Abstract
Fetal exposure to gestational diabetes mellitus (GDM) and poor postnatal diet are strong risk factors for type 2 diabetes development later in life, but the mechanisms connecting GDM exposure to offspring metabolic health remains unclear. In this study, we aimed to determine how GDM interacts with the postnatal diet to affect islet function in the offspring as well as characterize the gene expression changes in the islets. GDM was induced in female rats using a high-fat, high-sucrose (HFS) diet, and litters from lean or GDM dams were weaned onto a low-fat (LF) or HFS diet. Compared with the lean control offspring, GDM exposure reduced glucose-stimulated insulin secretion in islets isolated from 15-week-old offspring, which was additively worsened when GDM exposure was combined with postnatal HFS diet consumption. In the HFS diet–fed offspring of lean dams, islet size and number increased, an adaptation that was not observed in the HFS diet–fed offspring of GDM dams. Islet gene expression in the offspring of GDM dams was altered in such categories as inflammation (e.g., Il1b, Ccl2), mitochondrial function/oxidative stress resistance (e.g., Atp5f1, Sod2), and ribosomal proteins (e.g., Rps6, Rps14). These results demonstrate that GDM exposure induced marked changes in gene expression in the male young adult rat offspring that cumulatively interact to worsen islet function, whole-body glucose homeostasis, and adaptations to HFS diets.
The incidence of gestational diabetes mellitus (GDM) is rising across all ethnicities and is estimated to affect 5% to 10% of pregnancies in the general population (1). Gestational and early postnatal environments are important determinants of adult health because the induction of epigenetic mechanisms have persistent effects on gene expression in the offspring [reviewed in (2)]. Impaired β-cell function is apparent in the offspring of women with diabetes during pregnancy by 7 years of age (3) and in adulthood (4, 5). β-Cells undergo major structural and functional changes during gestation and early postnatal life (6). Evidence from animal models shows that maternal hyperglycemia and overnutrition can trigger maladaptive β-cell growth [reviewed in (7)]. Although a connection between maternal diabetes exposure, islet dysfunction, and metabolic health outcomes of adults is apparent, the mechanisms and gene expression changes that influence islet development and function following GDM exposure have not been fully characterized. It is also unclear how diabetes during pregnancy influences the susceptibility of islets to postnatal environmental stressors such as dietary fat and sugars that are prevalent in modern society.
In this study, we used a Sprague-Dawley rat model of high-fat, high-sucrose (HFS) diet–induced GDM to investigate the effects of GDM on islet structure and function in the offspring. This model is characterized by maternal obesity, glucose intolerance, and mild hyperglycemia during pregnancy (8). We previously reported that the 15-week-old male offspring of GDM dams were more obese, insulin resistant, and exhibited hepatic steatosis when compared with their lean controls (8). In the present study, we report that GDM exposure impaired islet function, which was worsened when combined with postnatal HFS diet stress. Additionally, the pancreas of the GDM offspring exhibited maladaptation to postnatal HFS feeding because islet number and β-cell area did not increase to compensate for insulin resistance. Finally, to our knowledge, we report the first transcriptomic analysis of the effects of GDM exposure on mRNA levels in the islets of rat offspring using RNA sequencing (RNA-seq). Our findings suggest that GDM triggers impaired insulin secretion via marked changes in the expression of genes involved in inflammation, mitochondrial function, oxidative stress resistance, and protein translation. Moreover, we observe that adaptive responses to an HFS diet are lost/impaired in islets exposed to GDM.
Materials and Methods
Diet-induced GDM model
All procedures were performed in accordance with the ARRIVE guidelines and approved by the Animal Welfare Committee of the University of Manitoba, which adheres to the principles for biomedical research involving animals developed by the Canadian Council on Animal Care and the Council for International Organizations of Medical Sciences. Rats were given ad libitum access to food and water and housed two per cage. The description of the GDM model and maternal characteristics were previously published (8). Briefly, female Sprague-Dawley rats were obtained at 3 weeks of age from the University of Manitoba colony and n = 12 were randomly assigned to a low-fat (LF) diet (10% fat, 70% carbohydrate with 33% from sucrose; Research Diets D12450B) and n = 12 were randomly assigned to an HFS diet (45% fat, 35% carbohydrate with 17% from sucrose; Research Diets D12451) for a period of 6 weeks. Notably, the HFS diet is higher in sucrose content than are other purified high-fat diets. After 6 weeks of dietary intervention, animals were mated with LF diet–fed males and diets were continued in the same dams throughout pregnancy and the suckling period. Food intake and body weight of the dams were measured weekly, and a glucose test was performed at midgestation. Dams were allowed to deliver naturally and at birth, and litters were reduced to eight pups to avoid competition for food. One-day-old neonatal pups were dissected and pancreata were isolated for histological analyses. The remaining offspring were weaned at 3 weeks of age, and male offspring from each litter were randomly assigned to either the LF or HFS postnatal diet for 12 weeks. Therefore, four different experimental groups were created: offspring from LF diet–fed (“lean”) dams that were fed a postweaning LF diet (Lean-LF), offspring from the lean dams receiving a postweaning HFS diet (Lean-HFS), offspring from HFS diet–fed dams (“GDM”) fed a postweaning LF diet (GDM-LF), and offspring from GDM dams fed a postweaning HFS diet (GDM-HFS). For all analyses, data from cage mates were averaged and the litter was used as the unit of analysis (i.e., a minimum of n = 6 litters per group were analyzed). Food intake and body weight were measured weekly for all rodents. Rats at 15 weeks of age were anesthetized by an IP injection of a sodium pentobarbital overdose and blood was collected by cardiac puncture. Blood samples were collected and placed on ice for a minimum of 10 minutes and plasma was separated by centrifugation (10 min at 4000 rpm, 4°C) and stored at −80°C.
