Abstract
Energetic demand from high-intensity exercise can easily exceed ATP synthesis rates of mitochondria leading to a reliance on anaerobic metabolism. The reliance on anaerobic metabolism results in the accumulation of intracellular metabolites, namely inorganic phosphate (Pi) and hydrogen (H+), that are closely associated with exercise-induced reductions in power. Cellular and molecular studies have revealed several steps where these metabolites impair contractile function demonstrating a causal role in fatigue. Elevated Pi or H+ directly inhibits force and power of the cross-bridge and decreases myofibrillar Ca2+ sensitivity, whereas Pi also inhibits Ca2+ release from the sarcoplasmic reticulum (SR). When both metabolites are elevated, they act synergistically to cause marked reductions in power, indicating that fatigue during high-intensity exercise has a bioenergetic basis.
Keywords: muscle fatigue, 31P-MRS, inorganic phosphate, acidosis, diprotonated phosphate, metabolism, cross-bridge mechanics, Ca2+ handling
Introduction
Skeletal muscle produces movement by converting chemical energy into mechanical energy through the hydrolysis of ATP. The total energetic demand of this process is the sum of the ATP hydrolyzed for ion transport (SR-Ca2+ and Na+/K+ ATPases) and the chemo-mechanical transduction of the myosin-actin interaction (myofibrillar ATPase). When the energetic demand of a motor task is low enough that ATP synthesis can be met primarily by oxidative phosphorylation, the mechanical power and force outputs necessary to perform the exercise can be sustained for hours. In contrast, as soon as the exercise intensity exceeds the ATP synthesis rates of oxidative phosphorylation requiring an increased reliance on anaerobic metabolism, force and power become impaired, i.e., fatigue develops, and failure to sustain the exercise occurs within seconds to minutes of the onset of contractile activity [1, 2]. Any alterations to the mechanics of the contraction, such as contractile frequency, shortening velocity or duty cycle, will inevitably change 1) the energetic demand and extent the exercise relies on anaerobic metabolism and 2) the rate at which fatigue develops [3]. The observation that the rate of fatigue development has a bioenergetic basis determined by the extent the exercise relies on anaerobic metabolism has been consistently documented in isometric and dynamic contractions [3–5], whole-body and isolated-limb exercises [1, 3, 6], and in men and women [2].
Although it is generally accepted that fatigue during high-intensity exercise has a bioenergetic basis, identifying the mechanisms by which a reliance on anaerobic metabolism causes fatigue has proven considerably more challenging. This is in large part because fatigue can originate at multiple locations along the motor pathway (Fig. 1), and the central nervous system is tightly tuned to the metabolic state of the muscle through sensory feedback from group III/IV afferents [7–9]. A preponderance of evidence, however, indicates that most of the fatigue during high-intensity exercise is due to impaired contractile function within the muscle [4, 6], and that this observation is true for healthy young and old men and women [10, 11]. Identifying how a reliance on anaerobic metabolism impairs muscle force and power in such a predictable manner is particularly important because in whole-body exercises, such as cycling, ~60–70% of the power outputs generated by the neuromuscular system are non-sustainable and elicit failure within seconds to minutes [1, 2]. In this brief review, we integrate findings from in vivo to isolated cellular and molecular studies to identify how a reliance on anaerobic metabolism causes fatigue by disrupting contractile function in the muscle.
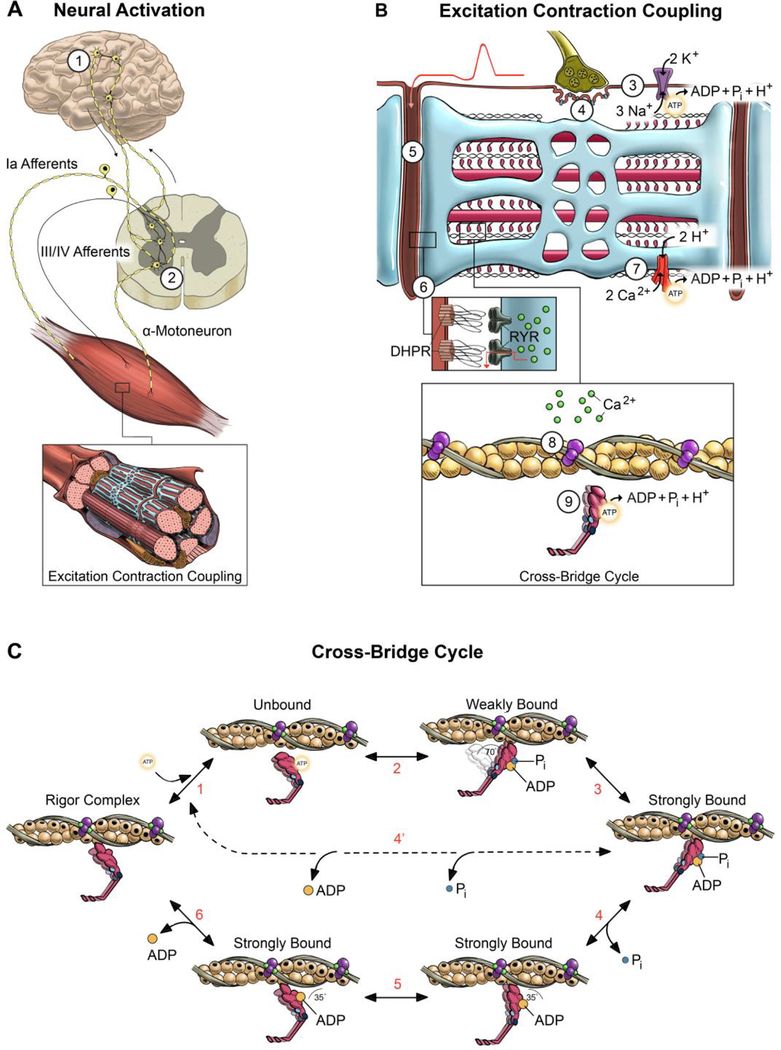
Figure 1. Schematic of potential sites of fatigue along the motor pathway.
