Abstract
Prevention strategies and clinical management of methicillin- resistant Staphylococcus aureus (MRSA) infections in ventilated patients who develop ventilator-associated pneumonia (VAP) are important. Since MRSA are the most frequently isolated bacteria in patients with VAP, and a significant cause of morbidity and mortality in intubated patients, rapid diagnosis and early treatment could reduce mortality. This review will examine preventive steps (i.e. screening ventilated patients for MRSA, decolonization, and hand washing), assessing clinical presentations before the results of culture are obtained to direct empiric treatment, and the appropriate antibiotic therapy upon culture confirmation of MRSA that could help in the management of VAP.
Keywords: Pneumonia, Staphylococcus aureus, ventilator-associated
PNEUMONIA
Pneumonia is a microbial infection with subsequent inflammation of the lungs that leads to a massive buildup of inflammatory cells and fluid in the terminal bronchioles and alveoli [1]. Pneumonia is prevalent in chronically ill and immune-compromised patients, smokers, and patients with interstitial lung diseases. However, the most vulnerable age group is children under age five. Almost 935,000 children under age five die each year from pneumonia worldwide [2].
Pneumonia can be presented in one of four ways: 1) Community–acquired pneumonia is a pneumonia that occurs outside hospital or health care facilities, 2) Hospital-acquired pneumonia (HAP) that is not seen at the time of admission at a hospital but occurs after 48 h of being admitted to the hospital, 3) Healthcare-associated pneumonia (HCAP) where the patient is not hospitalized but contracts pneumonia after contact with a healthcare professional, and 4) Ventilator-associated pneumonia (VAP) that occurs 48–72 h after endotracheal intubation [1].
VENTILATOR-ASSOCIATED PNEUMONIA
Ventilator-associated pneumonia usually occurs after 48 h of intubation and mechanical ventilation. The pneumonia that results is characterized by fever, chest congestion, and dyspnea. VAP pneumonia should have new or progressive pulmonary infiltrate as well as any of the following symptoms: Fever, decreased oxygenation, purulent tracheobronchial secretions, and increased respiratory rate. Symptoms of pneumonia can develop gradually or can develop suddenly with a cough that contains blood, wheezing, and increased expiratory time. Fever, cough, and fast breathing frequently occur in children, whereas elderly patients typically display confusion and delirium as the presenting symptoms[3, 4].
A number of bacterial species can cause VAP including Staphylococcus aureus (28.8%), Pseudomonas aeruginosa (21.8%), Klebsiella species (9.8%), Escherichia coli (6.9%), Acinetobacter species (6.8%), and Enterobacter species (6.3%). These species are responsible for almost 80% of VAP. The remaining 20% of VAP is caused by Serratia species, Stenotrophomonas maltophilia, Streptococcus pneumoniae, and Haemophilus influenzae [5].
VAP can occur either early within 48–96 h of intubation, or it can occur late, after four days of intubation. The species found during the early and late phases of VAP vary [6]. Early onset VAP is usually caused by antibiotic susceptible bacteria, whereas late onset VAP is mostly caused by multidrug resistant bacteria [7]. The different types of species associated with early and late onset VAP is linked to previous antibiotic administration, time on mechanical ventilation, and local factors which are institutional specific. Methicillin-sensitive S. aureus (MSSA), S. pneumoniae, H. influenzae, Proteus spp, Serratia marcescens, Klebsiella pneumoniae, and E. coli are typically found to be associated with the early phase of pneumonia; while methicillin-resistant S. aureus (MRSA), Acinetobacter spp., P. aeruginosa, and Enterobacter species are more regularly associated with late onset VAP [8].
Intubation increases the chances of acquiring VAP as it increases bacterial adherence and colonization of airways and acts as a reservoir for bacterial proliferation [9]. In a prospective study that compared patients who were intubated and patients who were not intubated at the time of admission and free of pneumonia when admitted, intubation increased the chance of developing pneumonia by 21-fold, [10, 11] and 9 to 27% of ventilated patients developed VAP > 48 h after being ventilated [12]. The most common mechanism of infection in intubated patients is direct contact with environmental reservoirs, including respiratory devices, contaminated water reservoirs, and disposable tubing used in respiratory circuits or endotracheal tubes contaminated by hospital personnels, improper handwashing [13]. Even after intense cleaning of ventilator equipment, there is still a possibility of VAP as a result of negligence in washing hands or not changing gloves between patients [8].
MRSA MEDIATED VAP
Various organisms can cause VAP despite taking the precautions to avoid the risk factors, although S. aureus is one of the most common causes of VAP [14]. Depending on the sensitivity to methicillin, S. aureus are either MSSA or MRSA. S. aureus strains without the mecA gene are referred to as MSSA and will display an MIC ≤ 2 μg/mL against methicillin, whereas S. aureus that carry the mecA gene have an MIC ≥ 4 μg/mL against methicillin are labeled MRSA [15]. The mecA gene encodes the production of an altered penicillin-binding protein PBP2a that does not allow for the binding of β-lactam drugs to the bacterial cell wall. PBP2a takes over the functions of PBPs and allow bacterial cell to grow in presence of antibiotics like methicillin [16].
Methicillin was introduced in 1959 and MRSA arose in hospitals around 1961. The first community-associated MRSA (CA-MRSA) was reported in the United States in 1980, but around 1990, the CA-MRSA pulse-field gel electrophoresis (PFGE) type USA400 lineage became widespread. In 2000, the PFGE type USA300 lineage arose and has replaced the USA400 lineage in the United States [17, 18]. As the USA300 MRSA variety supplanted other strains, the incidence of HA-MRSA infections by USA300 MRSA also rose. In 2005, the USA300 MRSA type represented 29% of invasive MRSA infections, including 67% of CA-MRSA and 22% of HA-MRSA. By 2011, the USA300 type accounted for 32% of HA-MRSA [19].
According to U.S. MRSA surveillance, it was estimated that there were 131,261 invasive MRSA infections in 2005 (8% had >1 infection), but only 80,461 invasive MRSA infections and 11,285 deaths in the USA in 2011. Data collected by the Active Bacterial Core surveillance (ABCs)/Emerging Infections Program Network showed that invasive MRSA infections from 2005 to 2011 decreased 27.7 % for healthcare-associated community-onset, 54.2 % for hospital- onset, and a 5 % decrease in community-associated infections [20].