Evaluation of glucose tolerance
Glucose tolerance tests (GTTs) were performed as described previously (8). A midgestational GTT (embryonic day 15.5) was performed in pregnant rats, following a 4-hour fast to prevent prolonged hypoglycemia in pregnancy that could influence the phenotype of the offspring. GTTs were also performed in 13-week-old offspring after an overnight fast. Additionally, blood was collected from the tail vein of the offspring at the 0-minute and 30-minute time points of the GTT to measure the serum insulin levels in response to glucose administration during the GTT. Circulating concentrations of insulin were determined in duplicate using a colorimetric ELISA assay (ALPCO, Salem, NH) according to the manufacturer’s instructions. The insulin secretion index was calculated as the fold increase above baseline.
Histology and immunofluorescence
The cryopreserved pancreatic tail was sectioned completely (10 μm) and mounted. Some sections were stained with cresyl violet for determination of islet area and numbers. Immunofluorescence (IF) was performed on other sections that were incubated with glucagon [RRID:AB_659831 (9)] or insulin [RRID:AB_659820 (10)] antibodies (Cell Signaling Technologies, Danvers, MA) to visualize α-cells and β-cells, respectively. Slides were incubated with insulin (1:200) or glucagon (1:300) primary antibodies at 4°C overnight and blocked in goat serum, followed by incubation in goat anti-rabbit biotin conjugate secondary antibody [1:400, Vector Laboratories, RRID:AB_2313606 (11)] for 2 hours and followed by incubation in Alexa Fluor 488–streptavidin conjugate [1:600, Thermo Fisher Scientific, Waltham, MA; RRID:AB_2336881 (12)] for 2 hours.
Islet morphometric analyses
Eight sections (240 μm apart) were analyzed per sample and averaged. Images were taken of slides using an Olympus IX70 inverted microscope with a QImaging Retiga-SRV Fast 1394 12-bit monochrome CCD camera. Numbers of islets were counted from cresyl violet sections. The total area of islets in each section and the pancreatic area on the slide were determined from cresyl violet–stained sections and quantified with computer-assisted measurements of slides using ImageJ software. The α-cell and β-cell fractions were calculated from the IF analyses as the respective ratio of the glucagon- or insulin-positive area to the total number of cells in the islet. The ratio of the glucagon- or insulin-positive area to total pancreatic tissue area was also calculated.
Islet isolation
Pancreatic islets were isolated from Rattus norvegicus (Sprague-Dawley) as described in Li et al. (13) with modifications to accommodate the features of the rat anatomy. Immediately after euthanizing the animal, the pancreas was localized and perfused via the bile duct in situ with ice-cold collagenase V solution (Sigma-Aldrich, St. Louis, MO). Pancreatectomy was performed and the excised pancreas was immersed into a 50-mL conical tube containing 5 mL of ice-cold collagenase V solution. The pancreas was digested at 37°C for 16 minutes with gentle manual shaking at 2- to 3-minute intervals. The solution was topped up to 30 to 35 mL with RPMI 1640 (11.1 mM glucose; Gibco; Thermo Fisher Scientific) supplemented with 10% FBS (Thermo Fisher), 1% penicillin/streptomycin (VWR, Radnor, PA), and 1% l-glutamine (Hyclone Laboratories; GE Healthcare, Logan, UT). Samples were inverted to create a homogeneous solution and then centrifuged at 290 × g for 30 seconds. The supernatant was decanted and the pellet was washed with 20 mL of 1× PBS by centrifuging at 290 × g for 30 seconds. After a 100-μm cell strainer (VWR) was pre-wet with 1 to 2 mL of 1× PBS, tissue suspension was resuspended to create a homogeneous solution and filtered by passing through the cell strainer over a 50-mL tube. The strainer was tapped to enhance liquid flow and subsequently washed with 3 mL of 1× PBS. Islets captured on the strainer were washed with 10 mL of 1× PBS. The cell strainer was inverted over a sterile 100-mm tissue culture plate and the strainer was rinsed with 15 mL of 1× PBS, collecting the released islets in the cell culture dish. Using a dissection microscope (Olympus SZ61; NCL 150 Illuminator), individual clean, healthy-looking islets were picked manually using a 20-mL or 100-mL pipette. Approximately 200 to 300 islets were picked into media and rested at 37°C overnight. The next day, 45 islets were removed for secretion assays, and the remaining islets were isolated and centrifuged at 290 × g for 30 seconds. The supernatant was removed and pellets were resuspended in 300 μL of RLT buffer (Qiagen, Valencia, CA) and stored at −80°C until needed.
