess correlations between maternal plasma concentration of visfatin, amniotic fluid WBC count, and amniotic fluid concentrations of glucose IL-6. Multiple linear regression analysis was used to determine which factors were significantly and independently associated with maternal plasma visfatin concentrations (after log transformation). The following parameters were included in the model: maternal age, gestational age at sampling, birth weight, first trimester body mass index (BMI) and the presence of IAI. A p value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 14 (SPSS Inc., Chicago, IL, USA).
Results
The demographic and clinical characteristics of women with a normal pregnancy and those with preterm labor are displayed in Table I. There was no significant difference in the median first trimester body mass index (BMI) or parity between the four groups. Women with a normal pregnancy had a lower gestational age at sampling than those with preterm labor without IAI who delivered preterm (p=0.02) but not than patients with preterm labor and IAI (p=0.4) or those with preterm labor who delivered at term (p=0.051). Women with a normal pregnancy had a higher median birth weight than any of the three preterm labor groups (p<0.001 for all comparison). Comparison of the demographics and clinical characteristics among patients with preterm labor is presented in Table I.
Table I.
Clinical and demographic characteristics of the study population
| Normal Pregnancy (n=123) |
PTL without IAI Term delivery (n=57) |
p1 | PTL without IAI Preterm delivery (n=47) |
p2 | PTL with IAI Preterm delivery (n=57) |
p3 | |
|---|---|---|---|---|---|---|---|
| Maternal age (years) | 25 (21–31) | 22 (19–26) | NS | 22 (19–27) | NS | 24 (20–27) | NS |
| Parity | 1 (0–2) | 1 (0–2) | NS | 1 (0–3) | NS | 1 (0–3) | NS |
| First trimester BMI (kg/m2) | 23.3 (21.6–26.2) | 23.1 (20.0–25.7) | NS | 23.4 (21.3–25.6) | NS | 24.8 (21.6–29.7) | NS |
| GA at blood sampling (weeks) | 27.7 (21.5–32.2) | 30.6 (26.3–32.7) | NS | 31.0 (28.9–32.3) | <0.01 | 25.1 (23.9–31.6) | NS |
| GA at delivery (weeks) | 39.8 (39.0–40.4) | 38.6 (37.6–39.3) | <0.01 | 34.2 (32.9–35.3) | <0.01 | 27.5 (24.4–32.8) | <0.01 |
| Birth weight (grams) | 3470 (3220–3730) | 2998 (2681–3250) | <0.01 | 2112 (1559–2358) | <0.01 | 920 (611–1742) | <0.01 |
p1: comparison between preterm labor who delivered at term and preterm labor without IAI
p2: comparison between preterm labor who delivered preterm without IAI and preterm labor with IAI
p3: comparison between preterm labor who delivered at term and preterm labor with IAI
Values are expressed as median and interquartile (IQR) range; PTL: preterm labor; GA: gestational age; BMI: body mass index; IAI: intra-amniotic infection/inflammation NS: not significant
Maternal plasma visfatin concentration in normal pregnant women and those with preterm labor
The median maternal plasma visfatin concentration was higher in patients with preterm labor with IAI than in those with preterm labor without IAI who delivered either preterm (median: 15.5 ng/mL, interquartile range [IQR] 14.2–19.0 vs. 14.4 ng/mL, IQR 11.3–15.9; p<0.001, Figure 1) or at term (15.5 ng/mL, IQR 14.2–19.0 vs. 14.4 ng/mL, IQR 12.2–17.2; p=0.002, Figure 1). Similarly, the median maternal plasma visfatin concentration was higher in patients with preterm labor with IAI than in those with a normal pregnancy (15.5 ng/mL, IQR 14.2–19.0 vs. 15.1 ng/mL, IQR 12.1–17.7; p=0.002, Figure 1).
Figure 1. Comparison of the median maternal plasma visfatin between women with normal pregnancies and patients with spontaneous PTL.
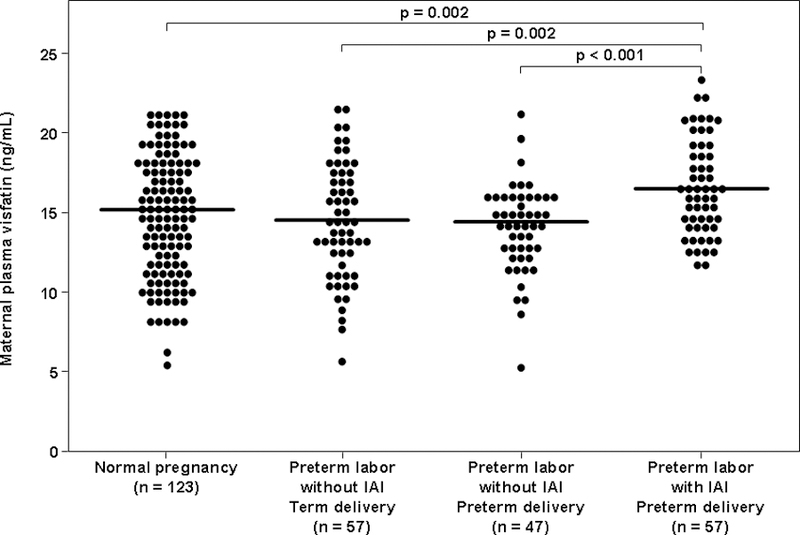
The median maternal plasma visfatin concentration was higher in patients with preterm labor with IAI than those with preterm labor without IAI who delivered either preterm or at term. Similarly, the median maternal plasma visfatin concentration was higher in patients with preterm labor with IAI than those with a normal pregnancy. The median maternal plasma visfatin concentration did not differ significantly between patients with preterm labor without IAI who delivered preterm and those who delivered at term. In addition, there was no significant difference in the median maternal plasma visfatin concentration between women with a normal pregnancy and those with preterm labor without IAI who delivered either preterm or at term.