Potential sites of fatigue are labeled 1–9 starting with the ability of the nervous system to activate the muscle (A) and progressing to excitation contraction coupling (B) and the cross-bridge cycle (C). During volitional contractions, skeletal muscle is activated via signals originating in the motor cortex (site 1) that are transmitted to the α-motoneurons in the spinal cord (site 2). The output of the polydendritic α-motoneuron is determined by the ensemble synaptic input from thousands of sensory and descending neural pathways, with the group III/IV afferents transmitting signals specific to the metabolic state of the muscle. The propagation of the action potential across the neuromuscular junction (site 4) and into the t-tubule (site 5) is detected by the dihydropyrodine receptor (DHPR) that initiates the release of Ca2+ (Site 6) through the ryanodine receptors (RYR) on the SR membrane. This process requires proper ion gradients and maintained excitability determined, at least in part, by the Na+/K+ pump (site 3) and SR-Ca2+ pump activity (site 7). The binding of Ca2+ to the troponin-tropomyosin complex induces a series of conformational changes in the regulatory proteins (site 8) that allows the interaction of myosin and actin to initiate the power stroke (site 9). Contemporary cross-bridge theory depicted in (C) suggests that the chemo-mechanical transduction of the myosin-actin interaction is partitioned into ~6-strain dependent structural transitions (steps 1–6) that make up the power stroke [32, 53, 72, 73]. Briefly, starting in the rigor complex, ATP binds to the catalytic site on myosin and dissociates myosin from actin (step 1). The hydrolysis of ATP reprimes and cocks the myosin head which attaches to actin in a weakly bound state with the hydrolysis metabolites still bound to the catalytic site (step 2). In the conventional power stroke, the weakly bound cross-bridge transitions to the strongly bound state through unknown mechanisms (step 3). Pi is released from the catalytic site initiating the power stroke where myosin pivots at the light chain domain (step 4). The power stroke continues with an isomerization step where ADP is still bound to the catalytic site (step 5), followed by the release of ADP (step 6). Sites where elevated H+ and Pi disrupt muscle contraction: A majority of the fatigue during high-intensity volitional exercise can be explained by the multifaceted and synergistic effects of increased levels of H+ and Pi. Elevated H+ disrupts contractile function by 1) reducing the sensitivity of the myofilaments to Ca2+ (site 8), 2) decreasing force of the cross-bridge by inhibiting the low- to high-force transition (step 3), and 3) slowing shortening velocity by inhibiting the ADP isomerization step (step 5) and/or the release of ADP (step 6). Elevated Pi disrupts contractile function by 1) decreasing the free [Ca2+] available for release from the SR (site 6), 2) reducing the sensitivity of the myofilaments to Ca2+ (site 8), and 3) decreasing force by inducing an unconventional power stroke where myosin dissociates from actin early in the high-force state prior to the release of Pi and ADP (step 4’).
Anaerobic metabolism impairs contractile function through the accumulation of intracellular metabolites
Intracellular concentrations of ATP ([ATP]) in quiescent skeletal muscle are low (~5–6 mmol/kg wet weight or ~8.2 mM) and could be depleted in less than 2 seconds during high-intensity exercise. The depletion of ATP would cause the contractile proteins to enter a state of rigor which does not occur in vivo; rather, intracellular [ATP] is maintained relatively stable via the synchronized activation of the creatine kinase and adenylate kinase reactions, glycolysis and oxidative phosphorylation. Under the most severe fatigue conditions, intracellular [ATP] rarely fall below 60–70% of resting values in the whole-muscle [12–14], with the most severely depleted muscle fibers reaching ~20% of resting [ATP], or ~1.6–1.8 mM [15]. Even in these severe conditions, the [ATP] has not reached the level necessary to observe impairments in contractile function [12, 13]. Thus, barring the unlikely event of a pronounced intracellular compartmentalization of ATP, there is little-to-no evidence that high-intensity exercise is limited by the rate at which ATP can be synthesized and supplied to the myofibrillar and ion transport ATPases. However, buffering the fall in [ATP] with glycolysis and the creatine kinase and adenylate kinase reactions results in marked disruptions in intracellular homeostasis through the accumulation of ATP hydrolysis metabolites, ADP, inorganic phosphate (Pi) and hydrogen (H+), the latter causing pH to decrease (pH = -log10[H+]).
Direct measures of intramuscular bioenergetics via phosphorus nuclear magnetic resonance spectroscopy (31P-MRS) have provided unprecedented detail of the time course and extent of intracellular metabolite accumulation that occurs during high-intensity exercise in vivo. In quiescent human skeletal muscle, intramuscular pH is ~7.0, [Pi] ~3–5 mM and [ADP] only a few μM [16]. At the onset of high-intensity exercise, ATP is synthesized primarily via the creatine kinase reaction resulting in an exponential decline in the concentration of phosphocreatine ([PCr]) with concomitant increases in [Pi], that can reach >30 mM in human skeletal muscle during volitional exercise [17]. Similarly, other than the first few seconds of exercise where the intracellular pH becomes more alkaline by ~0.1 pH units due to PCr hydrolysis (PCr + ADP + H+ ↔ ATP + Cr) [18], pH declines precipitously and can reach levels between 6.5 to 6.2 during high-intensity exercise [17, 19, 20], with more severe acidic states possible within regions of the muscle. In contrast, due to the creatine kinase and adenylate kinase reactions, [ADP] remains relatively stable until [PCr] is nearly depleted, after which [ADP] increases by a few hundred μM, which are levels that do not appear to significantly disrupt contractile function [12]. It is important to note that other compounds accumulate during high-intensity exercise, such as, AMP, IMP, creatine, lactate, extracellular K+, Mg2+, and reactive oxygen and nitrogen species, that have been implicated in fatigue; however, with the possible exception of K+ at high-firing frequencies [21], either 1) do not impair contractile function or do not reach the concentrations during volitional exercise where impaired contractile function occurs, 2) are not correlated with the reductions in force or power, or 3) cause structural damage to proteins that requires days to recover from and arguably can no longer be considered a mechanism of fatigue [13, 21–24]. Below we present evidence to suggest that a majority of the fatigue during high-intensity volitional exercise can be explained by the multifaceted and synergistic effects of elevated H+ and Pi.
Although in vivo studies are unable to determine whether the accumulation of H+ and/or Pi causes fatigue, two primary observations suggest that these metabolites play a major role in limiting force and power production during high-intensity exercise. The first is the further the exercise intensity exceeds the level that can be supported by oxidative phosphorylation, the more rapidly H+ and Pi accumulate in the muscle [5, 25] and the decrements in contractile function occur [4]. Accordingly, reducing O2 availability through inspired hypoxic gas [26] or blood flow occlusion [27] results in a more rapid accumulation of intracellular H+ and Pi and more rapid impairments in contractile function [28, 29]. The second, and perhaps more important observation, is that the extent of intracellular H+ and Pi accumulation is closely associated with the decrements in 1) force production during volitional [20, 30] and electrically-evoked isometric contractions [17] and 2) power production during volitional dynamic exercise (Fig. 2) [31]. Although these observations do not indicate a causal role for H+ and Pi in fatigue, they do provide the premise for mechanistic studies employing isolated cellular and molecular preparations. In the remainder of the review, we focus primarily on recent discoveries of the effects of H+ and Pi on contractile function from experiments using the chemically skinned fiber preparation and the in vitro motility and laser trap assays [10, 32–34]. The advantage of these approaches is they permit precise control over the milieu surrounding the contractile proteins to systematically study both the individual and combined effects of elevated metabolite levels on contractile function.
