Abstract
Purpose
To determine the influence of compression factor upon changes in axial length and choroidal thickness during and following orthokeratology treatment.
Methods
Orthokeratology lenses of different compression factors (one eye with 0.75 D and the fellow eye with 1.75 D) were randomly assigned to 28 subjects (median [range] age: 9.3 [7.8–11.0] years). Ocular biometrics were measured weekly for 1 month of lens wear and after lens cessation until the refraction stabilized (mean duration: 2.8 ± 0.4 weeks). Changes between eyes, and the associations between axial shortening and choroidal thickening with other ocular biometrics were analyzed.
Results
There were no significant between-eye differences in the changes of ocular biometrics (all P > 0.05). After adjusting for paired-eye data, axial length initially decreased by 26 ± 41 μm (P = 0.03) at week 1, then gradually returned to its original length. An approximate antiphase relationship of choroidal thickness (mean change: 9 ± 12 μm, P < 0.001) with axial length was observed. A significant rebound in axial length, but not choroidal thickness, occurred during the cessation period. Central corneal thinning and choroidal thickening accounted for 70% of initial axial shortening.
Conclusions
Increasing the compression factor by 1.00 D did not affect changes in ocular biometrics in short-term orthokeratology. Significant axial shortening and choroidal thickening were observed during early treatment period. Axial shortening could not be entirely explained by central corneal thinning and choroidal thickening, which warrants further investigation.
Translational Relevance
Initial axial shortening in orthokeratology is transient and therefore axial length remains useful for long-term monitoring of axial elongation in children.
Keywords: orthokeratology, compression factor, axial length, choroidal thickness, myopia control
Introduction
Myopia is a major cause of vision impairment and its prevalence is estimated to double to almost 5 billion people by 2050.1 Its progression is associated with axial elongation, which is characterized by stretching of the sclera.2 Higher risks of cataract, glaucoma, macular, and chorio-retinal complications, particularly in high myopia (>6 D), have also been reported.3 Both the direct and indirect costs associated with the diagnosis, treatment, and management of myopia will substantially increase the economic burden for health care systems.4 Therefore, significant research has been undertaken examining different pharmacological and optical interventions5 to slow myopia progression and to address the associated ocular complications.
Orthokeratology, one of the most effective and popular treatments for myopia control,6–8 utilizes overnight reverse geometry rigid gas permeable contact lenses that flatten the central cornea and steepen the mid-peripheral cornea.9 When fitting an orthokeratology lens, a compression factor (also known as the Jessen factor, usually of 0.75 D) is incorporated to counteract the daytime regression (steepening) of the central corneal shape and hence refractive correction.10 However, a retrospective study11 indicated that the conventional compression factor (CCF; 0.75 D) might not be sufficient to account for typical daytime regression and suggested the use of an increased compression factor (ICF; 1.75 D). Increasing the compression factor would lead to relatively more myopic correction, but could also induce more corneal and ocular higher-order aberrations. This could also lead to different outcomes in the myopia control effect of orthokeratology in young myopic children.12
Equation 1. Determination of back optic zone radius of the lens:
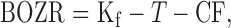 |
where BOZR is the back optic zone radius of the lens, Kf is the flattest keratometry reading, T is the target myopia reduction, and CF is the compression factor.
Axial shortening has been observed in some subjects undergoing orthokeratology treatment. Cho and Cheung6 found that 14% of older subjects (9–12 years) displayed an axial shortening even after 2 years of lens wear, and a similar phenomenon was also reported in adolescents wearing orthokeratology lenses for 6 months.13 This axial shortening was thought to be attributable mainly to central corneal thinning and choroidal thickening.6,13 Central corneal thinning in orthokeratology-treated subjects is a well-recognized phenomenon, which occurs as a result of corneal epithelial redistribution14 and usually stabilizes when the optimal correction is achieved.15 In contrast, changes in choroidal thickness after orthokeratology treatment remain equivocal, with various studies reporting either no significant changes during the first 9 months of lens wear,16 or an increase of approximately 20 μm in subjects wearing orthokeratology lenses for 3 weeks,17 6 months, and 1 year.18 However, some of these studies did not control for the influence of diurnal variation and performed measurements after cycloplegia, which is known to affect choroidal thickness and its response to defocus.19–21 To date, only two studies have analyzed the correlation between changes in axial length and choroidal thickness in subjects treated with orthokeratology. Chen et al.17 showed that the association between choroidal thickening and axial shortening was weak (correlation coefficient of −0.35 over a 3-week treatment period), while Li et al.22 found stronger correlations (correlation coefficients of −0.64 and −0.67 at 1- and 6-month visits, respectively). However, neither study accounted for the changes in central corneal thickness or controlled for possible confounding factors (e.g., baseline age, refractive error) in their analysis and could not provide a thorough explanation or identify other possible predictors for this apparent axial shortening.
Hence, this study aimed to examine the effect of different orthokeratology compression factors on weekly changes in axial length, choroidal thickness, and other ocular biometrics in young myopic children for 1 month of treatment and for 2 to 3 weeks after the cessation of lens wear. The association between the changes in axial length and other ocular parameters, including but not limited to central corneal thickness and choroidal thickness, were also investigated to examine possible contributors to the axial length changes in early orthokeratology treatment.
Methods
Study Design
This was a randomized, double-masked, contralateral clinical trial investigating the changes in ocular biometrics in children treated with orthokeratology lenses of different compression factors (CCF: 0.75 D; ICF: 1.75 D) in the two eyes for 1 month followed by lens cessation until corneal topography and noncycloplegic subjective refractive error were stabilized. This study followed the tenets of the Declaration of Helsinki and was approved by the ethics committee (Departmental Research Committee) of the School of Optometry of The Hong Kong Polytechnic University. Written informed consent, after explaining the nature and possible consequences of the study, was obtained from the parents before the commencement of the study. The study was registered at ClinicalTrials.gov (NCT02643875).
Subjects
Subject recruitment was conducted from February to June 2016. Telephone interviews and examinations were arranged to screen for children aged between 6 and 10 years (inclusive), with myopia between 0.50 to 4.00 D (inclusive), astigmatism of less than or equal to 1.25 D, anisometropia of less than or equal to 1.00 D, and corneal toricity of less than 2.00 D. Those with prior myopia control treatment (atropine, orthokeratology, bifocal contact lenses, or spectacle lenses), ocular diseases (e.g., amblyopia, strabismus, ocular inflammation, trauma, or surgery), ocular or systemic conditions affecting refractive development or lens wear, or poor compliance to lens wear, examination, or follow-up, were excluded. Both the children and their parents were taught lens handling and disinfection procedures. Randomization and lens ordering were performed for those who had passed the lens handling training. All subjects were required to wear their lenses on a daily basis during overnight sleep and to use the Menicon solutions (daily cleaner: Menicon Spray and Clean; disinfecting solution: MeniCare Plus; weekly protein removal: Menicon Progent A & B; NKL Contactlenzen, Emmen, The Netherlands) to clean their lenses. Saline (Ophtecs cleadew; Ophtecs Co., Tokyo, Japan) and artificial tears (Precilens Aquadrop+; Precilens, Creteil, France) were also provided to improve compliance with lens wear. Subjects were excluded in cases of unacceptable lens fitting, or adverse ocular surface changes after lens wear. Subjects exited the trial upon re-stabilization of corneal shape and refractive errors after lens cessation following completion of the 1-month lens wear period.