Maternal plasma visfatin concentration in normal pregnant women and those with preterm labor without IAI
The median maternal plasma visfatin concentration did not differ significantly between patients with preterm labor without IAI who delivered preterm and those who delivered at term (p=0.3). In addition, there was no significant difference in the median maternal plasma visfatin concentration between women with a normal pregnancy and those with preterm labor without IAI who delivered either preterm (p=0.6) or at term (p=0.07).
Multiple regression analysis was employed to examine the relationship between the plasma concentrations of visfatin and preterm labor adjusting for maternal age, maternal first trimester body mass index, gestational age at blood sampling, birthweight and the presence of IAI. The final regression model suggested that the presence of IAI (p=0.001) and birthweight (p=0.04) was independently associated with a higher maternal plasma visfatin concentration. In addition, gestational age at blood sampling (p=0.001) was independently associated with a lower maternal plasma visfatin concentrations.
A significant correlation was observed between maternal plasma concentrations of visfatin and amniotic fluid concentrations of IL-6 (Spearman rho coefficient: 0.2, p=0.008).
Discussion
Principal findings of the study:
1) Patients with preterm labor with IAI leading to preterm delivery had a significantly higher median maternal plasma concentration of visfatin than those with normal pregnancy; 2) among patients with preterm labor, those with IAI had the highest median maternal concentration of visfatin; 3) There was a relationship between the maternal plasma concentration of visfatin and the amniotic fluid concentration of IL-6.
What is the physiologic role of visfatin?
Visfatin, a 52 kDa molecule, was originally reported to enhance the effect of IL-7 and stem cell factor on pre-B-cell colony formation, thus named pre-B-cell enhancing factor (PBEF).136 Subsequently, visfatin was reported to be produced by adipose tissue,139;152–156 preferentially by visceral fat depot,138;139;157 as well as in the placenta, fetal membranes,118;158–164 myometrium,165 bone marrow, liver, muscle,136 heart, lung, kidney,136 macrophages,166 and neutrophils.117;136;137
The physiologic role of visfatin in humans has not been completely elucidated, however, a growing body of evidence suggests that this adipokine plays a regulatory role in energy homeostasis and in the inflammatory response. The evidence concerning the immunoregulatory role of visfatin includes: 1) treatment of human monocytes with visfatin results in an increased secretion of. IL-6, TNF-α, IL-1β in a dose dependent manner;167 2) acute lung injury in humans137 and animals142 is associated with increased visfatin expression from cells retrieved by bronchoalveolar lavage from the affected subjects; 3) visfatin expression increases in neutrophils retrieved from septic patients;168 and 4) chronic inflammatory disorders (e.g. inflammatory bowel disease,167 rheumatoid arthritis169) have associated with a higher circulating visfatin concentrations than normal subjects.
The following findings support the role of visfatin in metabolic regulation: 1) treatment of adipocytes with glucose results in increased secretion of visfatin.170 Consistent with this observation, administration of glucose to human subjects results in increase circulating visfatin concentration;170 2) obesity is associated with increased circulating visfatin concentration,117;152;171–175 3) human serum concentration of visfatin are positively correlated with the amount of intra-visceral fat;175 5) patients with type-2 DM175–178 or metabolic syndrome179;180 have higher plasma concentrations of visfatin.
Visfatin in normal gestation and in complications of pregnancy:
Visfatin also plays a role in normal gestation, as well as in pregnancy complications. The evidence for that includes: 1) normal pregnancy is associated with altered maternal circulating visfatin concentrations;114;181–183 2) gestational diabetes mellitus (GDM) is associated with higher maternal concentrations of visfatin113;184;185 than non-diabetic pregnant women ; 3) preeclampsia186 and fetal growth restriction187 are associated with higher concentrations of visfatin than normal pregnancy; and 5) IAI is associated with higher amniotic fluid concentrations of visfatin than the absence of infection.57 Collectively, these data support a role for visfatin in the physiologic adaptations to pregnancy as well as in metabolic- and inflammatory-associated complications of pregnancy.
Intra amniotic infection/inflammation is characterized by high circulating maternal visfatin concentrations:
Preterm labor with IAI was associated with a higher maternal plasma visfatin concentration that preterm labor without IAI either with preterm or term delivery. Preterm labor with IAI was also associated with a higher maternal plasma visfatin than normal pregnant women. These findings are novel. Indeed, to date, there are no reports concerning the association between visfatin and preterm labor or any other infection-related conditions during pregnancy. The findings reported herein are consistent with our previous study in which median amniotic fluid concentration of visfatin found to be in patients with preterm labor with IAI, as well as with those reported by Bryant-Greenwood group in which expression and secretion of visfatin from amniotic epithelial were increased in response to various inflammatory stimuli.118;161;163;164;188;189
There are paucity of data concerning the association between visfatin and acute infection and/or inflammation.190 Transcription of the visfatin gene is increased in neutrophils obtained from critically ill, septic patients. Moreover, this increased transcription is mitigated through the use of an antisense oligonucleotide. Of note, neutrophils harvested from the circulation of septic patients are characterized by a marked inhibition of the apoptosis and enhanced respiratory burst capacity.190;191 Jia et al.117 have proposed that visfatin plays a crucial role in these processes. This suggestion is supported by the finding that incubation of non-activated neutrophils (taken from healthy subjects) with visfatin results in dose-dependent inhibition of apoptosis.117 Anther acute condition in which the role of visfatin was investigated is acute lung injury. Ye et al.137;142 reported that there is an increased expression of visfatin in cells retrieved by bronchoalveolar lavage from patients with this condition137 and in lung tissue of animals with acute lung injury.142 Moreover, patients with the −1001G allele in the visfatin gene have increased risk of developing ARDS than wild-type homozygotes, while the −1543T allele is associated with decreased risk of developing ARDS in septic shock patients.192
Why is preterm labor with IAI associated with increased maternal plasma concentrations of visfatin?