Islet hormone secretion assays
Forty-five islets per condition (15 islets by three replicates) were preincubated at 37°C in Krebs–Ringer bicarbonate (KRB) buffer supplemented with 2.8 mM glucose for 1 hour. Then KRB buffer was replaced with 2.8 or 16.7 mM glucose KRB buffer and islets were incubated for 30 minutes at 37°C (14, 15). Insulin concentration in the media was measured using a mouse insulin ELISA kit (ALPCO, Salem, NH). Glucagon secretion was measured using an RIA kit (Millipore, Burlington, MA). Respective insulin and glucagon secretion indexes were calculated as the fold change in hormone level from baseline. Total insulin and glucagon content was measured from islets following acid-ethanol extraction, speed vacuuming, and resuspension in water using the respective ELISA or RIA analysis above. Data were normalized to DNA content measured using a NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific).
RNA-seq library preparation and analysis of mRNA
Total RNA from the islets was extracted using an RNAeasy Micro kit (Qiagen) as per the manufacturer’s instructions. Subsequently, rRNA was removed from 2 μg of total RNA by using a Ribo-Zero rRNA removal kit (human/mouse/rat) (Illumina). Total RNA and mRNA purity and integrity were assessed using Agilent 2000 Bioanalyzer total RNA Pico and mRNA Pico assays, respectively (Agilent Technologies, Santa Clara CA). The library was prepared according to an NEB Next Ultra II directional RNA library prep kit for Illumina (New England BioLabs, Ipswich, MA). RNA (10 to 100 ng) was fragmented, random primed, converted to cDNA, and ligated to sequencing adaptors. PCR enrichment of adaptor-ligated libraries was performed and libraries were analyzed and measured using an Agilent 2000 Bioanalyzer-2100 DNA 1000 chip (Agilent Technologies). The RNA sequencing was performed at the Genome Quebec Innovation Centre using HiSeq 4000. The base calls are made by the RTA software on the computer that runs the instrument. The RTA for HiSeq 4000 is v0.2.7.7 using default parameters. The fastq files obtained from sequencing were aligned to R. norvegicus (Rnor_5) genome assembly using STAR v2.5.1b (10) using default parameters. The statistics for the obtained reads are provided in Table 1. All data generated during this study are included in this published article, and the raw data were submitted in the GEO database (no. GSE116663). All of the “chrUn” sequences were not considered in the downstream analysis. The aligned bam files were then used for finding the differentially regulated genes for which we used HOMER (v4.9) (16). First, using the HOMER script makeTagDirectory, tag directories were made, which contain basic configuration information, such as the total number of reads and the total number of unique positions with aligned reads. Then, the HOMER script analyzeRepeats.pl was used to generate raw read counts based on exons, and the counts were not adjusted and were condensed to genes. Furthermore, the raw reads were subjected to the HOMER script getDiffExpression.pl where we used DESeq2 (17) to obtain the differentially regulated genes. We used a cutoff of ≥1.5-fold and a false discovery rate (FDR) of ≤0.05 to find the significant genes. The Venn diagrams were prepared using the online tool Venny v2.1 (http://bioinfogp.cnb.csic.es/tools/venny/index.html). For quantitative PCR (qPCR) analysis, cDNA was synthesized using the ProtoScript kit (New England BioLabs). The QuantiTect SYBR Green PCR kit (Qiagen) was used to monitor amplification of cDNA on an ABI 7500 real-time PCR detection machine (Applied Biosystems, Foster City, CA). Expression of genes was assessed in duplicate using 2−ΔΔCT, and data were normalized by the geometric averaging of multiple control genes (18), including eukaryotic initiation factor 2a (eIF2a), cyclophilin A, and β-actin expression as the reference genes that were constant across all groups of offspring. Primer sequences were validated and are reported in Table 2.
Table 1.
