Abstract
Objective
To determine the relationship between the intensity of the intra-amniotic inflammatory response and the gestational age at the time of diagnosis in cases with preterm premature rupture of membranes (PROM) and intra-amniotic infection caused by Ureaplasma spp.
Methods
A retrospective cohort study was conducted which included 71 women with preterm PROM and a positive amniotic fluid culture with Ureaplasma spp. Women with mixed intra-amniotic infections were excluded. The study population was classified into 3 groups according to gestational age: group 1, <26 weeks (extreme preterm PROM, n=17); group 2, 26.0–33.9 weeks (moderate preterm PROM, n=39); group 3, 34.0–36.9 weeks (late preterm PROM, n=15). The intensity of the intra-amniotic and maternal inflammatory response was compared among 3 groups. The intensity of the intra-amniotic inflammatory response was assessed by the concentration of amniotic fluid matrix metalloproteinase-8 (MMP-8) and white blood cell (WBC) count. The maternal inflammatory response was assessed by the concentration of C-reactive protein (CRP) and WBC count in maternal blood at the time of amniocentesis.
Results
1) The median values of amniotic fluid MMP-8 concentration and WBC count were the highest in extreme preterm PROM group and the lowest in the late preterm PROM group (P < 0.001 and P=0.01, respectively); 2) the intensity of the maternal inflammatory response measured by maternal blood WBC count and CRP concentration was not significantly associated with gestational age at the time of diagnosis.
Conclusion
The earlier the gestational age at the time of PROM, the higher the intensity of the intra-amniotic inflammatory response in women with preterm PROM and intra-amniotic infection caused by Ureaplasma spp.
Keywords: Ureaplasma, preterm premature rupture of membranes, microbial invasion of amniotic cavity, intra-amniotic inflammation, preterm labor, chorioamnionitis, prematurity, intra-amniotic infection, matrix metalloproteinase-8
Introduction
Ureaplasma spp. are the most common microorganisms isolated from the amniotic fluid of women with preterm premature rupture of membranes (PROM) [1–14], preterm labor with intact membranes [15–17], acute cervical insufficiency [18–20], clinical chorioamnionitis at preterm [21] and term [22], PROM at term [23], and idiopathic vaginal bleeding [24].
A strong body of evidence supports that intra-amniotic infection with Ureaplasma spp. is associated with a robust host response in the fetal, amniotic, and maternal compartments [4, 6, 9, 16, 25–27] and that these organisms are associated with neonatal morbidity including neonatal bacteremia [28, 29], intraventricular hemorrhage [30, 31], chronic lung disease [32–36], meningitis [37], and adverse neuromotor development [38].
Ureaplasma spp. are frequently detected in amniotic fluid of asymptomatic women at early mid-trimester [39–41], which are often associated with preterm delivery, but not in all cases [40, 41]. These findings suggest that not only microbial invasion of the amniotic cavity but also the host response to microorganisms may be an important factor in determining the outcome of pregnancy.
Previous studies showed that the lower the gestational age, the higher the frequency of intra-amniotic infection and an intra-amniotic inflammation in symptomatic women with preterm PROM [42–44], and preterm labor [45, 46]. We hypothesized that the earlier the gestational age of PROM, the more intense the host-inflammatory response in women with intra-amniotic infection by Ureaplasma spp.
The purpose of this study was to determine the relationship between the intensity of intra-amniotic inflammation response and the gestational age in women with preterm PROM and intra-amniotic infection caused by Ureaplasma spp.
Material and Methods
Study Design
This study population consisted of 71 consecutive patients admitted to Seoul National University Hospital between 1993 and 2012 with the diagnosis of preterm PROM (<37 weeks of gestation) who met the following criteria: (1) singleton pregnancy; (2) amniotic fluid obtained for microbiologic studies by transabdominal amniocentesis or at the time of cesarean delivery, and (3) proven intra-amniotic infection by Ureaplasma spp. using cultivation techniques. Women with a mixed intra-amniotic infection (Ureaplasma spp. and other microorganisms) were excluded.
Patients were divided into 3 groups according to the gestational age at amniocentesis: group 1, gestational age <26 weeks (extreme preterm, n=17); group 2, gestational age between 26.0–33.9 weeks (moderate preterm, n=39); group 3, gestational age between 34.0–36.9 weeks (late preterm, n=15). The intensity of the intra-amniotic inflammatory response was determined by the amniotic fluid matrix metalloproteinase-8 (MMP-8) concentration and white blood cell (WBC) count, and that of the maternal inflammatory response was determined by C-reactive protein (CRP) and WBC count in maternal blood at the time of amniocentesis. Amniocentesis is routinely offered to all patients who are admitted with the diagnosis of preterm PROM for microbiologic studies and/or assessment of fetal lung maturity. Retrieval of amniotic fluid and maternal blood was performed after written informed consent was obtained.
We followed the ethical standards for human experimentation established in the Declaration of Helsinki. The Institutional Review Board of Seoul National University Hospital approved the collection and use of these samples and information for research purposes. The University has a Federal Wide Assurance with the Office for Human Research Protection (OHRP) of the Department of Health and Human Services (DHHS) of the United States.
Amniotic fluid analysis
Amniotic fluid was retrieved by transabdominal amniocentesis or at the time of cesarean delivery. The fluid was collected and transferred into commercially available culture media and transported immediately to the Department of Laboratory Medicine of our hospital and cultured for genital mycoplasmas (Ureaplasma spp. and Mycoplasma hominis) as well as aerobic and anaerobic bacteria. An aliquot of amniotic fluid was examined in a hemocytometer chamber to determine the WBC count. Fluid not used for clinical purposes was centrifuged and stored in polypropylene tubes at −70°C. The stored amniotic fluid was analyzed for MMP-8, which was measured using a commercially available enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis, MN, USA). Each measurement was performed in duplicate. Intra- and inter-assay coefficients were <10% each. Intra-amniotic inflammation was defined as an elevated concentration of MMP-8 (>23 ng/mL), as previously reported [19, 42, 47–54].
Diagnosis of chorioamnionitis and neonatal morbidity
Acute histologic chorioamnionitis was defined as the presence of acute inflammatory changes in the choriodecidua and amnion, respectively; acute funisitis was diagnosed by the presence of neutrophil infiltration into umbilical vessel walls or Wharton’s jelly using criteria previously published [55–57]. Clinical chorioamnionitis was diagnosed in the presence of a maternal temperature of ≥37.8°C and ≥2 of the following criteria: (1) uterine tenderness; (2) malodorous vaginal discharge; (3) maternal leukocytosis (WBC count >15,000 cells/mm3); (4) maternal tachycardia (>100 beats/min); and (5) fetal tachycardia (>160 beats/min) [22, 58–67]. Significant neonatal morbidity was defined as the presence of any of the following conditions: respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage (grade ≥ II), proven congenital neonatal sepsis, and necrotizing enterocolitis. These conditions were diagnosed according to definitions previously described in detail [55].