We can not ascertain from the current study whether increased maternal concentration of visfatin is causally related to preterm labor with IAI due to the cross-sectional nature of this report. However, several possibilities can be entertained to explain the increased concentration of visfatin, including the presence of labor along with the increased metabolic burden associated with infection.
Labor is associated with a dramatic increase in energy demands as manifested by increased maternal blood glucose,193;194 free fatty acids,195 ketone bodies196, high glucose turnover.197 It is possible that the acute nature of preterm labor requires prompt metabolic rearrangements to ensure constant flux of macronutrients to the fetus and the mother. Visfatin has been implicated in the pathophysiology of insulin resistance. It is tempting to suggest that the high maternal concentration of visfatin is part of the metabolic adaptation to preterm labor associated with infection. Indeed, the median maternal visfatin concentrations did not differ between patients in labor (albeit preterm) and those not in labor. Thus, prima facia, it seems that increased circulating visfatin is not associated to the labor per se rather than to the state of infection. However, the median gestational age of patients with preterm labor without IAI was significantly higher that this of those with normal pregnancy (not in labor). In addition, acute infection/inflammation is associated with hypermetabolism, enhanced energy expenditure, and insulin resistance.198–200 Thus, it is likely that the presence of IAI (independent of labor) is associated with increased metabolic demands. Increase in production and/or secretion of visfatin may be part of the adaptive response to the metabolic dysregulation associated with the state of infection.
An additional explanation can be that the alterations in circulating maternal visfatin concentration are aimed at regulating the immune response. Compelling evidence suggests that visfatin has an important role in the regulation of the innate immune limb. In vitro, visfatin induces, in a dose dependent manner, the production of proinflammatory cytokines (e.g. IL-6, TNF-α, IL-1β) by human monocytes.167 Moreover, the following pro-inflammatory mediators were reported to increase visfatin expression: TNF-α in monocytes,166 macrophages201 and neutrophils,117 IL-6 in synovial202 and amniotic epithelial cells162 and IL-8 and granulocyte/macrophage colony stimulating factor in neutrophils.117 Similarly, expression of visfatin can be induced by endotoxin/LPS from neutrophils117 amniotic epithelial cells162 and lung microvascular endothelial cells.142 Thus, we hypothesize that the increased concentrations of visfatin in patients with preterm labor and IAI can be part of the innate immune response (i.e. inflammation).
In conclusion, the present study is the first to demonstrate that maternal plasma visfatin concentrations are higher in patients with preterm labor and intra-amniotic infection. The findings reported herein together with our previous report focusing on amniotic fluid visfatin suggest that this adipokine may have a role hitherto unrecognized in the pathogenesis of preterm labor associated with infection. The data of the present study do not allow us to determine whether the increase in maternal visfatin concentration is secondary to preterm labor with IAI or whether it is present already before establishment of the disease. However, since visfatin has been implicated in the regulation of metabolic adaptations to insults, as well as in the regulation of the innate immune limb, we suggest that the perturbation in visfatin homeostasis is an adaptive response to the insult imposed by preterm labor with IAI.
Acknowledgment:
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Minkoff H Prematurity: infection as an etiologic factor. Obstet.Gynecol 1983;62:137–44. [PubMed] [Google Scholar]
- 2.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD et al. Infection in the pathogenesis of preterm labor. Semin.Perinatol 1988;12:262–79. [PubMed] [Google Scholar]
- 3.Romero R, Mazor M. Infection and preterm labor. Clin.Obstet.Gynecol 1988;31:553–84. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–23. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–41. [DOI] [PubMed] [Google Scholar]
- 6.Ledger WJ. Infection and premature labor. Am.J.Perinatol 1989;6:234–36. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am.J.Obstet.Gynecol 1992;166:1515–28. [DOI] [PubMed] [Google Scholar]
- 8.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin.Perinatol 1995;22:281–342. [PubMed] [Google Scholar]
- 9.Brocklehurst P. Infection and preterm delivery. BMJ 1999;318:548–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. In: Critchely H, Bennett P, Thornton S, editors. Preterm Birth London: RCOG Press; 2004. p. 28–60. [Google Scholar]
- 11.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O et al. The preterm parturition syndrome. BJOG 2006;113 Suppl 3:17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Pineles BL, Gotsch F et al. Recurrent preterm birth. Semin. Perinatol 2007;31:142–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O et al. The fetal inflammatory response syndrome. Clin.Obstet.Gynecol 2007;50:652–83. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J.Matern.Fetal Neonatal Med 2008;21:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc.Gynecol Investig 2005;12:145–55. [DOI] [PubMed] [Google Scholar]
- 16.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm.Dis 1983;10:294–302. [PubMed] [Google Scholar]
- 17.Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am.J.Obstet.Gynecol 1987;157:815–19. [DOI] [PubMed] [Google Scholar]
- 18.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–66. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–24. [DOI] [PubMed] [Google Scholar]