Statistics of the Reads Obtained by Paired End Sequencing of the RNA Obtained From the Rat Samples
| Samples | Total Reads | Perfect Aligned Reads | Unmapped Reads | Read Pairs Examined | Mapped Pairs | Read Pair Duplicates | Percent Duplication | Estimated Library Size |
|---|---|---|---|---|---|---|---|---|
| 158G | 128,148,148 | 115,475,656 | 12,672,492 | 57,737,828 | 57,707,793 | 43,161,777 | 0.748 | 68,378,267 |
| 142G | 91,905,008 | 78,527,282 | 13,377,726 | 39,263,641 | 39,238,047 | 32,446,901 | 0.826 | 15,882,057 |
| 134G | 81,069,290 | 70,214,564 | 10,854,726 | 35,107,282 | 35,085,322 | 25,954,462 | 0.739 | 28,343,479 |
| 148G | 135,543,578 | 105,544,524 | 29,999,054 | 52,772,262 | 52,749,645 | 45,150,041 | 0.856 | 19,726,848 |
| 132G | 110,441,692 | 96,329,066 | 14,112,626 | 48,164,533 | 48,138,634 | 41,651,397 | 0.865 | 16,615,525 |
| 162G | 94,046,658 | 71,730,984 | 22,315,674 | 35,865,492 | 35,846,748 | 28,919,259 | 0.806 | 16,940,302 |
| 182G | 93,082,432 | 82,906,460 | 10,175,972 | 41,453,230 | 41,439,208 | 32,018,284 | 0.772 | 35,284,987 |
| 172K | 106,697,132 | 90,359,718 | 16,337,414 | 45,179,859 | 45,163,611 | 35,617,113 | 0.788 | 37,305,967 |
| 165K | 105,785,330 | 90,542,428 | 15,242,902 | 45,271,214 | 45,258,694 | 37,691,552 | 0.833 | 33,661,465 |
| 124G | 113,209,968 | 93,893,166 | 19,316,802 | 46,946,583 | 46,931,326 | 35,051,256 | 0.747 | 54,799,251 |
| 191K | 82,950,650 | 75,912,510 | 7,038,140 | 37,956,255 | 37,946,780 | 33,483,321 | 0.882 | 14,819,573 |
| 179G | 93,775,616 | 72,288,872 | 21,486,744 | 36,144,436 | 36,132,221 | 25,707,886 | 0.711 | 50,967,837 |
Table 2.
Real-Time qPCR Primer Pair Sequences
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| Ccl2 | GTCTCAGCCAGATGCAGTTAAT | CTGCTGGTGATTCTCTTGTAGTT |
| Il1b | CAGTGAGGAGAATGACCTGTTC | CGAGATGCTGCTGTGAGATT |
| Sod2 | CTGACCTGCCTTACGACTATG | CTCGGTGACGTTCAGATTGT |
| Rab32 | AATGTGACCAGAAGAAGGACAG | GTTATCCTTGGCAGAGGTTTCA |
| Pdx-1 | GCAGAACCGGAGGAGAATAAG | GGCCGGGAGATGTATTTGTTA |
| Hnf1-A | GCCTGCCTGGTAAGTGTAAA | GTTTGCCTCCTGCGTAGTTA |
| Snap25 | TCAGTATTGGGACACTGTCAAG | GTGTGTGTGTGTGTGTGTAAAG |
| Eif2A | CCTGAAGTGTGATCCTGTGTTT | CCAAATCCAGCCAGCACTAATA |
| B-actin | ACCTTCTTGCAGCTCCTCCGT | TGCCTCTCTTGCTCTGGGCCTC |
| Cyclophilin A | TCCAAAGACAGCAGAAAACTTTCG | TCTTCTTGCTGGTCTTGCCATTCC |
Statistical analysis
Data are presented as mean ± SEM. Comparisons between two groups were evaluated with an unpaired t test. Differences in measurements performed among four groups were analyzed using two-way ANOVA and a Bonferroni post hoc test with both diet and GDM as sources of variation. P < 0.05 was considered statistically significant. For RNA-seq analyses, statistical analysis was performed using R package DESeq2 where the P value correction was done using Benjamini–Hochberg analysis. The significant genes were selected with a cutoff of ≥1.5-fold and FDRs of ≤0.05. For RNA-seq, fold change analyses for all 14,407 genes were obtained, and cutoffs of −2-fold or fewer changes and 2-fold or more changes were used to obtain the differentially expressed genes. The RNA-seq data were normalized to the total read counts. The fragments per kilobase of transcript per million mapped reads values were used for making the partial least squares discriminant analysis (PLS-DA) plot.
Results
Effects of GDM on maternal and offspring body weight and litters
Consistent with our previous report (8), GDM increased maternal body weight, gestational weight gain, and midgestational hyperglycemia (Table 3). GDM did not impact litter size, although the average weight of newborn pups was significantly higher than for the lean controls (Table 3).
Table 3.
