Abstract
Cobalt-chromium-molybdenum (CoCrMo) alloys are widely used in load-bearing implants; specifically, in hip, knee, and spinal applications due to their excellent wear resistance. However, due to in vivo corrosion and mechanically assisted corrosion, metal ion release occurs and accounts for poor biocompatibility. Therefore, a significant interest to find an alternative to CoCrMo alloy exists. In the present work we hypothesize that calcium phosphate (CaP) will behave as a solid lubricant in CoCrMo alloy under tribological testing, thereby minimizing wear and metal ion release concerns associated with CoCrMo alloy. CoCrMo-CaP composite coatings were processed using laser engineered net shaping (LENS™) system. After LENS™ processing, CoCrMo alloy was subjected to laser surface melting (LSM) using the same LENS™ set-up. Samples were investigated for microstructural features, phase identification, and biocompatibility. It was found that LSM treated CoCrMo improved wear resistance by 5 times. CoCrMo-CaP composites displayed the formation of a phosphorus-based tribofilm. In vitro cell-material interactions study showed no cytotoxic effect. Sprague-Dawley rat and rabbit in vivo study displayed increased osteoid formation for CoCrMo-CaP composites, up to 2 wt.% CaP. Our results show that careful surface modification treatments can simultaneously improve wear resistance and in vivo biocompatibility of CoCrMo alloy, which can correlate to a reduction of metal ion release in vivo.
Keywords: CoCrMo alloys, load-bearing implants, metal ion release, tribofilms, surface modification
Graphical abstract

1. Introduction
While titanium alloys are known for their lower Young’s modulus and excellent biocompatibility in load-bearing implants,1,2 CoCrMo alloys are known more for applications in articulating surfaces of hip and knee joint implants.3-5 CoCrMo alloys are widely used in such articulating load-bearing implant applications due to their excellent wear resistance.6-8 Dental applications have also been seen such as crowns, abutments, and screw-based restoration devices.9 Demand for all of these implants is growing due to the increase in the median age of our population and a more active lifestyle.10,11 Although load-bearing implants are popular and dependable, revision surgeries are also common. Several reasons for revision surgeries are due to wear-induced osteolysis, ascetic loosening, metal ion release due to bio-tribocorrosion, and mismatch of modulus between the implant and bone tissue.11-14 Moreover, it is worth noting that wear and corrosion induced metal ion release from CoCrMo alloy can cause cancer and metallosis.2,11,12,15,16 A recent study, with 8343 patients over 9 years, showed that risk of cancer, not just bone but prostate, kidney and skin cancers as well and is significantly higher for patients with total hip arthroplasty (THA) that have either metal-on-metal (MOM) or metal-on-polymer (MOP) device.17 Other studies have also linked leukemic cancers and lymphomas for patients with THAs and total knee arthroplasties (TKAs).18 Almost in all cases, these cancers were linked to Co2+ and Cr3+ ion release due to wear and biocorrosion of the metal implants. Moreover, toxicity from Co2+ can cause neurological related symptoms (deafness and blindness), endocrine (hypothyroidism), hematological and cardiological issues as well.19 Coating of biomedical implants with bioactive ceramics offers improved biocompatibility,20 and also can suppress the leaching of metal ions.
It has been reported that coating of titanium with tricalcium phosphate (TCP) by laser engineering net shaping (LENS™) improved osteoprecursor cell-material interaction and increased hardness.21 LENS™ and radio frequency induction plasma spraying processing for graded hydroxyapatite coatings has also shown an increase in hardness and improved human fetal osteoblast cellular activity.22 Coating biphasic calcium phosphate (CaP) consisting of 72% hydroxyapatite (HA)/28% beta-tri-CaP on Ti6Al4V by neodymium-doped yttrium aluminum garnet laser technique also increased osteoblast-like cell proliferation 23 Borrowing that idea, we have shown that addition of CaP in CoCrMo can improve wear resistance.24 Apart from that, significant research is being conducted by various groups to address minimizing metal ion release using heat treatment to remelt the surface,25,26 improve surface properties through nitriding,27,28 and use of another alloy system to create compositional gradient coatings.29-31 Use of DC magnetron sputtering-based coating of TiC on silicon (001) improved biocompatibility as a potential for improvement in the surface of Ti and Ti alloys.32 However, further corrosion tests of these TiC nanocomposite coatings on CoCrMo alloy showed inferior corrosion resistance when compared to the uncoated alloy.33 Use of in situ biocompatible coatings or forming a biocompatible coating using simulated body fluids has also been done.34-36 Nevertheless, simultaneous improvement of wear resistance and inherent biocompatibility of CoCrMo alloys have not been accomplished using these techniques, which is the key objective of the present work.
HA is one of the main phases of CaP-based ceramics, which closely matches the chemistry of bone. HA is known for its applications in medicine as a synthetic bone substitute. HA can be produced from various sources such as from deproteinized human bone, eggshells, seashells, and inorganic substances,37,38 thereby decreasing processing complexity by reducing exotic raw material requirements. Thus, considering HA’s abundance and positive results from previous studies, addition of CaP in the form of HA to CoCrMo alloy was considered for this study. In addition, the effects of laser surface melting (LSM) on CoCrMo was also investigated. Previously, it was reported that tribological properties of alloys can be altered and improved using LSM due to surface hardening.39 It was reported that the wear rate decreased from ~7×10−4 mm3/Nm to ~3.5×10−4 mm3/Nm in Ti6Al4V due to LSM.40 In the present study to measure the biocompatibility issues of CoCrMo-CaP composites, CoCrMo alloys were surface treated with CaP ceramics followed by in vitro cell material interactions study using human osteoblast cells, and in vivo studies using both rat and rabbit distal femur models. In addition, microstructure, phase analysis, and tribological properties were studied via SEM, X-Ray diffraction (XRD) and a single station fully automated bio-tribometer, respectively. We hypothesized that laser surface melting or addition of calcium phosphate (CaP) in CoCrMo would reduce metal ion release. We further hypothesize that such addition of CaP in CoCrMo would also improve in vitro and in vivo biocompatibility.
2. Materials and Methods
2.1. Fabrication of CoCrMo-CaP composites using LENS™:
Several differing powder compositions were prepared for sample fabrication from stock powders. CoCrMo alloy (Stellite 6B) powder was attained from Corrosion Materials, Inc., (Houston, TX). Hydroxyapatite (CaP) powder was attained from Berkeley Advanced Biomaterials Inc., (Berkeley, CA). Initially, CaP powder was of <500 nm, which was heat treated to allow for agglomeration and then both CoCrMo and HA powder were sieved to particle size distribution of 49 to 145 μm. The prepared feedstock powder compositions were of pure CoCrMo alloy powder (CCM0), and 1 (CCM1), 2 (CCM2) and 3 (CCM3) wt.% CaP. Premixed feedstock powders were then processed using a commercial Laser Engineered Net Shaping system (LENS™ 750, Optomec Inc., Albuquerque, NM). The CoCrMo powder used for in vitro and in vivo work as well as the CoCrMo (CCM) substrate were of medical grade (Biodur® CCM Plus® Alloy) and attained from CarTech Micromelt®, (Carpenter (CRS) Inc., Philadelphia, PA). Fabricated in vitro samples were 12 mm diameter × 2 mm thick disks. In vivo rat samples were of 3 mm diameter × 5 mm in length cylinders and in vivo rabbit samples were of 5.5 mm diameter × 8 mm in length. Tribological and mechanical testing samples were of 12 × 10 mm geometry. Laser power of 400W and a raster scan speed of 45-60 cm/min were used while depositing the CoCrMo-CaP composites along with a powder feed rate of 60 g/min. Processing parameter optimization was conducted in the previous study and has been carried forward to the present study.24 In vitro and in vivo samples were sterilized by autoclaving. Further detail is discussed under the respective in vitro and in vivo sections. LSM sample preparation consisted of surface melting medical grade commercially available CoCrMo (CCM1LP). The surface melt geometry was of 10 mm diameter at 8.5 mm/s scan velocity using 450 W laser power for single laser pass samples. The laser surface melting experiments were conducted in an argon environment having oxygen content under 10 ppm.