Statistical analysis
Non-parametric analyses were used. Proportions were compared using the Fisher’s exact test. The Kruskal-Wallis test and Jonckheere-Terpstra test were used for comparison of continuous variables and linear-by-linear association was used for comparison of the proportions among the three groups. A P value < 0.05 was considered as significant. SPSS 22.0 for Windows (IBM, Armonk, NY, USA) was used for statistical analyses.
Results
A total of 73 patients met the inclusion criteria. Amniotic fluid was not available for MMP-8 determination in 2 cases; therefore, these patients were excluded from further analysis. Seventeen women comprised group 1 (extreme preterm PROM group, gestational age <26 weeks), 39 comprised group 2 (moderate preterm PROM group, gestational age from 26.0 weeks to 33.9 weeks), and 15 comprised group 3 (late preterm PROM group, gestational age from 34.0 weeks to 36.9 weeks). Table 1 displays the clinical characteristics and pregnancy outcomes according to gestational age at amniocentesis. There were no significant differences in maternal age, parity, rate of cesarean delivery, antibiotics use and neonatal gender among the three groups. Gestational age at delivery and use of antenatal corticosteroids were significantly different among groups. The prevalence of clinical chorioamnionitis was highest in extreme preterm PROM group (12% [2/17]), followed by moderate preterm PROM group (5% [2/39]) and by late preterm PROM group (0% [0/15]). However, the difference was not statistically significant (P>0.1).
Table 1.
Clinical characteristics and pregnancy outcomes according to gestational age at amniocentesis
| Gestational age at amniocentesis | ||||
|---|---|---|---|---|
| Characteristics | −25.9 weeks (n=17) |
26–33.9 weeks (n=39) |
34–36.9 weeks (n=15) |
P value |
| Maternal age, years | 31.5 ± 4.3 | 30.3 ± 3.6 | 29.1 ± 5.2 | .390 |
| Nulliparity | 65% (6/17) | 51% (20/39) | 40% (6/15) | .492 |
| Gestational age at amniocentesis, weeks | 23.3 ± 2.9 | 30.3 ± 2.4 | 35.4 ± 0.6 | <.001 |
| Cervical dilatation, cm | 0.5 ± 0.9 | 0.7 ± 0.8 | 2.2 ± 2.2 | .017 |
| Gestational age at delivery, weeks | 25.0 ± 3.6 | 31.6 ± 2.3 | 35.5 ± 0.7 | <.001 |
| Birth weight, gram | 742 ± 430 | 1645 ± 513 | 2441 ± 303 | <.001 |
| Male newbornsa | 67% (5/15) | 62% (23/37) | 43% (6/14) | .363 |
| Cesarean deliveryb | 31% (5/16) | 42% (16/38) | 20% (3/15) | .297 |
| Antenatal corticosteroids use | 71% (12/17) | 69% (27/39) | 20% (3/15) | .002 |
| Antenatal antibiotics use | 100% (17/17) | 97% (38/39) | 87% (13/15) | .129 |
| Clinical chorioamnionitis | 12% (2/17) | 5% (2/39) | 0% (0/15) | .347 |
GA, gestational age.
Data was presents as mean ± standard deviation or % (n/N).
Five cases with unavailable data were excluded from analysis.
Two cases with unavailable data were excluded from analysis.
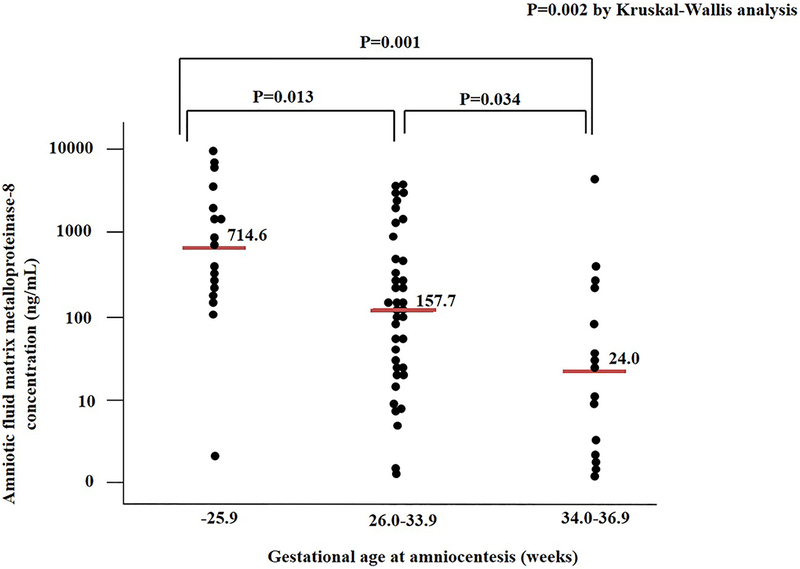
Figure 1 shows the intensity of intra-amniotic inflammatory response assessed by the amniotic fluid MMP-8 concentrations according to the gestational age at amniocentesis. The median amniotic fluid MMP-8 concentration in extreme preterm PROM group was 714.6 ng/mL (interquartile range [IQR], 165.8–2681.2 ng/mL), which was significantly higher than that of moderate preterm PROM group (median: 157.7 ng/mL, IQR: 24.9–870.8 ng/ml) and that of late preterm PROM group (median: 24.0 ng/ml, IQR 1.8–272.3 ng/ml) (P<0.05, respectively).
Figure 1.
Amniotic fluid matrix metalloproteinase-8 concentrations according to gestational age at amniocentesis (i.e., −25.9 weeks, 26.0–33.9 weeks, and 34.0–36.9 weeks) in women with intra-amniotic infection by Ureaplasma spp. (median: 714.6 ng/mL, interquartile range [IQR]: 165.8–2681.2 ng/mL vs. median: 157.7 ng/mL, IQR: 24.9–870.8 ng/mL vs. median: 24.0 ng/mL, IQR: 1.8–272.3 ng/mL; P=.002 by Kruskal-Wallis analysis).