Maternal and Offspring Characteristics
| Lean | GDM | |||
|---|---|---|---|---|
| Maternal | ||||
| Body weight, g | 411.0 ± 5.5 | 511.7 ± 7.9a | ||
| Gestational weight gain, g | 88.5 ± 15.8 | 128.6 ± 6.7a | ||
| Midgestation fasting blood glucose, mM | 5.3 ± 0.1 | 8.5 ± 0.2a | ||
| Midgestation fasting serum insulin, ng/mL | 2.18 ± 0.20 | 5.12 ± 0.51a | ||
| Offspring | ||||
| Litter size | 13 ± 1 | 13 ± 1 | ||
| Neonate weight at 1 d, g | 6.1 ± 0.2 | 7.2 ± 0.2b | ||
| Postnatal diet | LF | HFS | LF | HFS |
| Body weight at 15 wk, g | 638 ± 23 | 720 ± 16b | 652 ± 7.5 | 784 ± 11a,b |
P < 0.05, lean vs GDM using a two-way ANOVA followed by a Bonferroni posttest (n = 6 by group).
P < 0.05, LF vs HFS using a two-way ANOVA followed by a Bonferroni posttest (n = 6 by group).
Impact of GDM exposure on neonatal islet structure and composition
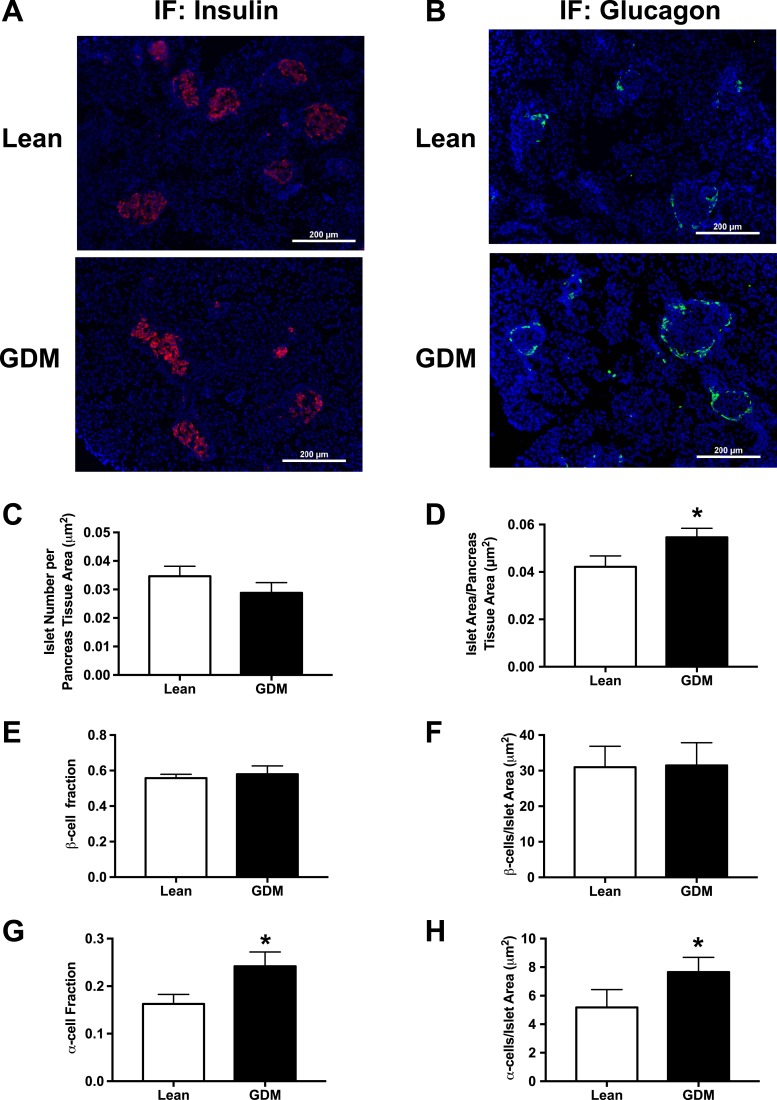
To determine how GDM exposure influences islet structure and composition at birth, we examined fixed pancreatic slices from neonates within 24 hours of birth that were born to lean and GDM dams. Slides were stained for insulin (Fig. 1A) or glucagon (Fig. 1B). Although no difference in islet number per pancreatic slice was observed (Fig. 1C), neonates born to GDM dams displayed significantly larger and more irregularly shaped islets (Fig. 1D). Immunofluorescent staining revealed that GDM did not influence the number of insulin-positive β-cells per islet (Fig. 1E and 1F) but rather increased the number of glucagon-positive α-cells per islet (Fig. 1G and 1H).
Figure 1.
One-day-old neonatal endocrine pancreas morphometry in the lean and GDM offspring. (A) Representative immunofluorescent images (original magnification, ×10) of insulin-stained pancreas sections. (B) Representative immunofluorescent images (original magnification, ×10) of glucagon-stained pancreas sections. (C) Number of islets per pancreas tissue area. (D) Islet area per pancreas tissue area. (E) β-Cell fraction. (F) Number of β-cells per islet area. (G) α-Cell fraction. (H) Number of α-cells per islet area. Data are mean ± SEM (n = 6). *P < 0.05, lean vs GDM using a Student t test.