2.2. Microstructure and phase analyses:
Characterization of CCM1LP microstructure was done transverse-plane cross-sectioning. Electrochemical polishing was conducted with 5% HCl aqueous solution at an applied potential of 3 V for 10 s. Microstructural imaging was done using a field emission scanning electron microscope (FESEM, FEI-SIRION, OR, USA), along with energy-dispersive x-ray spectroscopy (EDS). Surface of CCM1LP and substrate were analyzed by X-ray diffraction (XRD) using Cu-Kα radiation (PANalytical X’pert Pro MPD XRD machine, Netherlands). XRD analyses were performed between 10 and 90 degrees.
2.3. Hardness and wear characterization:
Hardness measurements were carried out using a Vickers microhardness tester with a load of 500 g with a dwell time of 15 s on the surface of the specimens. For reporting the hardness values, at least 6 measurements were taken. Ball-on-flat wear testing was conducted using a fully automated tribometer (NANOVEA, Microphotonics Inc., CA USA) in deionized (DI) water at room temperature. Wear tests were carried out using a 3 mm diameter hardened chrome steel ball (Boca Bearing, Boynton Beach, FL, USA). Wear tests were conducted with a normal load of 7N for CCM1LP and 5N for CCM samples with a total sliding distance of 1000 m. Electron micrographs were attained from the worn surfaces (wear scar) for characterization of the surface topography. At the end of every wear tests, wear media was collected and analyzed to determine the released ion concentration of Co2+ and Cr3+ via ICP-MS (Agilent 7700 ICP-MS, OR). Nanoindentation tests of the control CoCrMo alloy (CCM), CCM0, CCM3, and CCM1LP were carried out using a Hysitron TI-900 Nanoindenter with a load rate of 1800 μN/s, max load of 9000 μN, dwell time of 12 s and an unloading rate of -1800 μN/s.
2.4. In vitro cell-materials interaction study:
Circular disc samples of 12 mm diameter and 2 mm thickness were used for bone cell-material interactions studies for a period of 11 days using human fetal osteoblast cells. Commercially pure Titanium (Cp-Ti) samples were used as a control. Further details of in vitro study protocol have been given in the supplemental data.
2.5. In vivo rat study:
22 male Sprague-Dawley rats of 300g weight each were used for the study. Implanted compositions of 3 mm diameter cylinders were of dense Ti, CCM0, CCM1, CCM2, CCM3. Bilateral surgery was performed on all rats. Further details of the surgical procedure are given in the supplemental data. Further details of in vivo rat study protocol have been given in the supplemental data.
2.6. In vivo rabbit study:
8 New Zealand white rabbits (Western Oregon Rabbit Company, Philomath, OR) with an approximate weight of 4 kg each were used. CoCrMo implants with 0 and 2% CaP were used for in vivo implantation, with CCM0 as control and CCM2 as treatment. These compositions were decided based on the results from the in vitro and in vivo rat study. Considering the increased regulation over rabbit research, it was deduced that the two compositions would provide meaningful results with a minimal number of rabbits needed. Apart from histology, two sets of samples were harvested post necropsy for push-out tests. Push-out tests were performed to determine the interfacial shear modulus between the implant and the surrounding bone-tissue area by use of a uniaxial compression tester coupled with a load cell and strain gauge. Further details of the in vivo rabbit surgical procedures are given in the supplemental data.
2.7. Histological analysis:
Post-euthanization, bone-implant samples were harvested for histological analysis from both rat and rabbit studies. For histological analysis, bone-implant samples were fixed in 10% formalin solution for 72 h followed by dehydration using ethanol, ethanol-acetone mixture, and acetone. After dehydration, samples were embedded in Spurrs’ resin. Thin sections of these samples were cut using a diamond blade, which were later mounted on glass slides. Mounted samples were then stained using modified Masson Goldner’s trichrome staining method. Post-staining, a 2-way light microscope (Olympus BH-2, Olympus America Inc., USA) was used to observe the sections. Attained optical images were used for histomorphometric analysis, in accordance with published standardization.41 Examples of published work using such standardized methods may be viewed here.42-44 Osteoid colours – red/orange – were isolated from other spectrum colours and surface area percent was calculated for region of interest (ROI), having a band width of 0.75mm. For consistency from image-to-image ROI was kept constant, as well as image magnification across all images. Osteoid percentage values are representative of osteoid surface over bone surface (OS/BS) and value rendering was done using Gimp 2.8 software. Electron micrographs of the same stained samples were then attained under low vacuum for interfacial bonding characterization between the implant and the surrounding bone tissue. Elemental imaging or EDS was also conducted of the stained samples for presence detection of Cr3+ and Co2+ ions at the implant-tissue interface and the surrounding tissue.
2.8. Statistical analysis:
To maintain protection of α = 0.05 significance value, ANOVA test was conducted first for all histology samples and MTT assay compositions. Statistical analysis was first done by ANOVA testing in where the null hypothesis (H0) and the alternative hypothesis (Hα) were- H0 no significant difference amongst sample means and (Hα): at least one mean differs. Upon rejection of the null hypothesis, that a difference in means exists, a pairwise comparison was done to find significant difference amongst pairs. Quantitative statistical analysis was carried out for 5-week in vivo, with a sample size of three, and six for the in vitro MTT assay data.
3. Results
3.1. Microstructure and phase analyses:
Fig. 1 displays electron micrographs of different zones of LSM treated CCM1LP sample. As received CoCrMo alloy has a coarse-grained microstructure with carbide precipitates near grain boundaries, as shown in Fig. 1b. Sahasrabudhe et al.,24 also demonstrated the presence of Cr7C3 precipitates in CCM0 and CCM3 conditions as well as the evolution of the microstructure with increasing CaP presence. However, after LSM and near the solidification front, presence of very fine honeycomb textured dendritic structure is evident, Fig. 1c. Moreover, dendritic structure at the edge of melt pool is coarser compared to those of other regions. It appears that dendritic grains are aligned along the radial direction of the melt pool. The microstructure of CCM0 consisted of equiaxed grains with random orientation and a secondary phase network. This network structure was irregular in the case of CCM3 samples, and the CaP phase can be seen as the bright spots.24 XRD pattern of CCM substrate and CCM1LP are shown in Fig. 2. Intensities of face-centered cubic (fcc) gamma phase – γ (111) and γ (200) peaks of CoCrMo increased when subjected to laser surface melting. Thus, laser treatment has stabilized the gamma phase in CCM1LP. Fig. 2 confirms that LSM does not trigger any phase transformation of fcc to hexagonal close packed (hcp).
Figure 1.
Electron micrographs of CCM1LP, a) low magnification view of entire melted cross section; b) heat affected zone (HAZ); c) interface of two solidification fronts; d) radial region of one solidification front and e) superficial surface of the melted region.
Figure 2.
X-Ray diffraction (XRD) spectra of CCM and CCM1LP samples.
3.2. Hardness and wear properties
Hardness profile as a function of cross-sectional depth of CCM1LP is presented in Fig. 3. Hardness values increase from the unmelted region (parent microstructure region) to HAZ to the surface of CCM1LP The hardness of the parent microstructure region is 314.5±13.0 HV while it is 425.5±6.0 HV at the surface. In the case of CCM0 and CCM3, hardness was reported to be 564±10 HV0.1 and 571±8 HV0.1, respectively.24 It was discussed by Sahasrabudhe et al.24 that no significant increase in hardness occurred with the addition of 3 wt.% CaP. Upon statistical analysis of the present sample, the findings hold true. Nanoindentation testing for determination of elastic modulus was also conducted. The modulus for CCM3, CCM0, CCM, and CCM1LP were determined to be 103.14±8.02 GPa, 122.01±9.81 GPa, 217.97±17.94 GPa, and 233.76±4.30 GPa, respectively. The effect LSM is seen towards an increase in modulus while the opposite is true for increased CaP presence in the CoCrMo metal matrix.
Figure 3.
Cross-sectional hardness profile of CCM1LP, from substrate to surface hardness.