- 20.Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol 1990;163:757–61. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann.N.Y.Acad.Sci 1991;622:355–75. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–20. [DOI] [PubMed] [Google Scholar]
- 23.Gray DJ, Robinson HB, Malone J, Thomson RB Jr. Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat.Diagn 1992;12:111–17. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am.J.Obstet.Gynecol 1992;167:1086–91. [DOI] [PubMed] [Google Scholar]
- 25.McDuffie RS Jr., Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J.Obstet.Gynecol 1992;167:1583–88. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am.J.Obstet.Gynecol 1992;166:129–33. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J.Obstet.Gynecol 1992;166:1382–88. [DOI] [PubMed] [Google Scholar]
- 28.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet.Gynecol 1992;79:351–57. [DOI] [PubMed] [Google Scholar]
- 29.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993;5:81–88. [DOI] [PubMed] [Google Scholar]
- 30.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod.Immunol 1994;32:200–10. [DOI] [PubMed] [Google Scholar]
- 31.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994;171:1660–67. [DOI] [PubMed] [Google Scholar]
- 32.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–12. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol 1995;172:1598–603. [DOI] [PubMed] [Google Scholar]
- 34.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J.Reprod.Med 1995;40:375–79. [PubMed] [Google Scholar]
- 35.Mazor M, Hershkovitz R, Ghezzi F, Maymon E, Horowitz S, Leiberman JR. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand 1996;75:624–27. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. In: Elder MG, Romero R, Lamont RF, editors. Preterm labor New York, NY: Churchill Livingstone; 1997. p. 29–49. [Google Scholar]
- 37.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am.J.Obstet.Gynecol 1997;177:825–30. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am.J.Obstet.Gynecol 1998;179:186–93. [DOI] [PubMed] [Google Scholar]
- 39.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur.J.Obstet.Gynecol.Reprod.Biol 1998;78:5–10. [DOI] [PubMed] [Google Scholar]
- 40.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet.Gynecol 1998;179:194–202. [DOI] [PubMed] [Google Scholar]
- 41.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am.J.Obstet.Gynecol 2000;183:1130–37. [DOI] [PubMed] [Google Scholar]
- 42.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH et al. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol 2000;183:1070–77. [DOI] [PubMed] [Google Scholar]
- 43.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS et al. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol 2000;183:1138–43. [DOI] [PubMed] [Google Scholar]
- 44.Challis JR, Lye SJ, Gibb W, Whittle W, Patel F, Alfaidy N. Understanding preterm labor. Ann N.Y.Acad.Sci 2001;943:225–34. [DOI] [PubMed] [Google Scholar]
- 45.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am.J.Obstet.Gynecol 2001;185:1162–67. [DOI] [PubMed] [Google Scholar]
- 46.Gibbs RS, Davies JK, McDuffie RS Jr., Leslie KK, Sherman MP, Centretto CA et al. Chronic intrauterine infection and inflammation in the preterm rabbit, despite antibiotic therapy. Am J Obstet Gynecol 2002;186:234–39. [DOI] [PubMed] [Google Scholar]
- 47.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr.Rev 2002;60:S19–S25. [DOI] [PubMed] [Google Scholar]
- 48.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin.Neonatol 2002;7:259–74. [DOI] [PubMed] [Google Scholar]
- 49.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am.J.Obstet.Gynecol 2003;189:919–24. [DOI] [PubMed] [Google Scholar]
- 50.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol.Metab 2004;15:479–87. [DOI] [PubMed] [Google Scholar]
- 51.Keelan JA, Yang J, Romero RJ, Chaiworapongsa T, Marvin KW, Sato TA et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biology of Reproduction 2004;70:253–59. [DOI] [PubMed] [Google Scholar]
- 52.Menon R, Fortunato SJ. Fetal membrane inflammatory cytokines: a switching mechanism between the preterm premature rupture of the membranes and preterm labor pathways. J Perinat.Med 2004;32:391–99. [DOI] [PubMed] [Google Scholar]
- 53.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil.Steril 2004;82:799–804. [DOI] [PubMed] [Google Scholar]
- 54.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern.Fetal Neonatal Med 2005;18:405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern.Fetal Neonatal Med 2005;17:365–73. [DOI] [PubMed] [Google Scholar]
- 56.Vogel I, Thorsen P, Curry A, Sandager P, Uldbjerg N. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand 2005;84:516–25. [DOI] [PubMed] [Google Scholar]
- 57.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J.Perinat.Med 2008. [DOI] [PMC free article] [PubMed]
- 58.Kusanovic JP, Romero R., Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F et al. Resistin in Amniotic Fluid and its Association with Intra-amniotic Infection and Inflammation. The Journal of Maternal-Fetal and Neonatal Medicine 2008. [DOI] [PMC free article] [PubMed]
- 59.Fidel PL Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994;170:1467–75. [DOI] [PubMed] [Google Scholar]
- 60.Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am.J.Obstet.Gynecol 1996;174:754–59. [DOI] [PubMed] [Google Scholar]
- 61.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J.Obstet.Gynecol 1991;165:969–71. [DOI] [PubMed] [Google Scholar]
- 62.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J.Obstet.Gynecol 1992;167:1041–45. [DOI] [PubMed] [Google Scholar]
- 63.Gilles HM, Lawson JB, Sibelas M, Voller A, Allan N. Malaria, anaemia and pregnancy. Ann.Trop.Med Parasitol 1969;63:245–63. [DOI] [PubMed] [Google Scholar]