Effects of GDM on islet structure and composition of 15-week-old offspring
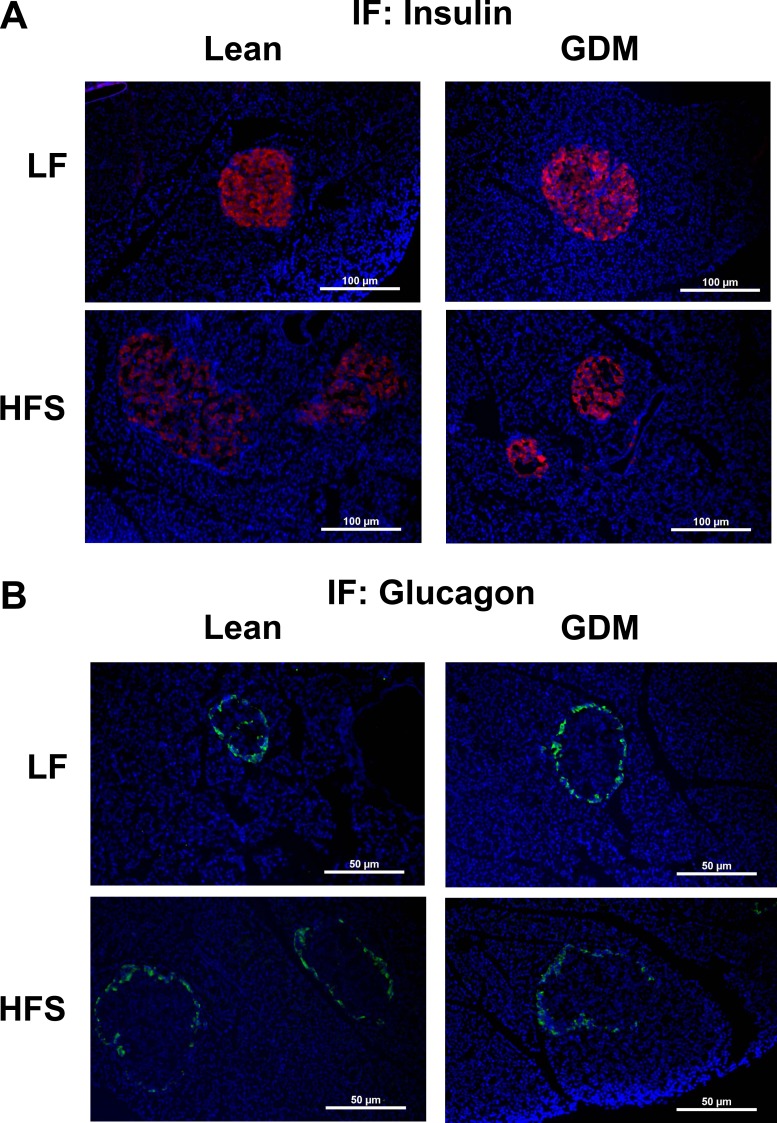
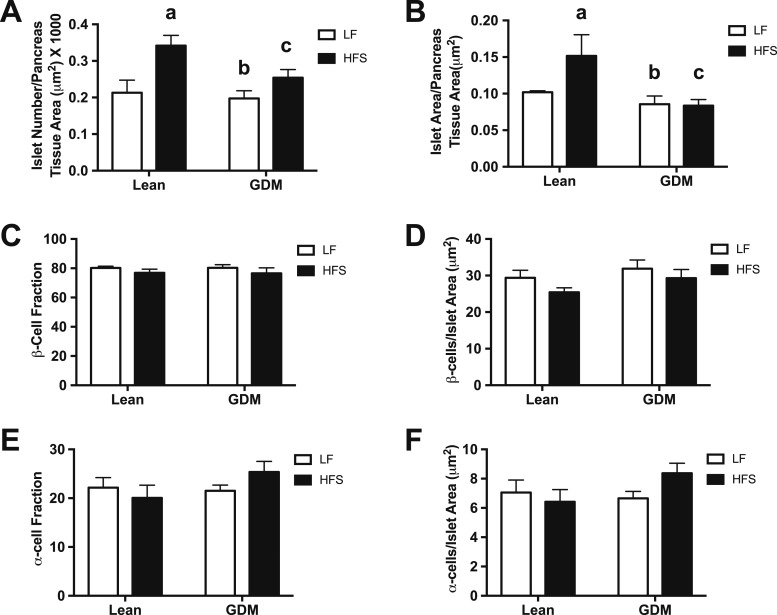
In rodents, islet development occurs late in fetal life and continues into the early neonatal stage. To determine whether the structural differences observed in the 1-day-old neonate persisted to 15 weeks of age, we examined islet structure and composition on fixed pancreatic slides. Representative images of insulin and glucagon IF are shown in Fig. 2. Similar to the neonatal stage, GDM did not influence islet numbers in the LF diet–fed 15-week-old rat offspring (Fig. 3A). Additionally, GDM exposure did not significantly affect mean islet size in LF diet–fed 15-week-old rats (Fig. 3B). In the offspring of lean dams, adaptation to postweaning HFS feeding resulted in an increase in islet number and the ratio of islet-to-pancreas area (Fig. 3A and 3B). In offspring exposed to GDM, these HFS-induced adaptive increases in the numbers of islets and islet area were not observed (Fig. 3A and 3B). Changes in β-cell or α-cell fractions and the ratio of these cell populations to the islet area were not observed (Fig. 3C–3F).