Figure 2. Associations between the accumulation of metabolites and the reductions in mechanical power during a fatiguing knee extension exercise.
Young (22.7 ± 1.2 yrs) and old participants (76.4 ± 6.0 yrs) performed a high-intensity fatiguing knee extension exercise consisting of 120 maximal velocity contractions (1 contraction per 2-s) lifting a load equivalent to 20% of the individual specific maximal voluntary isometric contraction. The reduction in power during the 4-min dynamic fatiguing exercise was closely associated with the [Pi] (A), pH (B), and concentration of diprotonated phosphate [H2PO4−] (C) measured over the final 64-s of the exercise. The greater fatigue in the old compared with young adults was accompanied by an ~30% greater increase in the [H+] (pH 6.61 vs. 6.73) and an ~42% greater increase in the [Pi] (32 vs. 23 mM Pi). The combination of the greater decrease in pH and increase in [Pi] resulted in an ~59% greater increase in the [H2PO4−] in the old compared with the young adults. The figure is reprinted with permission from [31].
Effects of acidosis, H+, on contractile function
Although the role of H+ in fatigue remains a topic of intense debate [35, 36], the argument against acidosis as a putative mechanism of fatigue is based primarily on the modest effect H+ has on isometric force under saturating Ca2+ conditions. Indeed, in saturating Ca2+ (pCa = 4.5 where pCa = -log10[Ca2+]) a pH of 6.2 elicits a relatively small, albeit still significant, reduction in peak isometric force of 4–18% in skinned rat and rabbit fibers at 30°C [37, 38]. These findings are consistent with both the 10% decline in peak isometric force elicited by a pH of ~6.67 in living mouse muscle fibers at 32°C [39] and the 20% decline in force elicited by a pH of 6.5 in a mini-ensemble of myosin studied in a laser trap assay at 30°C [34]. The observation that elevated H+ inhibits force production in both the laser trap assay with an unregulated thin filament [34] and in the skinned fiber preparation in saturating Ca2+ [37, 38] suggests that acidosis elicits decrements in force production, at least in part, by directly inhibiting the cross-bridge. While the mechanism remains unresolved, the acidosis induced decrements in isometric force may involve a reduction in the number of bound cross-bridges and/or the force generated per cross-bridge. The finding that the rate of force redevelopment (ktr) following a slack re-extension maneuver of maximally Ca2+-activated human fibers was slowed by elevated H+ and Pi (pH 6.2 + 30 mM Pi) compared with a control condition (pH 7.0 + 4 mM Pi) suggests that acidosis may reduce the force per cross-bridge by inhibiting the low- to high-force state of the cross-bridge cycle [10]. This hypothesis is supported by the decreased high-force generating events and prolonged cross-bridge attachment times elicited by a pH of 6.5 in a mini-ensemble of myosin studied in a laser trap assay [34, 40]. Thus, acidosis appears to inhibit peak isometric force in saturating Ca2+ primarily by reducing the force per cross-bridge via slowing the low- to high-force transition (step 3 Fig. 1C) rather than reducing the number of bound cross-bridges.
In addition to the effect H+ has on peak isometric force in saturating Ca2+, intracellular acidosis contributes to fatigue by decreasing the sensitivity of the myofilament to Ca2+. Because the relationship between the [Ca2+] and isometric force is sigmoidal, the acidosis-induced decrease in myofibrillar Ca2+ sensitivity manifests as a rightward shift in the force-pCa relationship and much greater reductions in isometric force when rat fibers were activated in pH 6.2 and submaximal compared with saturating Ca2+ [41]. The mechanisms for the decreased Ca2+ sensitivity are not fully elucidated; however, there is compelling evidence from skinned rabbit fibers that elevated H+ reduces the affinity of the binding sites on troponin C (TnC) to Ca2+ [42, 43] (site 8 Fig. 1B). A subsequent study using the in vitro motility assay and a specific mutation in TnC that slows the release of Ca2+ from the binding sites revealed that acidosis slows the rate that Ca2+ binds to TnC rather than accelerating the rate at which it’s released [44]. In addition to the decreased Ca2+ affinity, there is also evidence that a H+-binding residue on troponin I (TnI) may contribute to the acidosis-induced decrease in Ca2+ sensitivity by altering the binding affinity of TnI to TnC [45]. Irrespective of the mechanism, the evidence that the free [Ca2+] decreases in the myoplasm during high-intensity contractions to subsaturating levels [46] suggests that the H+-induced decrements in isometric force studied in saturating Ca2+ likely underestimate the depressive effects of acidosis on force production during fatigue in vivo.
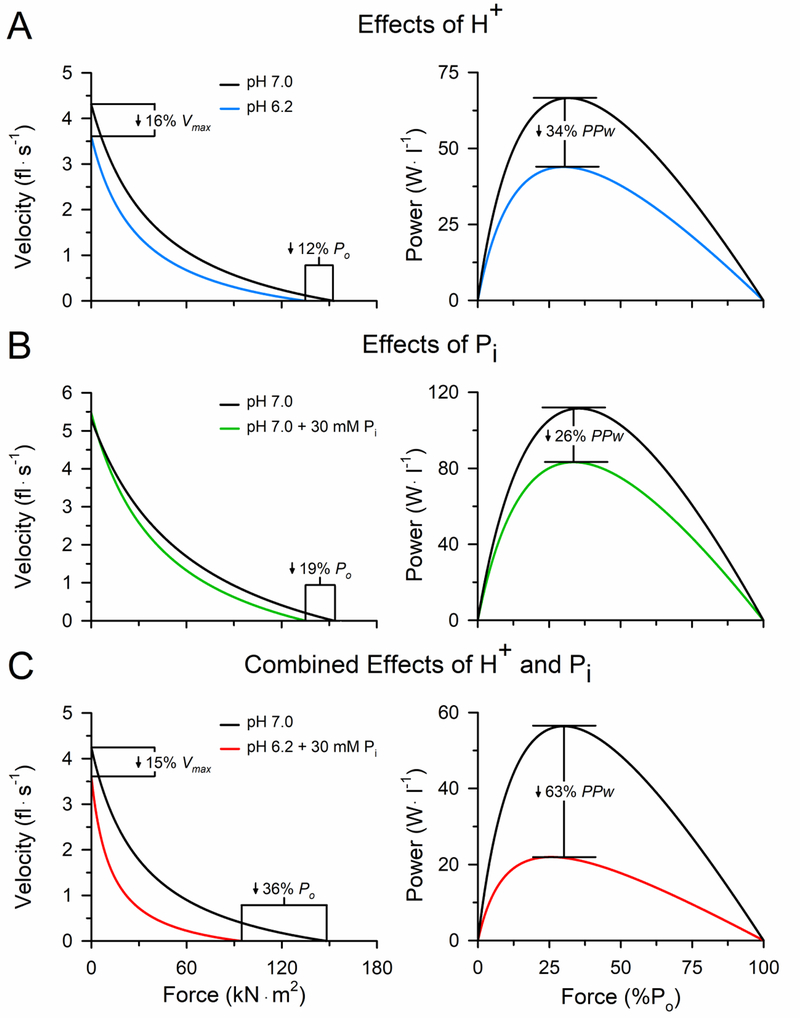
Perhaps more important than the effect acidosis has on isometric force is the effect it has on shortening velocity and the ability to generate mechanical power (Fig. 3). In saturating Ca2+, a pH of 6.2 inhibits the maximal shortening velocity of skinned rat and rabbit fibers by 11–30% at 30°C, regardless of whether the velocity was measured with the slack test or extrapolation of the force-velocity curve [38, 47]. These findings were corroborated by the observation that the actin filament velocity slowed markedly under acidic conditions in the in vitro motility assay at 20–30°C, which could be explained quantitatively by the acidosis-induced increase in the cross-bridge attachment times measured in the single molecule laser trap assay [33, 48]. The increased attachment times and slowed shortening velocity is thought to be due primarily to an inhibition of the ADP isomerization step of the cross-bridge cycle and/or the rate of ADP release [32, 44] (steps 5 & 6 Fig. 1C). The combination of the acidosis-induced decrease in both force and velocity resulted in an 18–34% reduction in peak power of rat fibers activated in pH 6.2 at 30°C [38]. Thus, acidosis has a detrimental effect on contractile function by directly inhibiting force, velocity and power of the cross-bridge and decreasing the sensitivity of the myofilament to Ca2+.
