Abstract
This review describes formation of the islet basement membrane and the function of extracellular matrix (ECM) components in β-cell proliferation and survival. Implications for islet transplantation are discussed. The insulin-producing β-cell is key for maintaining glucose homeostasis. The islet microenvironment greatly influences β-cell survival and proliferation. Within the islet, β-cells contact the ECM, which is deposited primarily by intraislet endothelial cells, and this interaction has been shown to modulate proliferation and survival. ECM-localized growth factors, such as vascular endothelial growth factor and cellular communication network 2, signal through specific receptors and integrins on the β-cell surface. Further understanding of how the ECM functions to influence β-cell proliferation and survival will provide targets for enhancing functional β-cell mass for the treatment of diabetes.
Diabetes is characterized by chronic hyperglycemia as a result of either loss or dysfunction of the insulin-producing β-cells. Diabetes is classified into two main types: type 1 diabetes (T1D) manifests as a result of the autoimmune destruction of β-cells, whereas type 2 diabetes (T2D) is caused by the dysfunction and loss of β-cells as a result of factors, such as insulin resistance and insulin secretory defects. Exogenous insulin is the only current treatment for T1D. Pharmacological treatments for T2D alleviate hyperglycemia by increasing insulin secretion, improving peripheral insulin resistance, or increasing glucose elimination. A cell-based approach for treating T1D is islet or β-cell transplantation, but to date, these strategies do not provide long-lasting results. Studies are ongoing to determine how to improve the longevity and functionality of islet grafts by altering the composition of the transplantation scaffold or cotransplanting the islets/β-cells with compounds that promote survival and function. There are many studies demonstrating that the extracellular matrix (ECM) is important for the health of islets in vivo and ex vivo, but the interactions between the ECM and islet cells are still not fully understood. Current islet isolation techniques disrupt the native islet ECM, leading to cell death and dysfunction. Here, we review the literature regarding how components of the islet ECM aid in β-cell survival and proliferation.
Pancreatic Islet Basement Membrane and Intraislet Endothelial Cell Contribution
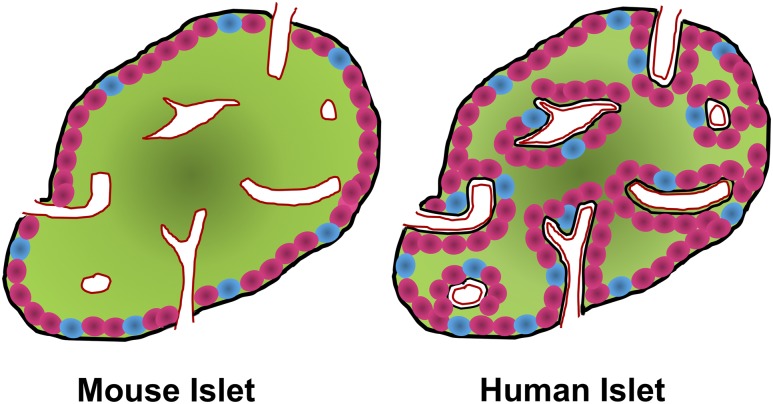
The basement membrane (BM) is a specialized ECM (1). Functionally, BMs provide structural support, divide tissues into separate compartments, and regulate cellular functions (2). Unlike the exocrine compartment of the pancreas and most other epithelial cell types, β-cells are incapable of forming their own vascular BM. The pancreatic islets of mice and humans have two distinct BMs: the peri-islet membrane and the vascular BM (Fig. 1). The peri-islet membrane covers the outer surface of the islet and is primarily involved in separating the islet endocrine cells from the exocrine compartment of the pancreas. It also acts as a barrier against infiltrating leukocytes in T1D (3).
Figure 1.
Comparison of mouse and human islet architecture and BM distribution. Illustration of the architecture of a (left) mouse and (right) human islet. Green represents insulin-producing β-cells; pink represents glucagon-producing α-cells; blue represents somatostatin-producing δ-cells. In mouse islets, β-cells usually make up the core of the islet, with α- and δ-cells on the periphery. The peri-islet BM (black) is located at the periphery, and the vascular BM (red) is mainly in contact with β-cells. Conversely, whereas human islet β-cells are mainly located in the core of the islet, α- and δ-cells are not confined to the periphery. Furthermore, human islets have a double vascular BM, which is primarily in contact with α- and δ-cells.
Structural differences and similarities exist between mouse and human endocrine cells in terms of BM interactions (Fig. 1). In mice, the endocrine cells are in contact with either the peri-islet membrane or the vascular BM. As a result of the architecture of mouse islets, where the islet core is comprised mainly of β-cells, the peri-islet membrane is primarily in contact with the cells on the perimeter or mantle of the islet, which are predominantly α-cells (Fig. 1) (3). In human islets, the endocrine cells are not organized into a strict core/mantle architecture, and thus, the peri-islet BM is in contact with different types of endocrine cells, including some β-cells. In contrast with mouse islets, human islets have intraislet septa that are formed by the peri-islet BM coinvaginating with islet blood vessels (Fig. 1) (4).
Human islet cells in the interior are in contact with a unique double BM, present around the intraislet blood vessels (Fig. 1) (4). This double BM consists of a vascular BM associated with the islet capillary, and an endocrine BM. Although β-cells are unable to secrete their own BM, they secrete vascular endothelial growth factor (VEGF) A, which binds to the VEGF receptor 2 (VEGFR2) on endothelial cells (ECs), attracting them to the forming islet (5). ECs are responsible for depositing the components of the vascular BM (5), such as collagen, laminin, fibronectin, and heparan sulfate, which play a role in β-cell proliferation and survival, described later in this review.