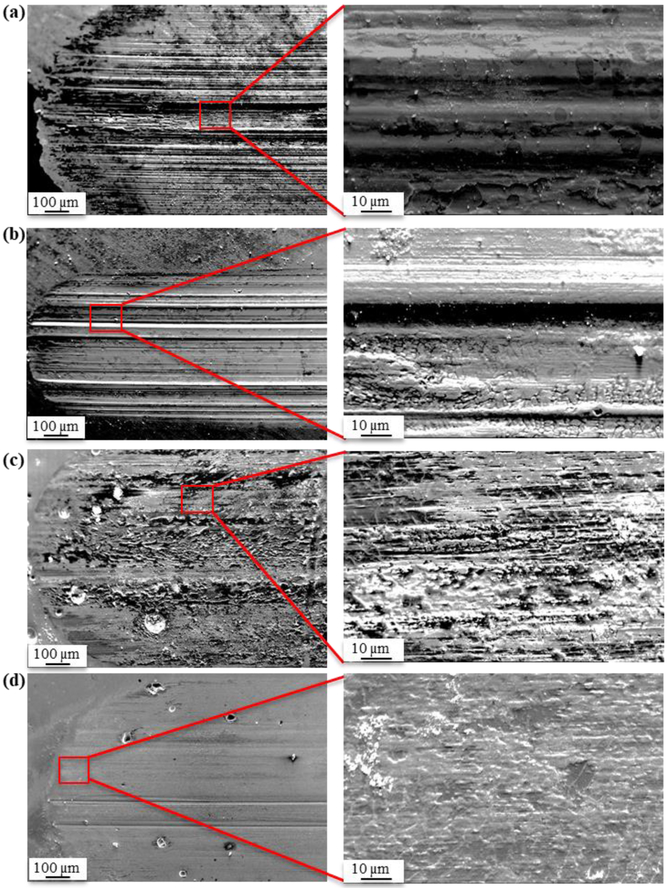
Instantaneous normalized wear rates of CCM, CCM0, CCM3. and CCM1LP are shown in Fig. 4. All LENS™ processed samples exhibit greatly reduced wear rates when compared to the CCM substrate. And, among the LENS™ processed samples, distinguishable is that the wear rate of CCM0 is the highest when compared against CCM1LP and CCM3 conditions. These results confirm that CoCrMo with CaP and LSM processing improves wear resistance compared to that of the control. As shown in Fig 4b, the contact resistance of CCM1LP and CCM3 increased compared to CCM0 confirming in situ formation of a passive tribofilm during articulation. Increase in contact resistance due to the formation of non-conducting tribofilm on the surface of CCM3 is also evident from the electron micrographs of the wear track. Fig. 5 displays the wear scar surface of CCM, CCM1LP, CCM0. and CCM3. By comparison, wear track of CCM appears deeper than CCM3. Secondary electron image of the wear track for CCM3 indicates that the wear track is very smooth and shallow. Furthermore, its grooves are parallel to the motion of the wear ball, which are just visible in low magnification compared to CCM and CCM1LP
Figure 4.
a) Instantaneous normalized wear rate of CCM0, CCM3, CCM1LP and CCM, b) instantaneous contact resistance of CCM0, CCM3 and CCM1LP during tribological testing.
Figure 5.
Electron micrograph of wear track scar for, a) CCM, b) CCM1LP, c) CCM0 and d) CCM3 samples.
The coefficient of friction (COF) data of CCM, CCM1LP, CCM0, and CCM3 were measured. COF of CCM was the highest among all the conditions and increased up to a sliding distance of 400 meters. For, CCM1LP and CCM3, COF values increased up to 600 m and 700 m, respectively and, then reached a steady state. Compound wear data was also examined. CoCrMo showed the highest compound wear. The increase in the compound wear of CCM0 was more severe from 400 m through 1000 m. Compound wear data of CCM1LP and CCM3 increased up to 400 m and then stabilized. However, the same compound wear for the CCM1LP and CCM3 at 1000 m were almost half of the CCM0.
All LENS™ processed sample wear tracks, Fig. 5b-c, display material adherence to the surface. Although the passivation layer was most predominantly for CCM1LP and CCM3. SEM image of CCM3 indicates that the wear track is very smooth and shallow. Fig. 5d shows that the wear track is covered by a superficial layer; which are also observed in the case of CCM0. Elemental mapping of the CCM0 and CCM3 was also conducted, elemental maps are given as supplemental information. EDS analysis results suggested that worn surface of CCM0 consist of Co, Cr, and Fe since debris coming from hardened chromium steel wear ball adhered on the surface of CoCrMo. Additionally, some microcracks were observed on the surface of CCM0. Phosphorous (P) elemental map indicated that CaP was successfully distributed on the surface of CoCrMo alloy via LENS™. Regarding the Mo and P elemental maps, it has been shown that regions with bright contrast are Mo and P rich, whereas the dark contrast mainly corresponds to Co-rich areas. No Fe was detectable on the CCM3 wear track surface, correlating to reduced abrasive wear mode characteristics.
After tribological testing was completed, the wear media was collected and analyzed by ICP-MS for measurement of Co2+ and Cr3+ ionic concentration. The released Co2+ and Cr3+ concentration for CCM1LP was 367±7.071 ppb and 25.5±3.536 ppb, respectively. In the case of CCM0 and CCM3, obtained results for the Co2+ ion release were 2393.40±315.78 ppb and 576.63±21.40 ppb, respectively. It was found that Co2+ ion release significantly decreased with LENS™ processing. Similarly, Cr3+ ion concentration was measured, and values were 861.00±159.89ppb and 210.85±12.81 ppb, respectively. Obtained results exhibit a significant drop in Cr3+ ion release in CCM1LP and CCM3 compositions compared to CCM and CCM0. Additional wear tests were conducted in Dulbecco's Modified Eagle Medium (DMEM) for CCM3, which is used to overcome the issues related to conventional simulated body fluids45 The released Co2+ and Cr3+ concentration were observed to only further decrease to 13.69±0.12 ppb and 4.34±0.06 ppb, respectively. Allowing for the proposed interpretation that only improved wear performance is to be expected when in the presence of a physiological environment.
3.3. In vitro cell materials interaction:
Fig. 6a shows the MTT assay results for CCM0, CCM1, and CCM3 with Cp-Ti as a positive control. The purpose of this in vitro study was to show that these materials are non-toxic due to CaP addition and even after laser-based processing. SEM images of the morphologies of osteoblast cells on CoCrMo and Ti samples were given as supplementary information. After day 3, adherence of cells on the surface was observed. CCM1 and CCM3 showed the formation of multilayer cells as compared to CCM0. Alkaline phosphatase (ALP) expression was observed for all compositions after 11 days. ALP is a protein used as a differentiation marker for osteoblast cells for its differentiation during the bone remodeling process. Day 11 when compared to Day 3 data displayed that the LENS™ processed samples did not suppress or inhibit osteoblast cell activity. Therefore, it can be seen from the MTT assay results that the cell density values have increased from day 3 to day 11 to confirm the non-toxic nature of these compositions. Fig. 6b shows confocal images after 11 days where the ALP protein is expressed by green fluorescence. Strong signals of ALP protein could be seen around the cell nuclei (indicated by blue fluorescence) which indicates osteoblast cell differentiation after 11 days and no cytotoxic effects to cells at later time points.
Figure 6.
a) MTT assay results showing the optical density values after 3 and 11 days for all samples measured at a wavelength of 570 nm, showing increase in cell density values up to 11 days indicating no cytotoxic effects. Note (*) p-value < 0.001. b-e) Confocal microscopy images showing the ALP expression after 11 days where protein expression is indicated by green fluorescence and cell nuclei by blue fluorescence for, b) Cp-Ti, c) CCM0, d) CCM1, e) CCM2.
3.4. In vivo rat study
Histological analysis:
For implant-bone interfacial bonding characterization, histological analysis and SEM characterization were performed. Histological evaluation of all samples including Cp-Ti as the control is shown in Figs. 7 & 8. Osteoid formation is represented in the form of orange color around the implants indicating the new bone formation. Signs of new bone formation could be seen as early as 5 weeks with good osteoid formation at the interface between the implant and the surrounding bone tissue region after 12 weeks. It could be observed from the images that CCM1, CCM2, and CCM3 showed better signs of osteoid formation compared to CCM0 and Cp-Ti. The means for the 5 week time point are: 7.68 ± 1.95%, 7.25 ± 0.73%, 6.45 ± 0.58%, 12.15 ± 1.62% and 37.0 ± 3.16% dense Ti, CCM0, CCM1, CCM2, and CCM3, respectively. That is a five-fold increase in osteoid presence when comparing CCM3 to CCM0. It is concluded that LENS™ processed CoCrMo did not show any signs of cytotoxicity and the addition of CaP improved in vivo biocompatibility of CoCrMo.