- 64.Herd N, Jordan T. An investigtion of malaria during pregnancy in Zimbabwe. Afr J Med 1981;27:62. [PubMed] [Google Scholar]
- 65.Kalanda BF, Verhoeff FH, Chimsuku L, Harper G, Brabin BJ. Adverse birth outcomes in a malarious area. Epidemiol.Infect 2005;1–8. [DOI] [PMC free article] [PubMed]
- 66.Hibbard L, Thrupp L, Summeril S, Smale M, Adams R. Treatment of pyelonephritis in pregnancy. Am J Obstet Gynecol 1967;98:609–15. [DOI] [PubMed] [Google Scholar]
- 67.Patrick MJ. Influence of maternal renal infection on the foetus and infant. Arch.Dis.Child 1967;42:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wren BG. Subclinical renal infection and prematurity. Med J.Aust 1969;2:596–600. [DOI] [PubMed] [Google Scholar]
- 69.Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: A clinical review. Obstet Gynecol 1973;42:112–17. [PubMed] [Google Scholar]
- 70.Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect.Immun 1999;67:5958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benedetti TJ, Valle R, Ledger WJ. Antepartum pneumonia in pregnancy. Am J Obstet.Gynecol 1982;144:413–17. [DOI] [PubMed] [Google Scholar]
- 72.Madinger NE, Greenspoon JS, Ellrodt AG. Pneumonia during pregnancy: has modern technology improved maternal and fetal outcome? Am J Obstet.Gynecol 1989;161:657–62. [DOI] [PubMed] [Google Scholar]
- 73.Munn MB, Groome LJ, Atterbury JL, Baker SL, Hoff C. Pneumonia as a complication of pregnancy. J.Matern.Fetal Med 1999;8:151–54. [DOI] [PubMed] [Google Scholar]
- 74.Offenbacher S, Beck JD, Lieff S, Slade G. Role of periodontitis in systemic health: spontaneous preterm birth. Journal Of Dental Education 1998;62:852–58. [PubMed] [Google Scholar]
- 75.Madianos PN, Lieff S, Murtha AP, Boggess KA, Auten RL Jr., Beck JD et al. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol 2001;6:175–82. [DOI] [PubMed] [Google Scholar]
- 76.Jeffcoat MK, Geurs NC, Reddy MS, Goldenberg RL, Hauth JC. Current evidence regarding periodontal disease as a risk factor in preterm birth. Ann.Periodontol 2001;6:183–88. [DOI] [PubMed] [Google Scholar]
- 77.Offenbacher S, Lieff S, Boggess KA, Murtha AP, Madianos PN, Champagne CM et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol 2001;6:164–74. [DOI] [PubMed] [Google Scholar]
- 78.Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: results of a prospective study. The Journal Of The American Dental Association 2001;132:875–80. [DOI] [PubMed] [Google Scholar]
- 79.Goepfert AR, Jeffcoat MK, Andrews WW, Faye-Petersen O, Cliver SP, Goldenberg RL et al. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet.Gynecol 2004;104:777–83. [DOI] [PubMed] [Google Scholar]
- 80.Jarjoura K, Devine PC, Perez-Delboy A, Herrera-Abreu M, D’Alton M, Papapanou PN. Markers of periodontal infection and preterm birth. Am.J.Obstet.Gynecol 2005;192:513–19. [DOI] [PubMed] [Google Scholar]
- 81.Khader YS, Ta’ani Q. Periodontal diseases and the risk of preterm birth and low birth weight: A meta-analysis. Journal of Periodontology 2005;76:161–65. [DOI] [PubMed] [Google Scholar]
- 82.Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG et al. Progressive periodontal disease and risk of very preterm delivery. Obstet.Gynecol 2006;107:29–36. [DOI] [PubMed] [Google Scholar]
- 83.Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG 2006;113:135–43. [DOI] [PubMed] [Google Scholar]
- 84.Johnson MJ, Petri M, Witter FR, Repke JT. Evaluation of preterm delivery in a systemic lupus erythematosus pregnancy clinic. Obstet.Gynecol 1995;86:396–99. [DOI] [PubMed] [Google Scholar]
- 85.Le HD, Wechsler B, Vauthier-Brouzes D, Seebacher J, Lefebvre G, Bletry O et al. Outcome of planned pregnancies in systemic lupus erythematosus: a prospective study on 62 pregnancies. Br.J.Rheumatol 1997;36:772–77. [DOI] [PubMed] [Google Scholar]
- 86.Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am.J.Gastroenterol 2002;97:641–48. [DOI] [PubMed] [Google Scholar]
- 87.Norgard B, Fonager K, Pedersen L, Jacobsen BA, Sorensen HT. Birth outcome in women exposed to 5-aminosalicylic acid during pregnancy: a Danish cohort study. Gut 2003;52:243–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kallen B, Rydhstroem H, Aberg A. Asthma during pregnancy--a population based study. Eur.J.Epidemiol 2000;16:167–71. [DOI] [PubMed] [Google Scholar]
- 89.Dombrowski MP, Schatz M, Wise R, Momirova V, Landon M, Mabie W et al. Asthma during pregnancy. Obstet.Gynecol 2004;103:5–12. [DOI] [PubMed] [Google Scholar]
- 90.Adams MM, Sarno AP, Harlass FE, Rawlings JS, Read JA. Risk factors for preterm delivery in a healthy cohort. Epidemiology 1995;6:525–32. [DOI] [PubMed] [Google Scholar]
- 91.Nohr EA, Bech BH, Vaeth M, Rasmussen KM, Henriksen TB, Olsen J. Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatr.Perinat.Epidemiol 2007;21:5–14. [DOI] [PubMed] [Google Scholar]
- 92.Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of Body Mass Index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC.Public Health 2007;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann.N.Y.Acad.Sci 1999;892:146–54. [DOI] [PubMed] [Google Scholar]
- 94.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–46. [DOI] [PubMed] [Google Scholar]
- 95.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat.Rev.Immunol 2006;6:772–83. [DOI] [PubMed] [Google Scholar]
- 96.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 2006;49:1677–85. [DOI] [PubMed] [Google Scholar]
- 97.Gurkan F, Atamer Y, Ece A, Kocyigit Y, Tuzun H, Mete N. Serum leptin levels in asthmatic children treated with an inhaled corticosteroid. Ann.Allergy Asthma Immunol 2004;93:277–80. [DOI] [PubMed] [Google Scholar]
- 98.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J.Allergy Clin.Immunol 2004;114:254–59. [DOI] [PubMed] [Google Scholar]
