Abstract
Primary sclerosing cholangitis (PSC) is a chronic, idiopathic, cholestatic liver disease characterized by inflammation and fibrosis of the intrahepatic and/or extrahepatic bile ducts. It can affect individuals of all age groups and gender, has no established pharmacotherapy, and is associated with a variety of neoplastic (e.g. cholangiocarcinoma) and non-neoplastic (e.g. dominant strictures) hepatobiliary complications. Given these considerations, endoscopy plays a major role in the care of patients with PSC. In this review, we discuss and provide updates regarding endoscopic considerations in the management of hepatobiliary manifestations and complications of PSC. Where evidence is limited, we suggest pragmatic approaches based on currently available data and expert opinion.
Keywords: Primary sclerosing cholangitis (PSC), Biliary tract disease, Balloon dilation, Cholangioscopy, Dominant stricture, Cholangiocarcinoma (CCA), Endoscopic retrograde, cholangiopancreatography (ERCP)
1. Introduction
Primary sclerosing cholangitis (PSC) is a chronic, idiopathic, cholestatic liver disease characterized cholangiographically and histopathologically by injury to, stricturing, and the destruction of the intrahepatic and/or extrahepatic bile ducts.1-4 In a majority of cases, it ultimately leads to end-stage liver disease and liver-related death.5,6 PSC is also associated with various other hepatobiliary complications and carries a particularly high risk of developing cholangiocarcinoma (CCA), reaching 400–1500 times higher risk than the general population.7-10 Despite extensive research over the last several decades, effective pharmacotherapies for PSC are lacking,2,5 and consequently, median survival is approximately 15–20 years.5,6 Liver transplantation (LT) is the only potentially curative intervention but is an option for only a small proportion of patients;11-13 moreover, many patients who undergo LT will experience recurrence of PSC or of PSC-associated malignancy.14-17 Due to the above mentioned reasons, endoscopy, especially endoscopic retrograde cholangiopancreatography (ERCP), often plays a critical role in the pre- and post-LT care of patients with PSC.18-20 Here we provide a focused review and clinical updates regarding the role of endoscopy in the management of biliary manifestations and complications of PSC.
2. Diagnosis of PSC
A diagnosis of PSC can usually be made based on a combination of a cholestatic serum biochemical pattern for at least 6 months, cholangiography with characteristic multifocal strictures and proximal segmental dilation, and the exclusion of mimics of PSC (Table 1).18,21,22 Although commonly required in the past, liver biopsy is now less frequently used to establish the diagnosis of PSC; instead, it is reserved primarily for patients who have a normal cholangiogram but are suspected to have small duct PSC (comprising 5% of PSC cases), to rule out an alternative etiology or overlap syndrome (e.g. autoimmune hepatitis), or when staging information is needed but not reliably obtainable by non-invasive methods.22-24
Table 1:
Mimics of PSC
| Categories | Examples |
|---|---|
| Infectious | AIDS cholangiopathy (e.g. C. parvum, CMV) |
| Helminthic infection (e.g. Clonorchis, Opisthorchis, Ascaris) | |
| Recurrent pyogenic cholangitis (i.e. “oriental cholangiohepatitis”) | |
| Neoplastic | Cholangiocarcinoma |
| Diffuse intrahepatic malignancy (primary or metastatic) | |
| Immunologic | IgG4-associated cholangiopathy |
| Eosinophilic cholangitis | |
| Mast cell cholangiopathy | |
| Histiocytosis X | |
| Systemic vasculitis | |
| Hepatic allograft rejection | |
| Primary biliary cirrhosis | |
| Ischemic | Post-transplant non-anastomotic strictures |
| Post-intraarterial chemotherapy | |
| Post-radiation therapy | |
| Inflammatory | Recurrent pyogenic cholangitis |
| Chronic pancreatitis | |
| Miscellaneous | Mirizzi syndrome |
| Compressive lymphadenopathy | |
| Portal hypertensive biliopathy | |
| Post-operative biliary strictures | |
| Choledochal cyst (e.g. Caroli’s disease) | |
| Progressive familial intrahepatic cholestasis |
Key: AIDS, acquired immune deficiency syndrome; CMV, cytomegalovirus; IgG4, immunoglobulin G subclass 4; PSC, primary sclerosing cholangitis.
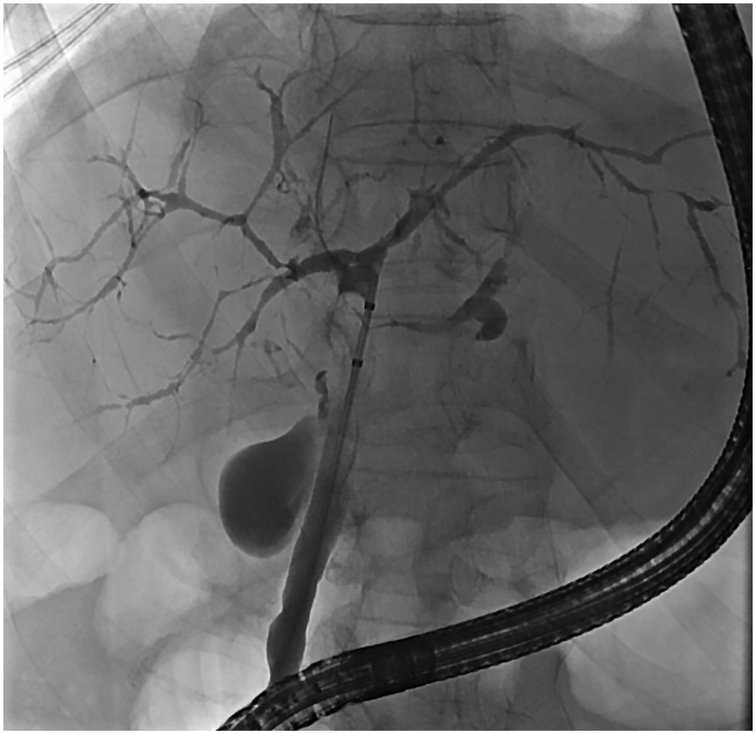
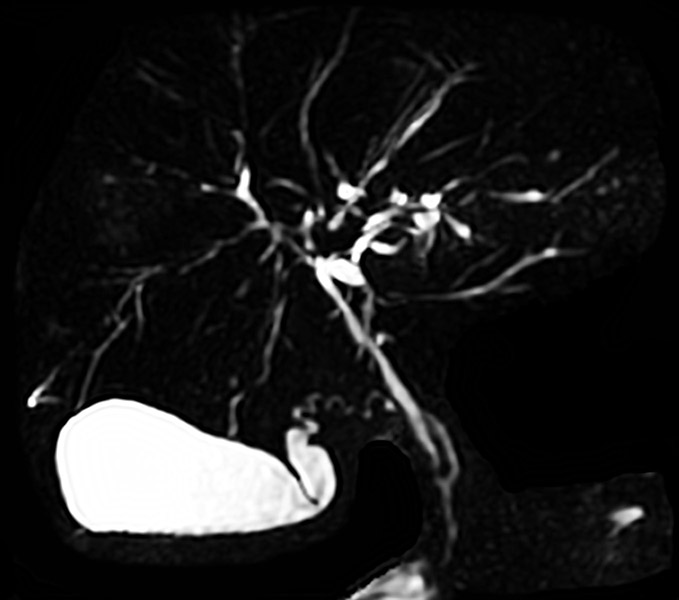
Cholangiography, on the other hand, be it through ERCP or magnetic resonance cholangiopancreatography (MRCP) plays an essential role in the diagnosis and monitoring of PSC. Historically, ERCP has been regarded as the gold standard in diagnosing PSC. In the 1970s, it was largely through the introduction and increased use of ERCP that facilitated the appreciation of the classic “beaded” appearance of the biliary tree in PSC (Fig. 1) and increased greater awareness of this disease.25 Due to advancements in magnetic resonance imaging (MRI), however, MRCP has essentially become the preferred initial cholangiographic modality for the diagnosis of PSC (Fig. 2).18,21 MRCP is non-invasive, accurate, and provides crosssectional images of the biliary tree and surrounding structures without the procedural risks and ionizing radiation associated with ERCP. A meta-analysis of 6 studies (456 patients) comparing MRCP to ERCP for the diagnosis of PSC found MRCP to have a high sensitivity and specificity (86% and 94%, respectively).26 Furthermore, cost-minimization analyses have found a significant cost savings by performing MRCP rather than ERCP in the initial diagnostic testing for PSC.27,28 However, there are limitations of MRCP which should be recognized, including: (i) decreased sensitivity for identifying subtle early changes of PSC (especially early intrahepatic disease), (ii) limited accuracy in the differentiation between secondary sclerosing cholangitis and CCA, and (iii) the inability to obtain tissue samples (e.g. biliary cytology brushings, intraductal biopsies).29-31 Thus, ERCP remains a useful diagnostic tool in certain scenarios, e.g. when MRCP is equivocal or infeasible, or when clinical suspicion for early PSC or related complications is high, and/or when biliary specimens are needed.18
Fig. 1. ERCP in a patient with PSC.
ERCP with balloon occlusion cholangiogram demonstrating diffusely irregular intrahepatic bile ducts consistent with PSC. Abbreviations: ERCP, endoscopic retrograde cholangiopancreatography; PSC, primary sclerosing cholangitis.
Fig. 2. MRCP in a patient with PSC.
MRCP demonstrating multifocal perihilar and intrahepatic ductal strictures consistent with PSC. Abbreviations: MRCP, magnetic resonance cholangiopancreatography; PSC, primary sclerosing cholangitis.
Endoscopic ultrasound (EUS) is another modality that may be useful in the diagnosis of suspected PSC (or its complications), with the advantage of a lower rate of complications than ERCP and ability to detect very early ductal changes in comparison to MRCP.32 It may be particularly useful in a subset of relatively asymptomatic patients with suspected early disease who are hesitant to undergo ERCP or liver biopsy due to the higher risk of morbidity and mortality associated with these diagnostic procedures.33 Although EUS is currently not a substitute for cholangiography by ERCP or MRCP, it can serve as a complement to routinely used diagnostic modalities, providing better clarity as to what the next best diagnostic step would be for patients with suspected PSC.34 Given the very few published studies on the use of EUS (and its associated interventions, e.g. EUS-guided liver biopsy, EUS hepatic elastography) in the diagnosis of PSC, further research is needed to clarify the role of EUS before it can be routinely recommended in clinical practice for this indication.