Figure 2.
Representative immunofluorescent images of endocrine pancreas of 15-wk-old lean and GDM offspring. (A) Insulin-stained pancreas (original magnification, ×10). (B) Glucagon-stained pancreas (original magnification, ×10).
Figure 3.
Endocrine pancreas morphometry of 15-wk-old lean and GDM offspring. (A) Number of islets per pancreas tissue area. (B) Islet area per pancreas tissue area. (C) β-Cell fraction. (D) Number of β-cells per islet area. (E) α-Cell fraction. (F) Number of α-cells per islet area. Data are mean ± SEM (n = 6 by group). The HFS diet included 45% fat (4.73 kcal/g); the LF diet included 10% fat (3.85 kcal/g). aP < 0.05, Lean-LF vs Lean-HFS; bP < 0.05, Lean-HFS vs GDM-LF; cP < 0.05, Lean-HFS vs GDM-HFS using a two-way ANOVA followed by a Bonferroni posttest.
Effect of GDM exposure on glucose homeostasis in vivo
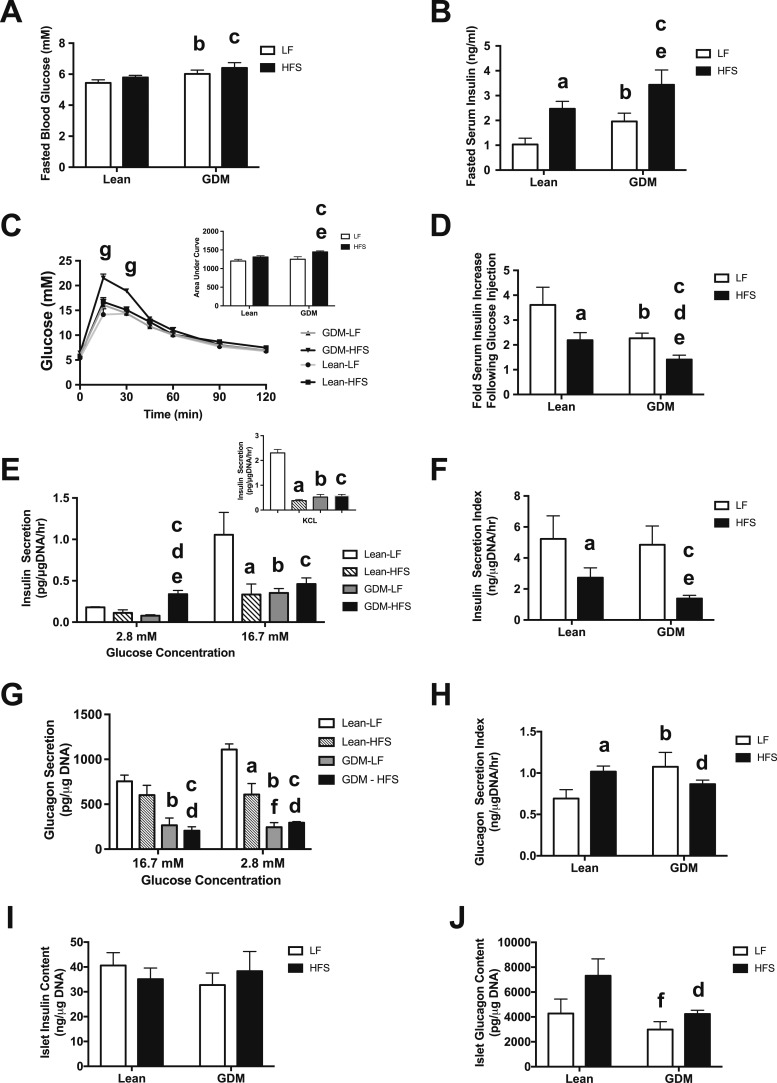
Next, we assessed the impact of GDM exposure on glucose homeostasis in vivo. The duration of the HFS diet at 15 weeks of age was not sufficient to trigger fasting hyperglycemia in lean offspring (Fig. 4A). Mild fasting hyperglycemia was found in both LF diet–fed and HFS diet–fed offspring of GDM dams (Fig. 4A), although the degree of fasting hyperglycemia was similar between the GDM-LF and GDM-HFS groups, suggesting that GDM exposure alone is responsible for the observed fasting hyperglycemia. As expected, the HFS diet triggered hyperinsulinemia in the Lean-HFS group (Fig. 4B), and the GDM-HFS group experienced hyperinsulinemia, which was not worsened by GDM exposure, suggesting that postnatal diet is the driving force behind the observed hyperinsulinemia. Next, we measured glucose excursions during 2 hours and circulating insulin levels 30 minutes after the glucose challenge. Glucose intolerance was present in the GDM-HFS group compared with all other groups (Fig. 4C, inset), which was accompanied by a significant decrease in circulating insulin levels after the glucose challenge (Fig. 4D). These findings suggest impaired glucose-stimulated insulin secretion (GSIS) in offspring born to GDM dams.