Another critical role of intraislet ECs is to maintain the high islet vascularity. Although islets comprise only 1% to 2% of the pancreas, they receive ∼10% to 15% of the blood flowing to the pancreas (6, 7). Proper vascularization of the islet is crucial both to preserve glucose homeostasis through the secretion of hormones into the bloodstream and for the maintenance of β-cell survival (8, 9). Intraislet ECs are highly fenestrated, which contributes to the ability of islets to detect changes sensitively in blood glucose levels and rapidly diffuse hormones into the bloodstream (10). The importance of intraislet ECs is prominently seen during islet transplantation. Revascularization of transplanted islets is crucial for their survival and function (11). Studies have shown that islet isolation and culture techniques completely sever islet vasculature (12) and perturb intraislet ECs (13).
Intraislet ECs also secrete many molecules that affect β-cell proliferation and survival. For example, ECs secrete hepatocyte growth factor (HGF) and cellular communication network 2 (CCN2; formerly known as connective tissue growth factor), which each enhances β-cell proliferation and survival (14–16). Intraislet ECs can also affect β-cell proliferation and survival by modulating the availability of growth factors in the ECM. They secrete matrix metalloproteinases that alter ECM integrity and release sequestered growth factors that affect β-cell proliferation and survival, such as fibroblast growth factor (FGF) and VEGF (discussed in more detail below).
Taken together, intraislet ECs are not simply a bystander cell type within the islet, solely acting as a conduit for nutrients and hormones. Rather, they are involved heavily in the promotion of islet health and survival by establishing the islet vascular BM while producing factors that influence β-cell proliferation and survival.
ECM Molecules, Growth Factors, and Matricellular Proteins that Contribute to β-Cell Proliferation and Survival
Multiple ECM proteins exist in the BM, but the most prominent are collagen, laminin, fibronectin, and heparan sulfate, which contribute to β-cell proliferation and survival through interaction with cell-surface integrin receptors (discussed further in Integrin–ECM Interactions Facilitate β-Cell Proliferation and Survival). In addition to the provision of structural support and an anchor for β-cells, interactions with the ECM lead to active intra-β-cell second messenger signaling that alters β-cell activity and gene expression. The ECM also acts as repository and reservoir to concentrate locally growth factors secreted by ECs and β-cells themselves. Table 1 contains a summary of all ECM components and ECM-localized growth factors known to play a role in the regulation of β-cell proliferation and survival.
Table 1.
ECM Components and ECM-Associated Growth Factors and Proteins Involved in β-Cell Proliferation and Survival
| Functions | References | |
|---|---|---|
| ECM components | ||
| Collagen | Promotes survival in β-cell lines and primary islets, both with or without encapsulation | (17–23) |
| Laminin | Promotes proliferation in primary human islets and β-cell lines; increases survival of primary mouse islets and β-cell lines | (19, 24, 25) |
| Fibronectin | Increases proliferation in rat islets; increases viability and decreases apoptosis in MIN6 β-cell line | (19, 21) |
| Heparan sulfate proteoglycans | Loss decreases β-cell proliferation and increases β-cell apoptosis; protects β-cells from reactive oxygen species-induced apoptosis | (26, 27) |
| Matricellular proteins and growth factors | ||
| CCN2 | Overexpression during embryogenesis induces β-cell proliferation; promotes β-cell proliferation of mouse islets ex vivo and in vivo after β-cell injury; loss disrupts compensatory β-cell mass increase during pregnancy | (28, 29) |
| FGF | Loss decreases β-cell number; protects against β-cell death during lipo- and glucotoxicity | (30, 31) |
| VEGF | Loss decreases β-cell proliferation; overexpression using the Pdx1 promoter increases β-cell proliferation and survival; overexpression using the RIP leads to β-cell loss | (10, 32–35) |
| HGF | Inactivation during embryogenesis results in decreased pancreatic insulin content; inactivation during pregnancy reduces maternal β-cell compensation and increases β-cell apoptosis; treatment increases β-cell survival after toxin exposure; inactivation decreases β-cell regeneration after injury | (36–38) |
Abbreviations: MIN6, mouse insulin-secreting cell line; RIP, rat insulin promoter.
Collagen
Collagen makes up a large portion of the BM in mammals and provides structural stiffness and cohesiveness to tissues (39, 40). To date, 28 different types of collagen have been identified, with the most common being types I, II, III, IV, and V (41). In islets, collagen IV and collagen VI are most abundant and are located in both the peri-islet BM and vascular BM (17, 18).
Collagen IV is critical for proper BM morphology, and mutations in collagen IV genes are embryonically lethal (42). Collagen IV significantly promotes survival in the mouse insulinoma-derived immortalized β-cell line (MIN6) and primary islets isolated from various species. MIN6 cells, grown in encapsulation platforms containing collagen IV, survived longer than MIN6 cells encapsulated without any ECM molecules (19). A study using another immortalized β-cell line—the rat-derived INS-1 line—demonstrated that cell survival and proliferation were significantly increased after being plated on collagen IV compared with control (20). Likewise, isolated primary rat islets plated on collagen IV had significantly higher survival compared with primary rat islets plated without ECM molecules (21).
Collagen VI has been less characterized than collagen IV but is known to be cytoprotective and regulate autophagy and cell differentiation in different tissue types (43). Currently, only one study has assessed the effects of collagen VI on β-cell survival. In this study, with the use of alginate-based, encapsulated primary human islets containing collagen VI in varying concentrations, islets exposed to collagen VI had enhanced viability and increased oxygen consumption (22).
Laminin
Laminins are glycoproteins composed of α, β, and γ polypeptide chains joined by disulfide bonds to create heterotrimers (44). To date, 16 laminin isoforms have been identified (24). Laminin expression and distribution in islets are still not well understood, and fewer studies about the effects of laminin on β-cell survival and proliferation have been performed compared with collagen. In the adult mouse, the primary isoform is laminin-511, which is comprised of the α5, β1, and γ1 isoforms. In human islets, laminin-411 (α4, β1, and γ1) and laminin-511 have been found to play roles in β-cell proliferation. In general, studies in immortalized cell lines and primary islets from rodents and human have found that culturing on laminin increases β-cell proliferation, decreases β-cell death, and prolongs β-cell survival (19, 21, 23–25). Laminin can also be used to prolong survival of transplanted islets. Human islets encapsulated in alginate containing laminin, in combination with collagen IV, had prolonged survival after exposure to cytokines (IL-1β, interferon-γ, and TNF-α) (13).