Figure 7.
a-d) Histological evaluation and a1-d1) SEM characterization of a) dense Ti, b) CCM0, c) CCM1, d) CCM2, e) CCM3, after 5 weeks in rat distal femur bone showing osteoid-like new bone formation and interfacial bonding between the implant and the surrounding bone tissue area.
Figure 8.
a-d) Histological evaluation and a1-d1) SEM characterization of a) dense Ti, b) CCM0, c) CCM1, d) CCM2, e) CCM3, after 12 weeks in rat distal femur bone showing osteoid like new bone formation and interfacial bonding between the implant and the surrounding bone-tissue area
To get a better understanding of the interfacial bonding between the implant and the surrounding bone-tissue area, SEM characterizations were performed at the interface between the implant and the surrounding tissue area. SEM images from the Figs. 7 & 8 (subscript 1) show signs of bonding at 5 weeks where bonding improved with increasing concentration of CaP. A better indication of bonding could be observed after 12 weeks for CoCrMo samples compared to the control Ti sample. Implant-tissue bonding also improved with increasing concentration of CaP, where CCM2 and CCM3 showing better signs of bonding than others. These results suggest that the bonding is directly influenced by the amount of CaP present in CoCrMo. EDS analysis was also performed to detect the presence of Co and Cr (Ti ions for control sample) ions at the interface of the bone-implant area and the surrounding tissue area for samples after 12 weeks. Figs. 9 show that no trace of Co2+ or Cr3+ ions are detected at the interface or the surrounding tissue area, indicating no detectable release of those ions within these time periods.
Figure 9.
EDS images of Cp-Ti control, CCM0, CCM1, CCM2 and CCM3 samples indicating absence of Ti, Co and Cr ions around the implant-tissue interface and the surrounding area, respectively.
In vivo study of wear tested samples:
Though as-processed samples did not show any signs of cytotoxicity, concerns could be raised regarding cytotoxicity of the wear tested samples. To alleviate such concern, another set of tests were conducted with wear tested CCM0 and CCM3 samples just to understand if there are any differential effects on wear tested samples. In Figs. 10 and 11, signs of osteoid formation can be seen at early stages with improved osteoid formation in the form of orange color around the implant after 12 weeks. Osteoid formation increased with CaP addition. No cytotoxic effects could be seen for CoCrMo samples due to wear induced damages.
Figure 10.
Histological evaluation and SEM characterization of CCM0 wear tested samples after 5 and 12 weeks showing osteoid like new bone formation and interfacial bonding between the implant and the surrounding bone-tissue area.
Figure 11.
Histological evaluation and SEM characterization of CCM3 wear tested samples after 5 and 12 weeks showing osteoid like new bone formation and interfacial bonding between the implant and the surrounding bone-tissue area.
3.5. In vivo rabbit study
Histological analyses:
In vivo rat study showed that LENS™ processed samples were nontoxic with or without wear testing. However, the effective surface area of the implants was very small in rat distal femur model. To further validate our results, only two selected compositions were tested in rabbit distal femur model – CCM0 and CCM2. CCM0 composition was selected as a control while CCM2 was selected as it showed excellent osteoid formation in the rat study. Histological evaluation of CCM0 and CCM2 showed the best early signs of osteoid formation in the form of orange color after 5 weeks in rabbit distal femur model as shown in Figs. 12 and 13. After 12 weeks, osteoid formation could still be observed indicating no cytotoxic effects but interfacial bonding for CCM0 was still poorer than CCM2. To get a better understanding of the interfacial bonding between the implant and the surrounding bone-tissue area, SEM characterization of the interface was performed. There are still some gaps between the implant and the surrounding bone-tissue area in the CCM0 sample as compared to CCM2. After 12 weeks, gaps closing indicated good signs of healing for CCM2.
Figure 12.
Histological evaluation and SEM characterization of CCM0 and CCM2 rabbit study samples after 5 weeks showing osteoid like new bone formation and interfacial bonding between the implant and the surrounding bone-tissue area.
Figure 13.
Histological evaluation and SEM characterization of CCM0 and CCM2 rabbit study samples after 12 weeks showing osteoid-like new bone formation and interfacial bonding between the implant and the surrounding bone-tissue area.
To further validate our findings, push-out tests were performed on selected samples to evaluate the shear modulus at the interface of the implant and surrounding bone-tissue area after the 12 weeks of implantation. The shear moduli of CCM0 and CCM2 were 48.02±2.00 MPa and 92.06±6.7 MPa, respectively. Higher shear modulus values for CCM2 than CCM0 indicates stronger interfacial bonding for CCM2, validating our observations in histology and SEM images.
4. Discussion
4.1. Microstructure and phase analyses:
Even though dendrites are randomly oriented, they were oriented according to the trend of heat flow in Figs. 1a, c, and d. Cooling rate affects the size of the dendritic structure. Increasing the cooling rate decreases the grain size of dendritic microstructure.46 The rapid cooling rate induces a limitation in the development of side branches and ends up with a fine dendritic structure. The dendritic structure at the edge of the melt pool in Figs. 1c and e are coarser compared to other regions where they exhibit dendritic microstructure due to the heat induced by laser treatment. LENS™ processing of CoCrMo may cause the formation of the different microstructure. String-like morphology belonging to a network of carbides has already been shown in CCM0.31,47 However, processing of CaP on CoCrMo via LENS™ triggers the formation of an uneven carbide network, presenting less discontinuous microstructure compared to CCM0. Formation of carbide network is known to contribute towards the improvement of wear resistance. Presence of carbide acts as a defending barrier and exhibit resistance to delamination of the adjacent matrix. Therefore, the excellent wear resistance of CCM1LP was attributed to the presence of carbides in addition to its very fine dendritic microstructure. Also, it was proposed that LSM processing promotes existing coarser carbide grain to dissolve and to reprecipitate as a fine structure during solidification 48 It is hypothesized that those fine carbides exist in the dendritic microstructure of CCM1LP, therefore contributing towards the improvement of wear resistance. Unlike the CCM1LP, the improvement in wear resistance of CCM3 is attributed to the formation of CaP-based in situ tribofilm at the wear track.
Based on XRD results, it can be easily distinguished that there are preferred solidification directions due to LENS™ processing. Relative peak intensities between γ (111) and γ (200) increased with LSM processing. Peak broadening was not observed with LENS™ processing indicating no significant rise in thermally-induced residual stresses. Addition of CaP to CoCrMo stabilized the hcp phase in CCM3.24 It has already been reported that formation of hcp phase in CoCrMo alloys enhance their mechanical properties such as hardness, tensile strength 49,50 and wear resistance while it causes detrimental effects towards ductility.4,51
4.2. Hardness and wear properties:
LENS™ processing of metallic materials causes an increase in hardness. For example, it has been reported that hardness of Cp-Ti increased from 185±2.7 HV to 794±93 HV.52 Similarly, the surface hardness of the CCM1LP increased to 426±6 HV from 324±14 HV, all within the same CCM substrate but after LSM. However, the increase in hardness is not significant for CCM3 compared to that of CCM0. The hardness of CoCrMo increased from 4.09 GPa (417 HV) to 5.15 GPa (525.1 HV) in plasma-vapor deposition TiN/AlN-coated CoCrMo,53 which is still lower than that of CCM1LP and CCM3. The hardness of hot forged CoCrMo alloy (428±11 HV) is comparable with that of CCM1LP, whereas it is lower than that of CCM3 (571±8 HV0.1).51 Similarly, the hardness of high-pressure torsion processed CoCrMo (503 HV) is greater than that of CCM1LP but is lower than that of CCM3.30 It has also been reported that hardness values over 800 HV can be accomplished via nitriding of a cobalt-based alloy.54 Such reported results give us an insight as to how significantly important the processing method is and its effects on hardness.