- 99.Barbier M, Vidal H, Desreumaux P, Dubuquoy L, Bourreille A, Colombel JF et al. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol.Clin.Biol 2003;27:987–91. [PubMed] [Google Scholar]
- 100.Tuzun A, Uygun A, Yesilova Z, Ozel AM, Erdil A, Yaman H et al. Leptin levels in the acute stage of ulcerative colitis. J.Gastroenterol.Hepatol 2004;19:429–32. [DOI] [PubMed] [Google Scholar]
- 101.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Scholmerich J et al. Adipocytokines in synovial fluid. JAMA 2003;290:1709–10. [DOI] [PubMed] [Google Scholar]
- 102.Bernotiene E, Palmer G, Talabot-Ayer D, Szalay-Quinodoz I, Aubert ML, Gabay C. Delayed resolution of acute inflammation during zymosan-induced arthritis in leptin-deficient mice. Arthritis Res.Ther 2004;6:R256–R263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matarese G, Sanna V, Di GA, Lord GM, Howard JK, Bloom SR et al. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur.J.Immunol 2001;31:1324–32. [DOI] [PubMed] [Google Scholar]
- 104.Batocchi AP, Rotondi M, Caggiula M, Frisullo G, Odoardi F, Nociti V et al. Leptin as a marker of multiple sclerosis activity in patients treated with interferon-beta. J.Neuroimmunol 2003;139:150–54. [DOI] [PubMed] [Google Scholar]
- 105.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol.Metab 2000;11:327–32. [DOI] [PubMed] [Google Scholar]
- 106.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J.Clin.Endocrinol.Metab 2001;86:3815–19. [DOI] [PubMed] [Google Scholar]
- 107.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003;289:1799–804. [DOI] [PubMed] [Google Scholar]
- 108.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J.Perinat.Med 2007;35:522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes.Rev 2007;8:21–34. [DOI] [PubMed] [Google Scholar]
- 110.Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F et al. Resistin: a hormone which induces insulin resistance is increased in normal pregnancy. J.Perinat.Med 2007;35:513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F et al. Adiponectin in severe preeclampsia. J.Perinat.Med 2007;35:503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F et al. Adiponectin multimers in maternal plasma. J.Matern.Fetal Neonatal Med 2008;21:796–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than GN et al. Maternal visfatin concentration in normal pregnancy. Journal Of Perinatal Medicine 2008. [DOI] [PMC free article] [PubMed]
- 114.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than GN et al. Visfatin in human pregnancy: maternal gestational diabetes vis-a-vis neonatal birthweight. Journal Of Perinatal Medicine 2008. [DOI] [PMC free article] [PubMed]
- 115.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation 2003;108:736–40. [DOI] [PubMed] [Google Scholar]
- 116.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J.Immunol 2005;174:5789–95. [DOI] [PubMed] [Google Scholar]
- 117.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J.Clin.Invest 2004;113:1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am.J.Obstet.Gynecol 2002;187:1051–58. [DOI] [PubMed] [Google Scholar]
- 119.Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc.Natl.Acad.Sci.U.S.A 1996;93:14564–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J.Immunol 2005;174:3137–42. [DOI] [PubMed] [Google Scholar]
- 121.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem.Biophys.Res.Commun 2002;298:297–302. [DOI] [PubMed] [Google Scholar]
- 122.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998;394:897–901. [DOI] [PubMed] [Google Scholar]
- 123.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem.Biophys.Res.Commun 2004;323:630–35. [DOI] [PubMed] [Google Scholar]
- 124.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett 2005;579:6821–26. [DOI] [PubMed] [Google Scholar]
- 125.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 2000;96:1723–32. [PubMed] [Google Scholar]
- 126.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ.Res 2008;102:218–25. [DOI] [PubMed] [Google Scholar]
- 127.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F et al. Maternal Adiponectin Multimers In Normal Pregnancy. The Journal of Maternal-Fetal and Neonatal Medicine 2008. [DOI] [PMC free article] [PubMed]
- 128.Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr.Diab.Rep 2005;5:278–81. [DOI] [PubMed] [Google Scholar]
- 129.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Schiff E, Sivan E. Cord blood adiponectin in large-for-gestational age newborns. Am.J.Obstet.Gynecol 2005;193:1238–42. [DOI] [PubMed] [Google Scholar]
- 130.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Wiser A, Schiff E et al. Maternal serum adiponectin levels during human pregnancy. J.Perinatol 2007;27:77–81. [DOI] [PubMed] [Google Scholar]
- 131.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E et al. Determining the source of fetal adiponectin. J.Reprod.Med 2007;52:774–78. [PubMed] [Google Scholar]
- 132.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Yinon Y, Wiser A et al. Adiponectin and leptin concentrations in dichorionic twins with discordant and concordant growth. J.Clin.Endocrinol.Metab 2008. [DOI] [PubMed]
- 133.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than NG et al. Visfatin in human pregnancy: maternal gestational diabetes vis-a-vis neonatal birthweight. J.Perinat.Med 2008. [DOI] [PMC free article] [PubMed]
- 134.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R et al. Adiponectin in human cord blood: relation to fetal birth weight and gender. J.Clin.Endocrinol.Metab 2003;88:5656–60. [DOI] [PubMed] [Google Scholar]
- 135.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol.Hum.Reprod 2002;8:399–408. [DOI] [PubMed] [Google Scholar]
- 136.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol.Cell Biol 1994;14:1431–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am.J.Respir.Crit Care Med 2005;171:361–70. [DOI] [PubMed] [Google Scholar]