Fibronectin
Fibronectin is a glycoprotein that exists as a dimer composed of two nearly identical, ∼250-kDa subunits linked by a pair of disulfide bonds. Fibronectin exists in both a soluble form that circulates in the plasma and an insoluble form that is located in the ECM (45). Fibronectin is critically important in vertebrate development, which is illustrated by the early embryonic lethality of mice with inactivation of the fibronectin gene (46). Of the three ECM glycoproteins discussed so far, the role of fibronectin in β-cell proliferation and survival has been least studied. However, it is known that the structure of fibronectin contains multiple protein-binding domains, where growth factors, such as FGF and VEGF, can bind (47). Thus, fibronectin plays a critical role in sequestering and regulating accessibility of critical β-cell mitogens and viability factors. In one study that examined the role of fibronectin in β-cell proliferation, DNA synthesis was significantly increased when neonatal rat islets were cultured on fibronectin (22). Finally, another study using MIN6 cells encapsulated with fibronectin, demonstrated that apoptosis was significantly inhibited in the encapsulated cells compared with controls (19).
Heparan sulfate proteoglycans
Heparan sulfate proteoglycans (HSPGs) are glycoproteins that contain one or more covalently attached heparan sulfate chains (48). There are 17 different HSPGs that are classified based on their location in the cell. For the purpose of this review, focus will be on the ECM-associated HSPGs that collaborate with other ECM components to define a BM structure. HSPGs can bind cytokines, chemokines, growth factors, and morphogens to protect these molecules from proteolysis. Through this action, HSPGs can act as a depot of sequestered factors that can affect cell function (49).
Little is known about the role of HSPGs in islet biology. HSPGs are scarcely expressed in islets during embryogenesis and during the first week after birth in mice, but the expression in islets greatly increases after 1 week postnatally (26). This finding suggests that HSPGs are involved in postnatal islet growth and maturation. To probe this hypothesis, mice with β-cell-specific HSPG inactivation were generated by inactivating the gene Extl3 that is involved in heparan sulfate synthesis. In this model, changes in islet morphology were observed after birth: β-cell area compared with total pancreatic area was reduced to 75% of control levels, and β-cell proliferation was significantly decreased 1 week after birth (26).
More recent studies have demonstrated a protective role of heparan sulfate. During islet isolation, extracellular heparan sulfate was reduced by 11% to 17% (26). Loss of intracellular heparan sulfate correlated with a two- to threefold increase in β-cell death in culture (27). Furthermore, addition of heparin, an analog of heparan sulfate, increased the viability of β-cells in culture after only 2 days. It was further found that β-cells were significantly protected from apoptosis in the face of reactive oxygen species-induced cell death when heparin was supplemented in the culture media.
ECM-sequestered growth factors
Some of the growth factors mentioned in this review, such as HGF, are made by the intraislet ECs, which are also responsible for deposition of the ECM-surrounding β-cells. Others are synthesized and secreted by other sources but become trapped in the ECM or bind to components of the ECM, where they are sequestered and locally concentrated to act on β-cells.
FGF
FGFs are a family of 23 proteins that can bind ECM HSPGs and interact with five distinct high-affinity tyrosine kinase receptors, FGF receptors (FGFRs) (50). FGF signaling affects many biological functions, such as proliferation, survival, migration, and differentiation (51). FGFs play a role in β-cell proliferation and survival from embryogenesis to the postnatal period (30).
Many FGF ligands are expressed in adult mouse β-cells, including FGF1, FGF2, FGF4, FGF5, FGF7, and FGF10, along with the receptors FGFR1 and FGFR2. In one study examining the roles of FGF1 and FGF2 in β-cell proliferation and survival, two mouse models were generated with a β-cell-specific inactivation of either FGFR1 or FGFR2 during embryogenesis. Whereas FGFR1-deficient mice had the same number of β-cells compared with control at birth, loss of FGFR1 resulted in a gradual, ∼25% decrease in the number of β-cells by postnatal day (P)27, demonstrating a role for FGF signaling in postnatal β-cell expansion (30). In settings of glucolipotoxicity and cytokine-induced apoptosis, FGF21 plays a protective role in both rat islets and INS-1E cells. Constant infusion of FGF21 for 8 weeks, beginning at 8 weeks of age in db/db mice, a mouse model of T2D, resulted in a greater number of insulin-positive cells per islet compared with control. This result indicates that FGF21 reduces the β-cell loss associated with disease progression in this model (31).
VEGF
VEGF-A, the prototypical member of the VEGF family, is secreted by β-cells and attracts ECs to the forming islet. VEGF binds to the tyrosine kinase receptors, VEGFR1, VEGFR2, and VEGFR3, to promote angiogenesis. VEGF is capable of binding to HSPGs and fibronectin in the ECM, where it can be cleaved and released as a soluble ligand to promote β-cell proliferation (52). VEGF-A expression is crucial for proper pancreatic and islet vascularization during embryogenesis, with inactivation leading to reduced vascularization, decreased β-cell proliferation, and overall reduced β-cell mass. In the islet, VEGFRs are expressed on ECs and not on β-cells, demonstrating that the effects of VEGF-A on β-cells are through the action of ECs (53). Inactivation of VEGF-A during adulthood causes reduced islet vascularization; however, β-cell mass and function were preserved, suggesting that VEGF-A has a lesser role in adult β-cell function and mass (32).
Conversely, overexpression of VEGF-A in the pancreas leads to very different outcomes, depending on the timing and magnitude of the induction. VEGF-A overexpression, driven by the Pdx1 promoter, which leads to increased production throughout the pancreas during embryogenesis and in β-cells in adulthood, increased pancreatic vascularization and islet hyperplasia when assessed at 2 months of age in mice (10). Likewise, VEGF-A overexpression in adult β-cells for 2 weeks stimulated islet angiogenesis and promoted β-cell proliferation, while protecting β-cells from apoptosis after exposure to the β-cell-specific toxin alloxan (33).