Of note, there is also a role for non-hepatobiliary endoscopy for patients with PSC (e.g. surveillance colonoscopy for colorectal cancer, surveillance upper endoscopy for esophageal varices in patients who progressed to cirrhosis); these modalities are beyond the scope of the present review but have been recently discussed elsewhere.19,23
3. Indications for endoscopic biliary intervention in PSC
3.1. Dominant strictures
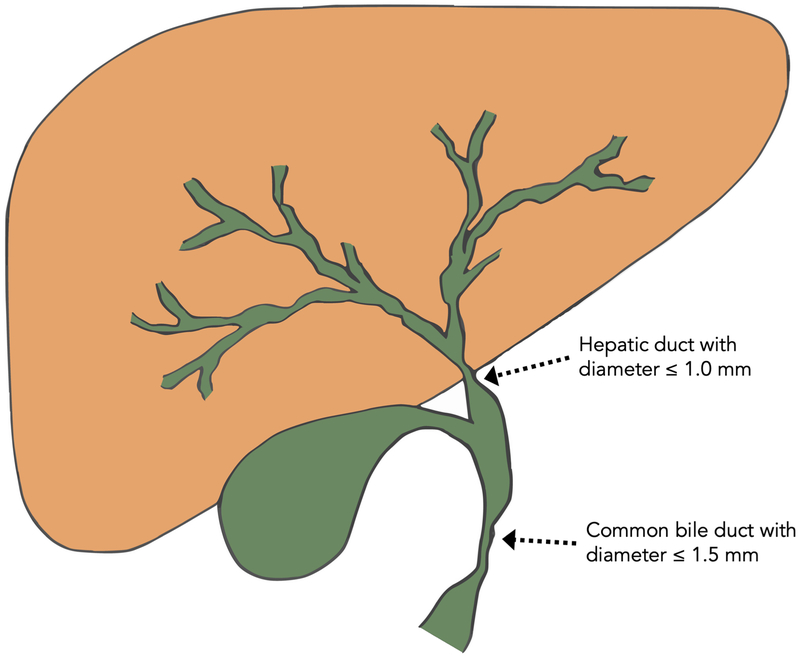
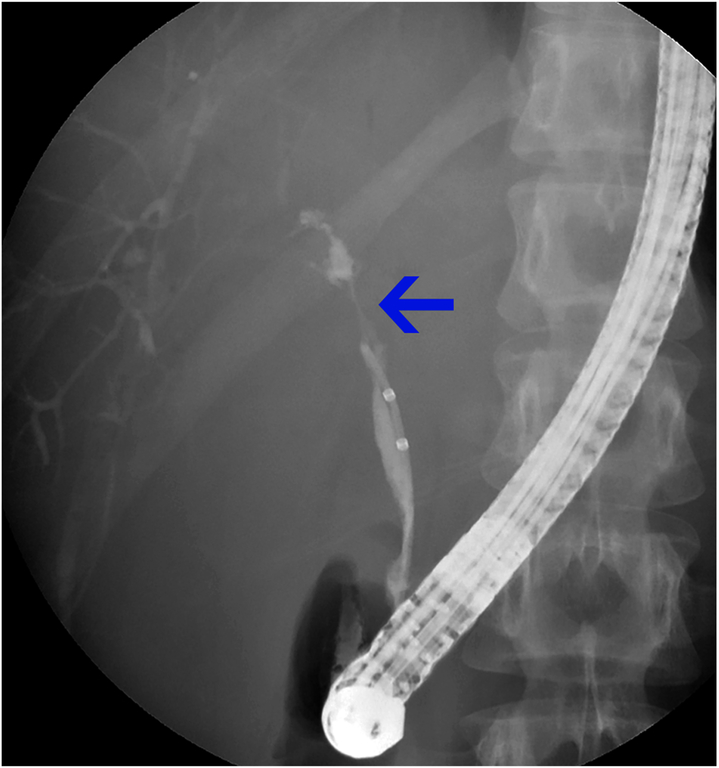
The most common indication for endoscopic intervention in PSC is to evaluate and/or treat “dominant strictures”.18,35 Dominant strictures on ERCP have been defined as a stenosis with a diameter of: (i) ≤ 1.5 mm in the common bile duct or (ii) ≤ 1.0 mm in the hepatic duct within 2 cm of the hepatic ductal confluence (Figs. 3 and 4).18,36,37 Though suspected dominant strictures can be visualized on MRCP as well as on other imaging modalities, the diameter criterion is considered by some to only be applicable to stenosis seen on ERCP due to the inability of other modalities to introduce hydrostatic pressure in the ducts.18,38 A large proportion of patients have dominant strictures (prevalence is estimated at 36–57% of patients with PSC), and patients can have multiple dominant strictures.36,39,40 Patients who develop a dominant stricture have been reported to have poorer long-term outcomes, largely due to the fact that a significant proportion of dominant strictures harbor CCA.39 In one longitudinal study of 128 patients with PSC, the mean survival of patients with dominant strictures was significantly worse than those without dominant strictures (13.7 vs. 23 years).41
Fig. 3. Schematic representation of diameter criteria for the diagnosis of a dominant stricture.
A dominant stricture is generally defined as a stenosis with a diameter of: (i) ≤ 1.5mm in the common bile duct or (ii) ≤ 1.0mm in the hepatic ducts within 2 cm of the hepatic ductal confluence.
Fig. 4. Dominant stricture during ERCP.
A dominant stricture in the region of the hepatic duct is seen on ERCP. Abbreviation: ERCP, endoscopic retrograde cholangiopancreatography.
CCA is arguably the most dreaded complication of PSC, responsible for approximately one-third of all-cause mortality in patients with PSC.7,42 This large proportion of deaths due to CCA can be attributed in part to the fact that CCA: (i) has a dearth of reliable predictors for developing CCA,8,10,43 (ii) frequently presents with non-specific symptoms (e.g. abdominal pain, weight loss, jaundice, fatigue), thus potentially delaying diagnosis,37,44 (iii) occurs with a relatively high incidence in PSC (400- to 1500-fold lifetime risk compared to the general population), 7,8 and (iv) is an aggressive malignancy, with up to 80% of patients who develop CCA dying within 1 year.8,45,46 Due to the association of dominant strictures and CCA, endoscopic therapy (discussed in later sections), in theory, could ostensibly further delay the diagnosis of CCA by alleviating its symptoms (e.g. via stenting).41 Thus, accurate and early distinction between a benign dominant stricture and CCA is vitally important.
3.2. Biliary stone disease
Biliary stones are another frequent indication for endoscopic biliary intervention. Right upper quadrant pain, new or worsening pruritus, new or worsening jaundice, rising serum liver enzymes or bilirubin, and unexplained fevers, when not related to an underlying dominant stricture, are often due to choledocholithiasis with or without acute cholangitis and/or acute cholecystitis.23,41,47 In a retrospective study of 117 patients with PSC, 51% of patients who underwent ERCP were found to have a stone.48 In a smaller prospective study of patients with PSC referred for cholangioscopy to evaluate for dominant strictures or stones, 56% of patients had stones, of which approximately one-third were missed on cholangiography.49 Interestingly, while extrahepatic bile duct stones are relatively common and intrahepatic bile duct stones very rare in the general population, in patients with PSC, both occur with relatively high frequency.50
Similar to the treatment of choledocholithiasis in the general population, choledocholithiasis in patients with PSC is often managed by ERCP with sphincterotomy, balloon sweeping of the bile ducts, and saline lavage. If a dominant stricture is present, balloon dilation and/or brushing can also be performed. Patients with cholecystitis or (resolved) choledocholithiasis may be referred for cholecystectomy, but caution should be taken as cholecystectomy in patients with PSC, particularly those with advanced liver disease, is associated with increased morbidity.51
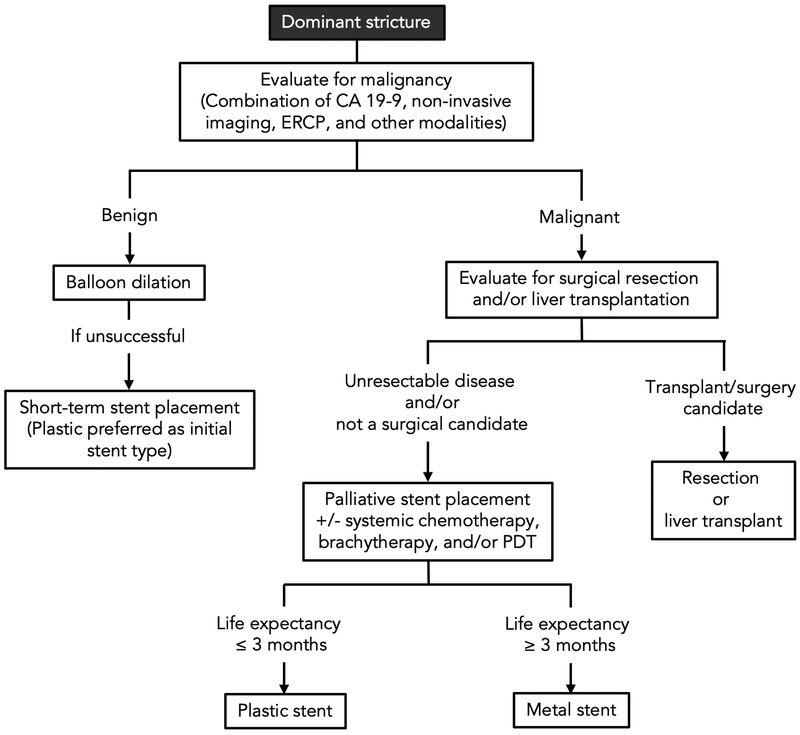
4. Initial evaluation and surveillance of dominant strictures
The finding or suspicion of a dominant stricture generally requires multi-modal testing to adequately assess for CCA, often entailing a combination of serologies, imaging, ERCP, and other endoscopic techniques (Fig. 5). Serologic testing with tumor marker carbohydrate antigen 19-9 (CA19-9) is often one of the first steps in ruling out CCA; however, the use of this biomarker is limited for several reasons, including its low sensitivity and specificity for CCA.52 Elevations in serum CA 19-9 levels can be seen not only in malignant conditions, but also in benign conditions, such as pancreatobiliary ductal obstruction or inflammation.18,23,47 Furthermore, not all individuals with malignancy synthesize CA19-9; for example, those who do not express the Lewis histo-blood group antigen (e.g. due to mutations in FUT3, the gene encoding fucosyltransferase 3 cannot effectively secrete CA 19-9, and thus will not mount elevated CA 19-9 levels even in the presence of CCA or other pancreatobiliary malignancy.53-55 Thus, the serum CA 19-9 level should be interpreted cautiously, and an undetectable level in the presence of (benign or malignant) biliary obstruction should be regarded as a possible clue to an individual patient’s inability to synthesize CA 19-9.53
Fig. 5. Evaluation of dominant strictures in patients with PSC: A multimodal undertaking.
Of note, ancillary modalities such as cholangioscopy and endoscopic ultrasound may be implemented in addition to ERCP with biliary brushings/biopsies, as discussed in the text, and serum liver tests including CA 19-9 should be monitored. Abbreviations: CA 19-9, carbohydrate antigen 19-9; CCA, cholangiocarcinoma; ERCP, endoscopic retrograde cholangiopancreatography; FISH, fluorescence in situ hybridization; MRCP, magnetic resonance cholangiopancreatography.