Lens Parameters
Subjects were fitted either with spherical or toric 4-zone lenses (Menicon Z Night or Menicon Z Night Toric lenses; NKL Contactlenzen B.V.) of hyperoxygen permeability material (Menicon Z; Dk 163 [ISO]). Lens fitting parameters were determined using the manufacturer's software (Easyfit, version 2013; NKL Contactlenzen B.V.), based on the ocular refraction, corneal topography, and horizontal visible iris diameter of each eye to minimize possible subjective bias in lens selection. The laterality of the compression factors for each subject was randomized. Full correction was ordered for the CCF eyes (with 0.75 D as the compression factor), while an extra 1.00 D was added to the targeted myopic correction for the ICF eyes (compression factor of 1.75 D). Since the eyes of our subjects were highly symmetrical, the same lens design (either spherical or toric) was suggested by the software and used in both eyes of each subject.
Data Collection Visits
The baseline visit was conducted at the time of delivery of orthokeratology lenses before commencing lens wear. All subjects were required to attend an orthokeratology aftercare after the first overnight lens wear and then weekly data collection visits over 4 weeks at the Optometry Clinic of The Hong Kong Polytechnic University. At the completion of the 1-month treatment period, the subjects ceased lens wear and were examined on a weekly basis until both subjective refractive error and apical corneal power (measured using a corneal topographer) regressed and stabilized (difference between consecutive visits ≤ 0.25 D). All regular follow-ups, except for the first overnight aftercare visit, which was scheduled in the early morning (2 hours after waking), were scheduled at a similar time to the baseline visit (± 2 hours) to minimize the influence of diurnal variation on ocular biometrics.19 Additional unscheduled visits were arranged when necessary to ensure good ocular health and vision throughout the study.
Examination
At each data collection visit, unaided and best-corrected logMAR visual acuities (Early Treatment Diabetic Retinopathy Study charts, 90% contrast; Precision Vision, Woodstock, IL), noncycloplegic subjective refraction, and ocular health were assessed by an unmasked examiner. Maximum plus for maximum visual acuity was used as the criterion for subjective refraction assessment, and ocular health was evaluated using the Efron grading system.23
The corneal topography measurement was performed by a masked examiner. The mean apical corneal power was obtained from the first four good images of corneal topography (E300 videokeratoscope; Medmont Pty. Ltd., Vermont, Victoria, Australia; score ≥ 98).
The primary outcomes of this study, which included the ocular biometrics (central corneal thickness, anterior chamber depth, crystalline lens thickness, axial length, and choroidal thickness), were also obtained by a masked examiner. An optical coherence interferometry device (Lenstar LS 900; Haag-Streit, Bern, Switzerland) with good reproducibility24 was used to measure central corneal thickness, anterior chamber depth, crystalline lens thickness, and axial length. The average of the first five measurements, with a maximum axial length difference of 0.02 mm between any of the readings, was used for analysis.
The Spectralis SD-OCT (Heidelberg Engineering, Inc., Heidelberg, Germany) was used to determine the subfoveal choroidal thickness. It provides cross-sectional images with axial resolution of 3.9 μm for chorio-retinal images. At the baseline visit, six foveal centered 30° long radial line scans (each consisting of 30 frames and separated by 30°) were acquired with the enhanced depth imaging mode and a high-speed scanning protocol, with automatic real-time tracking. These baseline scans served as the reference images for all follow-up scans at future visits to ensure the same retinal location was evaluated. The images were then exported for semiautomated segmentation using customized software.25 Only the horizontal scans were used for analysis. The base of the foveal pit was marked and manual correction of segmentation was performed when necessary. The subfoveal choroidal thickness was determined as the thickness between the outer retinal pigment epithelium/Bruch's membrane complex and the inner chorioscleral interface.
Sample Size Calculation
The sample size calculation was based on the apical corneal power as different compression factors (0.75 and 1.75 D) were estimated to induce at least a 0.50-D difference between eyes. Based on a previous study, the within-group SD of apical corneal power was approximately 0.70 D.18 To achieve a power of 80% with a significance level of 0.05 and allowing for a 20% dropout rate, including missing follow-ups, poor lens fitting, and broken lens issues, a sample size of at least 23 subjects was required to complete the study.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 23; IBM Corp., Armonk, NY). The normality of the data was assessed using Shapiro-Wilk tests. Paired t-tests or Wilcoxon tests, when appropriate, were used to compare the baseline characteristics, unaided visual acuities, and spherical equivalent refractions at the 1-month visit between eyes. Linear mixed model approaches were applied to examine the influence of different compression factors on ocular biometric changes (central corneal thickness, anterior chamber depth, crystalline lens thickness, axial length, choroidal thickness, and the overall eye length [sum of axial length and choroidal thickness]) over time (visit), with restricted maximum likelihood estimation assuming a first-order autoregressive covariance model. The compression factor was added as a within-subject correction to avoid inflation of the degrees of freedom and reduction in P values when using two eyes.26 Pairwise comparisons of estimated marginal means of each eye with Bonferroni corrections were used to compare the effects of different compression factors on ocular biometric changes between visits. If a significant difference was observed for a biometric outcome measure between eyes, the estimated marginal mean changes for each eye are presented. If no effect of compression factor was observed, the results are presented using paired-eye data with an appropriate adjustment.
Since axial shortening was observed during early orthokeratology treatment, a linear mixed model was also used to examine the association between ocular demographics (baseline age, sex, and different compression factors), refractive correction (in terms of apical power changes), changes in various ocular biometrics (central corneal thickness, anterior chamber depth, crystalline lens thickness, and choroidal thickness), and axial length changes to identify significant contributors to axial shortening during the early stage of orthokeratology treatment. Variables were gradually added to the model in a forward stepwise approach with the smallest Akaike information criterion as the quality metric for model fitting. As choroidal thickening was also observed during early lens wear, a similar approach was used to examine the association between ocular demographics (baseline age, sex, and different compression factors), refractive correction (in terms of apical power changes), and its relationship with axial length. In both analyses of predictors for axial shortening and choroidal thickening after 1 week of lens wear, Pearson or Spearman correlations, when appropriate, were used to check for multicollinearity. Beta coefficients (β) were reported for significant predictors found in the models for axial shortening and choroidal thickening. For all analyses, a P value of less than 0.05 was considered significant. Data are presented as the mean and SD.