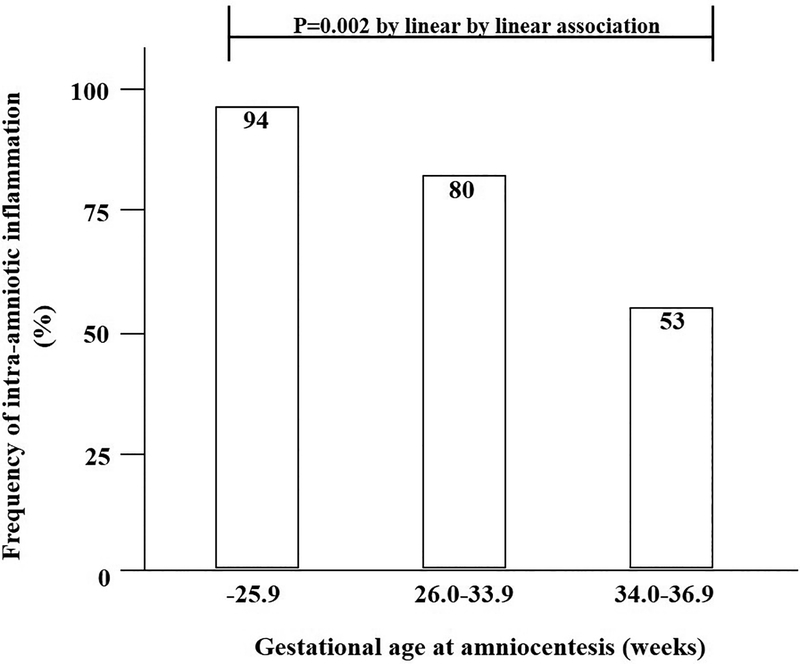
Table 2 shows that the amniotic fluid MMP-8 concentrations and the WBC counts decrease as a function of advancing gestational age at the time of amniocentesis (P<.001 for amniotic fluid MMP-8 concentrations and P=.010 for amniotic fluid WBC count by Jonckheere-Terpstra test). Figure 2 shows the rate of intra-amniotic inflammation defined as amniotic fluid MMP-8 concentration of > 23 ng/mL according to the gestational age at amniocentesis. The rate of intra-amniotic inflammation was 94% (16/17) in the extreme preterm PROM group, 80% [31/39] in moderate preterm PROM group, and 53% [8/15] in late preterm PROM group. It decreases with advancing gestational age (P=.002 by linear by linear association).
Table 2.
The amniotic fluid matrix metalloproteinase-8 concentration and amniotic white blood cell count according to gestational age at amniocentesis
| Gestational age at amniocentesis (weeks) | Amniotic fluid matrix metalloproteinase-8 (ng/ml) | P-valuea | Amniotic fluid white blood cell (cells/mm3) | P-valuea |
|---|---|---|---|---|
| −25.9 (n=17) | 714.6 (165.8–2681.2) | <.001 | >1000 (384 – >1000) | .010 |
| 26.0–33.9 (n=39) | 157.7 (24.9–870.8) | 372 (22 – >1000) | ||
| 34.0–36.9 (n=15) | 24.0 (1.8–272.3) | 101 (3 – >1000) |
Jonckheere–Terpstra test.
Values are given as median (interquartile range).
Figure 2.
The frequency of intra-amniotic inflammation (defined as amniotic fluid matrix metalloproteinase-8 concentration > 23 ng/mL) according to the gestational age (P=0.002 by linear by linear association).
Table 3 displays the maternal WBC counts and the CRP concentrations according to the gestational age at the time of amniocentesis. There were no significant differences in these maternal inflammatory markers among groups (P=0.186 for maternal blood WBC count and P=0.146 for serum CRP concentrations by Jonckheere-Terpstra test).
Table 3.
The maternal blood white blood cell count and serum C-reactive protein concentration according to gestational age at amniocentesis
| Gestational age at amniocentesis (weeks) | Maternal blood white blood cell (cells/mm3) | P-valuea | Maternal serum C-reactive protein (mg/dl) | P-valuea |
|---|---|---|---|---|
| −25.9 (n=17) | 13,730 (9845–17,890) | .186 | 1.3 (0.7–3.1) | .146 |
| 26.0–33.9 (n=39) | 11,495 (9318–14,043) | 0.5 (0.2–1.6) | ||
| 34.0–36.9 (n=15) | 12,335 (8363–15,200) | 0.9 (0.2–2.0) |
Jonckheere–Terpstra test.
Values are given as median (interquartile range).
Table 4 presents the correlation between markers of amniotic fluid and a systemic maternal inflammatory response, and gestational age at amniocentesis. The intensity of the amniotic fluid inflammatory response markers was inversely correlated with gestational age (P<.001 for amniotic fluid MMP-8 and P=.003 for amniotic fluid WBC count). However, the maternal inflammatory markers were not significantly associated with gestational age at the time of amniocentesis (P=.103 for maternal blood CRP and P=.055 for maternal blood WBC count).
Table 4.
Correlation between amniotic fluid and maternal blood inflammatory markers and gestational age at amniocentesis
| Correlation coefficients in Spearman’s rank correlation test | P value | |
|---|---|---|
| Amniotic fluid | ||
| Matrix metalloproteinase-8 | −0.467 | <.001 |
| White blood cell count | −0.355 | .003 |
| Maternal blood | ||
| C-reactive protein | −0.204 | .103 |
| White blood cell count | −0.247 | .055 |
Table 5 shows the neonatal outcome according to gestational age at amniocentesis. The lower the gestational age, the higher the frequency of low Apgar scores of <7 for 1 and 5 minutes, neonatal death, and significant morbidity.
Table 5.
Neonatal outcome according to gestational age at amniocentesis
| Characteristics | −25.9 weeks (n=16)a |
26–33.9 weeks (n=37)b |
34–36.9 weeks (n=15) |
P-value |
|---|---|---|---|---|
| Birth weight | 742 ± 430 | 1645 ± 513 | 2441 ± 303 | <.001 |
| Apgar score at 1 min <7 | 100% (16/16) | 64.9% (24/37) | 20.0% (3/15) | <.001 |
| Apgar score at 5 min <7 | 81.3% (13/16) | 16.2% (6/37) | 0% (0/15) | <.001 |
| Neonatal death | 37.5% (6/16) | 13.5% (5/37) | 0% (0/15) | .015 |
| Significant morbidityc | 91.7% (11/12) | 62.2% (23/37) | 26.7% (4/15) | .003 |
| Respiratory distress syndrome | 41.7% (5/12) | 18.9% (7/37) | 0% (0/15) | .022 |
| Bronchopulmonary dysplasia | 72.7% (8/11) | 18.8% (6/32) | 0% (0/15) | <.001 |
| Intraventricular hemorrhage | 25.0% (3/12) | 32.4% (12/37) | 14.3% (2/14) | .422 |
| Necrotizing enterocolitis | 25.0% (3/12) | 2.7% (1/37) | 0% (0/15) | .011 |
| Proven early neonatal sepsis | 8.3% (1/12) | 0% (0/37) | 6.7% (1/15) | .236 |
One case with unavailable data were excluded from analysis
Two cases with unavailable data were excluded from analysis
Four neonates who died in utero or immediately after birth because of extreme prematurity and thus could not be evaluated with respect to the presence or absence of complications were excluded from the analysis.
Discussion
Principal findings of this study
(1) The lower the gestational age at the time of PROM, the higher the intensity of the intra-amniotic inflammatory response in women with preterm PROM and intra-amniotic infection caused by Ureaplasma spp.; (2) the frequency of intra-amniotic inflammation was 94% (16/17) in the extreme preterm PROM group (gestational age <26 weeks), 80% (31/39) in the moderate preterm PROM group (gestational age between 26.0–33.9 weeks) and 53% (8/15) in the late preterm PROM group (gestational age between 34.0–36.9 weeks); 3) the intensity of the maternal inflammatory response was not significantly associated with gestational age at amniocentesis.