S. aureus is typically the number one cause of VAP in the United States, representing 14– 24 % of all VAP cases [21]. Other countries or regions of the world have variable rates of S. aureus-associated VAP. For instance, S. aureus is the third leading cause of VAP in Latin America and Asian ICUs [22].
In the United States, MRSA is responsible for 50% of ICU infections caused by S. aureus [23]. The prevalence of VAP due to S. aureus can vary based on the type of ICU where the patient is staying. Patients in ICUs have more VAPs compared to patients in other parts of a hospital, and MRSA is a common cause of VAP [24]. S. aureus causes approximately one third of all VAP cases in neurological and neurosurgical ICUs, likely due to the presence of a higher number of patients having greater aspiration of nasopharyngeal flora due to neurotrauma. On the other hand, S. aureus is responsible for fewer cases of VAP in patients residing in cardiothoracic ICUs.
RISK FACTORS FOR MRSA-ASSOCIATED VAP
Because MRSA-associated VAP is a significant medical concern, it is important to understand the multiple predisposing factors that are associated with the onset of MRSA- associated VAP. The predominant risk factors tied to MRSA-associated VAP are age, race, severity of underlying conditions like trauma, presence of endotracheal tube, longer duration of mechanical ventilation, prior antibiotic treatment, and aspiration from either the digestive or the respiratory tract in ventilated patients [25].
The most significant risk factor linked to MRSA-associated VAP is the presence of an endotracheal tube (ETT). An ETT will disrupt the patient’s innate defense mechanisms, like a cough reflex and mucocilliary clearance [8]. Intubation for longer periods is also associated with a higher risk of MRSA-associated VAP, increasing the risk of occurrence by 6 to 21-fold as opposed to patients who are not intubated [24]. The risk of developing pneumonia increases between 1% and 3% each day a patients requires intubation [26]. Patients ventilated for a longer time are more likely to have an increase in biofilm formation and an increase in colonizing bacterial numbers in their lungs. Similarly, reintubation or unplanned extubating puts the patient at a higher risk of contracting MRSA-associated VAP [27]. According to univariate analysis by Bouza et al, that compared the 111 MRSA-VAP episodes with 363 episodes due to other microorganisms, significant differences were found in the median age (68 vs 62 years; P = 0.003), presurgical antibiotic prophylaxis (38.7% versus 24%; P = 0.003), receiving any antibiotic at the present admission before VAP (82.9% versus 64.5%; P < 0.001), and abdominal surgery (35% versus 19%, P = 0.001, see Table 1) [25].
Table 1.
Clinical characteristics of MRSA-VAP patients compared with those with VAP due to other microorganisms
| Demographic | MRSA-VAP | Non-MRSA-VAP | P-value |
|---|---|---|---|
| Age | 68 (59–74) | 62 (44–73) | 0.03 |
| Antibiotic treatment | 92 (82.9%) | 234 (64.5%) | <0.001 |
| Abdominal surgery | 39 (35.1%) | 70 (19.3%) | <0.001 |
| Presurgical antibiotic prophylaxis | 43 (38.7%) | 87 (24%) | 0.003 |
Besides intubation, another significant risk factor linked to MRSA-associated VAP is the use of broad spectrum antibiotics in the preceding seven days. One study has shown that a patient’s previous exposure to antibiotic therapy will significantly increasing their chance of acquiring MRSA-associated VAP (19.7%) as compared to a patient that has not been treated with antibiotics (3.1%) [28]. Another study showed VAP was observed more than three times more frequently among patients who had prior exposure to antibiotics compared to patients that did not have prior antibiotic treatment [29].
CLINICAL PRESENTATIONS OF MRSA-ASSOCIATED VAP
An association with any of the risk factors detailed above will make a patient be more likely to contract MRSA-associated VAP. After a patient has developed MRSA-associated VAP, clinical presentations can differ from one patient to another, ranging from minor inflammation to a more fulminant infection. Early on, the clinical manifestations of pneumonia caused by MRSA are indistinguishable from pneumonia resulting from infection with MSSA or other pathogens [30]. However if CA-MRSA is involved, then a necrotizing VAP will develop with ensuing tissue destruction and cavitation. In these patients afflicted with necrotizing VAP, parenchymal infiltrations are commonly bilateral and multilobar. Abscesses and pleural empyema often occur in CA-MRSA-associated VAP [31].
DIAGNOSING MRSA INFECTIONS IN PATIENTS WITH VAP
Mortality due to VAP is high, so suspected cases of VAP need to be diagnosed quickly to start proper treatment. The key diagnostic strategies include careful examination of clinical features, radiological assessment of the lungs, and laboratory biomedical tests. The American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) 2005 guidelines outline that pneumonia is suspected if a new or progressive radiographic infiltrate with proof that the infiltrate is of an infectious origin (e.g. fever, leukocytosis or leukopenia, purulent secretions, and decline in oxygenation in ventilated patients) is seen in ventilated patient [32].
To confirm the patient with abnormal chest x-rays has VAP, samples are collected from the alveoli and airways. Respiratory samples can be collected from these patients either by non-bronchoscopic or bronchoscopic techniques. Non-bronchoscopic samples include tracheal bronchial aspirations and mini bronchioalveolar lavage (BAL) specimens. To collect tracheal bronchial aspiration samples, a catheter is inserted through an endotracheal tube, suction is applied, and collected specimens are sent to the laboratory for identification. The mini BAL is a procedure for collection of respiratory samples for culture. A mini BAL sample is collected by inserting a catheter through the endotracheal tube followed by a saline infusion into the lungs, and finally a mucus trap is connected to aspirate out the sample via suction for collection into a specimen cup [33].