Imaging, including abdominal ultrasound, computed tomography, and MRCP, also play a role in the workup and/or surveillance of dominant strictures.21,22 Currently, many large-volume centers perform yearly or biennial MRI/MRCP for patients with PSC,56 which has a reported sensitivity and specificity of 89% and 75%, respectively.52 The addition of CA 19-9 levels greater than 20 U/mL to suspicious findings on MRI/MRCP increases the sensitivity of detecting CCA to near 100%; however, this is at the expensive of decreased specificity (38%).52 Transabdominal ultrasound may be considered in lieu of MRCP given its lower cost, increased availability, and greater patient acceptability (e.g. quicker and less claustrophobia-inciting), but in some studies its sensitivity is seemingly lower at 57% (though specificity is higher at 94%).52 Analogous to MRI/MRCP, the use of CA 19-9 increases the sensitivity of ultrasound to 91%, but again, at the expensive of decreasing specificity to 67%.52 Thus, while imaging constitutes a vital tool, many patients will require ERCP with tissue sampling or other endoscopic techniques for accurate diagnosis.57,58
In the following sections, we discuss the role of various endoscopic techniques used in the evaluation of dominant strictures in PSC.
4.1. Biliary brush cytology and advanced cytologic techniques
Bile duct brushings are routinely obtained for tissue sampling during ERCP. This technique has a specificity of greater than 95% for detecting malignant lesions; however, its sensitivity for malignant lesions is low, ranging from 5% to 40%, with a systematic review and meta-analysis reporting a pooled sensitivity of 43%.59-62 A weighted scoring system, termed atypical biliary brushing score (ABBS), has been created to help risk stratify individuals (with or without PSC) with atypical brush cytologies. This tool assigns point values to seven variables (1 point for age over 60, procedure indication of pancreatic mass, stricture in the distal common bile duct, CA 19-9 over 300 U/mL; 2 points for endoscopic impression of malignancy, common hepatic duct stricture, and the presence of PSC), with scores over 4 associated with higher risks of malignancy.63 However, this tool has not been validated.
Although regular cytology has low sensitivity for detecting malignant lesions, fluorescence in situ hybridization (FISH) analysis can be added to enhance the sensitivity and improve the diagnostic yield of brush cytology. This advanced cytologic technique uses fluorescent deoxyribonucleic acid (DNA) probes to evaluate for chromosomal duplications or regional structural abnormalities, findings that may suggest a malignant process through chromosomal instability.64-68 Studies report that FISH polysomy combined with cytology can improve the sensitivity for malignant lesions to 45–59%, while keeping the specificity near 100%,60 and evaluating for the deletion of the 9p21 locus (which codes for the tumor suppressor gene p16 involved in cell cycle entry) in addition to FISH can further increase the sensitivity to 76–89%.60,69 Furthermore, the detection of polysomy during subsequent ERCPs (i.e. serial polysomy) or in multiple areas of the biliary tree (i.e. multifocal polysomy) is associated with a higher risk of CCA than isolated or unifocal polysomy.70,71 Digital image analysis (DIA), a technique that quantifies abnormalities of nuclear DNA, has also been shown to have a higher sensitivity than conventional cytology, but in part due to its lower specificity, has fallen out of favor.72
4.2. EUS
EUS can be a useful technique in distinguishing between malignant and benign biliary strictures, particularly for distal (extrahepatic) strictures.61,73-75 As mentioned earlier in this review, EUS is a safer technique than ERCP given its ability to provide data without cannulating the common bile duct. On sonography, the presence of a pancreatic mass (causing a stricture from extrinsic compression) and/or an irregular bile duct wall has a reported sensitivity for malignancy of 88% and specificity of 100%, while bile duct wall thickness greater than 3 mm has a reported sensitivity for malignancy of 79% and specificity of 79%.76 In a meta-analysis of 36 studies comprising 3532 individuals, overall pooled sensitivity and specificity of EUS for diagnosing malignancy was found to be 78% and 84%, respectively.77 The addition of fine needle aspiration (FNA) provides an even higher diagnostic yield, with a separate meta-analysis of 9 studies (with 284 patients), demonstrating a sensitivity and specificity of 84% and 100%, respectively.78 However, it should be noted that FNA carries a possible risk of seeding malignant cells along the needle track (particularly along the hepatoduodenal ligament), an area that may not be resected in subsequent surgical intervention (e.g. LT).79,80 Evidence for this risk is limited, with one study of 191 patients with CCA that underwent transperitoneal FNA prior to LT to have a higher likelihood of peritoneal metastases versus patients who did not undergo FNA biopsy,79 while a subsequent retrospective study found that preoperative EUS-FNA in patients with CCA did not affect overall survival.81 Due to this uncertainty, patients are currently excluded from LT evaluation per the Mayo Clinic protocol if they have undergone EUS-FNA.82
4.3. Intraductal ultrasound (IDUS)
IDUS is a technique that provides real-time high-resolution cross-sectional characterization of biliary strictures, typically performed during ERCP.83-85 By inserting a high-frequency (12–30 MHz) ultrasound transducer over a wire into the pancreatobiliary system under fluoroscopic guidance, the endoscopist can visualize the bile duct and its surrounding tissues.86,87 Disruption of normal bile duct wall echo layers, irregular thickening or irregularity of the bile duct wall, hypoechoic sessile masses with signs of adjacent tissue or vascular invasion, and the presence of enlarged lymph nodes are findings on IDUS that have been reported to suggest malignancy.84,87-90 Although not often used in clinical practice (in part due to highly variable exposure and training in fellowship programs), published literature suggests that IDUS is a highly accurate technique in evaluating strictures, with significantly higher sensitivity, specificity, and accuracy over ERCP or EUS.84,91,92 A retrospective study of 397 patients with indeterminate biliary strictures found the use of IDUS to provide a sensitivity, specificity, and accuracy of 93%, 90%, and 91%, respectively for distinguishing between malignant and benign strictures.93 A subsequent study of 193 patients found similar results, with IDUS providing high sensitivity, specificity, and accuracy for distinguishing malignant from benign bile duct obstruction (97%, 79%, 88%, respectively).90 Given the ability to visualize various layers of the bile duct wall, IDUS has also been considered a tool for tumor staging. A cohort study with 174 patients with malignant diseases found the accuracy of identifying T1, T2, and T3 stages to be 84%, 73%, and 71%, respectively; and the accuracy rates for N0 and N1 to be 69%.93 The low accuracy with N staging is likely due to limited depth of ultrasound penetration. Despite these favorable reports, IDUS is infrequently needed and not widely utilized for the evaluation of dominant strictures.
4.4. Cholangioscopy
Cholangioscopy is a technique that uses a small-caliber flexible endoscope to directly inspect the inner part of the biliary tree. First described in 1976, the first generation of direct peroral cholangioscopy was expensive, fragile, and required two highly skilled endoscopists to perform the procedure, preventing it from gaining widespread acceptance.94,95 Subsequently, new advances in technology have allowed for crucial improvements in visualization and technical tools, leading to revived interest in the field of cholangioscopy. In 2007, the SpyGlass® system (Boston Scientific Corp., Natick, MA, USA) was introduced, allowing for a single operator to perform the procedure.96 However, optical quality was still subpar. Most recently, in 2015, high-resolution cholangioscopy (SpyGlass DS™, Boston Scientific Corp., Natick, MA, USA) was introduced, providing significantly improved high-definition imaging of the bile ducts.94 In the past, most studies on single-operator cholangioscopy (SOC) have been performed using the earlier generation of the SpyGlass system, thus little is known about the strengths and weaknesses of the new system. However, preliminary research suggests that this tool will have a positive impact on our ability to accurately diagnose biliary strictures. Of note, there are other cholangioscopy systems, but they are not commonly used, and thus will not be discussed in this review.
The SpyGlass DS™ platform consists of a reusable fiber optic probe within a disposable delivery catheter (SpyScope), which is passed through the working channel of and attached to the duodenoscope.97 By means of this catheter, tasks such as irrigation of the bile duct, small tissue biopsies, as well as therapeutic procedures, such as lithotripsy and ablation, can be performed.98 Cholangioscopy has the unique ability to examine biliary epithelial vascular patterns for abnormalities (e.g. irregularly dilated tortuous vessels, also known as “tumor vessels”), which are highly sensitive and specific for malignancy when found in conjunction with abnormal targeted biopsies.99,100 Identification and biopsy of nodules, ulcers, or papillary/villous mucosal projections, all of which suggest malignancy, is also possible with cholangioscopy.101 A recent meta-analysis of 21 studies found that the pooled sensitivity and specificity of SOC for the diagnosis of CCA in patients with PSC was 65% and 97%, respectively.102 SOC with targeted biopsies had an accuracy rate of 96%, higher than the accuracy rate of bile duct brushings (87%), FISH (69% for polysomy, 47% for trisomy), probe-based confocal laser endomicroscopy (pCLE) (75%), or the combination of SOC and any of the aforementioned other ERCP-based modalities (ranging from 73 to 90%).102 However, this technique comes with tradeoffs; there are several reports suggesting a higher post-procedural adverse event rate when cholangioscopy is used (up to 20%).103-105 Furthermore, the cost-effectiveness of this system with a single-use disposable access and delivery catheter is unclear, though there is suggestion of cost savings.106-108 Additional large comparative diagnostic studies are warranted to validate the accuracy of SOC, evaluate the true rate of adverse events, and assess for cost savings.
4.5. pCLE
pCLE is an emerging imaging modality performed during ERCP that provides real-time high-resolution in vivo microscopic imaging of the biliary epithelium. By illuminating tissue (after administration of intravenous or topical contrast, typically fluorescein) and measuring the reflected fluorescent light, cellular and subcellular features can be identified, allowing for differentiation of normal architecture from neoplastic changes.109 Through the use of pCLE, unnecessary biopsies can be avoided, and if biopsy is necessary, it can be done with higher precision and efficiency. To standardize terminology for describing pCLE findings in the pancreaticobiliary ducts, a classification system was created (i.e., Miami classification), with the following criteria suggestive for malignancy: the presence of (i) thick, dark bands (>40 μm), (ii) thick, white bands (>20 μm), (iii) dark clumps, (iv) visualized epithelium (villi, glands), and (v) fluorescein leakage.110 These criteria have been shown to have an overall sensitivity, specificity, and accuracy of 98%, 67%, and 81%, respectively, for the diagnosis of malignancy in indeterminant strictures.111 The classification was subsequently validated, with a consensus definition that the combination of two or more of the Miami criteria (except fluorescein leakage) was suggestive of malignancy, providing a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 97%, 33%, 80%, and 80%, respectively, compared with 48%, 100%, 100%, and 41% for standard tissue sampling.112 Subsequently, a new set of criteria (i.e., Paris classification) has been proposed in an effort to improve the low specificity of pCLE when using the Miami classification.113,114 These criteria build off of the Miami classification and provides additional guidance to distinguish between malignant and benign inflammatory strictures by evaluating for vascular congestion, dark granular patterns, increased interglandular space, and thickened reticular structures.113 A prospective, international, multicenter study found the Paris classification to have a sensitivity, specificity, and accuracy of 89%, 71%, and 82%, respectively, compared with 56%, 100%, and 72% with standard tissue sampling alone.115 This represents an improvement over prior classification systems, but the accuracy is still less than desired. Establishment of a reliable and accurate classification system for diagnosing bile duct lesions with pCLE remains an ongoing effort.