Intra-amniotic infection by Ureaplasma spp.
Ureaplasma spp. are the most frequent isolates from amniotic fluid in patients with preterm labor [15–17, 68–70], preterm PROM [1–11], clinical chorioamnionitis [21, 22], acute cervical insufficiency [18, 20], and a short cervix [71]. Even in asymptomatic mid-trimester patients, isolation of Ureaplasma spp. is linked to early preterm delivery [40, 41, 72]. In a primate model of intra-amniotic infection, inoculation of Ureaplasma spp. in the amniotic fluid results in initiation of intra-amniotic pro-inflammatory cascade, and spontaneous preterm birth [73, 74]. Previous studies showed that intra-amniotic infection caused by Ureaplasma spp. has a similar or more intense inflammatory response compared to that caused by other microorganisms [4, 9, 75].
Several studies based on culture methods showed that cervicovaginal colonization of these organisms is associated with preterm birth [76–81]. However, cervico-vaginal colonization of these organisms is found in 30–60% of pregnant women [76, 77, 79, 82] and there is no evidence that detection of Ureaplasma spp. in cervico-vaginal fluid can be helpful in preventing preterm birth. In our previous study [83], bacterial vaginosis but not a positive culture for genital mycoplasmas is a risk factor for spontaneous preterm birth. This is consistent with previous studies [79, 82]. Interestingly, about a half of Trichomonas vaginalis isolates from vaginal samples of patients with purulent vaginitis harbor intra-cellular genital Mycoplasmas including M. hominis and Ureaplasma spp. [84].
It is unclear why most intra-amniotic infections are caused by Ureaplasma spp. [7, 26]. In this process, it is thought that the host defense system, such as the cervical canal and cervical mucus, plays an important role [85–88]. A solid body of evidence indicates that a short cervical length is associated with intra-amniotic infection/inflammation [68, 71, 89–91] and development of spontaneous preterm birth [68, 90, 92–112]. A recent microbiome study has found considerable greater complexity in the placental membrane community [113–115]. Virulent microorganisms including Escherichia coli and Enterobacter spp. are frequently found and similar in abundance between term and preterm subjects with and without chorioamnionitis [114]. Of interest is that preterm subjects with severe chorioamnionitis had high abundances of Ureaplasma spp. and Fusobacterium nucleatum [114, 116]. In our previous study which was directed at twins, Ureaplasma spp. were found in both amniotic cavities in all cases with intra-amniotic infections with Ureaplasma spp. Contrariwise, most of other microorganisms were found only in presenting amniotic cavity [117]. Collectively, these findings suggest that Ureaplasma spp. could more easily cross the chorioamniotic membrane, thereby reaching the amniotic fluid than did other bacteria.
Host inflammatory response to intra-amniotic infection caused by Ureaplasma spp.
In the current study, the intensity of the intra-amniotic inflammatory response was different in cases with intra-amniotic infections with Ureaplasma spp. as a function of gestational age. This may be due to a difference in inoculum size, the host’s immunological response, timing and duration of infection, co-infected microorganisms and the presence or absence of any other inflammatory modifiers such as prior immune priming or viral exposure [118]. Previous studies showed that the inoculum size plays a role in determining the intensity of the inflammatory response [2, 119, 120]. Therefore, the differences in the host-inflammatory response may largely depend on the burden of these organisms. Another reason may be due to different host-microbe interactions. Some investigators have reported that host genetic background and racial disparity impact the disease outcome during intrauterine infection with Ureaplasma spp. [121, 122]. However, these findings cannot explain the result of the current study that the earlier the gestational age, the greater the intensity of the intra-amniotic inflammatory response in women with preterm PROM and intra-amniotic infections with Ureaplasma spp.
It is well-known that oxytocin play a central role in spontaneous human labor. Progesterone and estradiol are thought as the primary regulators of oxytocin receptor expression [123, 124]. However, expression of the oxytocin receptor is also regulated by interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF) in uterine smooth muscle cells [125–127]. Other investigators showed that IL-1β and TNF upregulate inflammation-related rapid genes [128], which regulate oxytocin receptor [129, 130]. The level of oxytocin receptor messenger RNA (mRNA) and oxytocin receptor in human myometrium at term is greater than that found in preterm myometrium [131]. Therefore, more intense inflammatory response may be required to induce labor at early gestation. Considering the survival of newborn, it may be more advantageous to maintain pregnancy in early preterm cases with mild infection/inflammation.
The maternal systemic and intrauterine local inflammation
A strong body of evidence suggests that preterm birth is associated with maternal systemic inflammatory conditions such as appendicitis [132–135], acute pyelonephritis [136–138], influenza [139–141], and sepsis [142]. The rates of spontaneous preterm birth in such conditions are 5.1% in appendicitis [133], 10.3% in acute pyelonephritis [138], and 5–30% in influenza [139]. Of note, among pregnancies with appendicectomy, the spontaneous preterm birth rate is associated with the gestational age at appendicectomy (4.4% with gestational age <24 weeks; 7.5% with gestational age between 24–28 weeks and 9.1% with gestational age between 29–36 weeks) [133]. This finding supports the view that a more intense inflammatory response is required to induce spontaneous preterm labor at earlier gestational ages.
Most pregnant women deliver at term despite maternal infections such as appendicitis, acute pyelonephritis and influenza. By contrast, most patients with intra- amniotic infection and/or inflammation deliver preterm [2–4, 15, 17, 42, 45, 46, 143–145]. One of unexpected findings in the current study is that the intensity of maternal systemic inflammatory response was not associated with the gestational age at PROM. These findings suggest that the local inflammatory response has a more important role in spontaneous preterm birth than did maternal systemic inflammatory response.
Previous studies have shown that the analysis of maternal inflammatory markers may help predict intra-amniotic infection. However, this does not appear to have clinical utility compared to the assessment of amniotic cavity [146–148]. In a series of studies, the maternal concentration of inflammatory markers including IL-6 and CRP had a sensitivity of 56–79% and specificity of 59–77% in the identification of intra-amniotic infection [146–148]. Clinical chorioamnionitis based on maternal fever also has only 20–30% sensitivity to detect intra-amniotic infection [4, 147]. In the current study, only 25% of patients had an elevated maternal blood WBC count of > 15000 cells/mm3. Therefore, it is unlikely that analysis of maternal systemic inflammatory response is able to replace the evaluation of intra-amniotic infection and inflammatory response in women with preterm PROM [149].
Strengths and limitations of the study
The strength of this study is that the relationship between the intensity of intra-amniotic inflammation and gestational age was analyzed in a relatively large population with intra-amniotic infections with Ureaplasma spp. A limitation of this study is that the intra-amniotic infection was determined by cultivation techniques. Therefore, some of the cases may have infections with other microorganisms not detected using cultivation techniques [17, 150].