Excess VEGF-A can also be deleterious to islets. Overexpression of VEGF-A using the much stronger rat insulin gene promoter resulted in a massive increase in intraislet ECs, which led to a decrease in β-cell proliferation and mass at P1 (35). VEGF-A induction in postnatal β-cells, from both P1 to P7 and later, from P21 to P28, resulted in a reduced β-cell number. However, this effect was transient. When VEGF-A induction was terminated, islet morphology and vascularization returned to normal within 6 weeks, mainly as a result of β-cell proliferation (34).
Taken together, these studies indicate that the effects of VEGF-A on β-cell proliferation are highly dependent on a multitude of factors, including the timing, duration, and magnitude of overexpression.
HGF
HGF, formerly known as scatter factor, is a mitogen for hepatocytes and promotes angiogenesis. HGF binds to its receptor tyrosine kinase, c-Met, and has regenerative effects on various tissues (36). Intraislet ECs secrete HGF, which can act on c-Met on β-cells to promote proliferation and survival. It has also been well documented that HGF can bind to ECM components, such as fibronectin, to create complexes with c-Met, subsequently enhancing signaling (54). In the islet, β-cell-specific transgenic misexpression of HGF resulted in increased β-cell proliferation. Furthermore, HGF attenuated the effects of the diabetogenic β-cell toxin streptozotocin (37). Conversely, β-cell-specific inactivation of c-Met during embryogenesis led to an overall decrease in islet area and insulin content. This finding implies that HGF signaling is needed for β-cell expansion and proper β-cell mass (38). c-Met is upregulated following partial pancreatectomy, implicating HGF signaling in β-cell regeneration. In support of this, intraperitoneal injection of HGF further increased β-cell regeneration in this model, whereas inactivation of c-Met impaired β-cell proliferation and regeneration after partial pancreatectomy (14).
HGF signaling also plays a role in maternal β-cell mass expansion and survival during pregnancy. Female mice with a pancreas-wide deletion of c-Met displayed decreased β-cell proliferation, reduced β-cell mass, and an increase in β-cell apoptosis during pregnancy, leading to gestational diabetes (38). In this same study, human islets treated with HGF had significantly less apoptotic cells in the presence of dexamethasone compared with control, indicating a role for HGF in human β-cell survival (37).
CCN2
CCN2 (formerly known as connective tissue growth factor) is part of the CCN matricellular protein family. It is involved in a multitude of cell processes, including adhesion, migration, angiogenesis, and proliferation (55). During embryogenesis, CCN2 is expressed in insulin-positive cells, the ductal epithelium, and the vascular endothelium and is required for β-cell differentiation and proliferation. Overexpression of CCN2, specifically in embryonic insulin-producing cells, increases the endocrine area, with a substantial increase in both α-cell and β-cell proliferation (28). There is no known receptor specific for CCN2; however, it can interact with integrins on other cell types and may also do so in β-cells, although this needs to be tested.
In adulthood, CCN2 is expressed in the pancreas, only in the vascular endothelium, and plays a role in compensatory maternal β-cell proliferation during pregnancy (29). CCN2 also promotes β-cell regeneration in vivo. In a mouse model of 50% β-cell ablation, β-cell-specific induction of CCN2 significantly increased β-cell proliferation after only 2 days of treatment, which was sustained for up to 4 weeks of CCN2 treatment (16). This increase in proliferation resulted in a substantial regeneration in β-cell mass after 4 weeks. Exogenous CCN2 also induces β-cell proliferation in islets from adult mice ex vivo. Islets treated with recombinant human CCN2 had significantly increased β-cell proliferation compared with control after 4 days (16). Preliminary data from our group indicate that CCN2 can also increase β-cell proliferation in human islets, but this needs to be examined further. Our group has shown that CCN2 induces expression of known β-cell mitogenic pathways (including HGF, serotonin synthesis, and integrins) (16), suggesting that CCN2 acts indirectly to induce β-cell proliferation. Further studies need to be performed to elucidate the mechanism by which CCN2 induces β-cell proliferation.
Integrin–ECM Interactions Facilitate β-Cell Proliferation and Survival
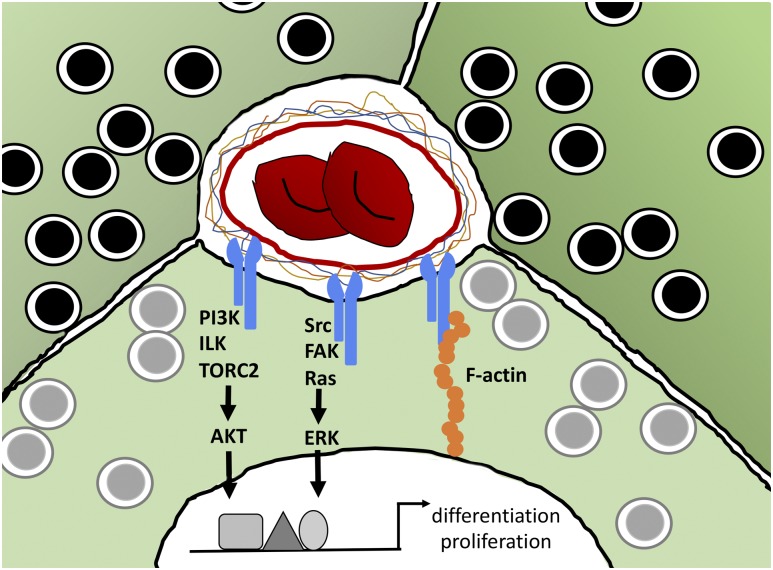
Integrins are transmembrane heterodimer receptors for ECM components and are expressed on virtually all cell types and composed of one α and one β subunit. Integrins affect a variety of cell processes, including adhesion, migration, differentiation, and cell growth. In mammals, 24 α and 9 β subunits have been identified, which combine to create 24 unique heterodimers. Both the α and β subunits are involved in the determination of ligand specificity; however, the β subunit facilitates adhesion and activates intracellular second messenger cascades (56). Most notably, stimulation of the β1 integrin leads to activation of focal adhesion kinase (FAK), extracellular-regulated kinase (ERK), protein kinase B (or AKT), and the steroid receptor coactivator (Src) family of kinases, all of which are involved in cell survival (Fig. 2). Ligands for integrins include fibronectin, laminin, collagen, fibrinogen, and thrombospondin.