Wear rate and COF values generally decrease with ceramic coatings on metals via LENS™ processing.35,55,56 Wear rate of CoCrMo decreased with CaP addition using LENS™ processing. All LENS™ processed samples exhibited a decrease in COF when compared to CCM. Although, CCM3 and CCM1LP exhibit a slight increase in COF towards the end of testing when compared to CCM0. For CCM1LP this can be due to the increased carbide or carbon dispersion near the surface. The intergranular carbide presence is lacking, and it seems is more homogenously present in the superficial surface of the laser melt surface, as seen in Fig. 1a. For CCM3, the slight increase in COF can be due to the presence of CaP. CaP is the selected phase subjected to abrasion during the wear test of CCM3. Wear debris of CaP accumulates and forms a superficial softer solid lubricating tribofilm. The same phenomena can be occurring for CCM1L,but with the increased dispersion of the carbides. In comparing with reported values, CCM1LP and CCM3 have higher COF than those of cast CoCrMo alloy,57 while they are lower compared to those of forged CoCrMo alloy and cast F75 CoCrMo alloy reported by Chiba et al.4
Wear rate graph in Fig. 4 indicates that wear resistance is improved with the addition of CaP in CoCrMo alloy. CaP is firstly worn out during the running-in wear regime at the start of linear reciprocating motion and forms a tribofilm on the surface; this film acts as a protective barrier reducing the physical contact between counter material and coating and subsequently reduces the abrasive wear mechanism.4 Since wear tests were conducted in DI water, it can be expressed that the formation of the tribofilm is dependent solely on CaP presence in the CoCrMo alloy and not protein surface adhesion, as is typically reported for DMEM or SBF testing. The contact resistance graph in Fig. 4b supports the formation of tribofilm in CCM3 in where contact resistance increased rapidly. This is in contrast to CCM1LP where wear resistance is attributed to its very fine dendritic microstructure produced by LENS™. One can distinguish that increase in the hardness, and a decrease in the grain size improves the mechanical properties, and therefore improves tribological properties.40 The slight difference in the wear resistance of CCM1LP and CCM3 is believed to be related to the grain size difference between the two compositions and the effect of the hcp phase on CCM3. Even though the hcp phase improves the mechanical properties hence the tribological properties, it also prompts a brittle behavior. The brittle phase may negatively affect the wear resistance in some cases.58 Moreover, CaP on the surface of CCM3 is softer, therefore, it can cause CCM3 to exhibit a lower initial wear resistance compared to that of CCM1LP By comparison, the final wears rates of CCM1LP (3.07×10−7 mm3/Nm) and CCM3 (5.16×10−7 mm3/Nm) are lower than CCM0 and of age treated CoCrMo (0.90×10−4 mm3/Nm), reported by Mantrala et al.59 However, the wear rate of CCM1LP and CCM3 are almost 2 to 3 fold higher by comparison to final wear rate of CCM1LP in DMEM medium (1.25 × 10−5 mm3/Nm). Also, the wear rates of CCM1LP and CCM3 are lower compared to that of LENS™ processed 100% CoCrMo alloy (3 × 10−5 mm3/Nm) reported by Dittrick et al.31 Finally, CCM1LP and CCM3 have lower wear rates compared to that of hot forged CoCrMo alloy (2.0×10−6 mm3/Nm) reported by Chiba et al.4 Besides, wear rate of CCM3 shows consistency with studies reported before regarding CaP addition.57,60
In discussing the wear track topography, CCM1LP displayed a smooth and shallow wear track when compared to CCM. It can be argued that the high wear resistance is attributed to its very fine dendritic microstructure shown in Fig. 1d. Adhered worn surface material in high magnification wear track images of CCM1LP display similarities to the case of carbide phase (Cr7C3) detachment during wear tests of pulsed laser CoCrMo alloy reported by Davila et al.48 This phenomenon also brings the idea that the carbide phase of CCM1LP might comprise the worn and adhered particles observed on the surface. The presence of hard phases and their uniform distribution improves mechanical properties such as hardness and therefore boosts the wear resistance producing a smooth and shallow wear track.48,61 The lighter regions apparent in Fig. 5c & d are of the protective tribofilm. Which was further validated by elemental mapping.
CaP addition to CoCrMo and LSM processing of CoCrMo alloy does not only promote an improvement in the wear resistance but also reduces Co2+ and Cr3+ ion leaching. Co2+ ion release decreased by almost seven times with LSM processing while it decreases around four times in CCM3. Formation of a protective tribofilm in CCM3 and very fine dendritic structure of CCM1LP allowed for a decrease in leached ion concentration during wear tests. Reported released Co2+ ion concentration by Dittrick et al.,31 was found to be around 250±120 ppb. Measured ion release concentration results in this study were much lower compared to those of pin-on-disk wear behavior of CoCrMo alloy where it was reported between 2000-13000 ppb.62
4.3. In vitro cell materials interactions:
As previously discussed, CoCrMo alloys have seen increased use in hip and knee replacements and dental applications;63,64 and this is due to their high wear resistance and superior mechanical properties. But, one major disadvantage concerning CoCrMo alloys is their poor biocompatibility and osseointegrative properties.9,64 Use of bioceramic coatings to overcome this disadvantage is being used as an alternative measure.65 In addition, the use of bioactive ceramics as coating material frequently enhances the biocompatibility of implant materials.66-68 Apart from bio-tribological wear testing, the main objective of this research was to evaluate the biocompatibility of these CoCrMo-CaP composites and observe if the addition of CaP improves the biocompatibility of these composites.
Cp-Ti was used as a control for the entire study, as titanium (Ti) is known to be more biocompatible than CoCrMo alloys.69-71 In vitro cell materials interaction was first studied for the CoCrMo-CaP composites to evaluate its biocompatibility. Human osteoblast cells were used and from the MTT assay results, an increase in the cell density for all CoCrMo samples indicated good signs of biocompatibility without any cytotoxic effects. ALP expression at day 11 was measured for all samples as ALP is a protein used as a differentiation marker for osteoblast cells. Mixed results were observed from the ALP expression data as CCM3 showed the weakest signals of ALP protein as compared to other samples. Literature has reported that a decrease in cell densities was observed at later time points for Co-based alloys. Different proliferation times is a possible reason.35,72,73 Although different results were observed in each case, good biocompatibility was also observed with no possible signs of cytotoxic effects.
4.4. In vivo rat and rabbit studies:
To understand the biocompatibility, in vivo studies were performed using rat and rabbit distal femur models. CCM2 composition was added to get a better understanding concerning the addition of CaP to CoCrMo alloys. Histological analysis revealed signs of osteoid formation as early as 5 weeks with improved osteoid formation after 12 weeks. From the histological evaluation, improved biocompatibility for CoCrMo-CaP composites could be observed, which imply no cytotoxic effects due to composition and processing history of the implants. CoCrMo-CaP implants showed better results than pure CoCrMo. SEM characterization gave a better idea about the interfacial bonding between the implant and the surrounding bone-tissue area where, with increasing concentration of CaP, the bonding improved for CoCrMo-CaP implants. This is derived from the observed decrease in the crevice region between the implant and host tissue. The reason for improved biocompatibility of CoCrMo-CaP implants is due to the passivation layer formed due to the presence of CaP. The occurrence of a passivation layer is a common phenomenon observed at the metal oxide interface which inhibits the release of metal ions.74-76 Similar findings can be observed for CoCrMo-CaP composites where a passivated CaP layer is formed due to the wear and inhibits the release of Co2+ and Cr3+ ions.
To ensure no Co2+ and Cr3+ ions were released at the surrounding bone-tissue area upon implantation, EDS analysis was performed at the interface between the implant and surrounding bone-tissue area. EDS analysis did not reveal any presence of Co2+ or Cr3+ ions at the surrounding tissue regions around the implant which also supports the claim of non-cytotoxicity of CoCrMo-CaP implants. As stated earlier, upon wear Co2+ and Cr3+ ions are released onto the surface of the implant which eventually gets released into the body which could prove to be toxic. To further support the inhibition of Co2+ and Cr3+ ion release due to CaP passivation layer formation, which we claim as the formation of tribofilm on the surface that prevents the release of these ions, wear tested samples were also studied for their biocompatibility and possible evaluation of cytotoxic effects using a rat distal femur model for 5 and 12 weeks. From the histological results, signs of osteoid formation were observed after 5 weeks itself and further improvement after 12 weeks.