Conclusion
The most important clinical implication of our study is that the intra-amniotic inflammatory response varies as a function of gestational age at the time of PROM in women with intra-amniotic infection caused by Ureaplasma spp. Specifically, the earlier the gestational age at which PROM occurs, the more intense the intra-amniotic inflammatory response.
Funding
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT & Future Planning, Republic of Korea (2017R1A2B2007958); federal funds from NICHD/NIH/DHHS (HHSN275201300006C) and the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice
Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS).
Footnotes
Conflicts of interest: The authors report no conflict of interest.
References
- 1.Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol 1992;167:1092–5. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51. [DOI] [PubMed] [Google Scholar]
- 3.Averbuch B, Mazor M, Shoham-Vardi I, Chaim W, Vardi H, Horowitz S, et al. Intra-uterine infection in women with preterm premature rupture of membranes: maternal and neonatal characteristics. Eur J Obstet Gynecol Reprod Biol 1995;62:25–9. [DOI] [PubMed] [Google Scholar]
- 4.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1998;179:1254–60. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. Interleukin-6 concentrations in cervical secretions in the prediction of intrauterine infection in preterm premature rupture of the membranes. Gynecol Obstet Invest 1998;46:91–5. [DOI] [PubMed] [Google Scholar]
- 6.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol 2000;183:1130–7. [DOI] [PubMed] [Google Scholar]
- 7.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002;8:3–13. [DOI] [PubMed] [Google Scholar]
- 8.Berger A, Witt A, Haiden N, Kretzer V, Heinze G, Pollak A. Amniotic cavity cultures, blood cultures, and surface swabs in preterm infants--useful tools for the management of early-onset sepsis? Journal of perinatal medicine 2004;32:446–52. [DOI] [PubMed] [Google Scholar]
- 9.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2010;203:211 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musilova I, Andrys C, Drahosova M, Soucek O, Pliskova L, Jacobsson B, et al. Cervical fluid interleukin 6 and intra-amniotic complications of preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2018;31:827–36. [DOI] [PubMed] [Google Scholar]
- 12.Tchirikov M, Schlabritz-Loutsevitch N, Maher J, Buchmann J, Naberezhnev Y, Winarno AS, et al. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. Journal of perinatal medicine 2018;46:465–88. [DOI] [PubMed] [Google Scholar]
- 13.Revello R, Alcaide MJ, Dudzik D, Abehsera D, Bartha JL. Differential amniotic fluid cytokine profile in women with chorioamnionitis with and without funisitis. J Matern Fetal Neonatal Med 2016;29:2161–5. [DOI] [PubMed] [Google Scholar]
- 14.Dudzik D, Revello R, Barbas C, Bartha JL. LC-MS-based metabolomics identification of novel biomarkers of chorioamnionitis and its associated perinatal neurological damage. J Proteome Res 2015;14:1432–44. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–24. [DOI] [PubMed] [Google Scholar]
- 16.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol 1998;92:77–82. [DOI] [PubMed] [Google Scholar]
- 17.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 1992;167:1086–91. [DOI] [PubMed] [Google Scholar]
- 19.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol 2008;198:633 e1–8. [DOI] [PubMed] [Google Scholar]
- 20.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. Journal of perinatal medicine 2010;38:261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh KJ, Kim SM, Hong JS, Maymon E, Erez O, Panaitescu B, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol 2017;216:604 e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. Journal of perinatal medicine 2015;43:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol 1992;166:129–33. [DOI] [PubMed] [Google Scholar]
- 24.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med 2005;18:31–7. [DOI] [PubMed] [Google Scholar]
- 25.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol 2003;189:919–24. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revello R, Alcaide MJ, Abehsera D, Martin-Camean M, Sousa EFGM, Alonso-Luque B, et al. Prediction of chorioamnionitis in cases of intraamniotic infection by ureaplasma urealyticum in women with very preterm premature rupture of membranes or preterm labour. J Matern Fetal Neonatal Med 2018;31:1839–44. [DOI] [PubMed] [Google Scholar]
- 28.Likitnukul S, Kusmiesz H, Nelson JD, McCracken GH Jr. Role of genital mycoplasmas in young infants with suspected sepsis. J Pediatr 1986;109:971–4. [DOI] [PubMed] [Google Scholar]
- 29.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol 2008;198:43 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viscardi RM, Hashmi N, Gross GW, Sun CC, Rodriguez A, Fairchild KD. Incidence of invasive ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. J Perinatol 2008;28:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasper DC, Mechtler TP, Bohm J, Petricevic L, Gleiss A, Spergser J, et al. In utero exposure to Ureaplasma spp. is associated with increased rate of bronchopulmonary dysplasia and intraventricular hemorrhage in preterm infants. Journal of perinatal medicine 2011;39:331–6. [DOI] [PubMed] [Google Scholar]
- 32.Cassell GH, Waites KB, Crouse DT, Rudd PT, Canupp KC, Stagno S, et al. Association of Ureaplasma urealyticum infection of the lower respiratory tract with chronic lung disease and death in very-low-birth-weight infants. Lancet 1988;2:240–5. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez PJ, Regan JA. Ureaplasma urealyticum colonization and chronic lung disease in low birth weight infants. Pediatr Infect Dis J 1988;7:542–6. [PubMed] [Google Scholar]
- 34.Payne NR, Steinberg SS, Ackerman P, Chrenka BA, Sane SM, Anderson KT, et al. New prospective studies of the association of Ureaplasma urealyticum colonization and chronic lung disease. Clin Infect Dis 1993;17 Suppl 1:S117–21. [DOI] [PubMed] [Google Scholar]
- 35.Lowe J, Watkins WJ, Edwards MO, Spiller OB, Jacqz-Aigrain E, Kotecha SJ, et al. Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. Pediatr Infect Dis J 2014;33:697–702. [DOI] [PubMed] [Google Scholar]
- 36.Viscardi RM, Kallapur SG. Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis: Current Concepts and Update. Clin Perinatol 2015;42:719–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hentschel J, Abele-Horn M, Peters J. Ureaplasma urealyticum in the cerebrospinal fluid of a premature infant. Acta Paediatr 1993;82:690–3. [DOI] [PubMed] [Google Scholar]
- 38.Berger A, Witt A, Haiden N, Kaider A, Klebermasz K, Fuiko R, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. Journal of perinatal medicine 2009;37:72–8. [DOI] [PubMed] [Google Scholar]