Figure 2.
Signaling pathways of ECM–integrin interactions in β-cells. Integrins (blue) on the surface of β-cells (green cells containing insulin granules) interact with ECM components surrounding the vasculature, initiating signal cascades, including activation of protein kinase B (AKT) and ERK, both of which then act on transcription factors to alter gene transcription. Activation of integrins also stimulates F-actin assembly to modulate adhesion and migration. ILK, integrin-linked kinase; PI3K, phosphatidylinositol 3-kinase; TORC2, target of rapamycin complex 2.
The specific α and β subunits expressed on islet cells differ among species. Integrin signaling is critical for pancreatic endocrine specification. In the developing pancreas, differentiation of endocrine cells from bipotent duct/endocrine progenitors within the pancreatic trunk epithelium requires decreased interaction between the α5β1 integrin and the ECM substrate (57). Likewise, inhibition of FAK or Src promotes endocrine differentiation from human embryonic stem cells (58).
Integrins also engage in crosstalk with growth factor receptors. The signals that are activated by the integrin–FAK–Src complex can be integrated with signals from growth factor receptor binding to subsequently activate the Ras–MAPK kinase–MAPK pathway, which is canonically associated with cell proliferation and survival (59). Some examples of growth factor receptors with which integrins act synergistically are VEGFR, c-Met, and endothelial growth factor receptor, all of which are involved in β-cell proliferation (60–62). In addition, integrins may act as the cell surface receptor for CCN2. Furthermore, integrins can regulate growth factor receptor availability by altering their rate of internalization and degradation, thus directly affecting signaling through these receptors (62). Integrins are also necessary for the response of anchorage-dependent cells to some growth factors, further providing evidence that components of the ECM play a role in growth factor signaling (63).
Although there are many integrin subunits, β1 integrin is one of the most studied. It is the most promiscuous integrin subunit in that it binds multiple α subunits and has been demonstrated to be important for embryonic development, as systemic deletion of β1 integrin results in embryonic lethality (64). β1 integrin, as a result of its ability to dimerize with multiple α subunits, is also capable of binding multiple ECM components, such as laminin, collagen, and fibronectin (25). This β1 integrin/ECM interaction facilitates β-cell proliferation and survival, as described in ECM Molecules, Growth Factors, and Matricellular Proteins that Contribute to β-Cell Proliferation and Survival.
In the pancreas, β1 integrin is highly expressed on the β-cell, promotes β-cell proliferation and survival, and facilitates the migration of endocrine progenitors during pancreas development (65). Studies using INS-1 and MIN6 cells demonstrated that the blockade of β1 integrin, using specific function-neutralizing antibodies, attenuates the beneficial effects that ECM components, such as collagen and laminin, have on β-cell proliferation and survival. The blockade of β1 integrin also decreases mRNA expression of insulin, elucidating another method by which β1 integrin can promote β-cell survival (20, 21). Similar findings have been demonstrated in primary rat β-cells and fetal human islets plated on laminin, where blockade of β1 integrin increased apoptosis and attenuated phosphorylation of both FAK and ERK, two kinases involved in the promotion of cell survival in general. Furthermore, insulin and Pdx1 mRNA were reduced under this condition (23, 66). These findings demonstrate that β1 integrin interaction with ECM components promotes β-cell proliferation and survival.
β1 Integrin inactivation has also been demonstrated to affect proliferation and survival of β-cells in vivo. Embryonic β-cell-specific inactivation of the β1 integrin resulted in significantly decreased β-cell proliferation both during embryogenesis and the postnatal period compared with control, leading to a significantly decreased β-cell area. This was attributed to a downregulation of cell-cycle genes, including Ccnd1, Ccnd2, and Ccne1 (67). The β1 integrin inactivation postnatally also generates similar results. With the use of a tamoxifen-inducible, β-cell-specific β1 integrin inactivation model, the β1 integrin was inactivated in mice at 4 weeks of age. Analysis of pancreata, 8 weeks later, revealed reduced β-cell mass as a result of a decrease in proliferation and an increase in apoptosis (68). These studies illustrate that the β1 integrin plays a key role in β-cell mass expansion and β-cell mass maintenance from embryogenesis to the postnatal period.
The ECM-Formed Scaffold and Its Role in β-Cell Proliferation and Survival
The incidence of both T1D and T2D is on the rise worldwide. It is estimated that 592 million people worldwide will have diabetes by 2035 (69). Transplantation of whole islets or stem cell-derived β-cells still remains one of the most promising treatments for T1D; however, the survival of transplanted islets rapidly declines as a result of many factors, including insufficient revascularization and loss of islet cell–ECM interactions. Here, we emphasize the importance of the three-dimensional ECM scaffold in vivo for β-cell proliferation and survival. Armed with this knowledge, many laboratories are generating ECM-containing scaffolds to prolong islet cell survival for transplantation.
Currently, there is great interest in the use of the decellularized pancreas as scaffolds for islet transplantation. In the process of decellularization, cells are removed from a tissue or organ, leaving behind a scaffold of ECM and structural proteins (70). Decellularization methods have been used to create ECM scaffolds from multiple organs, including the pancreas. These scaffolds can then be processed into hydrogels or left as they are. In one study, decellularized, healthy adult human pancreata were used to characterize the composition of the ECM scaffold and create hydrogels. These hydrogels were enriched in collagen I, collagen IV, and laminin, which highly reflect the composition of the native islet ECM. Furthermore, assessment of the microarchitecture of the scaffold and hydrogel, using electron microscopy, revealed preservation of the fibrillar bundles present in the native islet ECM (71). Similar results have been obtained using porcine pancreas, where decellularization results in an ECM scaffold that retains high amounts of ECM proteins, along with some of the vasculature (72).