With CCM2 showing the best results, it was considered as the treatment group for the in vivo study using a rabbit distal femur model. Push out data showed a higher interfacial shear modulus for CCM2 than CCM0. To confirm the biocompatibility and interfacial bonding of these implants, histological analysis, and SEM characterization was performed where CCM2 showed improved osteoid formation than CCM0 with better bonding between the implant and the surrounding bone-tissue area. Thus, from our results, we can conclude CoCrMo-CaP composites not only improve the bio-tribological behavior but also improve in vivo biocompatibility and show improved signs of interfacial bonding.
5. Conclusions
CoCrMo alloys are still widely used in load-bearing articulating surface applications due to their excellent wear resistance. However, the release of Co2+ and Cr3+ ions in vivo is a major concern for these alloys. In this study, we have explored the idea of adding CaP into CoCrMo to simultaneously improve wear resistance and biocompatibility. LSM processing demonstrated a reduction in abrasive wear mode. The formation of in situ tribofilm layer was observed in CCM3 – reducing wear rate as well as Co2+ and Cr3+ ions release in vitro. Biocompatibility was tested via in vitro cell material interactions using human osteoblast cells where good cell spreading and ALP protein expression was observed for CoCrMo-CaP composites with no obvious cytotoxic effects. Furthermore, in vivo studies using rat and rabbit distal femur models for 5 and 12 weeks were conducted resulting in good interfacial bonding between the implant and the bone-tissue area. And, no measurable release of metal ions was observed in all in vivo samples. A 3wt% CaP addition to CoCrMo was found to increase osteoid formation by 5 times. It can be concluded that CoCrMo-CaP composites help not only to improve the wear properties and reduce metal ion release but also enhance both in vitro and in vivo biocompatibility.
Supplementary Material
Acknowledgments
Authors like to acknowledge financial support from Life Sciences Discovery Fund (LSFD), Seattle, WA, USA for the research reported in this publication. The authors would like to thank Himanshu Sahasrabudhe for his help with fabricating the samples. The content is solely the responsibility of the authors and does not necessarily represent the official views of LSFD. Authors would also like to acknowledge financial support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR067306-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References
- (1).Liu H; Niinomi M; Nakai M; Cho K; Narita K; Şen M; Shiku H; Matsue T Mechanical properties and cytocompatibility of oxygen-modified β-type Ti-Cr alloys for spinal fixation devices. Acta Biomater. 2015, 12 (1), 352–361. [DOI] [PubMed] [Google Scholar]
- (2).Niinomi M Fatigue Performance and Cyto-Toxicity of Low Rigidity Titanium Alloy, Ti- 29Nb-13Ta-4.6Zr. Biomaterials 2003, 24 (16), 2673–2683. [DOI] [PubMed] [Google Scholar]
- (3).Atar E Sliding Wear Performances of 316 L, Ti6Al4V, and CoCrMo Alloys. 2013, No. January 2013, 183–188. [Google Scholar]
- (4).Chiba A; Kumagai K; Nomura N; Miyakawa S Pin-on-Disk Wear Behavior in a like-on-like Configuration in a Biological Environment of High Carbon Cast and Low Carbon Forged Co-29Cr-6Mo Alloys. Acta Mater. 2007, 55, 1309–1318. [Google Scholar]
- (5).Poljak-Guberina R; Knezović-Zlatarić D; Katunaric M Dental Alloys and Corrosion Resistance. Acta Stomatol. Croat. 2002, 36 (4), 447–450. [Google Scholar]
- (6).Jakobsen SS; Baas J; Jakobsen T; Soballe K Biomechanical Implant Fixation of CoCrMo Coating Inferior to Titanium Coating in a Canine Implant Model. J. Biomed. Mater. Res. - Part A 2010, 94 (1), 180–186. [DOI] [PubMed] [Google Scholar]
- (7).Hedberg YS; Pettersson M; Pradhan S; Odnevall Wallinder I; Rutland MW; Persson C Can Cobalt(II) and Chromium(III) Ions Released from Joint Prostheses Influence the Friction Coefficient? ACS Biomater. Sci. Eng. 2015, 1 (8), 617–620. [DOI] [PubMed] [Google Scholar]
- (8).Meyer JN; Mathew MT; Wimmer M. a.; Lesuer RJ Effect of Tribolayer Formation on Corrosion of CoCrMo Alloys Investigated Using Scanning Electrochemical Microscopy. Anal. Chem. 2013, 85 (15), 7159–7166. [DOI] [PubMed] [Google Scholar]
- (9).Hedberg YS; Qian B; Shen Z; Virtanen S; Odnevall Wallinder I In Vitro Biocompatibility of CoCrMo Dental Alloys Fabricated by Selective Laser Melting. Dent. Mater. 2014, 30 (5), 525–534. [DOI] [PubMed] [Google Scholar]
- (10).Bose S, Ke D, Sahasrabudhe H, Bandyopadhyay A, “Additive Manufacturing of Biomaterials,” Progress in Materials Science, 2018, 93, 45–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Heneghan C; Langton D; Thompson M Ongoing Problems with Metal-on-Metal Hip Implants. BMJ 2012, 344 (7846), 13–16. [DOI] [PubMed] [Google Scholar]
- (12).Fisher J; Hu XQ; Stewart TD; Williams S; Tipper JL; Ingham E; Stone MH; Davies C; Hatto P; Bolton J; et al. Wear of Surface Engineered Metal-on-Metal Hip Prostheses. J. Mater. Sci. Mater. Med. 2004, 15 (3), 225–235. [DOI] [PubMed] [Google Scholar]
- (13).Ulrich SD; Seyler TM; Bennett D; Delanois RE; Saleh KJ; Thongtrangan I; Kuskowski M; Cheng EY; Sharkey PF; Parvizi J; et al. Total Hip Arthroplasties: What Are the Reasons for Revision? Int. Orthop. 2008, 32 (5), 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sundfeldt M; Carlsson LV; Johansson CB; Thomsen P; Gretzer C Aseptic Loosening, Not Only a Question of Wear: A Review of Different Theories. Acta Orthop. 2006, 77(2), 177–197. [DOI] [PubMed] [Google Scholar]
- (15).Pritchett JW Adverse Reaction to Metal Debris: Metallosis of the Resurfaced Hip. Curr. Orthop. Pract. 2012, 23 (1). [Google Scholar]
- (16).Sansone V; Pagani D; Melato M The Effects on Bone Cells of Metal Ions Released from Orthopaedic Implants. A Review. Clin. Cases Miner. Bone Metab. 2013, 10 (1), 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Levašič V; Milošev I; Zadnik V Risk of Cancer after Primary Total Hip Replacement: The Influence of Bearings, Cementation and the Material of the Stem. Acta Orthop. 2018, 89 (2), 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Visuri T; Koskenvuo M Cancer Risk after McKee-Farrar Total Hip Replacement. Orthopedics 1991, 14 (2), 137–142. [DOI] [PubMed] [Google Scholar]
- (19).Posada O; Tate R; Meek RM; Grant M; Posada OM; Tate RJ; Meek RMD; Grant MH In Vitro Analyses of the Toxicity, Immunological, and Gene Expression Effects of Cobalt-Chromium Alloy Wear Debris and Co Ions Derived from Metal-on-Metal Hip Implants. Lubricants 2015, 3 (3), 539–568. [Google Scholar]