- 39.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med 1995;40:375–9. [PubMed] [Google Scholar]
- 40.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis 2003;187:518–21. [DOI] [PubMed] [Google Scholar]
- 41.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol 2004;191:1382–6. [DOI] [PubMed] [Google Scholar]
- 42.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–45. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kacerovsky M, Musilova I, Khatibi A, Skogstrand K, Hougaard DM, Tambor V, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2012;25:2014–9. [DOI] [PubMed] [Google Scholar]
- 45.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–6. [DOI] [PubMed] [Google Scholar]
- 46.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125 e1–e15. [DOI] [PubMed] [Google Scholar]
- 47.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol 2001;185:1156–61. [DOI] [PubMed] [Google Scholar]
- 48.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2007;197:294 e1–6. [DOI] [PubMed] [Google Scholar]
- 49.Lee J, Oh KJ, Yang HJ, Park JS, Romero R, Yoon BH. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med 2009;22:917–23. [DOI] [PubMed] [Google Scholar]
- 50.Kim SM, Romero R, Lee J, Mi Lee S, Park CW, Shin Park J, et al. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J Matern Fetal Neonatal Med 2012;25:1212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SM, Romero R, Park JW, Oh KJ, Jun JK, Yoon BH. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med 2015;28:1500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J, Romero R, Kim SM, Chaemsaithong P, Park CW, Park JS, et al. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J Matern Fetal Neonatal Med 2016;29:707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SM, Romero R, Lee J, Chaemsaithong P, Docheva N, Yoon BH. Gastric fluid versus amniotic fluid analysis for the identification of intra-amniotic infection due to Ureaplasma species. J Matern Fetal Neonatal Med 2016;29:2579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh KJ, Park JY, Lee J, Hong JS, Romero R, Yoon BH. The combined exposure to intra-amniotic inflammation and neonatal respiratory distress syndrome increases the risk of intraventricular hemorrhage in preterm neonates. Journal of perinatal medicine 2018;46:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70. [DOI] [PubMed] [Google Scholar]
- 56.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213:S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med 2006;19:693–7. [DOI] [PubMed] [Google Scholar]
- 58.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis 1982;145:1–8. [DOI] [PubMed] [Google Scholar]
- 59.Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol 1980;136:709–13. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. Journal of perinatal medicine 2016;44:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim SM, Romero R, Lee J, Chaemsaithong P, Lee MW, Chaiyasit N, et al. About one-half of early spontaneous preterm deliveries can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. J Matern Fetal Neonatal Med 2016;29:2414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J, Romero R, Kim SM, Chaemsaithong P, Yoon BH. A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM. J Matern Fetal Neonatal Med 2016;29:2727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. Journal of perinatal medicine 2016;44:53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Kusanovic JP, Yoon BH, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. Journal of perinatal medicine 2016;44:33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. Journal of perinatal medicine 2017;45:523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaiyasit N, Romero R, Chaemsaithong P, Docheva N, Bhatti G, Kusanovic JP, et al. Clinical chorioamnionitis at term VIII: a rapid MMP-8 test for the identification of intra-amniotic inflammation. Journal of perinatal medicine 2017;45:539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Lopez N, Romero R, Maymon E, Kusanovic JP, Panaitescu B, Miller D, et al. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. J Perinat Med 2018. November 9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomez R, Romero R, Nien JK, Chaiworapongsa T, Medina L, Kim YM, et al. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2005;192:678–89. [DOI] [PubMed] [Google Scholar]
- 69.Romero R, Espinoza J, Rogers WT, Moser A, Nien JK, Kusanovic JP, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med 2008;21:367–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. Journal of perinatal medicine 2006;34:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon BH, Romero R, Moon JB, Oh SY, Han SY, Kim JC, et al. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol 2001;185:1137–42. [DOI] [PubMed] [Google Scholar]
- 73.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009;16:56–70. [DOI] [PubMed] [Google Scholar]
- 74.Grigsby PL, Novy MJ, Sadowsky DW, Morgan TK, Long M, Acosta E, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol 2012;207:475 e1–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witt A, Berger A, Gruber CJ, Petricevic L, Apfalter P, Husslein P. IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. Journal of perinatal medicine 2005;33:22–6. [DOI] [PubMed] [Google Scholar]
- 76.Horowitz S, Horowitz J, Mazor M, Porath A, Glezerman M. Ureaplasma urealyticum cervical colonization as a marker for pregnancy complications. Int J Gynaecol Obstet 1995;48:15–9. [DOI] [PubMed] [Google Scholar]
- 77.Abele-Horn M, Scholz M, Wolff C, Kolben M. High-density vaginal Ureaplasma urealyticum colonization as a risk factor for chorioamnionitis and preterm delivery. Acta Obstet Gynecol Scand 2000;79:973–8. [PubMed] [Google Scholar]
- 78.Harada K, Tanaka H, Komori S, Tsuji Y, Nagata K, Tsutsui H, et al. Vaginal infection with Ureaplasma urealyticum accounts for preterm delivery via induction of inflammatory responses. Microbiol Immunol 2008;52:297–304. [DOI] [PubMed] [Google Scholar]
- 79.Breugelmans M, Vancutsem E, Naessens A, Laubach M, Foulon W. Association of abnormal vaginal flora and Ureaplasma species as risk factors for preterm birth: a cohort study. Acta Obstet Gynecol Scand 2010;89:256–60. [DOI] [PubMed] [Google Scholar]
- 80.Kwak DW, Hwang HS, Kwon JY, Park YW, Kim YH. Co-infection with vaginal Ureaplasma urealyticum and Mycoplasma hominis increases adverse pregnancy outcomes in patients with preterm labor or preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2014;27:333–7. [DOI] [PubMed] [Google Scholar]
- 81.Donders GGG, Ruban K, Bellen G, Petricevic L. Mycoplasma/Ureaplasma infection in pregnancy: to screen or not to screen. Journal of perinatal medicine 2017;45:505–15. [DOI] [PubMed] [Google Scholar]
- 82.Carey JC, Blackwelder WC, Nugent RP, Matteson MA, Rao AV, Eschenbach DA, et al. Antepartum cultures for Ureaplasma urealyticum are not useful in predicting pregnancy outcome. The Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 1991;164:728–33. [DOI] [PubMed] [Google Scholar]