4.6. Optical coherence tomography (OCT) and volumetric laser endomicroscopy (VLE)
OCT is another investigative tool that can provide real-time in vivo cross-sectional imaging of the ductal wall at a microscopic level. This technique is similar to EUS in principle but uses lowintensity infrared light (at a wavelength ranging 750–1300 nm) instead of sound, thus allowing for significantly higher resolution images.116,117 With OCT, visualization of layer architecture (and structures such as blood vessels, lymphoid aggregates, crypts, submucosal glands) is possible at a level of detail approaching that of histopathology.118-120 Although large studies comparing the accuracy of OCT to other endoscopic modalities are lacking, several small studies have shown that OCT can distinguish benign from malignant lesions with superior accuracy to that of brush cytology.121,122
Recently, VLE, a second-generation technology of OCT, has been developed, improving on OCT by allowing for 360 degree rotation, a wider field of view, and faster imaging processing.123 Early reports on this technology are encouraging, but additional studies are needed to evaluate the roles of OCT and VLE in evaluating dominant strictures in or surveilling patients with PSC.
5. Therapeutic endoscopic interventions for dominant strictures
Biliary obstruction of dominant strictures is a major cause of morbidity and mortality in patients with PSC. Although the optimal frequency, type of intervention, and degree to which endoscopic intervention delays the progression of PSC has been largely unclear, ERCP remains an important tool for the management of dominant strictures, with the goal of relieving biliary obstruction and reducing serum alkaline phosphatase level to below 1.5 times the upper limit of normal (which has been shown to be associated with improved survival and a reduced risk of CCA in patients with PSC).124,125 Patients with symptoms of biliary obstruction, such as jaundice, pruritus, right upper quadrant pain, worsening biochemical profile, and cholangitis are generally thought to be appropriate candidates for endoscopic therapy, with therapeutic options including balloon dilation and stent placement, both of which can be utilized either alone or in combination. A percutaneous approach is also an alternative to endoscopic therapy for relieving biliary obstruction, but is associated with increased morbidity and mortality, and thus reserved for the patients who have failed an endoscopic approach.126 In the following sections, we discuss the roles of various endoscopic methods currently employed in relieving biliary obstruction in patients with PSC (Fig. 6).
Fig. 6. Simplified overall management algorithm of dominant strictures in patients with PSC.
The overall management of dominant strictures depends on whether malignancy is found. Balloon dilation is the preferred initial treatment modality for benign strictures, while palliative stenting is the preferred initial treatment for (unresectable) malignant strictures or for benign dominant strictures that are refractory to balloon dilation. Abbreviations: CA 19-9, carbohydrate antigen 19-9; ERCP, endoscopic retrograde cholangiopancreatography; PDT, photodynamic therapy.
5.1. Balloon dilation
The American Association for the Study of Liver Diseases (AASLD) currently recommends that endoscopic biliary stricture dilation be the initial treatment of dominant strictures.127 In balloon dilation, a balloon catheter is introduced into the stricture, inflated for 30–60 seconds until the stricture opens, and then deflated and withdrawn (Fig. 7A). Strictures are typically dilated up to the maximum diameter of the ducts, and may require several (on average 2–3 times) serial dilations 1–4 weeks apart for technical success (no narrowing or obstruction of contrast medium through the previously stenosed biliary segment on fluoroscopy).18,36,128 In a large prospective study of 500 endoscopic balloon dilations in 96 patients, symptoms of biliary obstruction as well as biochemical profile were improved with balloon dilation, and transplant-free survival rates after five and ten years were 81% and 52%, respectively.129
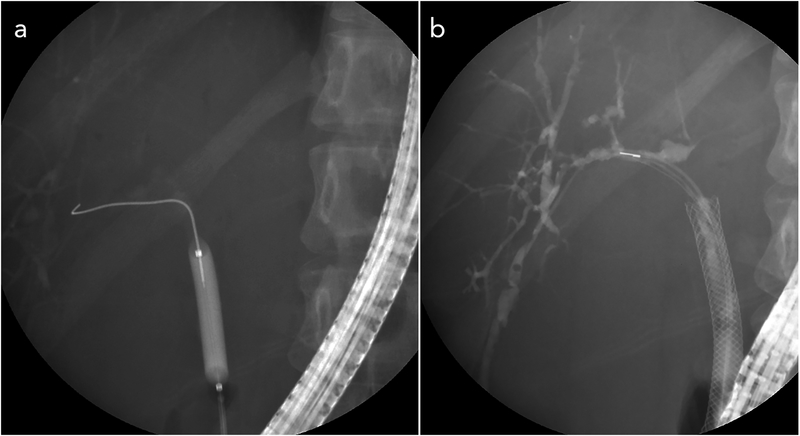
Fig. 7. Treatment of a dominant stricture in a patient with PSC.
Description: (A) Endoscopic balloon dilation of a dominant stricture. (B) Placement of a self-expandable metallic stent (SEMS) in a patient who experienced rapid stricture recurrence following balloon dilation alone and also following balloon dilation with plastic stent placement. Abbreviation: PSC, primary sclerosing cholangitis.
5.2. Stenting
Stenting is another option to open up a dominant stricture, though often reserved for cases wherein dilation alone appears to be inadequate or unable to provide durable benefit (Fig. 7B).18 In most large studies of endoscopic treatment using stent, plastic stents measuring 7 to 10 Fr in diameter have been used.18 A shorter stent duration (approximately 1 –2 weeks) is generally preferred over a longer stent duration (8–12 weeks) due to the increased risk of premature stent occlusion over time (which would require “early repeat” therapeutic ERCP) in patients with PSC; furthermore, similar efficacy has been shown regardless of the duration of stenting.130-132 In a retrospective study of 32 patients with PSC that underwent short term stenting (mean duration of 11 days), symptoms of pruritus, fatigue, and right upper quadrant abdominal pain improved in 83% of patients after 2 months. Furthermore, at 1 and 3 years, 80% and 60% of patients, respectively, did not require further interventions.132 Despite the potential ability to maintain dilation and prevent rapid re-occlusion of a stenosed bile duct, stenting carries multiple disadvantages, including: (i) the need for repeating ERCP for stent removal, (ii) the risk of worsening cholestasis and the development of cholangitis if re-occlusions are to occur, and (iii) an increased risk for bacterial translocation and colonization of the biliary tree.133 Microbiological studies have found rates of bacterobilia as high as 98% in patients with biliary stent placement compared with 55% in patients without a stent.134 Furthermore, patients with stents are more likely to have polymicrobial cultures with high-grade pathogens.134 This is especially troublesome as patients with PSC appear to have difficulty clearing biliary infections even after courses of antibiotics.135
Historically, the use of stenting has been based on endoscopist’s preference and expertise. In fact, the European Association for the Study of the Liver (EASL) and European Society for Gastrointestinal Endoscopy (ESGE) guidelines also suggest that the choice between stenting and balloon dilation should be left to the endoscopist’s discretion.18 However, only balloon dilation has been reported to show significant improvement in LT-free survival when compared to predicted revised Mayo Risk Score.36,48,136,137 Furthermore, a recently published multicenter randomized trial (DILSTENT2) of 65 patients with PSC and a dominant stricture found that short-term stents were not superior to balloon dilation and were associated with a significantly higher occurrence of adverse effects.138 Thus, we currently recommend balloon dilation as the first line therapy in the majority of cases.
5.3. Topical mitomycin C
Mitomycin C is an aziridine-containing chemotherapeutic agent that is currently used intravenously and topically for its antitumor activity.139 It has also been reported to have a role in preventing scar formation following various surgical procedures by slowing down fibroblast cell division and proliferation.140,141 Thus, it is hypothesized that mitomycin may be able to slow down the progression of biliary strictures in patients with PSC.142 A phase 2 study is currently underway to evaluate the efficacy of intrabiliary installation of mitomycin C during ERCP (ClinicalTrials.gov identifier: ).
6. Palliative endoscopic interventions for PSC-associated CCA
Surgical resection offers the only potentially curative therapy for malignant biliary obstruction. However, a majority of patients present with unresectable lesions, prompting the role of palliative endoscopic intervention.143 From a palliative perspective, endoscopic biliary drainage with biliary stent placement can relieve acute biliary obstruction and its associated symptoms (pruritus, jaundice, malaise, fat malabsorption), prevent liver failure due to progressive biliary obstruction, improve overall quality of life, and reduce the number of repeated hospitalizations and health care costs due to the aforementioned sequelae of untreated obstructive cholestasis.144 While percutaneous biliary drainage with or without percutaneous stenting is another option in relieving biliary obstruction, the endoscopic route is typically preferred, as it is less invasive, more comfortable for patients (eliminating the need for an external bag), and avoids the risk of tube-related complications, such as hemorrhage, infection, bile leakage, and pleural complications.143 Both plastic stents and self-expandable metallic stents (SEMSs) can be used for the palliation of distal bile duct obstruction, with various benefits and limitations. Plastic stents are less expensive compared with SEMSs (in patients surviving 3–6 months or less) and can be more easily replaced if they become occluded,145 but they have limited patency due to their narrow lumen limited up to 12 Fr, compared to SEMSs with diameters as large as 30 Fr or 10 mm. Comparison studies between plastic and SEMSs have shown that the patency rates of SEMSs were superior to those of plastic stents for distal biliary obstruction (10–12 months vs. 3–4 months, respectively).146-149 Thus, despite its high costs, the insertion of SEMSs is generally recommended in patients with a life expectancy greater than 3–6 months, as the higher costs are offset by lower incidences of re-intervention and hospitalizations for complications.150,151
In addition to stenting, there are other modalities of palliation which show promise. These include endoscopic photodynamic therapy, radiofrequency ablation, and high-intensity ultrasound therapy.152-156 It remains to be determined whether these modalities will improve survival in patients with advanced unresectable CCA.