Figure 3. Individual and combined effects of elevated H+ and Pi on force, velocity and power of rat slow MHC I fibers at 30°C.
Force–velocity and force–power curves obtained from rat type I fibers in the control pH 7.0 condition compared with pH 6.2 (A), 30 mM Pi (B), and combined pH 6.2 and 30 mM Pi (C) at 30°C. Shortening velocity (fiber lengths per second) and power (W·l −1) are plotted as a function of the force expressed relative to the fiber cross-sectional area (kN·m2) and as a percentage of the peak isometric force (%Po), respectively. Error bars around the mean curves have been omitted for clarity. Findings from fast fibers are qualitatively similar to those presented here. The figure is reprinted with permission from [32] and the data obtained from [38, 50, 63].
Effects of inorganic phosphate, Pi, on contractile function
Similar to elevated H+, activating rat and rabbit fibers in saturating Ca2+ and 25–30 mM Pi reduced peak isometric force by 5–19% at 30°C [49, 50]. However, the observation that the rate of force redevelopment following a slack re-extension maneuver of a maximally activated fiber (ktr) is accelerated in the presence of elevated Pi [51, 52] but slowed when H+ and Pi are elevated together [10] suggests that the mechanisms for the reduction in force differ for Pi compared with H+. While the mechanism is not fully elucidated, elevated Pi is thought to inhibit force, at least in part, by inducing an unconventional power stroke where myosin dissociates from actin early in the high-force state of the cross-bridge cycle prior to the release of Pi and ADP [32, 53, 54] (step 4’ Fig. 1C). This mechanism is consistent with the increased ATP cost of contraction observed in skinned fibers that occurs from the Pi-induced decrease in isometric force but a maintained myofibrillar ATP hydrolysis rate [54, 55]. Further support for this mechanism comes from the observation that 30 mM Pi elicited a 65% decrease in the cross-bridge attachment times of a mini-ensemble of myosin studied in a laser trap assay at 30°C [56]. Thus, elevated Pi appears to inhibit peak isometric force in saturating Ca2+ primarily by accelerating the detachment of myosin from actin, which reduces the number of bound cross-bridges.
In addition to the effect Pi has on isometric force in saturating Ca2+, elevated Pi contributes to fatigue by decreasing the sensitivity of the myofilament to Ca2+ [57] and inhibiting Ca2+ release from the sarcoplasmic reticulum (SR) [13, 58, 59]. The mechanism for the Pi-induced decrease in myofibrillar Ca2+sensitivity is unknown and needs further investigation; however, it is clear that, unlike H+, elevated Pi does not alter the affinity of the binding sites on TnC to Ca2+ [42]. In contrast to the lack of understanding of how elevated Pi decreases myofibrillar Ca2+ sensitivity, there is more evidence describing the mechanism by which elevated Pi inhibits Ca2+ release from the SR [13, 58, 60]. Briefly, Pi is thought to reduce the amount of free [Ca2+] available for release from the SR by diffusing into the SR through a Pi -permeable channel [61] and binding to Ca2+ to form a precipitate [58–60, 62] (site 6 Fig. 1B). Similar to H+, the reduced free [Ca2+] available in the myoplasm to subsaturating levels coupled with the decreased myofibrillar Ca2+ sensitivity suggests that the Pi -induced decrements in isometric force studied in saturating Ca2+ likely underestimate the depressive effects of Pi on force production during fatigue in vivo.
Unlike elevated H+, Pi does not alter the shortening velocity of rat, rabbit or human fibers activated at 30°C [10, 47, 50]. However, athletic prowess and the ability to perform daily activities is determined more by the muscle’s ability to generate mechanical power, and 30 mM Pi elicited an 18–26% reduction in peak power in rat fibers activated at 30°C [50] (Fig. 3). Thus, elevated Pi has a detrimental effect on contractile function by directly inhibiting force and power of the cross-bridge and by decreasing both the free [Ca2+] available for release from the SR and the sensitivity of the myofilament to Ca2+.
Synergistic effects of H+ and Pi on contractile function
Although studies on the individual effects of H+ and Pi are important, studying their effects when elevated together is more pertinent to understanding how these metabolites contribute to the decrements in contractile function that occur during high-intensity fatiguing exercise in vivo [17, 19, 20, 31]. Given the evidence that elevated H+ and Pi contribute to fatigue by different mechanisms, it is perhaps not surprising that when studied in combination their depressive effects on contractile function are additive. For example, a combined pH 6.2 and 30 mM Pi condition decreased peak isometric force of rat fibers by 36–46% in saturating Ca2+ at 30°C [63] and caused a considerably greater decrease in the sensitivity of the myofilament to Ca2+ than either metabolite alone [41, 57]. Interestingly, the combined condition had a synergistic effect where the decrements were greater than would be predicted from the sum of the individual metabolite effects, particularly for peak power of rat and rabbit fast fibers [38, 47, 50, 63]. The combined pH 6.2 and 30 mM Pi condition decreased peak power by 55–63% in rabbit and rat fibers at 30°C (Fig. 3), which was exacerbated to a 70% decline in power in rabbit fast fibers when the myosin regulatory light chain was phosphorylated [47, 63]. Extrapolating these findings to the whole-muscle suggests that ~55–70% of the reduction in the ability to generate power can be attributed to the synergistic effects of elevated H+ and Pi directly inhibiting the cross-bridge, which is likely exacerbated by the effects these metabolites also have on myofibrillar Ca2+ sensitivity and the release of Ca2+ from the SR. The mechanisms to explain the synergistic effects are unknown [34, 40], but may include alterations in the binding affinities of the myosin-actin interaction at different steps in the cross-bridge cycle [64].