Results
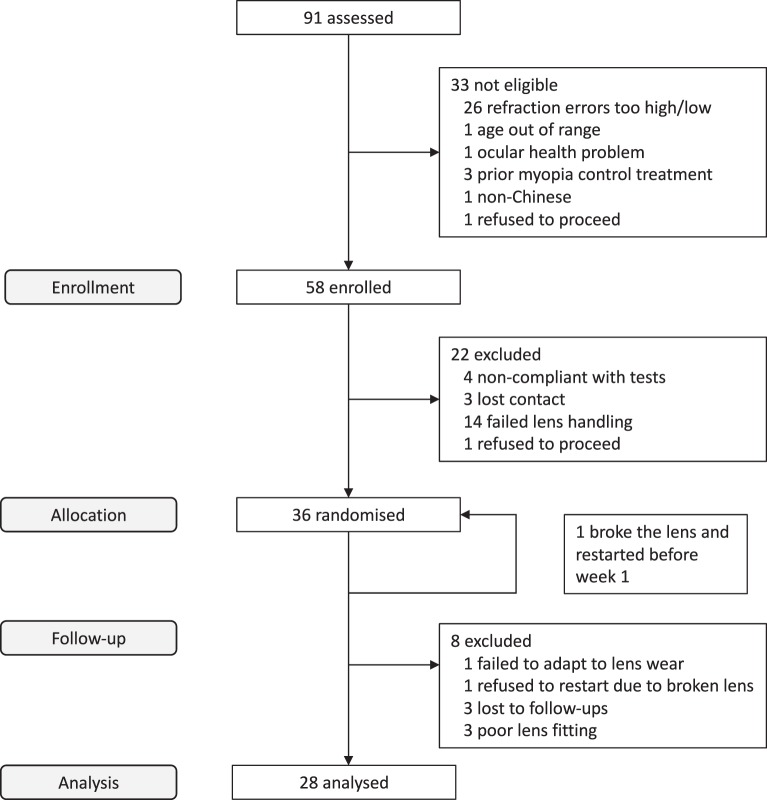
Of 91 subjects screened, 58 subjects deemed eligible were enrolled, of whom 36 were randomized and completed the baseline data collection (Fig. 1). Eight subjects were excluded at different stages of the study: one subject could not adapt to lens wear (before lens delivery), one subject refused to restart the study because of breaking a lens during cleaning procedures (before week 2), one subject discontinued due to poor vision resulting from significant undercorrection (before week 4), three subjects showed poor lens fittings (significant decentration from the corneal apex with induced astigmatism), and two subjects were lost to follow-up (one before week 4 and one before the cessation period). Therefore, the data from a total of 28 subjects (16 females, 12 males) were analyzed. These subjects completed 1 month of treatment followed by a lens cessation period of 2.8 ± 0.4 weeks (2 weeks: six subjects; 3 weeks: 22 subjects). Eighteen subjects wore spherical lenses and 10 wore toric lenses.
Figure 1.
Flowchart of the study.
Table 1 shows the baseline demographics and ocular biometrics of the analyzed subjects. The mean ± SD age of the subjects was 9.5 ± 1.0 years. There were no significant differences in the baseline demographics and ocular biometrics between eyes randomly assigned to ICF and CCF lenses (all P > 0.05).
Table 1.
Baseline Demographics and Ocular Biometrics (Mean ± SD or Median [Minimum, Maximum]) of the Eyes Fitted With Orthokeratology Lenses of ICF and CCF
| ICF (1.75 D) |
CCF (0.75 D) |
P Value |
|
| Unaided visual acuity, logMAR | 0.63 ± 0.29 | 0.65 ± 0.31 | 0.39a |
| Best-corrected visual acuity, logMAR | 0.00 ± 0.04 | 0.00 ± 0.05 | 0.68a |
| Myopia, D | −2.09 ± 0.97 | −2.12 ± 0.94 | 0.71a |
| Astigmatism, D | −0.50 (−1.25 to 0.00) | 0.00 (−1.25 to 0.00) | 0.08b |
| Spherical equivalent refraction, D | −2.30 ± 1.03 | −2.27 ± 0.99 | 0.65a |
| Apical corneal power, D | 43.83 ± 1.21 | 43.81 ± 1.16 | 0.80a |
| Central corneal thickness, μm | 553 ± 36 | 551 ± 35 | 0.12a |
| Anterior chamber depth, mm | 3.15 ± 0.17 | 3.15 ± 0.17 | 0.92a |
| Lens thickness, mm | 3.35 ± 0.12 | 3.36 ± 0.14 | 0.51a |
| Axial length, mm | 24.26 ± 0.61 | 24.29 ± 0.61 | 0.58a |
| Choroidal thickness, μm | 240 (153 to 551) | 250 (193 to 504) | 0.34b |
| Overall eye length, mm | 24.53 ± 0.60 | 24.56 ± 0.60 | 0.47a |
Overall eye length: sum of axial length and choroidal thickness.
Paired t-test.
Wilcoxon signed test.
Effect of Different Compression Factors on Ocular Biometrics
No significant differences were observed with respect to compression factor for the changes in apical corneal power, ocular biometrics (central corneal thickness, anterior chamber depth, crystalline lens thickness, axial length, and choroidal thickness), and the overall eye length (all P > 0.05; Table 2). Therefore, the changes in ocular biometrics are presented following adjustment for using paired-eye data. Table 3 shows the changes in apical corneal power, and other ocular biometrics compared with baseline during the treatment and cessation periods.
Table 2.
Changes (Mean ± SD) in the Ocular Parameters of Eyes Fitted With Lenses of ICF (1.75 D) and CCF (0.75 D) Compared With the Baseline
| Changes in Ocular Parameters |
Treatment Period |
|||
| Week 1 |
Week 2 |
|||
| ICF |
CCF |
ICF |
CCF |
|
| Apical corneal power, D | −2.10 ± 0.62 | −1.80 ± 0.62 | −2.27 ± 0.71 | −1.91 ± 0.71 |
| Central corneal thickness, μm | −9 ± 6 | −10 ± 6 | −10 ± 7 | −8 ± 7 |
| Anterior chamber depth, μm | −41 ± 72 | −41 ± 72 | −48 ± 73 | −45 ± 73 |
| Lens thickness, μm | 32 ± 85 | 31 ± 87 | 20 ± 82 | 26 ± 84 |
| Axial length, μm | −29 ± 59 | −22 ± 59 | −31 ± 83 | −17 ± 83 |
| Choroidal thickness, μm | 7 ± 17 | 11 ± 17 | 4 ± 23 | 5 ± 23 |
| Overall eye length, μm | −24 ± 59 | −12 ± 59 | −26 ± 82 | −13 ± 81 |
Overall eye length: sum of axial length and choroidal thickness.
Table 3.