Pancreatic ECM scaffolds have also been constructed from decellularized mouse pancreata (73). The three-dimensional ECM scaffold created in this study was nearly identical to the native pancreas in terms of fibrillar structure, and content of collagens I and IV, fibronectin, and laminin was preserved. In vitro studies with the scaffold were performed by recellularizing the scaffold with the mouse MIN6-immortalized β-cell line. After 5 days in culture, the MIN6 cells showed minimal apoptosis, as measured by TUNEL stain and retained insulin expression. Furthermore, insulin expression was elevated in MIN6 cells infused into the native pancreas ECM scaffold compared with MIN6 cells plated on single ECM components. The effects of decellularized pancreatic ECM scaffolds on β-cell survival have only been demonstrated in vitro thus far. Further research must be done to assess the effects of these scaffolds on β-cells in vivo before they can potentially be used as a therapeutic tool in islet transplantation.
Discussion
Islet transplantation is a very promising technique for the treatment of T1D. However, islet grafts do not remain functional long term, whether transplanted via the portal vein into the liver or within encapsulation devices (74–76). Identification of factors that increase islet graft survival would greatly benefit the development of cell-based therapies to treat diabetes. The ECM provides structural and biochemical support to cells, including β-cells in the islet. With the advent of pancreatic decellularization protocols, preservation of the native structure of the ECM can provide scaffolds that can be used for islet or β-cell transplantation to increase β-cell survival. Furthermore, the ECM acts as a depot for matricellular proteins and growth factors that can enhance β-cell proliferation and survival. Further research needs to be conducted to optimize islet transplantation using pancreas-derived ECM scaffolds and to provide selected ECM-localized growth factors as supplements.
It has been established that β1 integrin is necessary for expansion of β-cell mass during embryogenesis, leading to maintenance of β-cell mass postnatally into adulthood (67, 68). It has also been established that β1 integrin is needed to prevent loss of β-cell mass, which promotes euglycemia. Thus, it would be beneficial to increase β1 integrin–ECM interactions in the setting of islet transplantation to increase survival and proliferation. Furthermore, integrins can modulate the availability and activity of growth factor receptors to influence their signaling. Strategies to enhance integrin–growth factor receptor interactions would increase β-cell survival and proliferation in vivo (60, 63). Taken together, the current body of evidence demonstrates that there is much potential in the modulation of the islet ECM to promote β-cell proliferation and survival under metabolic stress.
Acknowledgments
Financial Support: S.E.T. was supported, in part, by the Vanderbilt University Training Program in Molecular Endocrinology (5T32 DK7563-30). M.G. was supported by grants from the American Diabetes Association (1-16-IBS-100) and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (R01 DK105689) and by a VA Merit Award (1 I01 BX003744-01).
Glossary
Abbreviations:
- BM
basement membrane
- CCN2
cellular communication network 2
- EC
endothelial cell
- ECM
extracellular matrix
- ERK
extracellular-regulated kinase
- FAK
focal adhesion kinase
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- HGF
hepatocyte growth factor
- HSPG
heparan sulfate proteoglycan
- INS-1
rat-derived
- immortalized β-cell line
MIN6
- mouse insulinoma-derived
immortalized β-cell line
- P
postnatal day
- Src
steroid receptor coactivator
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. [DOI] [PubMed] [Google Scholar]
- 2. Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131(10):2247–2256. [DOI] [PubMed] [Google Scholar]
- 3. Korpos É, Kadri N, Kappelhoff R, Wegner J, Overall CM, Weber E, Holmberg D, Cardell S, Sorokin L. The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human [published comment and response to comment appear in Diabetes. 2013;62(8):e14]. Diabetes. 2013;62(2):531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT, Otonkoski T. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51(7):1181–1191. [DOI] [PubMed] [Google Scholar]
- 5. Narayanan S, Loganathan G, Dhanasekaran M, Tucker W, Patel A, Subhashree V, Mokshagundam S, Hughes MG, Williams SK, Balamurugan AN. Intra-islet endothelial cell and β-cell crosstalk: implication for islet cell transplantation. World J Transplant. 2017;7(2):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53(5):1318–1325. [DOI] [PubMed] [Google Scholar]
- 7. Chandra R, Liddle RA. Neural and hormonal regulation of pancreatic secretion. Curr Opin Gastroenterol. 2009;25(5):441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brissova M, Powers AC. Revascularization of transplanted islets: can it be improved? Diabetes. 2008;57(9):2269–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(2):H560–H576. [DOI] [PubMed] [Google Scholar]
- 10. Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13(12):1070–1074. [DOI] [PubMed] [Google Scholar]
- 11. Nyqvist D, Speier S, Rodriguez-Diaz R, Molano RD, Lipovsek S, Rupnik M, Dicker A, Ilegems E, Zahr-Akrawi E, Molina J, Lopez-Cabeza M, Villate S, Abdulreda MH, Ricordi C, Caicedo A, Pileggi A, Berggren PO. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes. 2011;60(10):2571–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao M, Choudhary P, Srinivasan P, Tang H, Heaton N, Fung M, Barthel A, Bornstein SR, Amiel SA, Huang GC. Modification of human islet preparation: an effective approach to improve graft outcome after islet transplantation? Horm Metab Res. 2015;47(1):72–77. [DOI] [PubMed] [Google Scholar]
- 13. Llacua LA, de Haan BJ, de Vos P. Laminin and collagen IV inclusion in immunoisolating microcapsules reduces cytokine-mediated cell death in human pancreatic islets. J Tissue Eng Regen Med. 2018;12(2):460–467. [DOI] [PubMed] [Google Scholar]