Translating the bioenergetics perspective to understanding fatigue in old adults
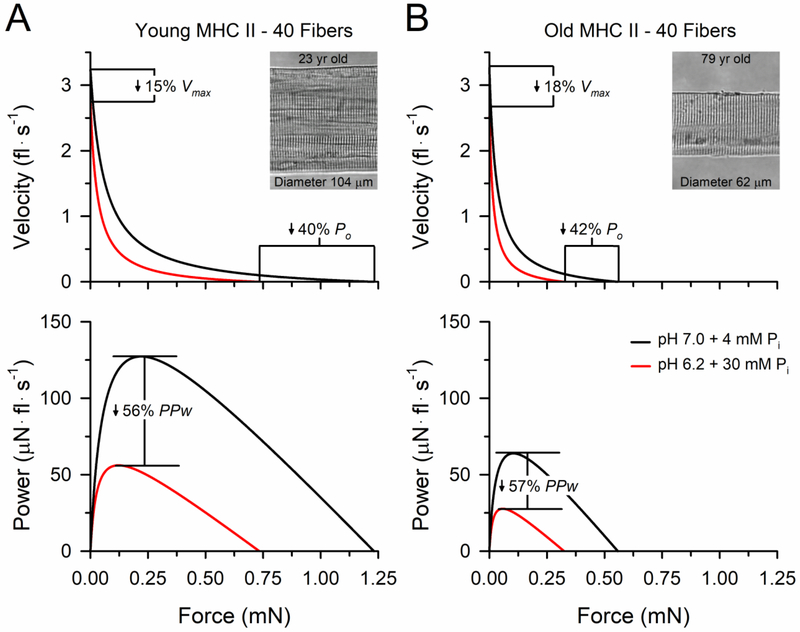
The ability of old adults to generate power is severely compromised by the combination of the atrophy of muscle fibers expressing the fast myosin heavy chain isoforms [10, 65, 66] and the increased fatigue that occurs when old adults perform moderate- to high-velocity contractions [11, 67–70]. The mechanisms for the age-related increase in fatigue are unresolved; however, studies employing non-invasive stimulation procedures to the intact neuromuscular system have localized the primary site to within the muscle rather than the nervous system [10, 11, 68, 69, 71]. Translating the understanding of the bioenergetic basis of fatigue to this problem, we tested whether age-related changes of the muscle resulted in either 1) an increased sensitivity of the cross-bridge to a given concentration of metabolite accumulation or 2) an increased production of metabolites due to a greater reliance on anaerobic metabolism. To test these hypotheses, we exposed fibers from the vastus lateralis of young and old men to a condition mimicking quiescent skeletal muscle (pH 7.0 + 4 mM Pi) and the combined pH 6.2 and 30 mM Pi condition (Fig. 4). While the data confirmed that these metabolites act synergistically to impair cross-bridge function, the decrements in force, velocity and power from the combined pH 6.2 and 30 mM Pi condition did not differ in the fibers isolated from young compared with old men [10]. In contrast, when we had young and old adults perform a dynamic fatiguing knee extension exercise while simultaneously measuring the intracellular metabolite accumulation with 31P-MRS, the greater fatigue in the old compared with young adults was accompanied by an ~30% greater increase in the [H+] (pH 6.61 vs. 6.73) and an ~42% greater increase in the [Pi] (32 vs. 23 mM Pi) [31]. Importantly, the reductions in power during the fatiguing exercise were closely associated with the intracellular metabolite accumulation (Fig. 2) suggesting that the increased fatigue in old adults during dynamic exercise has a bioenergetic basis explained by an increased accumulation of metabolites within the muscle [31].
Figure 4. Force-velocity and force-power curves of fast MHC II fibers from young and old men at 15°C.
Absolute peak power (PPw) and peak isometric force (Po) of the fast MHC II fibers from young adults (A) were ~2-fold greater than in fibers from the old adults (B). The age differences in absolute PPw and Po were explained entirely by differences in the size of the fibers between young and old adults. The insets are digital images taken of young and old adult fast fibers at 800x magnification that are representative of the average size of the fast MHC II fibers studied in the young and old adults. The combined pH 6.2 and 30 mM Pi condition caused significant decreases in Vmax, Po and PPw compared to the condition mimicking quiescent human skeletal muscle (pH 7.0 + 4 mM Pi) in fibers from young and old adults; however, the relative reductions did not differ with age. The variances around the mean curves were omitted for clarity. The figure was modified with permission from [10].
Concluding remarks
Integrating findings from in vivo and isolated cellular and molecular studies has provided considerable advancements in our understanding of how a reliance on anaerobic metabolism disrupts contractile function during high-intensity exercise. While many of the mechanisms are not fully resolved, the data suggest that fatigue during high-intensity exercise is determined, in large part, by the rate and extent of intracellular metabolite accumulation. We conclude that a majority of the fatigue in healthy young and old adults performing high-intensity volitional exercise can be explained by the multifaceted and synergistic effects of elevated H+ and Pi acting to impair contractile function within the muscle. Translating this understanding of the bioenergetic basis of fatigue to clinical populations may help guide studies aimed at identifying the mechanisms of fatigue and designing targeted therapies to offset the detrimental effects of fatigue in these alternative populations. Future studies are needed to identify how elevated H+ and Pi act synergistically to impair cross-bridge function and decrease myofibrillar Ca2+ sensitivity so that we may ultimately develop treatments to attenuate the effects of these metabolites on contractile function.
HIGHLIGHTS.
The reliance on anaerobic metabolism causes fatigue during high-intensity exercise
Metabolic pathways maintain ATP at levels that do not impair contractile function
Intracellular homeostasis is disrupted by metabolite accumulation, namely H+ and Pi
Multifaceted and synergistic effects of H+ and Pi markedly impair muscle contraction
Fatigue has a bioenergetic basis determined by the rate and extent of metabolite accumulation
Acknowledgements
Because of journal restrictions on manuscript length and references, the literature on neuromuscular fatigue and skeletal muscle bioenergetics could not be incorporated and acknowledged to the extent warranted by the existing scholarship. We are sincerely grateful to Ethan Claunch for the illustration in Fig. 1.