Changes (Mean ± SD) in the Ocular Parameters, Adjusted for Using Data From Both Eyes, Compared With the Baseline
| Changes in Ocular Parameters |
Treatment Period |
|||
| Week 1 |
Week 2 |
Week 3 |
Week 4 |
|
| Apical corneal power, D | −1.95 ± 0.44*** | −2.09 ± 0.51*** | −1.84 ± 0.51*** | −2.02 ± 0.51*** |
| Central corneal thickness, μm | −9 ± 4*** | −9 ± 5*** | −9 ± 5*** | −9 ± 5*** |
| Anterior chamber depth, μm | −41 ± 51*** | −47 ± 51*** | −35 ± 51** | −54 ± 50*** |
| Lens thickness, μm | 32 ± 61 | 23 ± 59 | 22 ± 59 | 42 ± 58** |
| Axial length, μm | −26 ± 41* | −24 ± 58 | −11 ± 69 | −5 ± 79 |
| Choroidal thickness, μm | 9 ± 12*** | 4 ± 16 | 3 ± 18 | −3 ± 20 |
| Overall eye length, μm | −18 ± 41 | −20 ± 57 | −8 ± 67 | −7 ± 75 |
Overall eye length: sum of axial length and choroidal thickness. Bold formatted numbers represent significant changes compared with the baseline (*P < 0.05, **P < 0.01, ***P < 0.001).
Table 2.
Extended
| Changes in Ocular Parameters |
Treatment Period |
|||
| Week 3 |
Week 4 |
|||
| ICF |
CCF |
ICF |
CCF |
|
| Apical corneal power, D | −1.95 ± 0.73 | −1.73 ± 0.73 | −2.12 ± 0.73 | −1.93 ± 0.73 |
| Central corneal thickness, μm | −9 ± 7 | −9 ± 7 | −10 ± 7 | −9 ± 7 |
| Anterior chamber depth, μm | −36 ± 72 | −35 ± 72 | −44 ± 71 | −64 ± 71 |
| Lens thickness, μm | 17 ± 82 | 27 ± 85 | 33 ± 82 | 51 ± 83 |
| Axial length, μm | −17 ± 100 | −4 ± 100 | −11 ± 115 | 2 ± 115 |
| Choroidal thickness, μm | 4 ± 27 | 3 ± 27 | −6 ± 30 | −1 ± 30 |
| Overall eye length, μm | −15 ± 97 | −2 ± 97 | −17 ± 111 | 2 ± 110 |
Table 2.
Extended
| Changes in Ocular Parameters |
Cessation Period |
|||||
| Week 5 |
Week 6 |
Week 7 |
||||
| ICF |
CCF |
ICF |
CCF |
ICF |
CCF |
|
| Apical corneal power, D | −0.48 ± 0.73 | −0.4 ± 0.73 | −0.34 ± 0.73 | −0.28 ± 0.73 | −0.14 ± 0.79 | −0.18 ± 0.80 |
| Central corneal thickness, μm | −2 ± 7 | −2 ± 7 | −1 ± 8 | 0 ± 8 | −1 ± 8 | 1 ± 8 |
| Anterior chamber depth, μm | 2 ± 73 | −9 ± 72 | 1 ± 73 | −1 ± 72 | −7 ± 77 | 0 ± 77 |
| Lens thickness, μm | −14 ± 83 | −7 ± 83 | −7 ± 83 | −3 ± 84 | 12 ± 88 | 8 ± 89 |
| Axial length, μm | 9 ± 127 | 19 ± 128 | 34 ± 139 | 39 ± 139 | 48 ± 150 | 54 ± 153 |
| Choroidal thickness, μm | −7 ± 32 | −3 ± 32 | −13 ± 34 | −12 ± 34 | −15 ± 36 | −13 ± 37 |
| Overall eye length, μm | 2 ± 121 | 16 ± 121 | 22 ± 131 | 27 ± 132 | 32 ± 141 | 41 ± 144 |
Table 3.
Extended
| Changes in Ocular Parameters |
Cessation Period |
||
| Week 5 |
Week 6 |
Week 7 |
|
| Apical corneal power, D | −0.44 ± 0.51 | −0.31 ± 0.51 | −0.16 ± 0.53 |
| Central corneal thickness, μm | −2 ± 5 | −1 ± 5 | 0 ± 5 |
| Anterior chamber depth, μm | −3 ± 51 | 0 ± 51 | −4 ± 55 |
| Lens thickness, μm | −11 ± 59 | −5 ± 59 | 10 ± 64 |
| Axial length, μm | 14 ± 88 | 36 ± 95 | 51 ± 103 |
| Choroidal thickness, μm | −5 ± 21 | −12 ± 22 | −14 ± 22* |
| Overall eye length, μm | 9 ± 81 | 25 ± 87 | 36 ± 93 |
At the 1-month visit, the median (range) unaided visual acuities of eyes fitted with ICF and CCF were 0.02 (−0.08 to 0.34) logMAR and −0.01 (−0.10 to 0.32) logMAR, respectively (P = 0.04). The median (range) manifest spherical equivalent refractions were +0.44 (−1.13 to +1.50) D and 0.00 (−1.00 to +1.00) D, respectively (P = 0.11).
Changes in Apical Corneal Power
The apical power was significantly reduced during the orthokeratology treatment (Table 3). After the first week of treatment, the power reduced by 1.95 ± 0.44 D (P < 0.001) and was stable throughout the remaining treatment period in both eyes (all P > 0.05). After ceasing treatment for 1 week, almost 80% of the refractive correction in terms of apical power changes, compared with week 4, had regressed (1.58 ± 0.44 D, P < 0.001), and no significant subsequent changes were found (all P > 0.05). The changes in apical corneal power at the end of the cessation period were not significantly different to baseline (difference: −0.16 ± 0.53 D, P = 1.00).
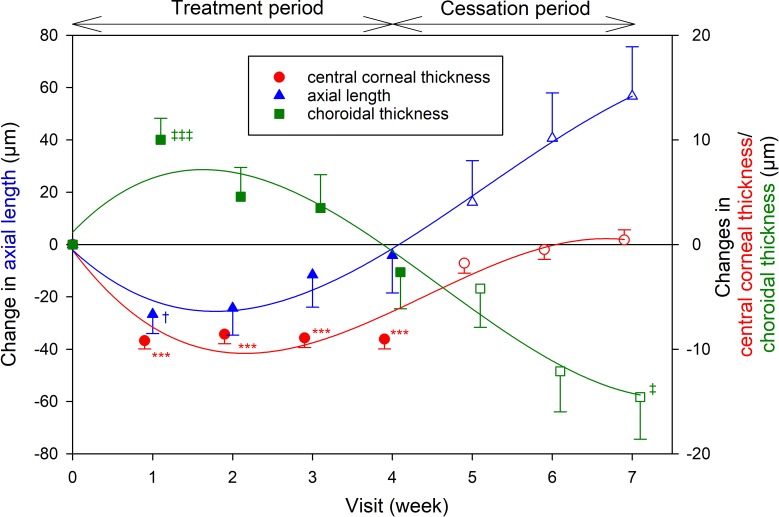
Changes in Axial Length
Significant axial shortening (−26 ± 41 μm, P = 0.03) was only observed at week 1 after commencing orthokeratology treatment (Table 3; Figure 2) and, on average, gradually returned to near baseline levels over the subsequent weeks (all P > 0.05). Following lens cessation, the mean axial length significantly increased by 41 ± 58 and 56 ± 73 μm, respectively, at week 6 and week 7, compared with the end of treatment period (week 4) (both P < 0.01); however, the change in axial length at the end of the study was not statistically significant compared with baseline (mean change: 51 ± 103 μm, P = 0.36).