Although non-bronchoscopic procedures are quicker and less invasive, there is sometimes a need for a bronchoscopic sampling technique. During a bronchoscopic procedure, a protected specimen brush (PSB) is employed. The PSB brushes the lower airways to allow for better specimen collection. Specimens are collected by brushing the airway wall, withdrawing the brush into the sheath, and then removing the sheath from the bronchoscope [32]. Once the specimens are collected in sterile containers and brought to a microbiology laboratory, they are vortexed for 1 min, pelleted by centrifugation for 10 min, and aliquots of the samples are then Gram stained. Samples containing S. aureus will display Gram-positive cocci in a mixture of arrangements. A negative predictive value (NPV) of 97% is associated with Gram staining an endotracheal aspirate. A NPV indicates if S. aureus was not seen from the Gram stain, there is a high chance the culture will not grow S. aureus. A culture result usually takes takes 72 h to be available, so a Gram stain can influence the empiric therapy [34].
After part of the specimen has been Gram stained, another part of the specimen is plated onto sheep blood agar, mannitol salt agar, a differential agar (e.g. eosin methylene blue or MacConkey agar), and chromogenic agar followed by incubation overnight at 37° C [35]. CHROMagar MRSA provides selection and differential properties tied to the rapid isolation of S. aureus. The chromogenic agar possesses MRSA selectivity by incorporating either methicillin or oxacillin into the agar as well as a chromogenic enzyme substrate that changes color in the presence of S. aureus. Colonies with mauve or pink color on CHROMagar MRSA are considered positive, indicating MRSA [36]. Chromogenic media has high false positive results, so positive results should be confirmed using other methods [37]. S. aureus with colony counts of ≥ 103 CFU/mL on the agar above are considered to be significant for PSBs, whereas S. aureus colony counts of ≥105 CFU/mL are considered significant for samples obtained by bronchoalveolar lavage. If the colony count is lower than 103 CFU/mL for a PSB and 105 CFU/mL for bronchoalveolar lavage, then treatment will only be started if the risk of missing VAP exceeds the risk of unnecessary treatment [35].
S. aureus displaying prototypic colony features (e.g., convex, medium to large, opaque, yellowed colored, and β-hemolytic on sheep blood agar) are then confirmed to be S. aureus using the tube coagulase test or one of several latex agglutination tests. Any colony showing a positive result is identified as S. aureus and negative results with these tests would indicate a coagulase negative Staphylococcus spp [38].
Once there is identification that the patient has S. aureus-associated VAP, antibiotic susceptibility testing is performed to detect whether the clinical isolate is MRSA or MSSA. A common, inexpensive way to screen for MRSA uses a cefoxitin saturated disk as part of a Kirby Bauer disk diffusion assay. According to the Clinical and Laboratory Standards Institute (CLSI), a zone of growth inhibition around the cefoxitin disk (30 μg) ≤ 19 mm in diameter indicates MRSA, while an inhibition zone size of ≥ 22 mm indicates MSSA instead [39]. S. aureus culture and antibiotic susceptibility testing for MRSA takes 48–72 h. VAP is associated with high morbidity and mortality, therefore, more rapid detection of MRSA is needed to avoid MRSA-associated VAP mortality. One such rapid detection methodology is the Xpert MRSA assay that is a real-time polymerase chain reaction technology. This assay is used for detecting an MRSA-specific DNA sequence within the SCC element in 2 h. The Xpert MRSA assay test uses a respiratory sample from the bronchiolalveolar lavage (BAL) or mini BAL. Since MRSA is found to have a high negative predictive value, this test can be used to exclude unnecessary antibiotic use in patients suspected of having MRSA-associated VAP [40]. Overuse of antibiotics will result in an increase in multidrug resistant pathogens, treatment-related side effects, and increase the cost of hospitalization. This test is not used for confirmatory diagnosis of MRSA from respiratory specimens because of a low positive predictive value. Since the Xpert MRSA test provides results within 2 h, it can be used to guide empiric therapy in VAP patients.
Other tests can be used for evaluating VAP, such as measuring procalcitonin or C- reactive protein levels, but they have very little role in the diagnosis of MRSA-associated VAP. Procalcitonin is a biological marker that can be used as a diagnostic tool to decide whether or not to start antibiotic in patients, but it is not used as a diagnostic marker in MRSA-associated VAP patients. This is because most VAP patients have developed systemic inflammation response syndromes, multiorgan failure, or conditions known to raise the procalcitonin level in the absence of active infection [41]. However, in patients who have confirmed VAP, serum calcitonin is useful to decide whether or not to initiate antibiotic therapy and the test can be used as a prognostic marker since increases in serum procalcitonin have been associated with septic shock and mortality [42]. Other biomarkers, such as C-reactive protein, are not used to discriminate between patients with and without VAP because their concentrations in BAL fluid have minimal diagnostic value for VAP [43, 44].
After a diagnosis of MRSA has been made in a patient with VAP, typing methods can be used to discriminate between different bacterial strains. Rapid and accurate epidemiologic typing systems are useful in controlling MRSA infections. Pulse-field gel electrophoresis is regarded as the gold standard genotyping method for MRSA, and it is a very useful method for tracking outbreaks associated with MRSA [45]. For PFGE analysis, MRSA genomic DNA is digested with SmaI and separated by PFGE. During outbreaks, DNA fingerprinting patterns are uploaded to the national database and each fingerprint pattern is investigated to see if one strain is causing a particular outbreak. These typing methods can help in both the early identification of outbreaks as well as the immediate institution of appropriate infection control measures [46]. However, PFGE is also a slow and time-consuming method that requires trained personnel and sophisticated equipment. In an outbreak, rapid and reliable results are needed as opposed to excellent discriminatory power. PCR-based typing methods are cheaper and can be used for faster molecular typing, but they are still insufficient to replace PFGE because of the high discriminatory power and reproducibility of PFGE [47].
MANAGEMENT OF MRSA INDUCED VAP
After a diagnosis of VAP, treatment should be started quickly, but the emergence of multidrug-resistant MRSA strains has created a problem in the management of MRSA- associated VAP. Treatment of MRSA-associated VAP has been challenging due to co-morbidity and complications. Currently, various prevention and treatment strategies have been adopted for the management of MRSA-associated VAP, such as taking preventive measures, rapid and appropriate antibiotic therapy, and treatment of complications associated with VAP.