- 14. Alvarez-Perez JC, Ernst S, Demirci C, Casinelli GP, Mellado-Gil JMD, Rausell-Palamos F, Vasavada RC, Garcia-Ocaña A. Hepatocyte growth factor/c-Met signaling is required for β-cell regeneration. Diabetes. 2014;63(1):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mellado-Gil J, Rosa TC, Demirci C, Gonzalez-Pertusa JA, Velazquez-Garcia S, Ernst S, Valle S, Vasavada RC, Stewart AF, Alonso LC, Garcia-Ocaña A. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic β-cell death and accelerates the onset of diabetes. Diabetes. 2011;60(2):525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riley KG, Pasek RC, Maulis MF, Peek J, Thorel F, Brigstock DR, Herrera PL, Gannon M. Connective tissue growth factor modulates adult β-cell maturity and proliferation to promote β-cell regeneration in mice. Diabetes. 2015;64(4):1284–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaido T, Yebra M, Cirulli V, Montgomery AM. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J Biol Chem. 2004;279(51):53762–53769. [DOI] [PubMed] [Google Scholar]
- 18. Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163(2):181–190. [DOI] [PubMed] [Google Scholar]
- 19. Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14(12):1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krishnamurthy M, Li J, Al-Masri M, Wang R. Expression and function of alphabeta1 integrins in pancretic beta (INS-1) cells. J Cell Commun Signal. 2008;2(3-4):67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pinkse GG, Bouwman WP, Jiawan-Lalai R, Terpstra OT, Bruijn JA, de Heer E. Integrin signaling via RGD peptides and anti-beta1 antibodies confers resistance to apoptosis in islets of Langerhans. Diabetes. 2006;55(2):312–317. [DOI] [PubMed] [Google Scholar]
- 22. Llacua A, de Haan BJ, Smink SA, de Vos P. Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes. J Biomed Mater Res A. 2016;104(7):1788–1796. [DOI] [PubMed] [Google Scholar]
- 23. Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, Rouiller DG, Halban PA. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53(8):2034–2041. [DOI] [PubMed] [Google Scholar]
- 24. Sigmundsson K, Ojala JRM, Öhman MK, Österholm AM, Moreno-Moral A, Domogatskaya A, Chong LY, Sun Y, Chai X, Steele JAM, George B, Patarroyo M, Nilsson AS, Rodin S, Ghosh S, Stevens MM, Petretto E, Tryggvason K. Culturing functional pancreatic islets on α5-laminins and curative transplantation to diabetic mice. Matrix Biol. 2018;70:5–19. [DOI] [PubMed] [Google Scholar]
- 25. Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takahashi I, Noguchi N, Nata K, Yamada S, Kaneiwa T, Mizumoto S, Ikeda T, Sugihara K, Asano M, Yoshikawa T, Yamauchi A, Shervani NJ, Uruno A, Kato I, Unno M, Sugahara K, Takasawa S, Okamoto H, Sugawara A. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem Biophys Res Commun. 2009;383(1):113–118. [DOI] [PubMed] [Google Scholar]
- 27. Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes. J Clin Invest. 2012;122(1):132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guney MA, Petersen CP, Boustani A, Duncan MR, Gunasekaran U, Menon R, Warfield C, Grotendorst GR, Means AL, Economides AN, Gannon M. Connective tissue growth factor acts within both endothelial cells and beta cells to promote proliferation of developing beta cells. Proc Natl Acad Sci USA. 2011;108(37):15242–15247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pasek RC, Dunn JC, Elsakr JM, Aramandla M, Matta AR, Gannon M. Vascular-derived connective tissue growth factor (Ctgf) is critical for pregnancy-induced β cell hyperplasia in adult mice. Islets. 2017;9(6):150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hart AW, Baeza N, Apelqvist A, Edlund H. Attenuation of FGF signalling in mouse beta-cells leads to diabetes. Nature. 2000;408(6814):864–868. [DOI] [PubMed] [Google Scholar]
- 31. Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55(9):2470–2478. [DOI] [PubMed] [Google Scholar]
- 32. Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, Hundemer GL, Kantz J, Thompson CS, Dai C, McGuinness OP, Powers AC. Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes. 2013;62(12):4154–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Leu N, Heremans Y, Coppens V, Van Gassen N, Cai Y, D’Hoker J, Magenheim J, Salpeter S, Swisa A, Khalaileh A, Arnold C, Gradwohl G, Van de Casteele M, Keshet E, Dor Y, Heimberg H. Short-term overexpression of VEGF-A in mouse beta cells indirectly stimulates their proliferation and protects against diabetes. Diabetologia. 2014;57(1):140–147. [DOI] [PubMed] [Google Scholar]
- 34. Brissova M, Aamodt K, Brahmachary P, Prasad N, Hong JY, Dai C, Mellati M, Shostak A, Poffenberger G, Aramandla R, Levy SE, Powers AC. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration [published correction appears in Cell Metab. 2015;22(4):750]. Cell Metab. 2014;19(3):498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, Shostak A, Radhika A, Poffenberger G, Quaggin SE, Jerome WG, Dumont DJ, Powers AC. Enhanced expression of VEGF-A in β cells increases endothelial cell number but impairs islet morphogenesis and β cell proliferation. Dev Biol. 2012;367(1):40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275(2):1226–1232. [DOI] [PubMed] [Google Scholar]
- 37. Dai C, Huh CG, Thorgeirsson SS, Liu Y. Beta-cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am J Pathol. 2005;167(2):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Demirci C, Ernst S, Alvarez-Perez JC, Rosa T, Valle S, Shridhar V, Casinelli GP, Alonso LC, Vasavada RC, García-Ocana A. Loss of HGF/c-Met signaling in pancreatic β-cells leads to incomplete maternal β-cell adaptation and gestational diabetes mellitus. Diabetes. 2012;61(5):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981;120(2):203–211. [DOI] [PubMed] [Google Scholar]
- 40. Yurchenco PD, Furthmayr H. Self-assembly of basement membrane collagen. Biochemistry. 1984;23(8):1839–1850. [DOI] [PubMed] [Google Scholar]