Figure 2.
Mean change in central corneal thickness (red circles), axial length (blue triangles), and choroidal thickness (green squares) compared with baseline during the treatment (weeks 1–4, filled symbols) and the cessation (weeks 5–7, empty symbols) periods, adjusted for paired-eye data. Solid lines represent the regression lines of the means. Left y-axis: changes in axial length; right y-axis: changes in central corneal thickness and choroidal thickness. The data have been separated along the x-axis at each time point to aid visualization. Each error bar represents 1 SEM. † or ‡: P < 0.05; *** or ‡‡‡: P < 0.001.
Changes in Choroidal Thickness
Significant changes in choroidal thickness were also noted during the study (P < 0.001; Table 3; Fig. 2). In contrast to axial length, choroidal thickness increased significantly by 9 ± 12 μm (P < 0.001) at week 1 and, on average, gradually reduced and returned to near baseline levels over the remaining 3 weeks of treatment (all P > 0.05). After ceasing lens wear, a progressive thinning (relative to baseline) was observed, with a mean thinning of 14 ± 22 μm observed at the end of the cessation period compared with baseline (P = 0.03).
Changes in Other Ocular Biometrics
As expected, central corneal thickness decreased by 9 ± 4 μm at week 1 (P < 0.001; Table 3; Fig. 2) but stabilized for the remaining period of lens wear (all P > 0.05). After ceasing lens wear for 1 week, central corneal thickness increased (rebounded) by a similar amount (7 ± 4 μm, P < 0.001). No further significant changes were found for the remaining cessation period (all P > 0.05).
A similar pattern of change was observed for anterior chamber depth, which decreased in depth by 41 ± 51 μm at week 1 of orthokeratology treatment (P < 0.001) and was stable thereafter (all P > 0.05; Table 3). The mean anterior chamber depth also rebounded by 51 ± 51 μm (P < 0.001) at week 1 of the cessation period, with no significant changes observed afterwards (all P > 0.05).
For the crystalline lens, although its thickness tended to increase during the treatment period, it only showed a significant mean thickening of 42 ± 58 μm at week 4 of the treatment when compared with baseline (P = 0.004; Table 3). No significant differences compared with baseline were observed thereafter (all P > 0.05).
The overall eye length also varied over time (P = 0.01). However, the changes in overall eye length during the treatment period were not significantly different compared with baseline (all P > 0.05; Table 3). At the end of the cessation period, the average overall eye length was 45 ± 78 μm longer than that at week 4 (P = 0.03). However, similar to the changes in axial length, the changes in overall eye length at the end of the study were not significantly different to baseline (P = 1.00).
Associations Between Axial Shortening and Choroidal Thickening With Other Ocular Biometrics
Since the majority of the ocular parameters exhibited their greatest changes after 1 week of treatment, only these changes at week 1 were used for investigating the associations between changes in ocular biometrics and axial length. As crystalline lens thickness was correlated with anterior chamber depth (Spearman coefficient r = −0.86, P < 0.001) and choroidal thickness (Spearman coefficient r = −0.30, P = 0.04), it was removed from the predictor analyses to minimize multicollinearity. It was observed that only the central corneal thickness (β = 4, P = 0.02; Fig. 3A) and choroidal thickness (β = −2, P < 0.001; Fig. 3B) were significantly associated with axial length changes (every micrometer change in central corneal thickness and choroidal thickness was associated with 4 μm more and 2 μm less in axial length, respectively), whereas baseline age, changes in apical corneal power, and anterior chamber depth were not significant (all P > 0.05), after adjustment for sex and inclusion of paired-eye data. After the first week of lens wear, central corneal thinning (9 ± 4 μm) and choroid thickening (9 ± 12 μm) contributed to approximately 70% of the axial shortening (26 ± 41 μm).
Figure 3.
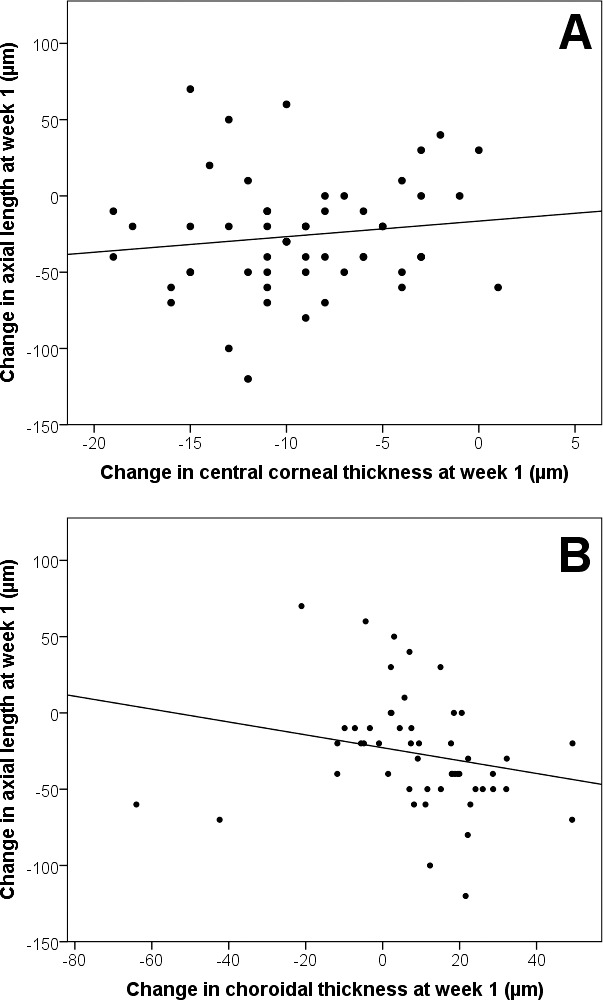
Scatterplots showing the relationships between (A) changes in central corneal thickness (β = 4, P = 0.02) and (B) changes in choroidal thickness (β = −2, P < 0.001), against changes in axial length at week 1, after adjusting for sex and paired-eye data.
When examining the association between the change in choroidal thickness with other ocular biometrics, choroidal thickening was associated with a greater change in apical corneal power (i.e., more myopic correction; apical corneal power, β = −3, P = 0.04). There was approximately 3 μm of choroidal thickening for every 1 D reduction in apical corneal power. Eyes with longer axial length exhibited significantly less choroidal thickening, but this association was small and not clinically significant (β < 1, P < 0.001). No significant association was observed between choroidal thickening and baseline age (P = 0.64), after adjusting for sex and paired-eye data.