7. Adverse events post-ERCP in PSC
Although commonly performed in clinical practice, ERCP is an invasive procedure that has significant risks. In patients without PSC, ERCP-specific adverse events are estimated to occur at a rate of 3–11%, with a systematic review of 21 surveys involving 16,855 patients revealing a complication rate of 6.85%.157,158 In patients with PSC, the reported rate of overall post-ERCP adverse events is thought to be higher (in most studies), with an estimated complication rate ranging from 7% to 18%, predominantly consisting of complications from post-ERCP pancreatitis (PEP) and post-ERCP cholangitis (PEC).48,159-163 The risks of perforation and bleeding, however, does not appear to be increased compared to patients without PSC.159 Despite these statistics, the overall risk of ERCP in PSC is thought to be acceptable when the procedure is performed by experienced pancreaticobiliary endoscopists.18,161,164,165
7.1. PEP
PEP is the most common and feared complication associated with ERCP, with published literature suggesting an occurrence rate of 5–10% in patients with PSC undergoing ERCP.159,163,166 PSC has also been shown to be an independent risk factor for PEP, with a two-fold increase in risk over patients without PSC.163 While the mechanism for this higher complication rate is unclear, it is hypothesized that the increased complexity of ERCP (and thus increased procedure times) in patients with PSC, the presence of distal biliary strictures leading to greater papillary manipulation, and the performance of therapeutic procedures (e.g. biliary brush cytology, sphincterotomy, stenting and dilation) lead to the increased risk of PEP in patients with PSC.18 Of note, biliary sphincterotomy performed during ERCP may increase the risk of PEP (as well as the risk of bleeding and perforation) immediately after the procedure, but is protective against PEP during subsequent ERCPs (which are commonly needed in patients with PSC) by facilitating easier biliary cannulation in future procedures.163
Several peri-procedural strategies have been shown to reduce the incidence and severity of PEP, including aggressive fluid hydration (particularly with lactated Ringer’s solution),167-169 routine use of rectal non-steroidal anti-inflammatory drugs (NSAIDs) (relative risk (RR) 0.36, number needed to treat (NNT) 15),170,171 and pancreatic duct stenting (in patients at high risk of PEP, absolute risk reduction of 12%).172-174 Both the administration of NSAIDs (particularly diclofenac or indomethacin administered rectally) immediately before or after ERCP (in patients without contraindications to NSAIDs) and the use of pancreatic duct stenting in high risk patients are strongly recommended by the ESGE and have been found to be cost-effective.175-177
7.2. PEC
The risk of PEC in patients with PSC has been difficult to assess as bacterial cholangitis and bacteriobilia are not infrequent findings among patients with PSC (regardless of whether ERCP has been recently performed).44 Furthermore, ERCP is often performed for patients with abdominal pain and abnormal serum liver tests, which may be the initial presentation of subclinical acute bacterial cholangitis; thus, it is often unclear whether bacterial cholangitis was already present prior to endoscopic intervention or is a complication of ERCP. Regardless, PEC has been reported to occur in 0.6–8% of patients, and proper preventative measures should be taken.48,136,159-163,165 Patients with PSC undergoing ERCP should receive prophylactic peri-procedural antibiotics as well as a short course of oral antibiotics that cover biliary flora (e.g. enteric gramnegative organisms and enterococci) for 3–7 days after the procedure to decrease the risk of PEC.18,21,178 ln a Cochrane meta-analysis of 9 randomized controlled trials (RCTs) including 1573 patients, the prophylactic use of antibiotics reduced the risk of cholangitis, sepsis, bacteremia, and pancreatitis (RR 0.54, RR 0.35, RR 0.50, RR 0.54, respectively).179 Other measures to reduce the risk of PEC include minimizing contrast injection during ERCP, aspirating bile immediately after biliary cannulation, and aspirating the injected contrast from the biliary tree at the end of the procedure to removal viscous fluid from the biliary tree, facilitating drainage of bile and reducing the risk of PEC.180
8. Cholangiographic classification of PSC and prognostic significance
PSC is a heterogenous disease, with a wide range of disease phenotypes. To better describe cholangiographic findings, Chen and Goldberg181 published the first ERCP criteria for ductal changes in 1984. Subsequently, the classification has been modified by Majoie et al.182 and Ponsioen et al.,6 with the classification of Ponsioen et al. now validated and shown to correlate with patient prognosis.183 The classification characterizes the radiographic appearances of the biliary tree based on severity (e.g. degree of narrowing/irregularity of the biliary ducts, visualization of dilated regions, degree of obliteration of the ducts) and then provides a resultant score based on the degree of intrahepatic and extrahepatic involvement.183 There are also other classifications that have recently been proposed. Robles-Medranda et al.184 proposed a novel classification system using a new set of definitions, which in a single-centered non-randomized study, appeared to improve sensitivity and specificity to 96% and 92%, respectively, with a PPV and NPV of 93% and 96%, respectively. Another group has proposed the Edmonton classification which attempts to stratify patients with PSC and extrahepatic dominant strictures based on differences in phenotypic expression seen on cholangioscopy.185 The clinical usefulness of these classification systems is yet to be determined.
9. Conclusions
PSC is a rare, premalignant cholangiopathy characterized by fibroinflammatory obliteration of the intrahepatic and/or extrahepatic biliary tree. Due to the nature of its many manifestations and complications, as well as the lack of effective pharmacotherapies, biliary endoscopy plays a significant role in the care of patients with PSC. Although ERCP has largely been replaced by MRI/MRCP and serum biomarkers for initial diagnosis, ERCP continues to be the primary modality for advanced diagnostics and treatment of PSC-related complications. Advanced endoscopic tools such as ERCP with FISH, EUS, IDUS, and cholangioscopy appear to be effective tools that have increased the diagnostic accuracy of evaluating dominant strictures for malignancy over the past several decades. In addition, the treatments of dominant strictures through the use of balloon dilation and stenting have significantly improved patient morbidity and quality of life. While endoscopic intervention is an invasive procedure that carries associated risks, we believe the results are generally favorable, especially when appropriate prophylactic strategies are taken and the patient is in experienced hands.
Acknowledgements
This work was completed in part through T32 fellowship funding support for Dr. J. H. Tabibian from the United States National Institutes of Health (grant DK007198).
Footnotes
Edited by Yuxia Jiang, Peiling Zhu and Genshu Wang.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med. 2016;375:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabibian JH, Lindor KD. Primary sclerosing cholangitis: a review and update on therapeutic developments. Expert Rev Gastroenterol Hepatol. 2013;7: 103–114. [DOI] [PubMed] [Google Scholar]
- 3.O’Hara SP, Tabibian JH, Splinter PL, LaRusso NF. The dynamic biliary epithelia: molecules, pathways, and disease. J Hepatol. 2013;58:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200–206. [PubMed] [Google Scholar]
- 5.Ali AH, Tabibian JH, Lindor KD. Update on pharmacotherapies for cholestatic liver disease. Hepatol Commun. 2016;1:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, et al. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51:562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hep-atology. 2013;58:2045–2055. [DOI] [PubMed] [Google Scholar]
- 8.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523–526. [DOI] [PubMed] [Google Scholar]
- 9.Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50: 158–164. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Baluyut A, Ismail A, et al. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7–11. [DOI] [PubMed] [Google Scholar]
- 11.United Network for Organ Sharing. Data Report Request. Liver Transplants Performed for Recipients Age 18þ at Time of Transplant in the US from January 1, 2005 to October 31,2014 by Primary Diagnosis at Time of Transplant and Year of Transplant. 2015.
- 12.Bjøro K, Brandsaeter B, Foss A, Schrumpf E. Liver transplantation in primary sclerosing cholangitis. Semin Liver Dis. 2006;26:69–79. [DOI] [PubMed] [Google Scholar]
- 13.Karlsen TH, Schrumpf E, Boberg KM. Update on primary sclerosing cholangitis. Dig Liver Dis. 2010;42:390–400. [DOI] [PubMed] [Google Scholar]
- 14.Alabraba E, Nightingale P, Gunson B, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330–340. [DOI] [PubMed] [Google Scholar]
- 15.Landaverde C, Ng V, Sato A, Tabibian J, Durazo F, Busuttil R. De-novo cholangiocarcinoma in native common bile duct remnant following OLT for primary sclerosing cholangitis. Ann Hepatol. 2009;8:379–383. [PubMed] [Google Scholar]
- 16.Hildebrand T, Pannicke N, Dechene A, et al. Biliary strictures and recurrence after liver transplantation for primary sclerosing cholangitis: a retrospective multicenter analysis. Liver Transpl. 2016;22:42–52. [DOI] [PubMed] [Google Scholar]
- 17.Graziadei IW. Live donor liver transplantation for primary sclerosing cholangitis: is disease recurrence increased? Curr Opin Gastroenterol. 2011;27: 301–305. [DOI] [PubMed] [Google Scholar]
- 18.Aabakken L, Karlsen TH, Albert J, et al. Role of endoscopy in primary sclerosing cholangitis: European society of gastrointestinal endoscopy (ESGE) and European association for the study of the liver (EASL) clinical guideline. Endoscopy. 2017;49:588–608. [DOI] [PubMed] [Google Scholar]
- 19.Tabibian JH, Baron TH. Endoscopic management of primary sclerosing cholangitis. Expert Rev Gastroenterol Hepatol. 2018;12:693–703. [DOI] [PubMed] [Google Scholar]
- 20.Tabibian JH, Visrodia KH, Levy MJ, Gostout CJ. Advanced endoscopic imaging of indeterminate biliary strictures. World J Gastrointest Endosc. 2015;7: 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindor KD, Kowdley KV, Harrison ME. ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol. 2015;110:646–659. [DOI] [PubMed] [Google Scholar]