- (20).Ke D; Robertson SF; Dernell WS; Bandyopadhyay A; Bose S Effects of MgO and SiO 2 on Plasma-Sprayed Hydroxyapatite Coating: An in Vivo Study in Rat Distal Femoral Defects. ACS Appl. Mater. Interfaces 2017, 9 (31), 25731–25737. [DOI] [PubMed] [Google Scholar]
- (21).Roy M; Balia VK; Bandyopadhyay A; Bose S Laser Processing of Bioactive Tricalcium Phosphate Coating on Titanium for Load-Bearing Implants. Acta Biomater. 2008, 4 (2), 324–333. [DOI] [PubMed] [Google Scholar]
- (22).Roy M; Balia VK; Bandyopadhyay A; Bose S Compositionally Graded Hydroxyapatite/Tricalcium Phosphate Coating on Ti by Laser and Induction Plasma. Acta Biomater. 2011, 7, 866–873. [DOI] [PubMed] [Google Scholar]
- (23).Zhang MY; Ye C; Erasquin UJ; Huynh T; Cai C; Cheng GJ Laser Engineered Multilayer Coating of Biphasic Calcium Phosphate/Titanium Nanocomposite on Metal Substrates. ACS Appl. Mater. Interfaces 2011, 3 (2), 339–350. [DOI] [PubMed] [Google Scholar]
- (24).Sahasrabudhe H; Bose S; Bandyopadhyay A Laser Processed Calcium Phosphate Reinforced CoCrMo for Load-Bearing Applications: Processing and Wear Induced Damage Evaluation. Acta Biomater. 2018, 66, 118–128. [DOI] [PubMed] [Google Scholar]
- (25).Escobedo JC; Ortiz JC; Almanza JM; Cortes DA Hydroxyapatite Coating on a Cobalt Base Alloy by Investment Casting. Scr. Mater. 2006, 54 (9), 1611–1615. [DOI] [PubMed] [Google Scholar]
- (26).Sheeja D; Tay BK; Lau S; Nung LN Tribological Characterisation of Diamond-like Carbon Coatings on Co-Cr-Mo Alloy for Orthopaedic Applications. Surf. Coatings Technol. 2001, 146–147, 410–416. [Google Scholar]
- (27).Martínez R; Escobedo JC; Cortes DA; Alves GG; Linhares ABR; Granjeiro JM; Prado M; Ortiz JC; Almanza JM; Muzquiz-Ramos EM In Vitro Bioactivity and Biocompatibility of a Co–Cr–Mo Alloy after Heat Treatment in Contact with Different Bioactive Systems. Ceram. Int. 2013, 39 (2), 2003–2011. [Google Scholar]
- (28).Walker JC; Cook RB; Murray J; T, C. A. Pulsed Electron Beam Surface Melting of CoCrMo Alloy for Biomedical Applications. Wear 2013, 301 (1–2), 250–256. [Google Scholar]
- (29).Schaaf P Laser Nitriding of Metals. Prog. Mater. Sci. 2002, 47 (1), 1–161. [Google Scholar]
- (30).Çelik A; Bayrak Ö; Alsaran A; Kaymaz I; Yetim AF Effects of Plasma Nitriding on Mechanical and Tribological Properties of CoCrMo Alloy. Surf. Coatings Technol. 2008, 202(11), 2433–2438. [Google Scholar]
- (31).Dittrick S; Balia VK; Davies NM; Bose S; Bandyopadhyay A In Vitro Wear Rate and Co Ion Release of Compositionally and Structurally Graded CoCrMo-Ti6Al4V Structures. Mater. Sci. Eng. C 2011, 31 (4), 809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Balázsi K; Vandrovcová M; Bačáková L; Balázsi C Structural and Biocompatible Characterization of TiC/a:C Nanocomposite Thin Films. Mater. Sci. Eng. C 2013, 33, 1671–1675. [DOI] [PubMed] [Google Scholar]
- (33).Oláh N; Fogarassy Z; Furkó M; Balázsi C; Balázsi K Sputtered Nanocrystalline Ceramic TiC/Amorphous C Thin Films as Potential Materials for Medical Applications. Ceram. Int. 2015, 41 (4), 5863–5871. [Google Scholar]
- (34).Balla VK; Xue W; Bose S; Bandyopadhyay A Functionally Graded Co-Cr-Mo Coating on Ti-6Al-4V Alloy Structures. Acta Biomater. 2008, 4 (3), 697–706. [DOI] [PubMed] [Google Scholar]
- (35).Balla VK; DeVasConCellos PD; Xue W; Bose S; Bandyopadhyay A Fabrication of Compositionally and Structurally Graded Ti–TiO2 Structures Using Laser Engineered Net Shaping (LENS). Acta Biomater. 2009, 5 (5), 1831–1837. [DOI] [PubMed] [Google Scholar]
- (36).Yan Y; Neville A; Dowson D Tribo-Corrosion Properties of Cobalt-Based Medical Implant Alloys in Simulated Biological Environments. Wear 2007, 263 (7–12 SPEC. ISS.), 1105–1111. [Google Scholar]
- (37).Lee SW; Balazsi C; Balazsi K; Seo D. hyun; Kim HS; Kim CH; Kim SG Comparative Study of Hydroxyapatite Prepared from Seashells and Eggshells as a Bone Graft Material. Tissue Eng. Regen. Med. 2014, 11 (2), 113–120. [Google Scholar]
- (38).Lee SW; Kim SG; Balázsi C; Chae WS; Lee HO Comparative Study of Hydroxyapatite from Eggshells and Synthetic Hydroxyapatite for Bone Regeneration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113 (3), 348–355. [DOI] [PubMed] [Google Scholar]
- (39).Ortiz JC; Cortés DA; Escobedo JC; Almanza JM; Muñiz CR; Luna JS; Rodríguez NA Bioactive Coating on a Cobalt Base Alloy by Heat Treatment. Mater. Lett. 2011, 65(2), 329–332. [Google Scholar]
- (40).Balla VK; Soderlind J; Bose S; Bandyopadhyay A Microstructure, Mechanical and Wear Properties of Laser Surface Melted Ti6Al4V Alloy. J. Mech. Behav. Biomed. Mater. 2014, 32, 335–344. [DOI] [PubMed] [Google Scholar]
- (41).Parfitt AM; Drezner MK; Glorieux FH; Kanis JA; Malluche H; Meunier PJ; Ott SM; Recker RR Bone Histomorphometry : Standardization. J. Bone Miner. Res. 1987, 2 (6), 595–610. [DOI] [PubMed] [Google Scholar]
- (42).He T; Cao C; Xu Z; Li G; Cao H; Liu X; Zhang C; Dong Y A Comparison of Micro-CT and Histomorphometry for Evaluation of Osseointegration of PEO-Coated Titanium Implants in a Rat Model. Sci. Rep. 2017, 7 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Vandeweghe S; Coelho PG; Vanhove C; Wennerberg A; Jimbo R Utilizing Micro-Computed Tomography to Evaluate Bone Structure Surrounding Dental Implants: A Comparison with Histomorphometry. J. Biomed. Mater. Res. - Part B Appl. Biomater. 2013, 101 (7), 1259–1266. [DOI] [PubMed] [Google Scholar]
- (44).Li K; Wang C; Yan J; Zhang Q; Dang B; Wang Z; Yao Y; Lin K; Guo Z; Bi L; et al. Evaluation of the Osteogenesis and Osseointegration of Titanium Alloys Coated with Graphene: An in Vivo Study. Sci. Rep. 2018, 8 (1), 1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Lee JTY; Leng Y; Chow KL; Ren F; Ge X; Wang K; Lu X Cell Culture Medium as an Alternative to Conventional Simulated Body Fluid. Acta Biomater. 2011, 7 (6), 2615–2622. [DOI] [PubMed] [Google Scholar]
- (46).Xiang W; Xuliang M; Xinlin L; Lihua D; Mingjia W Effect of Boron Addition on Microstructure and Mechanical Properties of TiC/Ti6Al4V Composites. Mater. Des. 2012, 36, 41–46. [Google Scholar]
- (47).España FA; Balla VK; Bose S; Bandyopadhyay A Design and Fabrication of CoCrMo Alloy Based Novel Structures for Load Bearing Implants Using Laser Engineered Net Shaping. Mater. Sci. Eng. C 2010, 30 (1), 50–57. [Google Scholar]