Discussion
This study is the first prospective study investigating the short-term effects of orthokeratology lenses of different compression factors on axial length, choroidal thickness, and other ocular biometrics in young myopic children. Surprisingly, the ICF (additional 1.00 D) did not induce further significant changes in refraction and other ocular biometrics during 1 month of lens wear.
According to the retrospective analysis by Chan et al.11 on 123 orthokeratology treated children, an extra compression factor of approximately 1.00 D should be considered to provide an overcorrection of 0.75 D during the daytime. The contralateral eye design in our study was applied to better control the intrinsic ocular properties, for example, corneal biomechanics,27 in affecting the outcomes between treatment groups. The lack of expected differences between eyes may be due to different lens designs used in this previous analysis and the current study, in which the lenses contained three 0.20-mm fenestrations at 120° intervals at the junction between the alignment and reverse curves. A relatively lower (approximately 0.50 D) and slower apical corneal flattening was observed in subjects using fenestrated lenses, especially in the early treatment period.28 In our study, slightly greater changes in refraction and ocular biometrics were observed in eyes fitted with the higher compression factor lens. However, a treatment period of 1 month may not be sufficient to induce statistically significant differences, and a longer study is warranted to confirm the effect of an ICF on refractive status, ocular biometrics, and effectiveness of myopia control.
Axial shortening occurred during early orthokeratology treatment, but this recovered over time. The axial shortening after orthokeratology treatment has been suggested to be partly attributable to central corneal thinning and choroidal thickening.6,13 The changes in axial length, corneal thickness, and choroidal thickness of the current and some of the previous orthokeratology studies16–18 are summarized in Table 4. In the current study, the mean corneal thinning (9 μm) was comparable to the previously reported results (mean thinning: approximately 10 μm).16,18 For choroidal thickness, the magnitude of changes in our subjects was smaller (approximately 10 μm), compared with previous studies17,18 (approximately 20 μm), which might possibly be due to the smaller refractive correction, as more choroidal thickening was associated with more reduction in apical corneal power in the current study. The choroid is an established short-term biomarker for longer term eye growth in various animals,29–31 and its response to defocus within a short period of time has been observed in some human studies.32–34 Li et al.18 also suggested that the subfoveal choroidal thickening during orthokeratology treatment probably served as a predictor for the long-term effectiveness of the treatment. However, despite the choroidal changes observed and their significance in the statistical model in this study, the interimage (examining two chorio-retinal images captured from the same eye in the same measurement session) coefficient of repeatability of the subfoveal choroidal thickness measurement using a semiautomated method in orthokeratology-treated subjects was only 10 μm.35 This implies that the maximum mean treatment group changes observed in this study approached the coefficient of interimage repeatability of our technique, and therefore there is some uncertainty whether the observed changes in choroidal thickness are genuine. The instrument used in this study has an axial resolution of 3.9 μm per pixel and the semiautomated image analysis software used in the current study, which was more repeatable than other studies solely using manual segmentation (coefficient of repeatability of approximately 35 μm),36,37 was applied to minimize the possible errors in choroidal thickness measurement. Automated software for choroidal thickness segmentation, which provides better repeatability, or averaging the choroid over a larger retinal region rather than a single subfoveal point to improve repeatability, may help to confirm if the changes are real physiological changes or simply measurement noise.
Table 4.
Results of Current and Previous Studies Investigating the Effect of Orthokeratology on Changes in Apical Corneal Power, Central Corneal Thickness, Choroidal Thickness, and Axial Length (Mean ± SD)
| Study |
Duration |
Age, y |
Change in: |
|||
| Apical Corneal Power, D |
Central Corneal Thickness, μm |
Choroidal Thickness, μm |
Axial Length, μm |
|||
| Current study | 1 wk | 9.5 ± 1.0 | −1.95 ± 0.44 | −9 ± 4 | 9 ± 12 | −26 ± 41 |
| Gardner et al.16 | 1 mo | 13.6 ± 1.3 | −2.01 ± 0.47a | −10 (SD not given) | 3 ± 34 | −40 ± 337 |
| Chen et al.17 | 1 wk | 10.6 ± 2.5 | −2.43 ± 0.87 | — | 15 ± 10 | — |
| 3 wk | — | — | 24 ± 11 | — | ||
| Li et al.18 | 1 mo | 12.3 ± 1.7 | −2.60 ± 0.70 | −12 ± 7 | 16 ± 11 | 20 ± 40 |
| 6 mo | −2.57 ± 0.70 | −13 ± 7 | 21 ± 13 | 60 ± 100 | ||
| 12 mo | −2.64 ± 0.77 | −9 ± 9 | 19 ± 14 | 170 ± 160 | ||
Change in spherical equivalent refraction (auto-refraction), not apical corneal power.
Since central corneal thinning and possible choroidal thickening would affect the axial length measurement (from anterior cornea to retinal pigment epithelium) during orthokeratology treatment, potentially leading to an underestimation of axial length changes, the overall eye length (from anterior cornea to posterior choroid) was also analyzed. Despite the persistent central corneal thinning and the possible involvement of choroidal thickness, the overall eye length did not significantly change throughout the 1-month treatment period. Li et al.22 applied a similar analysis and showed that the overall eye length was not different between their orthokeratology and control groups at the 1-month visit. The results of the current study, in agreement to that of Li et al.,22 indicate that the changes in ocular biometrics were mainly due to the effect of orthokeratology and not axial eye growth.
It may be argued that the myopia control effect of orthokeratology lenses could be overestimated due to axial shortening from central corneal thinning and choroidal thickening. However, in this study, the axial length was only significantly shortened at week 1, and the changes thereafter were almost negligible. The influence of other ocular biometrics on axial shortening appeared to be minimal after 1 month of treatment. In addition, the effect of short-term orthokeratology treatment on axial length and choroidal thickness are likely to be transient. Observed changes after stabilization may be related to the growth of the eyeball, rather than the influence of central corneal thinning and possible choroidal thickening. In clinical practice, the manifest refraction will be temporarily corrected after starting orthokeratology treatment. In order to track the progression of axial eye growth, using axial length as an indicator is rational, and this study validated the application of axial length in orthokeratology-treated subjects with no persistent axial shortening observed or overestimation of orthokeratology effectiveness on myopia control.
In other studies, investigating the relationship between changes in axial length and choroidal thickness following exposure to short-term imposed defocus,32–34 greater axial length changes were observed when compared with choroidal thickness changes, particularly under myopic defocus conditions. This was similar to the current study's findings in that only 70% of axial shortening could be explained by the central corneal thinning, even after taking the choroidal thickening into account. Other ocular biometrics, such as the sclera, may also contribute to the changes in axial length, as it is a viscoelastic tissue and might be capable of altering ocular size in response to the visual environment as observed in chicks.30 However, the changes in scleral thickness or its position were not measured in the current study and thus warrants further investigation of its role on axial length changes, particularly during early orthokeratology treatment.