- 22.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. [DOI] [PubMed] [Google Scholar]
- 23.Tabibian JH, Bowlus CL. Primary sclerosing cholangitis: a review and update. Liver Res. 2017;1:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angulo P, Maor-Kendler Y, Lindor KD. Small-duct primary sclerosing cholangitis: a long-term follow-up study. Hepatology. 2002;35:1494–1500. [DOI] [PubMed] [Google Scholar]
- 25.MacCarty RL, LaRusso NF, Wiesner RH, Ludwig J. Primary sclerosing cholangitis: findings on cholangiography and pancreatography. Radiology. 1983;149:39–44. [DOI] [PubMed] [Google Scholar]
- 26.Dave M, Elmunzer BJ, Dwamena BA, Higgins PD. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology. 2010;256:387–396. [DOI] [PubMed] [Google Scholar]
- 27.Talwalkar JA, Angulo P, Johnson CD, Petersen BT, Lindor KD. Cost-minimization analysis of MRC versus ERCP for the diagnosis of primary sclerosing cholangitis. Hepatology. 2004;40:39–45. [DOI] [PubMed] [Google Scholar]
- 28.Meagher S, Yusoff I, Kennedy W, Martel M, Adam V, Barkun A. The roles of magnetic resonance and endoscopic retrograde cholangiopancreatography (MRCP and ERCP) in the diagnosis of patients with suspected sclerosing cholangitis: a cost-effectiveness analysis. Endoscopy. 2007;39:222–228. [DOI] [PubMed] [Google Scholar]
- 29.Berstad AE, Aabakken L, Smith HJ, Aasen S, Boberg KM, Schrumpf E. Diagnostic accuracy of magnetic resonance and endoscopic retrograde cholangiography in primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2006;4:514–520. [DOI] [PubMed] [Google Scholar]
- 30.Moff SL, Kamel IR, Eustace J, et al. Diagnosis of primary sclerosing cholangitis: a blinded comparative study using magnetic resonance cholangiography and endoscopic retrograde cholangiography. Gastrointest Endosc. 2006;64: 219–223. [DOI] [PubMed] [Google Scholar]
- 31.Weber C, Kuhlencordt R, Grotelueschen R, et al. Magnetic resonance cholangiopancreatography in the diagnosis of primary sclerosing cholangitis. Endoscopy. 2008;40:739–745. [DOI] [PubMed] [Google Scholar]
- 32.Rustemovic N, Cukovic-Cavka S, Opacic M, et al. Endoscopic ultrasound elastography as a method for screening the patients with suspected primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2010;22:748–753. [DOI] [PubMed] [Google Scholar]
- 33.Mesenas S, Vu C, Doig L, Meenan J. Duodenal EUS to identify thickening of the extrahepatic biliary tree wall in primary sclerosing cholangitis. Gastrointest Endosc. 2006;63:403–408. [DOI] [PubMed] [Google Scholar]
- 34.Lutz HH, Wasmuth HE, Streetz K, et al. Endoscopic ultrasound as an early diagnostic tool for primary sclerosing cholangitis: a prospective pilot study. Endoscopy. 2012;44:934–939. [DOI] [PubMed] [Google Scholar]
- 35.European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51: 237–267. [DOI] [PubMed] [Google Scholar]
- 36.Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151–156. [DOI] [PubMed] [Google Scholar]
- 37.Hilscher MB, Tabibian JH, Carey EJ, Gostout CJ, Lindor KD. Dominant strictures in primary sclerosing cholangitis: a multicenter survey of clinical definitions and practices. Hepatol Commun. 2018;2:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz A, Lemoinne S, Carrat F, Corpechot C, Chazouillères O, Arrivé L. Radiologic course of primary sclerosing cholangitis: assessment by three-dimensional magnetic resonance cholangiography and predictive features of progression. Hepatology. 2014;59:242–250. [DOI] [PubMed] [Google Scholar]
- 39.Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102:107–114. [DOI] [PubMed] [Google Scholar]
- 40.Björnsson E, Lindqvist-Ottosson J, Asztely M, Olsson R. Dominant strictures in patients with primary sclerosing holangitis. Am J Gastroenterol. 2004;99: 502–508. [DOI] [PubMed] [Google Scholar]
- 41.Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis; a 25 year single centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergquist A, Glaumann H, Persson B, Broomé U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998;27:311–316. [DOI] [PubMed] [Google Scholar]
- 44.Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67:1298–1323. [DOI] [PubMed] [Google Scholar]
- 45.Takakura WR, Tabibian JH, Bowlus CL. The evolution of natural history of primary sclerosing cholangitis. Curr Opin Gastroenterol. 2017;33:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fevery J, Verslype C, Lai G, Aerts R, Van Steenbergen W. Incidence, diagnosis, and therapy of cholangiocarcinoma in patients with primary sclerosing cholangitis. Dig Dis Sci. 2007;52:3123–3135. [DOI] [PubMed] [Google Scholar]
- 47.Tabibian J, Lazaridis K, LaRusso N. Primary sclerosing cholangitis In: Jarnagin, ed. Blumgart’s surgery of the liver, biliary tract and pancreas. 6th ed. Philadelphia, PA: Elsevier; 2017:663–674. [Google Scholar]
- 48.Gluck M, Cantone NR, Brandabur JJ, Patterson DJ, Bredfeldt JE, Kozarek RA. A twenty-year experience with endoscopic therapy for symptomatic primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42:1032–1039. [DOI] [PubMed] [Google Scholar]
- 49.Awadallah NS, Chen YK, Piraka C, Antillon MR, Shah RJ. Is there a role for cholangioscopy in patients with primary sclerosing cholangitis? Am J Gastroenterol. 2006;101:284–291. [DOI] [PubMed] [Google Scholar]
- 50.Dodd GD 3rd, Niedzwiecki GA, Campbell WL, Baron RL. Bile duct calculi in patients with primary sclerosing cholangitis. Radiology. 1997;203:443–447. [DOI] [PubMed] [Google Scholar]
- 51.Eaton JE, Thackeray EW, Lindor KD. Likelihood of malignancy in gallbladder polyps and outcomes following holecystectomy in primary sclerosing cholangitis. Am J Gastroenterol. 2012;107:431–439. [DOI] [PubMed] [Google Scholar]
- 52.Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. [DOI] [PubMed] [Google Scholar]
- 53.Parra-Robert M, Santos VM, Canis SM, Pla XF, Fradera JMA, Porto RM. Relationship between CA 19.9 and the lewis phenotype: options to improve diagnostic efficiency. Anticancer Res. 2018;38:5883–5888. [DOI] [PubMed] [Google Scholar]
- 54.Luo G, Guo M, Jin K, et al. Optimize CA19-9 in detecting pancreatic cancer by Lewis and Secretor genotyping. Pancreatology. 2016;16:1057–1062. [DOI] [PubMed] [Google Scholar]
- 55.Vestergaard EM, Hein HO, Meyer H, et al. Reference values and biological variation for tumor marker CA 19-9 in serum for different Lewis and secretor genotypes and evaluation of secretor and Lewis genotyping in a Caucasian population. Clin Chem. 1999;45:54–61. [PubMed] [Google Scholar]
- 56.Schramm C, Eaton J, Ringe KI, Venkatesh S, Yamamura J. Recommendations on the use of magnetic resonance imaging in PSC-A position statement from the International PSC Study Group. Hepatology. 2017;66:1675–1688. [DOI] [PubMed] [Google Scholar]
- 57.Tabibian JH, Lindor KD. Challenges of cholangiocarcinoma detection in patients with primary sclerosing cholangitis. J Anal Oncol. 2012;1:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ali AH, Tabibian JH, Nasser-Ghodsi N, et al. Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis. Hepatology. 2018;67: 2338–2351. [DOI] [PubMed] [Google Scholar]
- 59.Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79: 783–789. [DOI] [PubMed] [Google Scholar]
- 60.Gonda TA, Glick MP, Sethi A, et al. Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointest Endosc. 2012;75:74–79. [DOI] [PubMed] [Google Scholar]
- 61.Rösch T, Hofrichter K, Frimberger E, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390–396. [DOI] [PubMed] [Google Scholar]
- 62.Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Witt BL, Hilden RN, Scaife C, et al. Identification of factors predictive of malignancy in patients with atypical biliary brushing results obtained via ERCP. Diagn Cytopathol. 2013;41:682–688. [DOI] [PubMed] [Google Scholar]
- 64.Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675–1681. [DOI] [PubMed] [Google Scholar]
- 65.Levy MJ, Baron TH, Clayton AC, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol. 2008;103:1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bangarulingam SY, Bjornsson E, Enders F, et al. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology. 2010;51:174–180. [DOI] [PubMed] [Google Scholar]
- 67.Fang X, Zhang P. Aneuploidy and tumorigenesis. Semin Cell Dev Biol. 2011;22: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang M, Cheng L, Jia Y, et al. Aneuploid embryonic stem cells exhibit impaired differentiation and increased neoplastic potential. EMBO J. 2016;35: 2285–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boldorini R, Paganotti A, Andorno S, et al. A multistep cytological approach for patients with jaundice and biliary strictures of indeterminate origin. J Clin Pathol. 2015;68:283–287. [DOI] [PubMed] [Google Scholar]
- 70.Barr Fritcher EG, Kipp BR, Voss JS, et al. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. Am J Gastroenterol. 2011;106: 2023–2028. [DOI] [PubMed] [Google Scholar]
- 71.Eaton JE, Barr Fritcher EG, Gores GJ, et al. Biliary multifocal chromosomal polysomy and cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2015;110:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baron TH, Harewood GC, Rumalla A, et al. A prospective comparison of digital image analysis and routine cytology for the identification of malignancy in biliary tract strictures. Clin Gastroenterol Hepatol. 2004;2:214–219. [DOI] [PubMed] [Google Scholar]
- 73.Mohamadnejad M, DeWitt JM, Sherman S, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71–78. [DOI] [PubMed] [Google Scholar]
- 74.Khashab MA, Fockens P, Al-Haddad MA. Utility of EUS in patients with indeterminate biliary strictures and suspected extrahepatic cholangiocarcinoma (with videos). Gastrointest Endosc. 2012;76:1024–1033. [DOI] [PubMed] [Google Scholar]
- 75.Eloubeidi MA, Chen VK, Jhala NC, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209–213. [DOI] [PubMed] [Google Scholar]
- 76.Lee JH, Salem R, Aslanian H, Chacho M, Topazian M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069–1073. [DOI] [PubMed] [Google Scholar]
- 77.Garrow D, Miller S, Sinha D, et al. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5:616–623 (e1). [DOI] [PubMed] [Google Scholar]
- 78.Wu LM, Jiang XX, Gu HY, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy in the evaluation of bile duct strictures and gallbladder masses: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:113–120. [DOI] [PubMed] [Google Scholar]
- 79.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011;13:356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Razumilava N, Gleeson FC, Gores GJ. Awareness of tract seeding with endoscopic ultrasound tissue acquisition in perihilar cholangiocarcinoma. Am J Gastroenterol. 2015;110:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chafic AH, Dewitt J, LeBlanc JK, et al. Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy. 2013;45:883–889. [DOI] [PubMed] [Google Scholar]