- 83.Lee SE, Romero R, Kim EC, Yoon BH. A high Nugent score but not a positive culture for genital mycoplasmas is a risk factor for spontaneous preterm birth. J Matern Fetal Neonatal Med 2009;22:212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ioannidis A, Papaioannou P, Magiorkinis E, Magana M, Ioannidou V, Tzanetou K, et al. Detecting the Diversity of Mycoplasma and Ureaplasma Endosymbionts Hosted by Trichomonas vaginalis Isolates. Front Microbiol 2017;8:1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakai A, Taniuchi Y, Miyake H, Nakai M, Yokota A, Takeshita T. Increased level of granulocyte elastase in cervical secretion is an independent predictive factor for preterm delivery. Gynecol Obstet Invest 2005;60:87–91. [DOI] [PubMed] [Google Scholar]
- 86.Becher N, Adams Waldorf K, Hein M, Uldbjerg N. The cervical mucus plug: structured review of the literature. Acta Obstet Gynecol Scand 2009;88:502–13. [DOI] [PubMed] [Google Scholar]
- 87.Smith-Dupont KB, Wagner CE, Witten J, Conroy K, Rudoltz H, Pagidas K, et al. Probing the potential of mucus permeability to signify preterm birth risk. Sci Rep 2017;7:10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vornhagen J, Quach P, Santana-Ufret V, Alishetti V, Brokaw A, Armistead B, et al. Human Cervical Mucus Plugs Exhibit Insufficiencies in Antimicrobial Activity Towards Group B Streptococcus. J Infect Dis 2018;217:1626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2014:1–17 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kiefer DG, Peltier MR, Keeler SM, Rust O, Ananth CV, Vintzileos AM, et al. Efficacy of midtrimester short cervix interventions is conditional on intraamniotic inflammation. Am J Obstet Gynecol 2016;214:276 e1–6. [DOI] [PubMed] [Google Scholar]
- 91.Tarca AL, Fitzgerald W, Chaemsaithong P, Xu Z, Hassan SS, Grivel JC, et al. The cytokine network in women with an asymptomatic short cervix and the risk of preterm delivery. Am J Reprod Immunol 2017;78:e12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med 1996;334:567–72. [DOI] [PubMed] [Google Scholar]
- 93.Iams JD, Goldenberg RL, Mercer BM, Moawad A, Thom E, Meis PJ, et al. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 1998;178:1035–40. [DOI] [PubMed] [Google Scholar]
- 94.Goldenberg RL, Iams JD, Das A, Mercer BM, Meis PJ, Moawad AH, et al. The Preterm Prediction Study: sequential cervical length and fetal fibronectin testing for the prediction of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 2000;182:636–43. [DOI] [PubMed] [Google Scholar]
- 95.Hibbard JU, Tart M, Moawad AH. Cervical length at 16–22 weeks’ gestation and risk for preterm delivery. Obstet Gynecol 2000;96:972–8. [DOI] [PubMed] [Google Scholar]
- 96.To MS, Skentou C, Cicero S, Liao AW, Nicolaides KH. Cervical length at 23 weeks in triplets: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol 2000;16:515–8. [DOI] [PubMed] [Google Scholar]
- 97.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol 2000;182:1458–67. [DOI] [PubMed] [Google Scholar]
- 98.Hadzi-Lega M, Markova AD, Stefanovic M, Tanturovski M. Correlation of cervical length, fetal fibronectin, phIGFBP-1, and cytokines in spontaneous preterm birth up to 14 days from sampling. Journal of perinatal medicine 2015;43:545–51. [DOI] [PubMed] [Google Scholar]
- 99.Nikolova T, Bayev O, Nikolova N, Di Renzo GC. Comparison of a novel test for placental alpha microglobulin-1 with fetal fibronectin and cervical length measurement for the prediction of imminent spontaneous preterm delivery in patients with threatened preterm labor. Journal of perinatal medicine 2015;43:395–402. [DOI] [PubMed] [Google Scholar]
- 100.Conde-Agudelo A, Romero R. Vaginal progesterone to prevent preterm birth in pregnant women with a sonographic short cervix: clinical and public health implications. Am J Obstet Gynecol 2016;214:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khalifeh A, Berghella V. Universal cervical length screening in singleton gestations without a previous preterm birth: ten reasons why it should be implemented. Am J Obstet Gynecol 2016;214:603 e1–5. [DOI] [PubMed] [Google Scholar]
- 102.Melamed N, Pittini A, Hiersch L, Yogev Y, Korzeniewski SJ, Romero R, et al. Do serial measurements of cervical length improve the prediction of preterm birth in asymptomatic women with twin gestations? Am J Obstet Gynecol 2016;215:616 e1–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol 2016;214:365 e1–5. [DOI] [PubMed] [Google Scholar]
- 104.Vintzileos AM, Visser GH. Interventions for women with mid-trimester short cervix: which ones work? Ultrasound Obstet Gynecol 2017;49:295–300. [DOI] [PubMed] [Google Scholar]
- 105.Sharvit M, Weiss R, Ganor Paz Y, Tzadikevitch Geffen K, Danielli Miller N, Biron-Shental T. Vaginal examination vs. cervical length - which is superior in predicting preterm birth? Journal of perinatal medicine 2017;45:977–83. [DOI] [PubMed] [Google Scholar]
- 106.Oturina V, Hammer K, Mollers M, Braun J, Falkenberg MK, de Murcia KO, et al. Assessment of cervical elastography strain pattern and its association with preterm birth. Journal of perinatal medicine 2017;45:925–32. [DOI] [PubMed] [Google Scholar]
- 107.Hernandez-Andrade E, Maymon E, Luewan S, Bhatti G, Mehrmohammadi M, Erez O, et al. A soft cervix, categorized by shear-wave elastography, in women with short or with normal cervical length at 18–24 weeks is associated with a higher prevalence of spontaneous preterm delivery. Journal of perinatal medicine 2018;46:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Conde-Agudelo A, Romero R, Da Fonseca E, O’Brien JM, Cetingoz E, Creasy GW, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol 2018;219:10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanchez-Ramos L Vaginal progesterone is an alternative to cervical cerclage in women with a short cervix and a history of preterm birth. Am J Obstet Gynecol 2018;219:5–9. [DOI] [PubMed] [Google Scholar]
- 110.Boelig RC, Berghella V. What’s new in preterm birth prediction and prevention? Journal of perinatal medicine 2018;46:455–6. [DOI] [PubMed] [Google Scholar]
- 111.Shivani D, Quek BH, Tan PL, Shephali T. Does rescue cerclage work? Journal of perinatal medicine 2018;46:876–80. [DOI] [PubMed] [Google Scholar]
- 112.Daskalakis G, Zacharakis D, Theodora M, Antsaklis P, Papantoniou N, Loutradis D, et al. Safety and efficacy of the cervical pessary combined with vaginal progesterone for the prevention of spontaneous preterm birth. Journal of perinatal medicine 2018;46:531–7. [DOI] [PubMed] [Google Scholar]
- 113.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014;6:237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol 2016;214:627 e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Latino MA, Botta G, Badino C, Maria D, Petrozziello A, Sensini A, et al. Association between genital mycoplasmas, acute chorioamnionitis and fetal pneumonia in spontaneous abortions. Journal of perinatal medicine 2018;46:503–8. [DOI] [PubMed] [Google Scholar]