A rebound effect in axial length during the cessation period, which was significant compared with week 4 of treatment, was observed, but the changes were statistically insignificant compared with baseline. A similar phenomenon has been reported in other myopia control interventions.38–40 When the application of 1% atropine was ceased, there was an evidence of accelerated myopic progression (rebound) by 1.14 D (0.35 mm) in 1 year (mean progression in placebo-treated eyes: 0.38 D [0.15 mm] per year).38 However, no rebound was observed following the cessation of other optical treatments for myopia control, such as contact lenses with positive spherical aberration39 or progressive addition spectacles.40 In a contralateral crossover study of orthokeratology, Swarbrick et al.13 did note a rebound effect in the eyes initially wearing orthokeratology, which were then switched to a control condition for 6 months. However, the change of axial length (approximately 0.03 to 0.05 mm in 1 month) was both statistically and clinically insignificant. In the current study, after 3 weeks of lens cessation, the changes in axial length were significantly different to those at the end of the treatment period. The potential for a rebound effect following longer treatment compared with a control group remains an important question for future research.
Following lens cessation, the change in axial length (mean change: +51 ± 103 μm) could not be entirely explained by the changes in central corneal thickness (mean change: 0 ± 5 μm) and choroidal thickness (mean change: −14 ± 22 μm) alone. While the central corneal thickness returned to its original thickness, the choroid continued to thin. A similar change in choroidal thickness upon the removal of imposed short-term myopic defocus (induced by spectacle lenses) was also noted by Wang et al.41; that is, the choroid thinned following the removal of optical defocus rather than thickening (approximately 10 times more thinning occurred during the recovery period than observed during imposed defocus) as anticipated based on animal and human studies.30,31,33 Since there was no spectacle-wearing control group in the current study (contralateral design), the historical axial eye growth of the spectacle-wearing control group from the retardation of myopia in orthokeratology (ROMIO) study42 (recalculated over a 7-week period) was investigated as this cohort is well matched with respect to baseline characteristics (age and myopia) to the subjects in the current study (both P > 0.05, unpaired t-tests). A comparable increase in axial length was observed between the two studies (ROMIO: 58 ± 32 μm; current study: 51 ± 103 μm; P = 0.49, unpaired t-test). Therefore, the change in axial length during the cessation period in the current study was postulated to be predominately related to the natural eye growth without any optical intervention.
Our subjects showed a reduction of approximately 54 μm in anterior chamber depth and an increase of 42 μm in crystalline lens thickness after 1 month of lens wear; however, some researchers43–45 have reported no change in anterior chamber depth over time, and a randomized 2-year clinical trial43 showed a nonsignificant increase in the crystalline lens thickness of approximately 10 μm after 24 months of lens wear. Since Read et al.46 found that the lens thickness increased by 160 μm when viewing a 3 D of accommodation stimulus (approximately 53 μm/D), it is reasonable that after orthokeratology treatment, treated eyes might tend to accommodate to compensate for a small amount of overcorrection (spherical equivalent refraction at week 4: 0.18 D), and therefore may account for some of the observed change in anterior chamber depth and crystalline lens thickness.
This study had various limitations. While a semiautomated segmentation software was used for the analysis to minimize any potential errors or bias in manual procedures,36,37 the magnitude of the mean change in choroidal thickness was similar to the interimage subfoveal choroidal thickness repeatability (10 μm). The use of a contralateral study design provided enhanced power to detect between-eye differences, but also prevented running the risk of interocular interactions in response to the treatment. By limiting the treatment period to 1 month, the long-term effects of the orthokeratology treatments on eye growth with different compression factors were not investigated.
In conclusion, examination of the changes in axial length, choroidal thickness, and other ocular biometrics in eyes fitted with orthokeratology lenses with different compression factors (0.75 and 1.75 D), indicated that increasing the compression factor by 1 D did not affect the changes in all ocular biometrics. Axial length decreased significantly during the early treatment period, but this change regressed during the remaining 3 weeks of lens wear. The axial shortening in the early orthokeratology treatment period appeared to result partly from central corneal thinning and possible choroidal thickening, but this requires further confirmation with the use of a more accurate and repeatable technique for determining subtle choroidal thickness changes.
Acknowledgments
The authors thank Michael Collins for his comments on the experiment and analysis, David Alonso-Caneiro for the software for choroidal thickness segmentation, Carmen Ka-Man Wong for data collection and choroidal thickness analysis, and Maureen Boost for her advice in the preparation of the manuscript.
This study was supported by a Collaborative Research Agreement between The Hong Kong Polytechnic University (PolyU), Menicon Co., Ltd, Japan (ZG3Z), and the Research Residency Scheme of the School of Optometry, PolyU, provided to J.K. Lau and K. Wan. Rinsing solutions and artificial tears were sponsored by Ophtecs Co., Tokyo, Japan, and Precilens, Creteil, France, respectively.