- (48).Acevedo-Dávila JL; Lõpez HF; Cepeda-Rodríguez F; Rodriguez-Reyes M; García-Vazquez F; Hernández-Garcia HM Microstructural Effects on the Wear Behavior of a Biomedical As-Cast Co-27Cr-5Mo-0.25C Alloy Exposed to Pulsed Laser Melting. J. Biomed. Mater. Res. - Part A 2014, 102 (6), 2008–2016. [DOI] [PubMed] [Google Scholar]
- (49).Isik M; Niinomi M; Cho K; Liu H; Nakai M; Yilmazer H; Horita Z; Narushima T Microstructural Evolution and Mechanical Properties of Biomedical Co-Cr-Mo Alloy Subjected to High-Pressure Torsion. J. Mech. Behav. Biomed. Mater. 2015, 59, 226–235. [DOI] [PubMed] [Google Scholar]
- (50).Isik M; Niinomi M; Liu H; Cho K; Nakai M; Horita Z; Narushima T; Ueda K Optimization of Microstructure and Mechanical Properties of Co-Cr-Mo Alloys by High- Pressure Torsion and Subsequent Short Annealing. Mater. Trans. 2016, 57 (11), 1887— 1896. [Google Scholar]
- (51).Koizumi Y; Chen Y; Li Y; Yamanaka K; Chiba A; Tanaka SI; Hagiwara Y Uneven Damage on Head and Liner Contact Surfaces of a Retrieved Co-Cr-Based Metal- on-Metal Hip Joint Bearing: An Important Reason for the High Failure Rate. Mater. Sci. Eng. C 2016, 62, 532–543. [DOI] [PubMed] [Google Scholar]
- (52).Roy M; Balia VK; Bandyopadhyay A; Bose S MgO-Doped Tantalum Coating on Ti: Microstructural Study and Biocompatibility Evaluation. ACS Appl. Mater. Interfaces 2012, 4 (2), 577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Goldberg JR; Gilbert JL The Electrochemical and Mechanical Behavior of Passivated and TiN/AlN-Coated CoCrMo and Ti6A14V Alloys. Biomaterials 2004, 25 (5), 851–864. [DOI] [PubMed] [Google Scholar]
- (54).Hiromoto S; Onodera E; Chiba A; Asami K; Hanawa K Microstructure and Corrosion Behaviour in Biological Environments of the New Forged Low-Ni Co-Cr-Mo Alloys. Biomaterials 2005, 26 (24), 4912–4923. [DOI] [PubMed] [Google Scholar]
- (55).Das M; Balia VK; Basu D; Manna I; Sampath Kumar TS; Bandyopadhyay A Laser Processing of in Situ Synthesized TiB-TiN-Reinforced Ti6A14V Alloy Coatings. Scr. Mater. 2012, 66(8), 578–581. [Google Scholar]
- (56).Bose S; Robertson SF; Bandy opadhyay A Surface Modification of Biomaterials and Biomedical Devices Using Additive Manufacturing. Acta Biomater. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Doni Z; Alves AC; Toptan F; Gomes JR; Ramalho A; Buciumeanu M; Palaghian L; Silva FS Dry Sliding and Tribocorrosion Behaviour of Hot Pressed CoCrMo Biomedical Alloy as Compared with the Cast CoCrMo and Ti6A14V Alloys. Mater. Des. 2013, 52, 47–57. [Google Scholar]
- (58).He DH; Manory R A Novel Electrical Contact Material with Improved Self-Lubrication for Railway Current Collectors. Wear 2001, 249 (7), 626–636. [Google Scholar]
- (59).Mantrala KM; Das M; Balia VK; Rao CS; Kesava Rao VVS Additive Manufacturing of Co-Cr-Mo Alloy: Influence of Heat Treatment on Microstructure, Tribological, and Electrochemical Properties. Front. Mech. Eng. 2015, 1, 2. [Google Scholar]
- (60).Balagna C; Spriano S; Faga MG Characterization of Co-Cr-Mo Alloys after a Thermal Treatment for High Wear Resistance. Mater. Sci. Eng. C 2012, 32 (7), 1868–1877. [DOI] [PubMed] [Google Scholar]
- (61).Valer VV Thermal Cooling Effects in the Microstructure and Properties of Cast Cobalt-Base Biomedical Alloys. 2014, No. August, 844. [Google Scholar]
- (62).St. John KR; Zardiackas LD; Poggie RA Wear Evaluation of Cobalt-Chromium Alloy for Use in a Metal-on-Metal Hip Prosthesis. J. Biomed. Mater. Res. 2004, 68B (1), 1–14. [DOI] [PubMed] [Google Scholar]
- (63).Patntirapong S; Habibovic P; Hauschka PV Effects of Soluble Cobalt and Cobalt Incorporated into Calcium Phosphate Layers on Osteoclast Differentiation and Activation. Biomaterials 2009, 30 (4), 548–555. [DOI] [PubMed] [Google Scholar]
- (64).Hodgson AWE; Kurz S; Virtanen S; Fervel V; Olsson COA; Mischler S Passive and Transpassive Behaviour of CoCrMo in Simulated Biological Solutions. Electrochim. Acta 2004, 49 (13), 2167–2178. [Google Scholar]
- (65).Huk OL; Bansal M; Betts F; Rimnac CM; Lieberman JR; Huo HH; Salvati EA Polyethylene and Metal Debris Generated by Non-Articulating Surfaces of Modular Acetabular Components. J. Bone Joint Surg. Br. 1994, 76 (4), 568–574. [PubMed] [Google Scholar]
- (66).Zhang X; Li XW; Li JG; Sun XD Preparation and Characterizations of Bioglass Ceramic Cement/ca-p Coating on Pure Magnesium for Biomedical Applications. ACSAppl. Mater. Interfaces 2014, 6 (1), 513–525. [DOI] [PubMed] [Google Scholar]
- (67).Li Y; Yang W; Li X; Zhang X; Wang C; Meng X; Pei Y; Fan X; Lan P; Wang C; et al. Improving Osteointegration and Osteogenesis of Three-Dimensional Porous Ti6A14V Scaffolds by Polydopamine-Assisted Biomimetic Hydroxyapatite Coating. ACS Appl. Mater. Interfaces 2015, 7 (10), 5715–5724. [DOI] [PubMed] [Google Scholar]
- (68).Yan L; Xiang Y; Yu J; Wang Y; Cui W Fabrication of Antibacterial and Antiwear Hydroxyapatite Coatings via in Situ Chitosan-Mediated Pulse Electrochemical Deposition. ACS Appl. Mater. Interfaces 2017, 9 (5), 5023–5030. [DOI] [PubMed] [Google Scholar]
- (69).Schmalzried TP; Jasty M; Harris WH Periprosthetic Bone Loss in Total Hip Arthroplasty. Polyethylene Wear Debris and the Concept of the Effective Joint Space. J. Bone Joint Surg. Am. 1992, 74 (6), 849–863. [PubMed] [Google Scholar]
- (70).Kokubo T; Kim HM; Kawashita M Novel Bioactive Materials with Different Mechanical Properties. Biomaterials 2003, 24 (13), 2161–2175. [DOI] [PubMed] [Google Scholar]
- (71).Bandyopadhyay A; Espana F; Balia VK; Bose S; Ohgami Y; Davies NM Influence of Porosity on Mechanical Properties and in Vivo Response of Ti6A14V Implants. Acta Biomater. 2010, 6(4), 1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Xue W; Balia VK; Bandyopadhyay A; Bose S Processing and Biocompatibility Evaluation of Laser Processed Porous Titanium. Acta Biomater. 2007, 2 (6), 1007–1018. [DOI] [PubMed] [Google Scholar]
- (73).Wang K The Use of Titanium for Medical Applications in the USA. Mater. Sci. Eng. A 1996, 212 (1–2), 134–137. [Google Scholar]
- (74).Qin L; Zeng Q; Wang W; Zhang Y; Dong G Response of MC3T3-E1 Osteoblast Cells to the Microenvironment Produced on Co-Cr-Mo Alloy Using Laser Surface Texturing. J. Mater. Sci. 2014, 49 (6), 2662–2671. [Google Scholar]
- (75).Tomashov ND; Chernova GP; Ruscol YS; Ayuyan GA The Passivation of Alloys on Titanium Bases. Electrochim. Acta 1974, 19 (4), 159–172. [Google Scholar]
- (76).Pickering HW Important Early Developments and Current Understanding of the IR Mechanism of Localized Corrosion. J. Electrochem. Soc. 2003, 150 (5), Kl. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.