- 82.Navaneethan U, Njei B, Venkatesh PG, Lourdusamy V, Sanaka MR. Endoscopic ultrasound in the diagnosis of cholangiocarcinoma as the etiology of biliary strictures: a systematic review and meta-analysis. Gastroenterol Rep (Oxf). 2015;3:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stavropoulos S, Larghi A, Verna E, Battezzati P, Stevens P. Intraductal ultrasound for the evaluation of patients with biliary Strictures and no bdominal mass on computed tomography. Endoscopy. 2005;37:715–721. [DOI] [PubMed] [Google Scholar]
- 84.Sun B, Hu B. The role of intraductal ultrasonography in pancreatobiliary diseases. Endosc Ultrasound. 2016;5:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vazquez-Sequeiros E, Baron TH, Clain JE, et al. Evaluation of indeterminate bile duct strictures by intraductal US. Gastrointest Endosc. 2002;56:372–379. [PubMed] [Google Scholar]
- 86.Tharian B, George NE, Tham TC. What is the current role of endoscopy in primary sclerosing cholangitis? World J Gastrointest Endosc. 2015;7:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujita N, Noda Y, Kobayashi G, et al. Intraductal ultrasonography (IDUS) for the diagnosis of biliopancreatic diseases. Best Pract Res Clin Gastroenterol. 2009;23:729–742. [DOI] [PubMed] [Google Scholar]
- 88.Tamada K, Tomiyama T, Wada S, et al. Endoscopic transpapillary bile duct biopsy with the combination of intraductal ultrasonography in the diagnosis of biliary strictures. Gut. 2002;50:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krishna NB, Saripalli S, Safdar R, Agarwal B. Intraductal US in evaluation of biliary strictures without a mass lesion on CT scan or magnetic resonance imaging: significance of focal wall thickening and extrinsic compression at the stricture site. Gastrointest Endosc. 2007;66:90–96. [DOI] [PubMed] [Google Scholar]
- 90.Chen L, Lu Y, Wu J, Bie L, Xia L, Gong B. Diagnostic utility of endoscopic retrograde cholangiography/intraductal ultrasound (ERC/IDUS) in distinguishing malignant from benign bile duct Obstruction. Dig Dis Sci. 2016;61: 610–617. [DOI] [PubMed] [Google Scholar]
- 91.Menzel J, Poremba C, Dietl KH, Domschke W. Preoperative diagnosis of bile duct strictures-comparison of intraductal ultrasonography with conventional endosonography. Scand J Gastroenterol. 2000;35:77–82. [DOI] [PubMed] [Google Scholar]
- 92.Tischendorf JJ, Meier PN, Schneider A, Manns MP, Krüger M. Transpapillary intraductal ultrasound in the evaluation of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Scand J Gastroenterol. 2007;42: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 93.Meister T, Heinzow HS, Woestmeyer C, et al. Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology. World J Gastroenterol. 2013;19:874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hüsing-Kabar A, Heinzow HS, Schmidt HH-J, et al. Single-operator cholangioscopy for biliary complications in liver transplant recipients. World J Gastroenterol. 2017;23:4064–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen NQ, Binmoeller KF, Shah JN. Cholangioscopy and pancreatoscopy (with videos). Gastrointest Endosc. 2009;70:1200–1210. [DOI] [PubMed] [Google Scholar]
- 96.Ghersi S, Fuccio L, Bassi M, Fabbri C, Cennamo V. Current status of peroral cholangioscopy in biliary tract diseases. World J Gastrointest Endosc. 2015;7: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mellemgaard F, Strandby RB, Blockmann J, Kofoed SC, Svendsen LB, Achiam MP. Single-operator cholangioscopy is useful for visual assessment of bile duct pathology. Dan Med J. 2018;65 pii: A5496. [PubMed] [Google Scholar]
- 98.Franzini TA, Moura RN, de Moura EGH. Advances in therapeutic cholangioscopy. Gastroenterol Res Pract. 2016;2016:5249152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Itoi T, Osanai M, Igarashi Y, et al. Diagnostic peroral video cholangioscopy is an accurate diagnostic tool for patients with bile duct lesions. Clin Gastroenterol Hepatol. 2010;8:934–938. [DOI] [PubMed] [Google Scholar]
- 100.Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000;52:635–638. [DOI] [PubMed] [Google Scholar]
- 101.Seo DW, Lee SK, Yoo KS, et al. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc. 2000;52:630–634. [DOI] [PubMed] [Google Scholar]
- 102.Njei B, McCarty TR, Varadarajulu S, Navaneethan U. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139–1151. [DOI] [PubMed] [Google Scholar]
- 103.Laleman W, Verraes K, Van Steenbergen W, et al. Usefulness of the single-operator cholangioscopy system SpyGlass in biliary disease: a single-center prospective cohort study and aggregated review. Surg Endosc. 2017;31: 2223–2232. [DOI] [PubMed] [Google Scholar]
- 104.Lübbe J, Arnelo U, Lundell L, et al. ERCP-guided cholangioscopy using a single-use system: nationwide register-based study of its use in clinical practice. Endoscopy. 2015;47:802–807. [DOI] [PubMed] [Google Scholar]
- 105.Hammerle CW, Haider S, Chung M, et al. Endoscopic retrograde cholangiopancreatography complications in the era of cholangioscopy: is there an increased risk? Dig Liver Dis. 2012;44:754–758. [DOI] [PubMed] [Google Scholar]
- 106.Hajj El II, Shah RJ. Digital single-operator cholangioscopy: fully disposable yet valuable. Gastrointest Endosc. 2016;84:656–658. [DOI] [PubMed] [Google Scholar]
- 107.Deprez PH, Duran R, Moreels T, et al. The economic impact of using single-operator cholangioscopy for the treatment of difficult bile duct stones and diagnosis of indeterminate bile duct strictures. Endoscopy. 2018;50:109–118. [DOI] [PubMed] [Google Scholar]
- 108.Derdeyn J, Laleman W. Current role of endoscopic cholangioscopy. Curr Opin Gastroenterol. 2018;34:301–308. [DOI] [PubMed] [Google Scholar]
- 109.Rizvi S, Eaton J, Yang JD, Chandrasekhara V, Gores GJ. Emerging technologies for the diagnosis of perihilar cholangiocarcinoma. Semin Liver Dis. 2018;38: 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen YK, Shah RJ, Pleskow DK, et al. 788c: Miami classification (MC) of probe-based confocal laser endomicroscopy (pCLE) findings in the pancreaticobiliary (PB) system for evaluation of indeterminate strictures: interim results from an international multicenter registry. Gastrointest Endosc. 2010;71:AB134. [Google Scholar]
- 111.Meining A, Chen YK, Pleskow D, et al. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest Endosc. 2011;74:961–968. [DOI] [PubMed] [Google Scholar]
- 112.Meining A, Shah RJ, Slivka A, et al. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy. 2012;44: 251–257. [DOI] [PubMed] [Google Scholar]
- 113.Caillol F, Filoche B, Gaidhane M, Kahaleh M. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the paris classification. Dig Dis Sci. 2013;58:1784–1789. [DOI] [PubMed] [Google Scholar]
- 114.Taunk P, Singh S, Lichtenstein D, Joshi V, Gold J, Sharma A. Improved classification of indeterminate biliary strictures by probe-based confocal laser endomicroscopy using the Paris Criteria following biliary stenting. J Gastroenterol Hepatol. 2017;32:1778–1783. [DOI] [PubMed] [Google Scholar]
- 115.Slivka A, Gan I, Jamidar P, et al. Validation of the diagnostic accuracy of probe-based confocal laser endomicroscopy for the characterization of indeterminate biliary strictures: results of a prospective multicenter international study. Gastrointest Endosc. 2015;81:282–290. [DOI] [PubMed] [Google Scholar]
- 116.Tyberg A, Xu MM, Gaidhane M, Kahaleh M. Second generation optical coherence tomography: preliminary experience in pancreatic and biliary strictures. Dig Liver Dis. 2018;50:1214–1217. [DOI] [PubMed] [Google Scholar]
- 117.Mosko JD, Pleskow D. Evaluation of NinePoint Medical’s Nvision VLE device for gastrointestinal applications. Expert Rev Med Devices. 2017;14:495–503. [DOI] [PubMed] [Google Scholar]
- 118.Testoni PA, Mangiavillano B. Optical coherence tomography in detection of dysplasia and cancer of the gastrointestinal tract and bilio-pancreatic ductal system. World J Gastroenterol. 2008;14:6444–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mahmud MS, May GR, Kamal MM, et al. Imaging pancreatobiliary ductal system with optical coherence tomography: a review. World J Gastrointest Endosc. 2013;5:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003;21:1361–1367. [DOI] [PubMed] [Google Scholar]
- 121.Testoni PA, Mariani A, Mangiavillano B, Arcidiacono PG, Di Pietro S, Masci E. Intraductal optical coherence tomography for investigating main pancreatic duct strictures. Am J Gastroenterol. 2007;102:269–274. [DOI] [PubMed] [Google Scholar]
- 122.Arvanitakis M, Hookey L, Tessier G, et al. Intraductal optical coherence tomography during endoscopic retrograde cholangiopancreatography for investigation of biliary strictures. Endoscopy. 2009;41:696–701. [DOI] [PubMed] [Google Scholar]
- 123.Corral JE, Mousa OY, Krishna M, et al. Volumetric laser endomicroscopy in the biliary and pancreatic ducts: a feasibility study with histological correlation. Endoscopy. 2018;50:1089–1094. [DOI] [PubMed] [Google Scholar]
- 124.Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329–334. [DOI] [PubMed] [Google Scholar]
- 125.Lindström L, Hultcrantz R, Boberg KM, Friis-Liby I, Bergquist A. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;11: 841–846. [DOI] [PubMed] [Google Scholar]
- 126.Coelen RJS, Roos E, Wiggers JK, et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: a multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3: 681–690. [DOI] [PubMed] [Google Scholar]
- 127.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. [DOI] [PubMed] [Google Scholar]
- 128.Wagner S, Gebel M, Meier P, et al. Endoscopic management of biliary tract strictures in primary sclerosing cholangitis. Endoscopy. 1996;28:546–551. [DOI] [PubMed] [Google Scholar]
- 129.Gotthardt DN, Rudolph G, Klöters-Plachky P, Kulaksiz H, Stiehl A. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527–534. [DOI] [PubMed] [Google Scholar]
- 130.van Milligen de Wit AW, Rauws EA, van Bracht J, et al. Lack of complications following short-term stent therapy for extrahepatic bile duct strictures in primary sclerosing cholangitis. Gastrointest Endosc. 1997;46:344–347. [DOI] [PubMed] [Google Scholar]
- 131.Kaya M, Petersen BT, Angulo P, et al. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1059–1066. [DOI] [PubMed] [Google Scholar]
- 132.Ponsioen CY, Lam K, van Milligen de Wit AW, Huibregtse K, Tytgat GN. Four years experience with short term stenting in primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:2403–2407. [DOI] [PubMed] [Google Scholar]