- 116.Cox C, Saxena N, Watt AP, Gannon C, McKenna JP, Fairley DJ, et al. The common vaginal commensal bacterium Ureaplasma parvum is associated with chorioamnionitis in extreme preterm labor. J Matern Fetal Neonatal Med 2016;29:3646–51. [DOI] [PubMed] [Google Scholar]
- 117.Oh KJ, Hong JS, Romero R, Yoon BH. The frequency and clinical significance of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2019;32:527–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keelan JA. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J Reprod Immunol 2018;125:89–99. [DOI] [PubMed] [Google Scholar]
- 119.Moss TJ, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol 2005;192:1179–86. [DOI] [PubMed] [Google Scholar]
- 120.Jacobsson B, Aaltonen R, Rantakokko-Jalava K, Morken NH, Alanen A. Quantification of Ureaplasma urealyticum DNA in the amniotic fluid from patients in PTL and pPROM and its relation to inflammatory cytokine levels. Acta Obstet Gynecol Scand 2009;88:63–70. [DOI] [PubMed] [Google Scholar]
- 121.von Chamier M, Allam A, Brown MB, Reinhard MK, Reyes L. Host genetic background impacts disease outcome during intrauterine infection with Ureaplasma parvum. PLoS One 2012;7:e44047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Modi BP, Teves ME, Pearson LN, Parikh HI, Haymond-Thornburg H, Tucker JL, et al. Mutations in fetal genes involved in innate immunity and host defense against microbes increase risk of preterm premature rupture of membranes (PPROM). Mol Genet Genomic Med 2017;5:720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol 1984;150:734–41. [DOI] [PubMed] [Google Scholar]
- 124.Bogacki M, Silvia WJ, Rekawiecki R, Kotwica J. Direct inhibitory effect of progesterone on oxytocin-induced secretion of prostaglandin F(2alpha) from bovine endometrial tissue. Biol Reprod 2002;67:184–8. [DOI] [PubMed] [Google Scholar]
- 125.Saji F, Samejima Y, Kamiura S, Sawai K, Shimoya K, Kimura T. Cytokine production in chorioamnionitis. J Reprod Immunol 2000;47:185–96. [DOI] [PubMed] [Google Scholar]
- 126.Mitchell BF, Schmid B. Oxytocin and its receptor in the process of parturition. J Soc Gynecol Investig 2001;8:122–33. [PubMed] [Google Scholar]
- 127.Schmid B, Wong S, Mitchell BF. Transcriptional regulation of oxytocin receptor by interleukin-1beta and interleukin-6. Endocrinology 2001;142:1380–5. [DOI] [PubMed] [Google Scholar]
- 128.Massrieh W, Derjuga A, Doualla-Bell F, Ku CY, Sanborn BM, Blank V. Regulation of the MAFF transcription factor by proinflammatory cytokines in myometrial cells. Biol Reprod 2006;74:699–705. [DOI] [PubMed] [Google Scholar]
- 129.Kimura T, Ivell R, Rust W, Mizumoto Y, Ogita K, Kusui C, et al. Molecular cloning of a human MafF homologue, which specifically binds to the oxytocin receptor gene in term myometrium. Biochem Biophys Res Commun 1999;264:86–92. [DOI] [PubMed] [Google Scholar]
- 130.Bethin KE, Nagai Y, Sladek R, Asada M, Sadovsky Y, Hudson TJ, et al. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol 2003;17:1454–69. [DOI] [PubMed] [Google Scholar]
- 131.Wathes DC, Borwick SC, Timmons PM, Leung ST, Thornton S. Oxytocin receptor expression in human term and preterm gestational tissues prior to and following the onset of labour. J Endocrinol 1999;161:143–51. [DOI] [PubMed] [Google Scholar]
- 132.Mazze RI, Kallen B. Appendectomy during pregnancy: a Swedish registry study of 778 cases. Obstet Gynecol 1991;77:835–40. [PubMed] [Google Scholar]
- 133.Ibiebele I, Schnitzler M, Nippita T, Ford JB. Appendicectomy during pregnancy and the risk of preterm birth: A population data linkage study. Aust N Z J Obstet Gynaecol 2019;59:45–53. [DOI] [PubMed] [Google Scholar]
- 134.Won RP, Friedlander S, Lee SL. Management and Outcomes of Appendectomy during Pregnancy. Am Surg 2017;83:1103–7. [PubMed] [Google Scholar]
- 135.Abbasi N, Patenaude V, Abenhaim HA. Evaluation of obstetrical and fetal outcomes in pregnancies complicated by acute appendicitis. Arch Gynecol Obstet 2014;290:661–7. [DOI] [PubMed] [Google Scholar]
- 136.Millar LK, DeBuque L, Wing DA. Uterine contraction frequency during treatment of pyelonephritis in pregnancy and subsequent risk of preterm birth. Journal of perinatal medicine 2003;31:41–6. [DOI] [PubMed] [Google Scholar]
- 137.Farkash E, Weintraub AY, Sergienko R, Wiznitzer A, Zlotnik A, Sheiner E. Acute antepartum pyelonephritis in pregnancy: a critical analysis of risk factors and outcomes. Eur J Obstet Gynecol Reprod Biol 2012;162:24–7. [DOI] [PubMed] [Google Scholar]
- 138.Wing DA, Fassett MJ, Getahun D. Acute pyelonephritis in pregnancy: an 18-year retrospective analysis. Am J Obstet Gynecol 2014;210:219 e1–6. [DOI] [PubMed] [Google Scholar]
- 139.Meijer WJ, van Noortwijk AG, Bruinse HW, Wensing AM. Influenza virus infection in pregnancy: a review. Acta Obstet Gynecol Scand 2015;94:797–819. [DOI] [PubMed] [Google Scholar]
- 140.Bayraktar MR, Ozerol IH, Gucluer N, Celik O. Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. Int J Infect Dis 2010;14:e90–5. [DOI] [PubMed] [Google Scholar]
- 141.Fell DB, Platt RW, Basso O, Wilson K, Kaufman JS, Buckeridge DL, et al. The Relationship Between 2009 Pandemic H1N1 Influenza During Pregnancy and Preterm Birth: A Population-based Cohort Study. Epidemiology 2018;29:107–16. [DOI] [PubMed] [Google Scholar]
- 142.Knowles SJ, O’Sullivan NP, Meenan AM, Hanniffy R, Robson M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG 2015;122:663–71. [DOI] [PubMed] [Google Scholar]
- 143.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol 1995;22:281–342. [PubMed] [Google Scholar]
- 144.Rodriguez N, Fernandez C, Zamora Y, Berdasquera D, Rivera JA. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: association with pregnancy outcomes. J Matern Fetal Neonatal Med 2011;24:47–50. [DOI] [PubMed] [Google Scholar]
- 145.Gervasi MT, Romero R, Bracalente G, Erez O, Dong Z, Hassan SS, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. Journal of perinatal medicine 2012;40:329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol 1996;88:1034–40. [DOI] [PubMed] [Google Scholar]
- 147.Dulay AT, Buhimschi IA, Zhao G, Bahtiyar MO, Thung SF, Cackovic M, et al. Compartmentalization of acute phase reactants Interleukin-6, C-Reactive Protein and Procalcitonin as biomarkers of intra-amniotic infection and chorioamnionitis. Cytokine 2015;76:236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stepan M, Cobo T, Musilova I, Hornychova H, Jacobsson B, Kacerovsky M. Maternal Serum C-Reactive Protein in Women with Preterm Prelabor Rupture of Membranes. PLoS One 2016;11:e0150217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017;217:693 e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]