Funding
This work was supported by an American Heart Association postdoctoral fellowship (19POST34380411) to Christopher Sundberg and a National Institute of Aging R01 (AG048262) to Robert Fitts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No conflicts of interests, financial or otherwise, are declared by the authors.
References and Recommended Reading
Papers of particular interest are highlighted as:
*of special interest
**of outstanding interest
- [1].Bundle MW, Weyand PG, Sprint Exercise Performance: Does Metabolic Power Matter?, Exer Sport Sc Rev 40(3) (2012) 174–182. [DOI] [PubMed] [Google Scholar]
- *[2].Sundberg CW, Hunter SK, Bundle MW, Rates of performance loss and neuromuscular activity in men and women during cycling: evidence for a common metabolic basis of muscle fatigue, J Appl Physiol (1985) 122(1) (2017) 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compared the neuromuscular activity and rates of power loss between men and women during multiple exercise bouts that spanned the full range of power outputs that could be generated by the neuromuscular system. The data revealed that while the absolute cycling power outputs differed considerably between the sexes, the rate-limiting mechanisms were similar between men and women and determined by the extent the exercise relied on anaerobic metabolism.
- [3].Sundberg CW, Bundle MW, Influence of duty cycle on the time course of muscle fatigue and the onset of neuromuscular compensation during exhaustive dynamic isolated limb exercise, Am J Physiol Regul Integr Comp Physiol 309(1) (2015) R51–R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Burnley M, Vanhatalo A, Jones AM, Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans, J Appl Physiol (1985) 113(2) (2012) 215–223. [DOI] [PubMed] [Google Scholar]
- [5].Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC, Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS, Am J Physiol Regul Integr Comp Physiol 294(2) (2008) R585–93. [DOI] [PubMed] [Google Scholar]
- [6].Burnley M, Jones AM, Power-duration relationship: Physiology, fatigue, and the limits of human performance, Eur J Sport Sci 18(1) (2018) 1–12. [DOI] [PubMed] [Google Scholar]
- [7].Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OCJ, Reflex origin for the slowing of motoneuron firing rates in fatigue of human voluntary contractions, J Physiol 379 (1986) 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA, Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans, J Physiol 589(Pt 21) (2011) 5299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gandevia SC, Spinal and supraspinal factors in human muscle fatigue, Physiol Rev 81(4) (2001) 1725–89. [DOI] [PubMed] [Google Scholar]
- **[10].Sundberg CW, Hunter SK, Trappe SW, Smith CS, Fitts RH, Effects of elevated H+ and Pi on the contractile mechanics of skeletal muscle fibres from young and old men: implications for muscle fatigue in humans, J Physiol 596(17) (2018) 3993–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to examine the combined effects of elevated H+ and Pi on contractile function of fibers isolated from human skeletal muscle. The data confirmed previous non-human studies that the metabolites act synergestically to impair cross-bridge function, but that the effects were similar in fibers isolated from young and old men. The findings highlight the importance of the metabolites in human muscle fatigue, while also suggesting that the age-related increase in fatigue during dynamic exercise cannot be explained by an increased sensitivity of the cross-bridge to H+ and Pi.
- [11].Sundberg CW, Kuplic A, Hassanlouei H, Hunter SK, Mechanisms for the age-related increase in fatigability of the knee extensors in old and very old adults, J Appl Physiol (1985) 125(1) (2018) 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cooke R, Modulation of the actomyosin interaction during fatigue of skeletal muscle, Muscle & Nerve 36(6) (2007) 756–777. [DOI] [PubMed] [Google Scholar]
- [13].Allen DG, Lamb GD, Westerblad H, Skeletal muscle fatigue: Cellular mechanisms, Physiol Rev 88(1) (2008) 287–332. [DOI] [PubMed] [Google Scholar]
- [14].Fitts RH, Cellular mechanisms of muscle fatigue, Physiol Rev 74(1) (1994) 49–94. [DOI] [PubMed] [Google Scholar]
- [15].Karatzaferi C, de Haan A, Ferguson RA, van Mechelen W, Sargeant AJ, Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise, Pflugers Arch 442(3) (2001) 467–74. [DOI] [PubMed] [Google Scholar]
- [16].Kemp GJ, Meyerspeer M, Moser E, Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review, NMR Biomed 20(6) (2007) 555–65. [DOI] [PubMed] [Google Scholar]
- *[17].Broxterman RM, Layec G, Hureau TJ, Amann M, Richardson RS, Skeletal muscle bioenergetics during all-out exercise: mechanistic insight into the oxygen uptake slow component and neuromuscular fatigue, J Appl Physiol (1985) 122(5) (2017) 1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows the association between the increase in intracellular metabolite accumulation and the reduction in the electrically-evoked twitch following an all out, isometric knee extension exercise. The associations provide compelling evidence that elevated H+ and Pi have an important role in limiting force production in vivo by disrupting contractile function.
- [18].Adams GR, Foley JM, Meyer RA, Muscle buffer capacity estimated from pH changes during rest-to-work transitions, J Appl Physiol (1985) 69(3) (1990) 968–72. [DOI] [PubMed] [Google Scholar]
- [19].Cady EB, Jones DA, Lynn J, Newham DJ, Changes in force and intracellular metabolites during fatigue of human skeletal muscle, J Physiol 418 (1989) 311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wilson JR, McCully KK, Mancini DM, Boden B, Chance B, Relationship of muscular fatigue to pH and diprotonated P in humans: a 31P-NMR study, J Appl Physiol (1985) 64(6) (1988) 2333–9. [DOI] [PubMed] [Google Scholar]
- **[21].Pedersen KK, Nielsen OB, Overgaard K, Contractile benefits of doublet-initiated low-frequency stimulation in rat EDL muscle exposed to high extracellular [K+] or fatiguing contractions, Am J Physiol Cell Physiol (2019). [DOI] [PubMed] [Google Scholar]; This study shows that elevated extracellular K+ (11 mM) does not alter the contractile response of the rat EDL muscle to high-frequency doublet stimulations (300 Hz) when elicited among a train of near in vivo firing frequencies (50 Hz) but inhibits power when elicited among a train of high-firing frequencies (150 Hz). The data suggest that elevated extracellular K+ may play a role in fatigue at high-firing frequencies but does not likely have a major role at the firing frequencies typically observed during volitional exercise in vivo.