Preventive Methods to Restrict MRSA-associated VAP
A VAP-associated disease outcome can be greatly reduced by adopting certain preventive measures. In 2011, most European countries had MRSA prevalence rates between 10% and 50%. However, four European countries (Sweden, Norway, Denmark, and the Netherlands) reported MRSA prevalence rates that were less than 5%. In these four countries, a low prevalence of MRSA was accomplished using preventive interventions, like screening by PCR or culture, isolation in a single room, contact precautions, decolonization with mupirocin, and proper antibiotic stewardship [48]. European countries where MRSA prevalence was high had preventive measures that were often not fully implemented [49].
Active Surveillance of MRSA
To prevent MRSA-associated VAP, patients who are asymptomatically colonized with MRSA need to be identified and decolonized. Active surveillance of MRSA in hospitals can be done by collecting samples from the anterior nares, rectum, throat, or skin of healthcare personnel and patients upon admission or during their stay in the hospital. Collection from the anterior nares is the most standard method of sampling [50]. Samples can be screened for MRSA as described above or by a PCR detection method within 48 h of admission. Unlike culturing, the PCR testing for MRSA in anterior nares samples takes less than 2 h with a sensitivity of 88% and a negative predictive value of 99.2% for MRSA-associated pneumonia [51]. Therefore, a negative result would exclude MRSA. The use of PCR-based detection methods could help reduce MRSA-directed empiric antibiotic therapy for patients hospitalized with lower respiratory tract infections [52].
A patient colonized with MRSA at the time of hospital admission will have a significantly greater risk of developing MRSA-associated VAP [53, 54]. In one cohort study, patients found to be negative for MRSA nasal colonization at the time of admission at the hospital rarely developed MRSA-associated VAP compared to most patients who had MRSA nasal colonization at admission who often developed MRSA-associated VAP. A high negative predictive value (94%) when screening for MRSA nasal colonization would indicate patients that did not have MRSA in their anterior nares on admission to the hospital would rarely develop MRSA-associated VAP [54].
Preventive Measures to Reduce MRSA
Besides active surveillance, appropriate hand hygiene remains an important preventive measure for VAP. Thorough hand washing should be done for 10 secs before and after contact with all patients. Additionally, contact precautions should be done before and after examining patients colonized with MRSA, including the use of protective gowns and gloves which should always be worn when there is contact with oral and endotracheal secretions [8].
Another method to prevent MRSA spread is by placing patients suspected of being colonized with MRSA in single rooms. If single rooms are not available, then patients could be placed in rooms with other patients who are at low risk of acquiring MRSA. Healthcare workers that have indirect contact with patients should also be screened for MRSA colonization [55]. Healthcare workers that rotate between hospitals or long-term care facilities and the community have a higher chance of being reservoirs, vectors or victims of MRSA cross- transmission. A routine screening of MRSA in healthcare patients may not be possible due to the cost [56]. Routine screening and decolonization of healthcare workers is recommended for those who work in high-risk units, such as burns wards, ICUs, and surgical wards. When there are nosocomial outbreaks or during the early stages of an institutional epidemic, a screening regimen will help to control these nosocomial-based MRSA outbreaks [57]. After screening, nasal decolonization of infected patients can control MRSA infection. Nasal decolonization can be done by using mupirocin, which is an antimicrobial used for decolonization of MRSA that inhibits the synthesis of bacterial proteins by inhibiting bacterial isoleucyl-tRNA synthetase. Mupirocin and it has been recommended for clearance of nasal MRSA carriage during outbreaks [58]. Since 1980, mupirocin has been considered as the standard to decolonize S. aureus from patients [59]. Several studies have shown that 81 to 100% of patients were effectively decolonized using a nasal mupirocin spray [60–62].
Besides screening and decolonization, another method for prevention of VAP is elevating the head of the bed. By elevating the head of the bed, VAP can be reduced in intubated patient by lessening the subsequent aspiration and development of VAP. Patients should be placed in a semi-recumbinant position with the head of bed elevated at an angle of 30 to 45 degree. This will help to prevent reflux and aspiration of bacteria from the stomach into the airways [63].
Because an ETT is a significant risk factor for VAP, the use of a silver-coated ETT was found to decrease VAP in intubated patients for more than 24 h by 40% compared to patients who were intubated with uncoated tubes [64]. In a randomized single-blinded trial that compared silver-coated ETTs with uncoated ETTs in patients undergoing endotracheal intubation, the use of silver-coated ETTs was also associated with a significant delay in the occurrence of VAP. Silver-coated ETTs slowly release silver cations that have strong antimicrobial effects, which has been shown to an effective means to prevent VAP [65].
Overall, preventive measures like screening, isolation, contact precautions, decolonization, and silver coated endotracheal tube, can reduce the occurrence of MRSA- associated VAP. These methods when implemented in the care of ventilated patients can save the people lives.
Treatment of Disease Antibiotic Therapy to Control VAP Caused by MRSA
Various preventive strategies can help stop the occurrence of VAP. However, if these precautionary measures fail, suspected VAP patients must be rapidly administered an appropriate antibiotic therapy to manage their VAP. The primary way MRSA-associated VAP is controlled is through antibiotic therapy. When MRSA is suspected in a VAP case, empiric coverage includes linezolid or vancomycin [32]. Because microbiological results take 24 to 48 h, empirical treatment based on clinical presentation is often done [66]. If multidrug-resistant pathogens are suspected, then empiric treatment for multidrug resistance strains is initiated. However, once culture and antibiotic susceptibility results are completed, the treatment of VAP may change. Patients who receive the correct treatment as soon as symptoms are displayed will have a lower mortality [67]. If a patient has been receiving antibiotics in a hospital where MRSA, P. aeruginosa, or other drug resistant gram-negative bacilli are prevalent, then specific antimicrobial treatment regimens should be considered [32].
Linezolid or Vancomycin.
If a patient is in a hospital where MRSA is prevalent or when the patient is diagnosed with MRSA-associated VAP, then a special antimicrobial regimen should include linezolid or vancomycin [32]. Linezolid is an effective antibiotic against S. aureus with an effective dosage of 600 mg twice a day, given orally or via intravenous (IV) drip if the patient is unable to receive oral medications. Pulmonary penetration of linezolid is good [68]. Linezolid is an effective alternative to vancomycin in people with renal impairment or that have poor intravenous access. On the other hand, linezolid is associated with more gastrointestinal side effects [66]. If the patient is taking selective serotonin reuptake inhibitors, then linezolid use can be associated with serotonin syndrome. Linezolid treatment is restricted to a maximum of 28 days [68]. Longer use of linezolid can cause adverse effects like cytopenias, induction of lactic acidosis blindness, and peripheral neuropathy. Linezolid is also found to be associated with thrombocytopenia and myelosuppression [69].