- 41. Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339(1):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–1628. [DOI] [PubMed] [Google Scholar]
- 43. Cescon M, Gattazzo F, Chen P, Bonaldo P. Collagen VI at a glance. J Cell Sci. 2015;128(19):3525–3531. [DOI] [PubMed] [Google Scholar]
- 44. Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20(1):255–284. [DOI] [PubMed] [Google Scholar]
- 45. Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115(Pt 20):3861–3863. [DOI] [PubMed] [Google Scholar]
- 46. George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119(4):1079–1091. [DOI] [PubMed] [Google Scholar]
- 47. Zhu J, Clark RAF. Fibronectin at select sites binds multiple growth factors and enhances their activity: expansion of the collaborative ECM-GF paradigm. J Invest Dermatol. 2014;134(4):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choong FJ, Freeman C, Parish CR, Simeonovic CJ. Islet heparan sulfate but not heparan sulfate proteoglycan core protein is lost during islet isolation and undergoes recovery post-islet transplantation. Am J Transplant. 2015;15(11):2851–2864. [DOI] [PubMed] [Google Scholar]
- 49. Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3(7):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kilkenny DM, Rocheleau JV. Fibroblast growth factor receptor-1 signaling in pancreatic islet beta-cells is modulated by the extracellular matrix. Mol Endocrinol. 2008;22(1):196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yun YR, Won JE, Jeon E, Lee S, Kang W, Jo H, Jang JH, Shin US, Kim HW. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. 2010;2010(1):218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, Patel Y, Suda Y, Humphries MJ, Sobel M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99(8):853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. Pancreatic islet production of vascular endothelial growth factor—a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–2985. [DOI] [PubMed] [Google Scholar]
- 54. Rahman S, Patel Y, Murray J, Patel KV, Sumathipala R, Sobel M, Wijelath ES. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005;6(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leask A, Parapuram SK, Shi-Wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal. 2009;3(2):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. [DOI] [PubMed] [Google Scholar]
- 57. Mamidi A, Prawiro C, Seymour PA, de Lichtenberg KH, Jackson A, Serup P, Semb H. Mechanosignalling via integrins directs fate decisions of pancreatic progenitors [published comment appears in Nature. 2018;564(7734):50–51]. Nature. 2018;564(7734):114–118. [DOI] [PubMed] [Google Scholar]
- 58. Afrikanova I, Yebra M, Simpkinson M, Xu Y, Hayek A, Montgomery A. Inhibitors of Src and focal adhesion kinase promote endocrine specification: impact on the derivation of β-cells from human pluripotent stem cells. J Biol Chem. 2011;286(41):36042–36052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122(Pt 2):159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213(3):649–653. [DOI] [PubMed] [Google Scholar]
- 61. Sridhar SC, Miranti CK. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin-dependent crosstalk with c-Met receptor and Src kinases. Oncogene. 2006;25(16):2367–2378. [DOI] [PubMed] [Google Scholar]
- 62. Bill HM, Knudsen B, Moores SL, Muthuswamy SK, Rao VR, Brugge JS, Miranti CK. Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol Cell Biol. 2004;24(19):8586–8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ivaska J, Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol. 2011;27(1):291–320. [DOI] [PubMed] [Google Scholar]
- 64. Fässler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9(15):1896–1908. [DOI] [PubMed] [Google Scholar]
- 65. Cirulli V, Beattie GM, Klier G, Ellisman M, Ricordi C, Quaranta V, Frasier F, Ishii JK, Hayek A, Salomon DR. Expression and function of alpha(v)beta(3) and alpha(v)beta(5) integrins in the developing pancreas: roles in the adhesion and migration of putative endocrine progenitor cells. J Cell Biol. 2000;150(6):1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Saleem S, Li J, Yee SP, Fellows GF, Goodyer CG, Wang R. beta1 Integrin/FAK/ERK signalling pathway is essential for human fetal islet cell differentiation and survival. J Pathol. 2009;219(2):182–192. [DOI] [PubMed] [Google Scholar]
- 67. Diaferia GR, Jimenez-Caliani AJ, Ranjitkar P, Yang W, Hardiman G, Rhodes CJ, Crisa L, Cirulli V. β1 Integrin is a crucial regulator of pancreatic β-cell expansion. Development. 2013;140(16):3360–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peart J, Li J, Lee H, Riopel M, Feng ZC, Wang R. Critical role of β1 integrin in postnatal beta-cell function and expansion. Oncotarget. 2017;8(38):62939–62952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. [DOI] [PubMed] [Google Scholar]
- 70. Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. [DOI] [PubMed] [Google Scholar]
- 71. Sackett SD, Tremmel DM, Ma F, Feeney AK, Maguire RM, Brown ME, Zhou Y, Li X, O’Brien C, Li L, Burlingham WJ, Odorico JS. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci Rep. 2018;8(1):10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Salvatori M, Katari R, Patel T, Peloso A, Mugweru J, Owusu K, Orlando G. Extracellular matrix scaffold technology for bioartifical pancreas engineering: state of the art and future challenges. J Diabetes Sci Technol. 2014;8(1):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, Badylak SF, Banerjee I. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34(28):6760–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dimitrioglou N, Kanelli M, Papageorgiou E, Karatzas T, Hatziavramidis D. Paving the way for successful islet encapsulation. Drug Discov Today. 2019;24(3):737–748. [DOI] [PubMed] [Google Scholar]
- 75. Farney AC, Sutherland DE, Opara EC. Evolution of islet transplantation for the last 30 years. Pancreas. 2016;45(1):8–20. [DOI] [PubMed] [Google Scholar]
- 76. Rickels MR, Roberston RP. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr Rev. 2019;40(2):631–668. [DOI] [PMC free article] [PubMed] [Google Scholar]