- [22].Cheng AJ, Yamada T, Rassier DE, Andersson DC, Westerblad H, Lanner JT, Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery, J Physiol 594(18) (2016) 5149–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McKenna MJ, Bangsbo J, Renaud JM, Muscle K+, Na+, and Cl disturbances and Na+-K+ pump inactivation: implications for fatigue, J Appl Physiol (1985) 104(1) (2008) 288–95. [DOI] [PubMed] [Google Scholar]
- [24].Brooks GA, The Science and Translation of Lactate Shuttle Theory, Cell Metab 27(4) (2018) 757–785. [DOI] [PubMed] [Google Scholar]
- [25].Vanhatalo A, Fulford J, DiMenna FJ, Jones AM, Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study, Exp Physiol 95(4) (2010) 528–540. [DOI] [PubMed] [Google Scholar]
- [26].Hogan MC, Richardson RS, Haseler LJ, Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study, J Appl Physiol (1985) 86(4) (1999) 1367–1373. [DOI] [PubMed] [Google Scholar]
- [27].Lanza IR, Larsen RG, Kent-Braun JA, Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow, J Physiol 583(Pt 3) (2007) 1093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Romer LM, Haverkamp HC, Lovering AT, Pegelow DF, Dempsey JA, Effect of exercise-induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans, Am J Physiol Regul Integr Comp Physiol 290(2) (2006) R365–75. [DOI] [PubMed] [Google Scholar]
- [29].Romer LM, Haverkamp HC, Amann M, Lovering AT, Pegelow DF, Dempsey JA, Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans, Am J Physiol Regul Integr Comp Physiol 292(1) (2007) R598–606. [DOI] [PubMed] [Google Scholar]
- [30].Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA, In vivo ATP production during free-flow and ischaemic muscle contractions in humans, J Physiol 577(Pt 1) (2006) 353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[31].Sundberg CW, Prost RW, Fitts RH, Hunter SK, Bioenergetic basis for the increased fatigability with ageing, J Physiol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows the association between the increase in intracellular metabolite accumulation and the reduction in power during an all-out, volitional knee extension exercise. The data also reveal that the age-related increase in fatigue during dynamic exercise is accompanied by an ~30% greater increase in the [H+] (pH 6.73 vs. 6.61) and an ~42% greater increase in the [Pi] (32 vs. 23 mM Pi), suggesting that the increased fatigue in old adults has a bioenergetic basis explained by an increased accumulation of H+ and Pi.
- [32].Debold EP, Fitts RH, Sundberg CW, Nosek TM, Muscle fatigue from the perspective of a single crossbridge, Med Sci Sports Exerc 48(11) (2016) 2270–2280. [DOI] [PubMed] [Google Scholar]
- **[33].Debold EP, Longyear TJ, Turner MA, The effects of phosphate and acidosis on regulated thin-filament velocity in an in vitro motility assay, J Appl Physiol (1985) 113(9) (2012) 1413–22. [DOI] [PubMed] [Google Scholar]; This study shows that pH 6.5 causes marked reductions in the actin filament velocity in the in vitro motility assay, which confirms findings from skinned fibers that acidosis inhibits velocity. The study also presents data suggesting that the mechanism by which acidosis slows shortening velocity is by inhibiting the ADP isomerization step of the cross-bridge cycle (step 5 Fig. 1C).
- [34].Woodward M, Debold EP, Acidosis and Phosphate Directly Reduce Myosin’s Force-Generating Capacity Through Distinct Molecular Mechanisms, Front Physiol 9 (2018) 862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fitts RH, The Role of Acidosis in Fatigue: Pro Perspective, Med Sci Sports Exerc 48(11) (2016) 2335–2338. [DOI] [PubMed] [Google Scholar]
- [36].Westerblad H, Acidosis Is Not a Significant Cause of Skeletal Muscle Fatigue, Med Sci Sports Exerc 48(11) (2016) 2339–2342. [DOI] [PubMed] [Google Scholar]
- [37].Pate E, Bhimani M, Franks-Skiba K, Cooke R, Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue, J Physiol 486 (Pt 3) (1995) 689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[38].Knuth ST, Dave H, Peters JR, Fitts RH, Low cell pH depresses peak power in rat skeletal muscle fibres at both 30°C and 15°C: implications for muscle fatigue, J Physiol 575(Pt 3) (2006) 887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study tested the effects of elevated H+ on force, velocity and power of rat slow and fast fibers at near in vivo temperatures of 30°C. The data revealed that pH 6.2 elicited a 4–12% decrease in isometric force, a 25–32% decrease in unloaded shortening velocity, and an 18–34% decrease in peak power, indicating that acidosis has an important role in fatigue by directly disrupting cross-bridge function.
- [39].Westerblad H, Bruton JD, Lannergren J, The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature, J Physiol 500 (Pt 1) (1997) 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jarvis K, Woodward M, Debold EP, Walcott S, Acidosis affects muscle contraction by slowing the rates myosin attaches to and detaches from actin, J Muscle Res Cell Motil (2018). [DOI] [PubMed] [Google Scholar]
- [41].Nelson CR, Fitts RH, Effects of low cell pH and elevated inorganic phosphate on the pCa-force relationship in single muscle fibers at near-physiological temperatures, Am J Physiol Cell Physiol 306(7) (2014) C670–8. [DOI] [PubMed] [Google Scholar]
- [42].Palmer S, Kentish JC, The role of troponin C in modulating the Ca2+ sensitivity of mammalian skinned cardiac and skeletal muscle fibres, J Physiol 480 (Pt 1) (1994) 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Parsons B, Szczesna D, Zhao J, Van Slooten G, Kerrick WG, Putkey JA, Potter JD, The effect of pH on the Ca2+ affinity of the Ca2+ regulatory sites of skeletal and cardiac troponin C in skinned muscle fibres, J Muscle Res Cell Motil 18(5) (1997) 599–609. [DOI] [PubMed] [Google Scholar]
- [44].Longyear TJ, Turner MA, Davis JP, Lopez J, Biesiadecki B, Debold EP, Ca++-sensitizing mutations in troponin, Pi, and 2-deoxyATP alter the depressive effect of acidosis on regulated thin-filament velocity, J Appl Physiol (1985) 116(9) (2014) 1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Robertson IM, Holmes PC, Li MX, Pineda-Sanabria SE, Baryshnikova OK, Sykes BD, Elucidation of isoform-dependent pH sensitivity of troponin i by NMR spectroscopy, J Biol Chem 287(7) (2012) 4996–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[46].Allen DG, Clugston E, Petersen Y, Roder IV, Chapman B, Rudolf R, Interactions between intracellular calcium and phosphate in intact mouse muscle during fatigue, J Appl Physiol (1985) 111(2) (2011) 358–66. [DOI] [PubMed] [Google Scholar]; This study is the first to show that both the myoplasmic free Ca2+ and SR Ca2+ decline from the onset of contractile activity and continue to decrease in conjunction with the decrease in force during a fatiguing protocol in an in situ mouse muscle preparation. The decrease in isometric force production was associated with the increase in the [Pi] suggesting that Pi may be responsible for the changes in free [Ca2+]. Importantly, the fatiguing protocol in this preparation elicited an exponential decline in force production, which is similar to what is observed in humans during all-out, volitional exercise.