Vancomycin has been the gold standard for the treatment of MRSA infections. Vancomycin treatment is 15 to 20 mg/kg administered IV every 8 to 12 h to patients who have normal renal function with a target serum trough concentration of 15 to 20 mg/ml [66]. Desirable properties of vancomycin include a low resistance rate, a relatively safe profile, and limited anti-MRSA regimens that are available. However, vancomycin has a slow bactericidal action, pulmonary penetration is poor, and renal damage may occur [68]. High trough levels are associated with nephrotoxicity, which may require discontinuation of drugs in a patient. Vancomycin can cause red man syndrome, leading to an erythematous rash on the face and upper body. Reducing the dosage of vancomycin can cure red man syndrome. Besides red man syndrome, vancomycin can cause ototoxicity, which require prompt discontinuation of the drug [70]. In patients with normal renal function, vancomycin is poorly metabolized and is excreted unchanged in urine. Patients who have impaired renal function, renal excretion of drug is impaired that cause drug accumulation in body. Vancomycin drug accumulation can cause toxic effects, so the dose needs to be adjusted if a patient has renal impairment [71]. Patients infected with MRSA strains possessing a vancomycin MIC of ≥ 2 μg/ml have a higher chance of treatment failure, thus, an alternate drug therapy is recommended. Vancomycin pharmokinetics may be different in patients depending on age, weight, and the underlying disease. It is recommended that the vancomycin dose be determined individually in VAP patients [72].
Various studies have been conducted to determine whether linezolid or vancomycin is superior in controlling MRSA-associated VAP. In one study, patients were treated with either linezolid or vancomycin and the outcomes were compared. Successful patient outcomes for MRSA-associated VAP patients treated with linezolid or vancomycin were 58% and 48%, respectively. Patient treated with both drugs had similar death rates, clinical responses, and MRSA eradication rates [66]. Respiratory specimen culturing found that 16 out of 92 VAP patients who received linezolid were still culture positive for MRSA, whereas 50 out of 109 patients who received vancomycin were culture positive for MSRA. Thus, linezolid treatment had statistically better clinical and microbiological results, but the patient mortality rate was the same as vancomycin treated patients. Although vancomycin treatment led to the same number of deaths as linezolid treatment, nephrotoxicity was more common in patients treated with vancomycin [73]. Other antibiotics that can treat MRSA-associated VAP are needed because of the limitations associated with both linezolid and vancomycin.
One newer approved drug is telavancin. Telavancin, approved by the FDA in 2013, is an alternative drug for the treatment of MRSA-associated HAP and VAP. The dose of telavancin is 10 mg/kg given IV every 24 h in patients who have normal renal function. According to the assessment of telavancin for treatment of hospital-acquired pneumonia (ATTAIN) study, the MRSA-associated VAP cure rate after telavancin treatment was 82%, whereas the vancomycin cure rate was 74% [74].
Because 15 to 20 % of patients with S. aureus-associated VAP have bloodstream co- infections, treatment with telavancin may be advantageous compared to either linezolid or vancomycin [75]. Linezoid has bacteriostatic activity and may not rapidly clear bloodstream infections. Vancomycin is less effective at treating S. aureus-associated VAP in patients with concurrent bacteremia [76]. Telavancin treatment has been found to be more effective than vancomycin as a treatment for MSSA-associated pneumonia because it has activity against several gram positive pathogens besides MRSA [75, 77].
Unfortunately, telavancin has many side effects in patients with severe renal impairment, including an increased chance of mortality as compared to treatment with vancomycin [78]. Minor side effects include taste disturbances, nausea, headache, vomiting, constipation, insomnia, and foamy urine. Telavancin drug therapy was associated with adverse fetal outcomes in animal studies [68]. and should be used only when the benefit to the patient outweighs the risks.
Other anti-staphylococcal drugs have been approved for the treatment of MRSA infections, but are not recommended for the treatment of VAP. Daptomycin cannot be used to treat pneumonia because it doesn’t achieve a sufficiently high concentration in the respiratory tract and is inhibited by pulmonary surfactant [79]. Another recently approved broad-spectrum drug is tigecycline, which can be used for CAP and HAP. However, this drug is not approved for VAP caused by MRSA because of an increased risk of mortality and clinical failure compared with other drugs [80]. Tigecycline has adverse effects such as fever, chills, headache, pain. In patients with baseline bacteremia, this drug is associated with high mortality and clinical failure due to delayed clearance of bacteremia [81].
In Europe, the broad-spectrum antibiotic ceftobiprole has been approved for treatment of MRSA-associated HAP and CAP as well as HAP and CAP caused by other gram-positive bacteria and gram-negative bacteria, but the drug is not recommended for VAP. The FDA has not approved ceftobiprole for use in the United States [82].
New Drugs and Monoclonal Antibodies in Pipeline
Although several drugs are approved for MRSA-associated VAP, there is still a need to find alternative drugs to use in treatment regimens. Two antibiotics in the pipeline that have been shown to be effective for treating MRSA infections include dalbavancin and oritavancin, but neither drug has been proven to treat pneumonia [83]. Other antibiotics in the pipeline to treat MRSA-associated CAP are radezolid and cethromycin, but neither drug has been evaluated in VAP. Phase II clinical trials were completed for CAP and uncomplicated skin and skin structure infections [84]. If cethromycin is shown to not cause hepatotoxicity, then the drug could show promise for treating community-associated pneumonia [85].
Because MRSA will continue to develop resistance to antibiotics, there is a need to develop novel therapeutics, like monoclonal antibodies, against MRSA infections. Many monoclonal antibodies have been developed for MRSA-associated pneumonia, but they have not been introduced into clinical practice because they failed to provide sufficient efficacy [86]. Two monoclonal antibodies, Aridis Pharmaceuticals candidate AR-301 and Medimmune’s MEDI4893, target S. aureus alpha toxin and are in clinical evaluation for the treatment of MRSA-associated VAP. MED14893 has completed phase I trials and is in phase II study, while AR-301 is in a phase clinical I/II clinical trial [84].