Disclosure: J.K. Lau, None; K. Wan, None; S.-W. Cheung, None; S.J. Vincent, None; P. Cho, None
References
- 1.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Saka N, Ohno-Matsui K, Shimada N, et al. Long-term changes in axial length in adult eyes with pathologic myopia. Am J Ophthalmol. 2010;150:562–568.e1. doi: 10.1016/j.ajo.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Ikuno Y. Overview of the complications of high myopia. Retina. 2017;37:2347–2351. doi: 10.1097/IAE.0000000000001489. [DOI] [PubMed] [Google Scholar]
- 4.Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123:697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Cho P, Cheung SW. Protective role of orthokeratology in reducing risk of rapid axial elongation: a reanalysis of data from the ROMIO and TO-SEE Studies. Invest Ophthalmol Vis Sci. 2017;58:1411–1416. doi: 10.1167/iovs.16-20594. [DOI] [PubMed] [Google Scholar]
- 7.Wolffsohn JS, Calossi A, Cho P, et al. Global trends in myopia management attitudes and strategies in clinical practice. Cont Lens Anterior Eye. 2016;39:106–116. doi: 10.1016/j.clae.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Cheung SW, Lam C, Cho P. Parents' knowledge and perspective of optical methods for myopia control in children. Optom Vis Sci. 2014;91:634–641. doi: 10.1097/OPX.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 9.Nichols JJ, Marsich MM, Nguyen M, Barr JT, Bullimore MA. Overnight orthokeratology. Optom Vis Sci. 2000;77:252–259. doi: 10.1097/00006324-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Mountford J. Retention and regression of orthokeratology with time. Int Contact Lens Clin. 1998;25:59–64. [Google Scholar]
- 11.Chan B, Cho P, Mountford J. The validity of the Jessen formula in overnight orthokeratology: a retrospective study. Ophthalmic Physiol Opt. 2008;28:265–268. doi: 10.1111/j.1475-1313.2008.00545.x. [DOI] [PubMed] [Google Scholar]
- 12.Hiraoka T, Kakita T, Okamoto F, Oshika T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology. 2015;122:93–100. doi: 10.1016/j.ophtha.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 13.Swarbrick HA, Alharbi A, Watt K, Lum E, Kang P. Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology. 2015;122:620–630. doi: 10.1016/j.ophtha.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Nieto-Bona A, Gonzalez-Mesa A, Nieto-Bona MP, Villa-Collar C, Lorente-Velazquez A. Short-term effects of overnight orthokeratology on corneal cell morphology and corneal thickness. Cornea. 2011;30:646–654. doi: 10.1097/ICO.0b013e31820009bc. [DOI] [PubMed] [Google Scholar]
- 15.Alharbi A, Swarbrick HA. The effects of overnight orthokeratology lens wear on corneal thickness. Invest Ophthalmol Vis Sci. 2003;44:2518–2523. doi: 10.1167/iovs.02-0680. [DOI] [PubMed] [Google Scholar]
- 16.Gardner DJ, Walline JJ, Mutti DO. Choroidal thickness and peripheral myopic defocus during orthokeratology. Optom Vis Sci. 2015;92:579–588. doi: 10.1097/OPX.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Xue F, Zhou J, Qu X, Zhou X. Effects of orthokeratology on choroidal thickness and axial length. Optom Vis Sci. 2016;93:1064–1071. doi: 10.1097/OPX.0000000000000894. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Hu Y, Cui D, Long W, He M, Yang X. Change in subfoveal choroidal thickness secondary to orthokeratology and its cessation: a predictor for the change in axial length. Acta Ophthalmol. 2019;97:e454–e459. doi: 10.1111/aos.13866. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- 20.Sander BP, Collins MJ, Read SA. The effect of topical adrenergic and anticholinergic agents on the choroidal thickness of young healthy adults. Exp Eye Res. 2014;128:181–189. doi: 10.1016/j.exer.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Sander BP, Collins MJ, Read SA. The interaction between homatropine and optical blur on choroidal thickness. Ophthalmic Physiol Opt. 2018;38:257–265. doi: 10.1111/opo.12450. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Cui D, Hu Y, Ao S, Zeng J, Yang X. Choroidal thickness and axial length changes in myopic children treated with orthokeratology. Cont Lens Anterior Eye. 2017;40:417–423. doi: 10.1016/j.clae.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Efron N. Grading scales for contact lens complications. Ophthalmic Physiol Opt. 1998;18:182–186. doi: 10.1016/s0275-5408(97)00066-5. [DOI] [PubMed] [Google Scholar]
- 24.Cruysberg LP, Doors M, Verbakel F, Berendschot TT, De Brabander J, Nuijts RM. Evaluation of the Lenstar LS 900 non-contact biometer. Br J Ophthalmol. 2010;94:106–110. doi: 10.1136/bjo.2009.161729. [DOI] [PubMed] [Google Scholar]
- 25.Alonso-Caneiro D, Read SA, Collins MJ. Automatic segmentation of choroidal thickness in optical coherence tomography. Biomed Opt Express. 2013;4:2795–2812. doi: 10.1364/BOE.4.002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2013;33:7–14. doi: 10.1111/opo.12009. [DOI] [PubMed] [Google Scholar]
- 27.Lam AK, Leung SY, Hon Y, et al. Influence of short-term orthokeratology to corneal tangent modulus: a randomized study. Curr Eye Res. 2018;43:474–481. doi: 10.1080/02713683.2017.1418895. [DOI] [PubMed] [Google Scholar]
- 28.Cho P, Chan B, Cheung SW, Mountford J. Do fenestrations affect the performance of orthokeratology lenses? Optom Vis Sci. 2012;89:401–410. doi: 10.1097/OPX.0b013e31824cb743. [DOI] [PubMed] [Google Scholar]
- 29.Zhu X, Park TW, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46:2238–2241. doi: 10.1167/iovs.04-0956. [DOI] [PubMed] [Google Scholar]
- 30.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 31.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 32.Read SA, Collins MJ, Sander BP. Human optical axial length and defocus. Invest Ophthalmol Vis Sci. 2010;51:6262–6269. doi: 10.1167/iovs.10-5457. [DOI] [PubMed] [Google Scholar]
- 33.Chakraborty R, Read SA, Collins MJ. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res. 2012;103:47–54. doi: 10.1016/j.exer.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Chakraborty R, Read SA, Collins MJ. Hyperopic defocus and diurnal changes in human choroid and axial length. Optom Vis Sci. 2013;90:1187–1198. doi: 10.1097/OPX.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 35.Lau JK, Cheung SW, Collins MJ, Cho P. Repeatability of choroidal thickness measurements with Spectralis OCT images. BMJ Open Ophthalmol. 2019;4:e000237. doi: 10.1136/bmjophth-2018-000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman W, Chen FK, Yeoh J, Patel P, Tufail A, Da Cruz L. Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:2267–2271. doi: 10.1167/iovs.10-6024. [DOI] [PubMed] [Google Scholar]
- 37.Hanumunthadu D, Ilginis T, Restori M, et al. Spectral-domain optical coherence tomography retinal and choroidal thickness metric repeatability in age-related macular degeneration. Am J Ophthalmol. 2016;166:154–161. doi: 10.1016/j.ajo.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 38.Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–579. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Cheng X, Xu J, Chehab K, Exford J, Brennan N. Soft contact lenses with positive spherical aberration for myopia control. Optom Vis Sci. 2016;93:353–366. doi: 10.1097/OPX.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 40.Berntsen DA, Sinnott LT, Mutti DO, Zadnik K. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci. 2012;53:640–649. doi: 10.1167/iovs.11-7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Chun RK, Liu M, et al. Optical defocus rapidly changes choroidal thickness in schoolchildren. PLoS One. 2016;11:e0161535. doi: 10.1371/journal.pone.0161535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho P, Cheung SW. Retardation of myopia in orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53:7077–7085. doi: 10.1167/iovs.12-10565. [DOI] [PubMed] [Google Scholar]
- 43.Cheung SW, Cho P. Long-term effect of orthokeratology on the anterior segment length. Cont Lens Anterior Eye. 2016;39:262–265. doi: 10.1016/j.clae.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009;93:1181–1185. doi: 10.1136/bjo.2008.151365. [DOI] [PubMed] [Google Scholar]
- 45.Tsukiyama J, Miyamoto Y, Higaki S, Fukuda M, Shimomura Y. Changes in the anterior and posterior radii of the corneal curvature and anterior chamber depth by orthokeratology. Eye Contact Lens. 2008;34:17–20. doi: 10.1097/ICL.0b013e3180515299. [DOI] [PubMed] [Google Scholar]
- 46.Read SA, Collins MJ, Woodman EC, Cheong SH. Axial length changes during accommodation in myopes and emmetropes. Optom Vis Sci. 2010;87:656–662. doi: 10.1097/OPX.0b013e3181e87dd3. [DOI] [PubMed] [Google Scholar]