- **[47].Karatzaferi C, Franks-Skiba K, Cooke R, Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue, Am J Physiol Regul Integr Comp Physiol 294(3) (2008) R948–55. [DOI] [PubMed] [Google Scholar]; This is the first study to demonstrate that elevated H+ and Pi act synergistically to inhibit peak power production in rabbit fast fibers at near in vivo temperatures of 30°C. Specifically, the combined pH 6.2 and 30 mM Pi condition elicited a 55% decrease in peak power that was exacerbated to a 70% reduction when the myosin regulatory light chain was phosphorylated.
- [48].Debold EP, Beck SE, Warshaw DM, Effect of low pH on single skeletal muscle myosin mechanics and kinetics, Am J Physiol Cell Physiol 295(1) (2008) C173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Coupland ME, Puchert E, Ranatunga KW, Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate, J Physiol 536(Pt 3) (2001) 879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Debold EP, Dave H, Fitts RH, Fiber type and temperature dependence of inorganic phosphate: implications for fatigue, Am J Physiol Cell Physiol 287(3) (2004) C673–81. [DOI] [PubMed] [Google Scholar]
- [51].Wahr PA, Cantor HC, Metzger JM, Nucleotide-dependent contractile properties of Ca2+-activated fast and slow skeletal muscle fibers, Biophys J 72(2 Pt 1) (1997) 822–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tesi C, Colomo F, Piroddi N, Poggesi C, Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles, J Physiol 541(Pt 1) (2002) 187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Caremani M, Melli L, Dolfi M, Lombardi V, Linari M, The working stroke of the myosin II motor in muscle is not tightly coupled to release of orthophosphate from its active site, J Physiol 591(20) (2013) 5187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[54].Linari M, Caremani M, Lombardi V, A kinetic model that explains the effect of inorganic phosphate on the mechanics and energetics of isometric contraction of fast skeletal muscle, Proc Biol Sci 277(1678) (2010) 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study developed a model that accurately describes the increased ATP cost of contraction with the increase in the [Pi] in skinned fibers. The model predicts that Pi inhibits isometric force production by inducing an unconventional power stroke where myosin detaches from actin early in the strongly bound state prior to the release of Pi and ADP (Step 4’ Fig. 1C).
- [55].Kerrick WG, Xu Y, Inorganic phosphate affects the pCa-force relationship more than the pCa-ATPase by increasing the rate of dissociation of force generating cross-bridges in skinned fibers from both EDL and soleus muscles of the rat, J Muscle Res Cell Motil 25(2) (2004) 107–17. [DOI] [PubMed] [Google Scholar]
- *[56].Debold EP, Walcott S, Woodward M, Turner MA, Direct observation of phosphate inhibiting the force-generating capacity of a miniensemble of Myosin molecules, Biophys J 105(10) (2013) 2374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that elevated Pi inhibits force in a miniensemble of myosin studied in the laser trap assay and decreases the cross-bridge attachment times by 65%. Using mechanical modeling of the cross-bridge cycle, the study was able to accurately predict the Pi-induced effects by incorporating an unconventional power stroke where myosin detaches from actin early in the strongly bound state prior to the release of Pi and ADP (Step 4’ Fig. 1C).
- [57].Debold EP, Romatowski J, Fitts RH, The depressive effect of Pi on the force-pCa relationship in skinned single muscle fibers is temperature dependent, Am J Physiol Cell Physiol 290(4) (2006) C1041–50. [DOI] [PubMed] [Google Scholar]
- [58].Allen DG, Trajanovska S, The multiple roles of phosphate in muscle fatigue, Front Physiol 3 (2012) 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fryer MW, Owen VJ, Lamb GD, Stephenson DG, Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres, J Physiol 482 (Pt 1) (1995) 123–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Allen DG, Westerblad H, Role of phosphate and calcium stores in muscle fatigue, Journal of Physiology-London 536(3) (2001) 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Laver DR, Lenz GK, Dulhunty AF, Phosphate ion channels in sarcoplasmic reticulum of rabbit skeletal muscle, J Physiol 535(Pt 3) (2001) 715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dutka TL, Cole L, Lamb GD, Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle, Am J Physiol Cell Physiol 289(6) (2005) C1502–12. [DOI] [PubMed] [Google Scholar]
- *[63].Nelson CR, Debold EP, Fitts RH, Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers, Am J Physiol Cell Physiol 307(10) (2014) C939–C950. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to demonstrate that elevated H+ and Pi act synergistically to inhibit peak power production in slow fibers at low (15°C) and near in vivo temperatures (30°C), and showed an ~60% decline in peak power in both slow and fast fibers at both temperatures.
- [64].Karatzaferi C, Adamek N, Geeves MA, Modulators of actin-myosin dissociation: basis for muscle type functional differences during fatigue, Am J Physiol Cell Physiol 313(6) (2017) C644–c654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lexell J, Human aging, muscle mass, and fiber type composition, J Gerontol A Biol Sci Med Sci 50 Spec No (1995) 11–6. [DOI] [PubMed] [Google Scholar]
- [66].Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ, The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size, Exp Gerontol 48(5) (2013) 492–8. [DOI] [PubMed] [Google Scholar]
- [67].McNeil CJ, Rice CL, Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors, J Gerontol A Biol Sci Med Sci 62(6) (2007) 624–9. [DOI] [PubMed] [Google Scholar]
- [68].Dalton BH, Power GA, Vandervoort AA, Rice CL, The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions, Exp Gerontol 47(1) (2012) 85–92. [DOI] [PubMed] [Google Scholar]
- [69].Dalton BH, Power GA, Vandervoort AA, Rice CL, Power loss is greater in old men than young men during fast plantar flexion contractions, J Appl Physiol (1985) 109(5) (2010) 1441–7. [DOI] [PubMed] [Google Scholar]
- [70].Callahan DM, Kent-Braun JA, Effect of old age on human skeletal muscle force-velocity and fatigue properties, J Appl Physiol (1985) 111(5) (2011) 1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Baudry S, Klass M, Pasquet B, Duchateau J, Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions, Eur J Appl Physiol 100(5) (2007) 515–25. [DOI] [PubMed] [Google Scholar]
- [72].Geeves MA, Fedorov R, Manstein DJ, Molecular mechanism of actomyosin-based motility, Cell Mol Life Sci 62(13) (2005) 1462–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Caremani M, Melli L, Dolfi M, Lombardi V, Linari M, Force and number of myosin motors during muscle shortening and the coupling with the release of the ATP hydrolysis products, J Physiol 593(15) (2015) 3313–32. [DOI] [PMC free article] [PubMed] [Google Scholar]