Complications of MRSA-Associated VAP
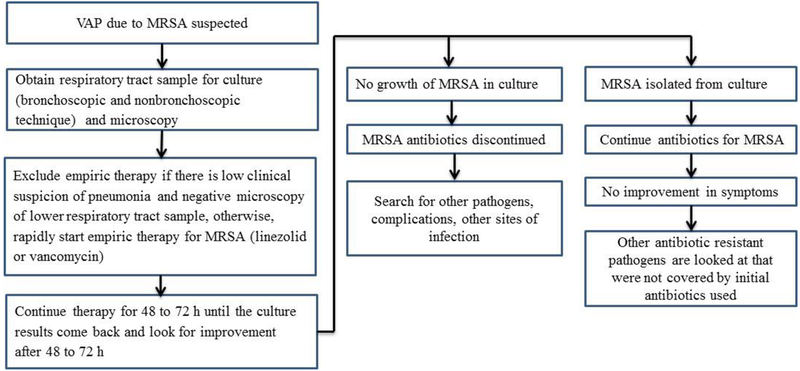
Patients usually improve within 3–5 days after starting antibiotic treatment for MRSA- associated VAP [87]. However, despite early use of antibiotics, resolution of MRSA-associated VAP is sometimes slow or incomplete. Life-threatening hemoptysis, septicemia, respiratory failure, empyema, and lung abscess can develop as a result of treatment failure and complications from the initial pneumonia [88]. Clinical failure can occur during treatment of VAP in which the patient may die or may not show improvement after treatment for more than 10 days. If the signs and symptoms of VAP still persist after 10 days, then the therapy should be changed [87]. The clinical failure rate of patients with MRSA-based VAP is 30 to 40%. Treatment failure may be because of several factors, including co-morbidities, age, and the severity of the VAP. Sometimes treatment failure is because of sequestered foci, like empyema or lung abscesses, which prevent the antibiotic from reaching the site of infection. When medical treatment fails and complications occur, surgical interventions like debridement or resection is done to decrease morbidity and mortality [89]. For patients who cannot tolerate resection, lung debridement is an option. The appropriate timing for surgery is unclear, although it is recommended that surgery be done if the patient did not improve or if they develop complications following the use of antibiotics or less invasive procedures [4, 90]. Clinical management of patients with suspected VAP is summarized below (See Figure 1 modified from [21]).
Figure 1.
Clinical management of a patient with suspected MRSA-based VAP (modification of figure in [21]).
CONCLUSION
MRSA-associated VAP can cause significant morbidity and mortality. Currently, the standard diagnostic technique for MRSA-associated VAP is respiratory culture, but diagnosis is moving towards the use of molecular techniques like PCR. PCR has not yet made a breakthrough as a diagnostic tool for MRSA from respiratory specimens, however, PCR provides a faster result and has a high negative predictive value for MRSA from respiratory specimen. A faster PCR result alleviates the wait-time for culture, therefore, it allows proper treatment to be started very early in ventilated patients. Ventilated patients may be better off with faster result from PCR as a diagnostic tool as oppose to more accurate result from culture in rush to save life. In the future, PCR will likely be the gold standard method for the rapid diagnosis of MRSA from respiratory specimens. A rapid diagnosis would be helpful to avoid empiric therapy for MRSA and instead begin proper treatment earlier.
Besides rapid diagnosis, preventive interventions such as screening, contact precautions decolonization, and antibiotic stewardship as well as educating healthcare providers about risk factors and preventive measures for VAP will also help to decrease incidence of VAP. Active surveillance of handwashing by assigned healthcare personnel, using early appropriate antibiotics and followed by de-escalation on the basis of microbial cultures will help in the management of VAP.
ACKNOWLEDGEMENTS
W. R.S. was supported by grant AI47801 from the National Institutes of Health.
REFERENCES
- 1.Hoare Z 2006. Br. Med. J, 332, 1077.16675815 [Google Scholar]
- 2.Ginsburg AS, Delarosa J, Brunette W, Levari S, Sundt M, Larson C, Anderson R 2015. PLOS One, 10, e0139625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meduri GU 1995. Clin. Chest Med, 16, 61. [PubMed] [Google Scholar]
- 4.Rello J 2007. Nosocomial pneumonia: Strategies for management, J. Wiley, Chichester, England. [Google Scholar]
- 5.Jones RN 2010. Clin. Infect. Dis, 51, S81. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society: Infectious Diseases Society of America. 2005. Am. J. Respir. Crit. Care Med, 171, 388.15699079 [Google Scholar]
- 7.Restrepo MI, Peterson J, Fernandez JF, Qin Z, Fisher AC and Nicholson SC 2013. Respir. Care, 58, 1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustyn B 2007. Crit. Care Nurse, 27, 32. [PubMed] [Google Scholar]
- 9.Alp E and Voss A 2006. Ann. Clin. Microbiol. Antimicrob, 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross AS and Roup B 1981. Am. J. Med, 70, 681. [DOI] [PubMed] [Google Scholar]
- 11.Usman S, James P and R M. 2014. Int. J. Res. Med. Sci, 2, 239. [Google Scholar]
- 12.Kalanuria AA, Ziai W and Mirski M 2014. Crit. Care , 18, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonten MJM, Kollef MH and Hall JB 2004. Clin. Infect. Dis, 38, 1141. [DOI] [PubMed] [Google Scholar]
- 14.Chan JD, Pham TN, Wong J, Hessel M, Cuschieri J, Neff M and Dellit TH 2011. J. Intensive Care Med, 26, 385. [DOI] [PubMed] [Google Scholar]
- 15.Dibah S, Arzanlou M, Jannati E and Shapouri R 2014. Iran. J. Microbiol, 6, 163. [PMC free article] [PubMed] [Google Scholar]