- 138.Hug C, Lodish HF. Medicine. Visfatin: a new adipokine. Science 2005;307:366–67. [DOI] [PubMed] [Google Scholar]
- 139.Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol.Med 2005;11:344–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tanaka M, Nozaki M, Fukuhara A, Segawa K, Aoki N, Matsuda M et al. Visfatin is released from 3T3-L1 adipocytes via a non-classical pathway. Biochem.Biophys.Res.Commun 2007;359:194–201. [DOI] [PubMed] [Google Scholar]
- 141.Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ et al. Insulin-like effects of visfatin on human osteoblasts. Calcif.Tissue Int 2007;80:201–10. [DOI] [PubMed] [Google Scholar]
- 142.Ye SQ, Zhang LQ, Adyshev D, Usatyuk PV, Garcia AN, Lavoie TL et al. Pre-B-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc.Res 2005;70:142–51. [DOI] [PubMed] [Google Scholar]
- 143.Bajwa EK, Yu CL, Gong MN, Thompson BT, Christiani DC. Pre-B-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome. Crit Care Med 2007;35:1290–95. [DOI] [PubMed] [Google Scholar]
- 144.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am.J.Obstet.Gynecol 1982;144:768–73. [DOI] [PubMed] [Google Scholar]
- 145.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet.Gynecol 2001;98:525–38. [PubMed] [Google Scholar]
- 146.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet.Gynecol 1996;87:163–68. [DOI] [PubMed] [Google Scholar]
- 147.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB et al. [A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000]. Rev.Med.Chil 2004;132:1155–65. [DOI] [PubMed] [Google Scholar]
- 148.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol 2001;185:1130–36. [DOI] [PubMed] [Google Scholar]
- 149.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am.J.Obstet.Gynecol 1988;159:114–19. [DOI] [PubMed] [Google Scholar]
- 150.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am.J.Obstet.Gynecol 1990;163:968–74. [DOI] [PubMed] [Google Scholar]
- 151.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am.J.Obstet.Gynecol 1991;165:821–30. [DOI] [PubMed] [Google Scholar]
- 152.Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 2005;54:2911–16. [DOI] [PubMed] [Google Scholar]
- 153.Choi KC, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG et al. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem.Biophys.Res.Commun 2005;336:747–53. [DOI] [PubMed] [Google Scholar]
- 154.Frydelund-Larsen L, Akerstrom T, Nielsen S, Keller P, Keller C, Pedersen BK. Visfatin mRNA expression in human subcutaneous adipose tissue is regulated by exercise. Am.J.Physiol Endocrinol.Metab 2007;292:E24–E31. [DOI] [PubMed] [Google Scholar]
- 155.Hammarstedt A, Pihlajamaki J, Rotter S, V, Gogg S, Jansson PA, Laakso M et al. Visfatin is an adipokine, but it is not regulated by thiazolidinediones. J.Clin.Endocrinol.Metab 2006;91:1181–84. [DOI] [PubMed] [Google Scholar]
- 156.Sethi JK. Is PBEF/visfatin/Nampt an authentic adipokine relevant to the metabolic syndrome? Curr.Hypertens.Rep 2007;9:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tanaka M, Nozaki M, Fukuhara A, Segawa K, Aoki N, Matsuda M et al. Visfatin is released from 3T3-L1 adipocytes via a non-classical pathway. Biochem.Biophys.Res.Commun 2007;359:194–201. [DOI] [PubMed] [Google Scholar]
- 158.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol.Hum.Reprod 2002;8:399–408. [DOI] [PubMed] [Google Scholar]
- 159.Nemeth E, Tashima LS, Yu Z, Bryant-Greenwood GD. Fetal membrane distention: I. Differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. Am.J.Obstet.Gynecol 2000;182:50–59. [DOI] [PubMed] [Google Scholar]
- 160.Nemeth E, Millar LK, Bryant-Greenwood G. Fetal membrane distention: II. Differentially expressed genes regulated by acute distention in vitro. Am.J.Obstet.Gynecol 2000;182:60–67. [DOI] [PubMed] [Google Scholar]
- 161.Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD. Chronic Stretching of Amniotic Epithelial Cells Increases Pre-B Cell Colony-Enhancing Factor (PBEF/Visfatin) Expression and Protects Them from Apoptosis. Placenta 2008. [DOI] [PubMed]
- 162.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J.Mol.Endocrinol 2001;26:107–17. [DOI] [PubMed] [Google Scholar]
- 163.Ognjanovic S, Tashima LS, Bryant-Greenwood GD. The effects of pre-B-cell colony-enhancing factor on the human fetal membranes by microarray analysis. Am.J.Obstet.Gynecol 2003;189:1187–95. [DOI] [PubMed] [Google Scholar]
- 164.Ognjanovic S, Ku TL, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor is a secreted cytokine-like protein from the human amniotic epithelium. Am.J.Obstet.Gynecol 2005;193:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Esplin MS, Fausett MB, Peltier MR, Hamblin S, Silver RM, Branch DW et al. The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am.J.Obstet.Gynecol 2005;193:404–13. [DOI] [PubMed] [Google Scholar]
- 166.Dahl TB, Yndestad A, Skjelland M, Oie E, Dahl A, Michelsen A et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation 2007;115:972–80. [DOI] [PubMed] [Google Scholar]
- 167.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J.Immunol 2007;178:1748–58. [DOI] [PubMed] [Google Scholar]
- 168.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J.Clin.Invest 2004;113:1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann.Rheum.Dis 2006;65:1198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia 2006;49:1909–14. [DOI] [PubMed] [Google Scholar]
- 171.Haider DG, Holzer G, Schaller G, Weghuber D, Widhalm K, Wagner O et al. The adipokine visfatin is markedly elevated in obese children. J.Pediatr.Gastroenterol.Nutr 2006;43:548–49. [DOI] [PubMed] [Google Scholar]
- 172.Zahorska-Markiewicz B, Olszanecka-Glinianowicz M, Janowska J, Kocelak P, Semik-Grabarczyk E, Holecki M et al. Serum concentration of visfatin in obese women. Metabolism 2007;56:1131–34. [DOI] [PubMed] [Google Scholar]