- 133.Thosani N, Banerjee S. Endoscopic retrograde cholangiopancreatography for primary sclerosing cholangitis. Clin Liver Dis. 2014;18:899–911. [DOI] [PubMed] [Google Scholar]
- 134.Rerknimitr R, Fogel EL, Kalayci C, Esber E, Lehman GA, Sherman S. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointest Endosc. 2002;56:885–889. [DOI] [PubMed] [Google Scholar]
- 135.Pohl J, Ring A, Stremmel W, Stiehl A. The role of dominant stenoses in bacterial infections of bile ducts in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2006;18:69–74. [DOI] [PubMed] [Google Scholar]
- 136.Baluyut AR, Sherman S, Lehman GA, Hoen H, Chalasani N. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308–312. [DOI] [PubMed] [Google Scholar]
- 137.van Milligen de Wit AW, van Bracht J, Rauws EAJ, Jones EA, Tytgat GN, Huibregtse K. Endoscopic stent therapy for dominant extrahepatic bile duct strictures in primary sclerosing cholangitis. Gastrointest Endosc. 1996;44: 293–299. [DOI] [PubMed] [Google Scholar]
- 138.Ponsioen CY, Arnelo U, Bergquist A, et al. No Superiority of stents vs balloon dilatation for dominant strictures in patients with primary sclerosing cholangitis. Gastroenterology. 2018;155:752–759 (e5). [DOI] [PubMed] [Google Scholar]
- 139.Williamson KD, Chapman RW. New therapeutic strategies for primary sclerosing cholangitis. Semin Liver Dis. 2016;36:5–14. [DOI] [PubMed] [Google Scholar]
- 140.Chung JH, Cosenza MJ, Rahbar R, Metson RB. Mitomycin C for the prevention of adhesion formation after endoscopic sinus surgery: a randomized, controlled study. Otolaryngol Head Neck Surg. 2002;126:468–474. [DOI] [PubMed] [Google Scholar]
- 141.Rahbar R, Jones DT, Nuss RC, et al. The Role of mitomycin in the prevention and treatment of scar formation in the pediatric aerodigestive tract: friend or foe? Arch Otolaryngol Head Neck Surg. 2002;128:401–406. [DOI] [PubMed] [Google Scholar]
- 142.Rodriguez EA, Carey EJ, Lindor KD. Emerging treatments for primary sclerosing cholangitis. Expert Rev Gastroenterol Hepatol. 2017;11:451–459. [DOI] [PubMed] [Google Scholar]
- 143.Kim JH. Endoscopic stent placement in the palliation of malignant biliary obstruction. Clin Endosc. 2011;44:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chahal P, Baron TH. Endoscopic palliation of cholangiocarcinoma. Curr Opin Gastroenterol. 2006;22:551–560. [DOI] [PubMed] [Google Scholar]
- 145.Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1–7. [DOI] [PubMed] [Google Scholar]
- 146.Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488–1492. [DOI] [PubMed] [Google Scholar]
- 147.Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178–182. [DOI] [PubMed] [Google Scholar]
- 148.Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, ontrolled Trtial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25:207–212. [DOI] [PubMed] [Google Scholar]
- 149.Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986–995. [DOI] [PubMed] [Google Scholar]
- 150.Irisawa A, Katanuma A, Itoi T. Otaru consensus on biliary stenting for unresectable distal malignant biliary obstruction. Dig Endosc. 2013;25:52–57. [DOI] [PubMed] [Google Scholar]
- 151.Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993;25:213–218. [DOI] [PubMed] [Google Scholar]
- 152.Laquière A, Boustiere C, Leblanc S, Penaranda G, Désilets E, Prat F. Safety and feasibility of endoscopic biliary radiofrequency ablation treatment of extrahepatic cholangiocarcinoma. Surg Endosc. 2016;30:1242–1248. [DOI] [PubMed] [Google Scholar]
- 153.Strand DS, Cosgrove ND, Patrie JT, et al. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014;80:794–804. [DOI] [PubMed] [Google Scholar]
- 154.Roque J, Ho SH, Reddy N, Goh KL. Endoscopic ablation therapy for biliopancreatic malignancies. Clin Endosc. 2015;48:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weiss MJ, Cosgrove D, Herman JM, Rastegar N, Kamel I, Pawlik TM. Multi-modal treatment strategies for advanced hilar cholangiocarcinoma. Langen-beck’s Arch Surg. 2014;399:679–692. [DOI] [PubMed] [Google Scholar]
- 156.Cosgrove ND, Al-Osaimi AM, Sanoff HK, et al. Photodynamic therapy provides local control of cholangiocarcinoma in patients awaiting liver transplantation. Am J Transplant. 2014;14:466–471. [DOI] [PubMed] [Google Scholar]
- 157.Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP omplications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781–1788. [DOI] [PubMed] [Google Scholar]
- 158.Vandervoort J, Soetikno RM, Tham TC, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652–656. [DOI] [PubMed] [Google Scholar]
- 159.Bangarulingam SY, Gossard AA, Petersen BT, Ott BJ, Lindor KD. Complications of endoscopic retrograde cholangiopancreatography in primary sclerosing cholangitis. Am J Gastroenterol. 2009;104:855–860. [DOI] [PubMed] [Google Scholar]
- 160.Enns R, Eloubeidi MA, Mergener K, Jowell PS, Branch MS, Baillie J. Predictors of successful clinical and laboratory outcomes in patients with primary sclerosing cholangitis undergoing endoscopic retrograde cholangiopancreatography. Can J Gastroenterol. 2003;17:243–248. [DOI] [PubMed] [Google Scholar]
- 161.Etzel JP, Eng SC, Ko CW, et al. Complications after ERCP in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2008;67:643–648. [DOI] [PubMed] [Google Scholar]
- 162.Van den Hazel SJ1, Wolfhagen EH, van Buuren HR, van de Meeberg PC, Van Leeuwen DJ. Prospective risk assessment of endoscopic retrograde cholangiography in patients with primary sclerosing cholangitis. Endoscopy. 2000;32: 779–782. [DOI] [PubMed] [Google Scholar]
- 163.Von Seth E, Arnelo U, Enochsson L, Bergquist A. Primary sclerosing cholangitis increases the risk for pancreatitis after endoscopic retrograde cholangiopancreatography. Liver Int. 2015;35:254–262. [DOI] [PubMed] [Google Scholar]
- 164.Ismail S, Kylänpää L, Mustonen H, et al. Risk factors for complications of ERCP in primary sclerosing cholangitis. Endoscopy. 2012;44:1133–1138. [DOI] [PubMed] [Google Scholar]
- 165.Navaneethan U, Jegadeesan R, Nayak S, et al. ERCP-related adverse events in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2015;81: 410–419. [DOI] [PubMed] [Google Scholar]
- 166.Barkin JA, Levy C, Souto EO. Endoscopic management of primary sclerosing cholangitis. Ann Hepatol. 2017;16:842–850. [DOI] [PubMed] [Google Scholar]
- 167.Buxbaum J, Yan A, Yeh K, Lane C, Nguyen N, Laine L. Aggressive hydration with lactated ringer’s solution reduces pancreatitis following endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol. 2014;12: 303–307 (e1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.DiMagno MJ, Wamsteker EJ, Rai J, et al. Do larger periprocedural fluid volumes reduce the severity of post-ERCP pancreatitis? Pancreas. 2014;43:642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sagi SV, Schmidt S, Fogel E, et al. Association of greater intravenous volume infusion with shorter hospitalization for patients with post-ERCP pancreatitis. J Gastroenterol Hepatol. 2014;29:1316–1320. [DOI] [PubMed] [Google Scholar]
- 170.Andrade-Dávila VF, Chávez-Tostado M, Dávalos-Cobián C, et al. Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC Gastroenterol. 2015;15:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shi N, Deng L, Altaf K, Huang W, Xue P, Xia Q. Rectal indomethacin for the prevention of post-ERCP pancreatitis: a meta-analysis of randomized controlled trials. Turk J Gastroenterol. 2015;26:236–240. [DOI] [PubMed] [Google Scholar]
- 172.Andriulli A, Forlano R, Napolitano G, et al. Pancreatic duct stents in the prophylaxis of pancreatic damage after endoscopic retrograde cholangiopancreatography: a systematic analysis of benefits and associated risks. Digestion. 2007;75:156–163. [DOI] [PubMed] [Google Scholar]
- 173.Singh P, Das A, Isenberg G, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544–550. [DOI] [PubMed] [Google Scholar]
- 174.Sofuni A, Maguchi H, Itoi T, et al. Prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis by an endoscopic pancreatic spontaneous dislodgement stent. Clin Gastroenterol Hepatol. 2007;5:1339–1346. [DOI] [PubMed] [Google Scholar]
- 175.Dumonceau JM, Andriulli A, Elmunzer B, et al. Prophylaxis of post-ERCP pancreatitis: European society of gastrointestinal endoscopy (ESGE) guideline-updated June 2014. Endoscopy. 2014;46:799–815. [DOI] [PubMed] [Google Scholar]
- 176.Nicolás-Pérez D, Castilla-Rodríguez I, Gimeno-García AZ, Romero-García R, Núiñez-Díaz V, Quintero E. Prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a cost-effectiveness analysis. Pancreas. 2015;44:204–210. [DOI] [PubMed] [Google Scholar]
- 177.Das A, Singh P, Sivak MV Jr, Chak A. Pancreatic-stent placement for prevention of post-ERCP pancreatitis: a cost-effectiveness analysis. Gastrointest Endosc. 2007;65:960–968. [DOI] [PubMed] [Google Scholar]
- 178.Anderson MA, Fisher L, Jain R, et al. Complications of ERCP. Gastrointest Endosc. 2012;75:467–473. [DOI] [PubMed] [Google Scholar]
- 179.Brand M, Bizos D, O’Farrell P Jr. Antibiotic prophylaxis for patients undergoing elective endoscopic retrograde cholangiopancreatography. Cochrane Database Syst Rev. 2010:CD007345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Navaneethan U, Lourdusamy D, Gutierrez NG, Zhu X, Vargo JJ, Parsi MA. New approach to decrease post-ERCP adverse events in patients with primary sclerosing cholangitis. Endosc Int Open. 2017;05:E710–E717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen LY, Goldberg HI. Sclerosing cholangitis: broad spectrum of radiographic features. Gastrointest Radiol. 1984;9:39–47. [DOI] [PubMed] [Google Scholar]
- 182.Majoie CB, Reeders JW, Sanders JB, Huibregtse K,Jansen PL. Primary sclerosing cholangitis: a modified classification of cholangiographic findings. AJR Am J Roentgenol. 1991;157:495–497. [DOI] [PubMed] [Google Scholar]
- 183.Ponsioen CY, Reitsma JB, Boberg KM, Aabakken L, Rauws EA, Schrumpf E. Validation of a cholangiographic prognostic model in primary sclerosing cholangitis. Endoscopy. 2010;42:742–747. [DOI] [PubMed] [Google Scholar]
- 184.Robles-Medranda C, Valero M, Soria-Alcivar M, et al. Reliability and accuracy of a novel classification system using peroral cholangioscopy for the diagnosis of bile duct lesions. Endoscopy. 2018;50:1059–1070. [DOI] [PubMed] [Google Scholar]
- 185.Sandha G, D’Souza P, Halloran B, Montano-Loza AJ. A cholangioscopy-based novel classification system for the phenotypic stratification of dominant bile duct strictures in primary sclerosing cholangitis-the edmonton classification. J Can Assoc Gastroenterol. 2018;1:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]