- 16.Llarrull LI, Fisher JF and Mobashery S 2009. Antimicrob. Agents Chemother, 53, 4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlemann A-C, Otto M, Lowy FD and DeLeo FR 2014. Infect., Genet. Evol, 21, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones MB, Montgomery CP, Boyle-Vavra S, Shatzkes K, Maybank R, Frank BC, Peterson SN and Daum RS 2014. BMC Genomics, 15, 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diekema DJ, Richter SS, Heilmann KP, Dohrn CL, Riahi F, Tendolkar S, McDaniel JS and Doern GV 2014. Infect. Control Hosp. Epidemiol, 35, 285. [DOI] [PubMed] [Google Scholar]
- 20.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R.m, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin Sand Emerging Infections Program-Active Bacterial Core Surveillance MRSA surveillance Investigators. 2013. JAMA Intern. Med, 173, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinstein E, Kollef MH and Nathwani D (2008). Clin. Infect. Dis, 46, S378. [DOI] [PubMed] [Google Scholar]
- 22.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK and National Healthcare Safety Network Team and Participating National Healthcare Safety Network Facilities. 2008. Infect. Control Hosp. Epidemiol, 29, 996. [DOI] [PubMed] [Google Scholar]
- 23.Poulakou G, Souto J, Kmet Lunacek N and Rello J 2012. Curr. Respir. Med. Rev, 8, 245. [Google Scholar]
- 24.Meyer E, Schwab F and Gastmeier P 2010. Eur. J. Med. Res, 15, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouza E, Giannella M, Bunsow E, Torres MV, Pérez Granda MJ, Martín-Rabadán P and Muñoz P 2012. J. Hosp. Infect, 80, 150. [DOI] [PubMed] [Google Scholar]
- 26.Rello J, Diaz E, Roque M and Vallé S J 1999. Am. J. Respir. Critical Care Med, 159, 1742. [DOI] [PubMed] [Google Scholar]
- 27.Ranjan N, Chaudhary U, Chaudhry D and Ranjan KP 2014. Indian J. Crit. Care Med, 18, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacconelli E, De Angelis G, Cataldo MA, Pozzi E and Cauda R 2007. J. Antimicrob.Chemother, 61, 26.17986491 [Google Scholar]
- 29.Wooten DA and Winston LG 2013. Respir. Med, 107, 1266. [DOI] [PubMed] [Google Scholar]
- 30.Tacconelli E and De Angelis G 2009. Curr. Opin. Pulmon. Med, 15, 218. [DOI] [PubMed] [Google Scholar]
- 31.Kreienbuehl L, Charbonney E and Eggimann P 2011. Ann. Intensive Care, 1, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratala J, El Solh AA, Fey PD, File TM Jr., Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL and Brozek JL 2016. Clin. Infect. Dis, 63, e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waghray P, Tummuru VR, Koteshwara Rao ANV, Veena V and Hasnani R 2015. Apollo Med, 12, 15. [Google Scholar]
- 34.O’Horo JC, Thompson D and Safdar N 2012. Clin. Infect. Dis, 55, 551. [DOI] [PubMed] [Google Scholar]
- 35.Bairy I and Dey A 2007. Ann. Thorac. Med, 2, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Micheel V, Hogan B, Köller T, Warnke P, Crusius S, Hinz R, Hagen RM, Schwarz NG and Frickmann H 2015. Mil. Med. Res, 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wendt C, Havill NL, Chapin KC, Boyce JM, Dickenson R, Eigner U, Schutt S and Fahr AM 2010. J. Clin. Microbiol, 48, 2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akcam FZ, Tinaz GB, Kaya O, Tigli A, Ture E and Hosoglu S 2009. Microbiol. Res, 164, 400. [DOI] [PubMed] [Google Scholar]
- 39.Anand KB, Agrawal P, Kumar S and Kapila K 2009. Indian J. Med. Microbiol, 27, 27. [PubMed] [Google Scholar]
- 40.Leone M, Malavieille F, Papazian L, Meyssignac B, Cassir N, Textoris J, Antonini F, La Scola B, Martin C, Allaouchiche B, Hraiech S and AzuRea Network. 2013. Crit. Care, 17, R170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luyt C-E, Combes A, Reynaud C, Hekimian G, Nieszkowska A, Tonnellier M, Aubry A, Trouillet JL and Chastre J 2008. Intensive Care Med, 34, 1434. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez P, Garcia MA, Ferrer M, Aznar J, Valencia M, Sahuquillo JM, Menendez R, Asenjo MA and Torres A 2008. Eur. Respir. J, 31, 356. [DOI] [PubMed] [Google Scholar]
- 43.Fagon J-Y 2011. Crit. Care, 15, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linssen CFM, Bekers O, Drent M and Jacobs JA 2008. Ann. Clin. Biochem, 45, 293. [DOI] [PubMed] [Google Scholar]
- 45.Pasquale TR, Jabrocki B, Salstrom S-J, Wiemken TL, Peyrani P, Haque NZ, Scerpella EG, Ford KD, Zervos MJ, Ramirez JA, File TM Jr. and IMPACT-HAP Study Group. 2013. Int. J. Infect. Dis, 17, e398. [DOI] [PubMed] [Google Scholar]
- 46.Rebic V, Budimir A, Aljicevic M, Bektas S, Vranic SM and Rebic D 2016. Acta Inform. Med, 24, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frickmann H, Gawlik P, Crusius S and Podbielski A 2012. Eur. J. Microbiol. Immunol, 2, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chastre J, Blasi F, Masterton RG, Relio J, Torres A and Welte T 2014Clin. Microbiol. Infect, 20, 19. [DOI] [PubMed] [Google Scholar]
- 49.Kock R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skow RL, Struelens MJ, Tacconelli E, Witte W and Friedrich AW 2014. Euro Surveill. 19, 20860. [DOI] [PubMed] [Google Scholar]
- 50.Lai PS, Liang L, Cibas ES, Liu AH, Gold DR, Baccarelli A and Phipatanakul W 2015. J. Allergy Clin. Immunol, 136, 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dangerfield B, Chung A, Webb B and Seville MT 2014. Antimicrob. Agents Chemother, 58, 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson JA, Wright ME, Sheperd LA, Musher DM and Dang BN 2015. Permanente J, 19, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baby N, Faust AC, Smith T, Sheperd LA, Knoll L and Goodman EL 2017. Antimicrob. Agents Chemother, 61, e02432–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langsjoen J, Brady C, Obenauf E and Kellie S 2014. Am. J. Infect. Control, 42, 1014. [DOI] [PubMed] [Google Scholar]