- 173.Filippatos TD, Derdemezis CS, Kiortsis DN, Tselepis AD, Elisaf MS. Increased plasma levels of visfatin/pre-B cell colony-enhancing factor in obese and overweight patients with metabolic syndrome. J.Endocrinol.Invest 2007;30:323–26. [DOI] [PubMed] [Google Scholar]
- 174.Filippatos TD, Derdemezis CS, Gazi IF, Lagos K, Kiortsis DN, Tselepis AD et al. Increased plasma visfatin levels in subjects with the metabolic syndrome. Eur.J.Clin.Invest 2008;38:71–72. [DOI] [PubMed] [Google Scholar]
- 175.Sandeep S, Velmurugan K, Deepa R, Mohan V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism 2007;56:565–70. [DOI] [PubMed] [Google Scholar]
- 176.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J.Clin.Endocrinol.Metab 2006;91:295–99. [DOI] [PubMed] [Google Scholar]
- 177.Fernandez-Real JM, Moreno JM, Chico B, Lopez-Bermejo A, Ricart W. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance. Diabetes Care 2007;30:616–21. [DOI] [PubMed] [Google Scholar]
- 178.Lopez-Bermejo A, Chico-Julia B, Fernandez-Balsells M, Recasens M, Esteve E, Casamitjana R et al. Serum visfatin increases with progressive beta-cell deterioration. Diabetes 2006;55:2871–75. [DOI] [PubMed] [Google Scholar]
- 179.Filippatos TD, Derdemezis CS, Kiortsis DN, Tselepis AD, Elisaf MS. Increased plasma levels of visfatin/pre-B cell colony-enhancing factor in obese and overweight patients with metabolic syndrome. J.Endocrinol.Invest 2007;30:323–26. [DOI] [PubMed] [Google Scholar]
- 180.Filippatos TD, Derdemezis CS, Gazi IF, Lagos K, Kiortsis DN, Tselepis AD et al. Increased plasma visfatin levels in subjects with the metabolic syndrome. Eur.J.Clin.Invest 2008;38:71–72. [DOI] [PubMed] [Google Scholar]
- 181.Mastorakos G, Valsamakis G, Papatheodorou DC, Barlas I, Margeli A, Boutsiadis A et al. The role of adipocytokines in insulin resistance in normal pregnancy: visfatin concentrations in early pregnancy predict insulin sensitivity. Clin.Chem 2007;53:1477–83. [DOI] [PubMed] [Google Scholar]
- 182.Morgan SA, Bringolf JB, Seidel ER. Visfatin expression is elevated in normal human pregnancy. Peptides 2008;29:1382–89. [DOI] [PubMed] [Google Scholar]
- 183.Katwa LC, Seidel ER. Visfatin in pregnancy: proposed mechanism of peptide delivery. Amino.Acids 2008. [DOI] [PubMed]
- 184.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N et al. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin.Sci.(Lond) 2006;110:605–09. [DOI] [PubMed] [Google Scholar]
- 185.Lewandowski KC, Stojanovic N, Press M, Tuck SM, Szosland K, Bienkiewicz M et al. Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance. Diabetologia 2007;50:1033–37. [DOI] [PubMed] [Google Scholar]
- 186.Fasshauer M, Waldeyer T, Seeger J, Schrey S, Ebert T, Kratzsch J et al. Serum levels of the adipokine visfatin are increased in preeclampsia. Clin.Endocrinol.(Oxf) 2007. [DOI] [PubMed]
- 187.Fasshauer M, Bluher M, Stumvoll M, Tonessen P, Faber R, Stepan H. Differential regulation of visfatin and adiponectin in pregnancies with normal and abnormal placental function. Clin.Endocrinol.(Oxf) 2007;66:434–39. [DOI] [PubMed] [Google Scholar]
- 188.Kendal-Wright CE. Stretching, mechanotransduction, and proinflammatory cytokines in the fetal membranes. Reprod.Sci 2007;14:35–41. [DOI] [PubMed] [Google Scholar]
- 189.Kendal CE, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor (PBEF/Visfatin) gene expression is modulated by NF-kappaB and AP-1 in human amniotic epithelial cells. Placenta 2007;28:305–14. [DOI] [PubMed] [Google Scholar]
- 190.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J.Leukoc.Biol 2008. [DOI] [PubMed]
- 191.Taneja R, Parodo J, Jia SH, Kapus A, Rotstein OD, Marshall JC. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med 2004;32:1460–69. [DOI] [PubMed] [Google Scholar]
- 192.Bajwa EK, Yu CL, Gong MN, Thompson BT, Christiani DC. Pre-B-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome. Crit Care Med 2007;35:1290–95. [DOI] [PubMed] [Google Scholar]
- 193.Kashyap ML, Sivasamboo R, Sothy SP, Cheah JS, Gartside PS. Carbohydrate and lipid metabolism during human labor: free fatty acids, glucose, insulin, and lactic acid metabolism during normal and oxytocin-induced labor for postmaturity. Metabolism 1976;25:865–75. [DOI] [PubMed] [Google Scholar]
- 194.Katz M, Kroll D, Shapiro Y, Cristal N, Meizner I. Energy expenditure in normal labor. Isr.J.Med Sci 1990;26:254–57. [PubMed] [Google Scholar]
- 195.Whaley WH, Zuspan FP, Nelson GH, Ahlquist RP. Alterations of plasma free fatty acids and glucose during labor. Am.J.Obstet.Gynecol 1967;97:875–80. [DOI] [PubMed] [Google Scholar]
- 196.Felig P, Lynch V. [Starvation in human pregnancy: hypoglycemia, hypoinsulinemia, and hyperketonemia.]. Science 1970;170:990–92. [DOI] [PubMed] [Google Scholar]
- 197.Maheux PC, Bonin B, Dizazo A, Guimond P, Monier D, Bourque J et al. Glucose homeostasis during spontaneous labor in normal human pregnancy. J.Clin.Endocrinol.Metab 1996;81:209–15. [DOI] [PubMed] [Google Scholar]
- 198.Goldstein SA, Elwyn DH. The effects of injury and sepsis on fuel utilization. Annu.Rev.Nutr 1989;9:445–73. [DOI] [PubMed] [Google Scholar]
- 199.Tappy L, Chiolero R. Substrate utilization in sepsis and multiple organ failure. Crit Care Med 2007;35:S531–S534. [DOI] [PubMed] [Google Scholar]
- 200.Trager K, DeBacker D, Radermacher P. Metabolic alterations in sepsis and vasoactive drug-related metabolic effects. Curr.Opin.Crit Care 2003;9:271–78. [DOI] [PubMed] [Google Scholar]
- 201.Iqbal J, Zaidi M. TNF regulates cellular NAD+ metabolism in primary macrophages. Biochem.Biophys.Res.Commun 2006;342:1312–18. [DOI] [PubMed] [Google Scholar]
- 202.Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum 2006;54:2084–95. [DOI] [PubMed] [Google Scholar]