- 55.Sadsad R, Sintchenko V, McDonnell GD and Gilbert GL 2013. PLoS ONE, 8, e83099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albrich WC and Harbarth S 2008. Lancet Infect. Dis, 8, 289. [DOI] [PubMed] [Google Scholar]
- 57.Dulon M, Peters C, Schablon A, and Nienhaus A 2014. BMC Infect. Dis,14, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babu T, Rekasius V, Parada JP, Schreckenberger P and Challapalli M 2009. J. Clin. Microbiol, 47, 2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coates T, Bax R and Coates A 2009. J. Antimicrob. Chemother, 64, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ammerlaan HSM, Kluytmans JAJW, Wertheim HFL, Nouwen JL and Bonten MJM 2009. Clin. Infect. Dis, 48, 922. [DOI] [PubMed] [Google Scholar]
- 61.Calfee DP, Salgado CD, Milstone AM, Harris AD, Kuhar DT, Moody J, Aureden K, Huang SS, Maragakis LL and Yokoe DS 2014. Infect. Control Hosp. Epidemiol, 35, 772. [DOI] [PubMed] [Google Scholar]
- 62.Mody L, Kauffman CA, McNeil SA, Galecki AT and Bradley SF 2003. Clin. Infect. Dis, 37, 1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klompas M, Branson R, Eichenwald EC, Greene LR, Howell MD, Lee G, Magill SS, Maragakis LL, Priebe GP, Speck K, Yokoe DS,Berenholtz SM and Society for Healthcare Epidemiology of America (SHEA). 2016. Infect. Control Hosp. Epidemiol, 35, 915. [DOI] [PubMed] [Google Scholar]
- 64.Kollef MH 2008. JAMA, 300, 805.18714060 [Google Scholar]
- 65.Tokmaji G, Vermeulen H, Müller MC, Kwakman PH, Schultz MJ and Zaat SA 2015. Silver-coated endotracheal tubes for prevention of ventilator-associated pneumonia in critically ill patients Cochrane Database of Systematic Reviews, The Cochrane Collaboration (Ed.), John Wiley & Sons, Ltd, Chichester, UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rivera AM and Boucher HW 2011. Mayo Clinic Proc, 86, 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park D 2005. Respir. Care, 50, 932. [PubMed] [Google Scholar]
- 68.Pletz MW, Burkhardt O and Welte T 2010. Eur. J. Med. Res, 15, 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plosker GL and Figgitt DP 2005. Pharmacoeconomics, 23, 945. [DOI] [PubMed] [Google Scholar]
- 70.Klibanov OM, Filicko JE, DeSimone JA and Tice DS 2003. Ann. Pharmacother, 37, 61. [DOI] [PubMed] [Google Scholar]
- 71.Launay-Vacher V, Izzedine H, Mercadel L and Deray G 2002. Crit. Care, 6, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elyasi S and Khalili H 2016. Eur. J. Clin. Pharmacol, 72, 777. [DOI] [PubMed] [Google Scholar]
- 73.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A and Chastre J 2012. Clin. Infect. Dis,54, 621. [DOI] [PubMed] [Google Scholar]
- 74.Barriere SL 2014. Future Microbiol, 9, 281. [DOI] [PubMed] [Google Scholar]
- 75.Sandrock CE and Shorr AF 2015. Clin. Infect. Dis, 61(Suppl 2), S79. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez C, Rubio M, Romero-Vivas J, Gonzalez M and Picazo JJ 1999. Clin. Infect. Dis, 29, 1171. [DOI] [PubMed] [Google Scholar]
- 77.Hooper C and Smith W 2012. Ther. Clin. Risk Manag, 8, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corey GR, Kollef MH, Shorr AF, Rubinstein E, Stryjewski ME, Hopkins A and Barriere SL 2014. Antimicrob. Agents Chemother, 58, 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJand Chambers HF. 2011. Clin. Infect. Dis, 52, 285. [DOI] [PubMed] [Google Scholar]
- 80.Montravers P, Harpan A and Guivarch E 2016. Adv. Ther, 33, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McGovern PC, Wible M, El-Tahtawy A, Biswas P and Meyer RD 2013. Int. J. Antimicrob. Agents, 41, 463. [DOI] [PubMed] [Google Scholar]
- 82.Awad SS, Rodriguez AH, Chuang Y-C, Marjanek Z, Pareigis AJ, Reis G, Scheeren TW, Sanchez AS, Zhou X, Saulay M and Engelhardt M 2014. Clin. Infect. Dis, 59, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramirez P, Fernández-Barat L and Torres A 2012. Curr. Opin. Infect. Dis, 25, 159. [DOI] [PubMed] [Google Scholar]
- 84.Vuong C, Yeh AJ, Cheung GY and Otto M 2016. Expert Opin. Investig. Drugs, 2, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kurosu M, Siricilla S and Mitachi K 2013. Exp. Opin. Drug Discov, 8, 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giersing BK, Dastgheyb SS, Modjarrad K and Moorthy V 2016. Vaccine, 34, 2962. [DOI] [PubMed] [Google Scholar]
- 87.Hamilton LA, Christopher Wood G, Magnotti LJ, Croce MA, Martin JB, Swanson JM, Boucher BA, and Fabian TC 2012. J. Trauma Acute Care Surg, 72, 1478. [DOI] [PubMed] [Google Scholar]
- 88.Domínguez AA, Arango MV and Torres A 2006. Sem. Respir. Crit. Care Med, 27, 104. [DOI] [PubMed] [Google Scholar]
- 89.Schweigert M, Dubecz A, Beron M, Ofner D and Stein H 2012. Thorac. Cardiovasc. Surg, 61, 636. [DOI] [PubMed] [Google Scholar]
- 90.Tsai Y-F and Ku Y-H 2012. Curr. Opin. Pulmon. Med, 18, 246. [DOI] [PubMed] [Google Scholar]