Figure 4.
Dysregulation of insulin and glucagon secretion in islets from 15-wk-old offspring of GDM dams. (A) Fasting blood glucose levels. (B) Fasting plasma insulin levels. (C) Glucose tolerance test; area under the curve (inset). (D) Insulin levels 30 min following IP glucose administration (2 g/kg body weight). (E) Ex vivo insulin secretion by isolated islets in low-glucose (2.8 mM) and high-glucose (16.7 mM) conditions; KCl (300 mM) stimulated insulin secretion (inset). (F) Glucose-stimulated insulin secretion (insulin secretion index) in islets. (G) Ex vivo glucagon secretion by isolated islets in low-glucose (2.8 mM) and high-glucose (16.7 mM) conditions. (H) Glucose inhibition of glucagon secretion (glucagon secretion index) in islets. (I) Total islet insulin content. (J) Total islet glucagon content. Data are mean ± SEM (n = 6 by group). The HFS diet included 45% fat (4.73 kcal/g); the LF diet included 10% fat (3.85 kcal/g). aP < 0.05, Lean-LF vs Lean-HFS; bP < 0.05, Lean-LF vs GDM-LF; cP < 0.05, Lean-LF vs GDM-HFS; dP < 0.05, Lean-HFS vs GDM-HFS; eP < 0.05, GDM-LF vs GDM-HFS; fP < 0.05, Lean-HFS vs GDM-LF using a two-way ANOVA followed by a Bonferroni posttest. gP < 0.05, comparing GDM-HFS offspring vs all other offspring groups, as calculated by a two-way repeated measures ANOVA with a Bonferroni posttest.
Impact of GDM exposure on islet function
To further examine how GDM exposure impacts islet hormone secretion capacities, we isolated islets from 15-week-old offspring from all four experimental groups of rats and performed static hormone (insulin and glucagon) secretion assays and measured islet hormone content. As expected, consumption of the HFS diet for 12 weeks (Lean-HFS) impaired GSIS compared with the Lean-LF offspring (Fig. 4E). Interestingly, offspring exposed to GDM that consumed a postnatal LF diet (GDM-LF) experienced the same degree of GSIS impairment as did the Lean-HFS group, despite not having consumed the HFS diet. In addition to impaired GSIS, offspring in the GDM-HFS group also experienced hypersecretion of insulin in the basal/low glucose state, dramatically reducing the insulin secretion index by 75% compared with the Lean-LF group (Fig. 4F). To assess the impact of GDM on insulin secretion events downstream of cellular depolarization, we stimulated isolated islets with KCl. We observed impaired insulin secretion in the Lean-HFS islets as well as in the GDM-LF and GDM-HFS islets (Fig. 4E, inset), suggesting that a defect in the secretion pathway downstream of depolarization at least in part contributes to the observed insulin secretion defects in these GDM-exposed groups. No differences were observed in insulin content of islets isolated from all four groups (Fig. 4I). These data demonstrate that exposure of the β-cell to GDM alone is sufficient to cause functional impairments along the β-cell insulin secretory pathway that persist into adult life and worsen the impact of subsequent environmental exposures (e.g., postnatal HFS diet) on β-cell function.
We also measured glucagon secretion capacity in isolated islets and, as expected, observed significant glucagon secretion at low glucose concentration by the islets from Lean-LF rats, which was suppressed by high glucose concentration (Fig. 4G). Exposure to a postnatal HFS diet alone impaired glucagon secretion when incubated in low glucose. Exposure to GDM worsened this impairment in both the GDM-LF and GDM-HFS groups and was accompanied by a loss of glucose-mediated suppression of glucagon secretion (Fig. 4H). Exposure to GDM did not affect the total islet glucagon content between Lean-LF and GDM-LF rats, but GDM-HFS offspring had lower islet glucagon levels compared with Lean-HFS rats (Fig. 4J). These findings suggest that GDM exposure impairs both the maximum capacity and glucose-mediated regulation of glucagon secretion.
Impact of GDM exposure on offspring islet gene expression
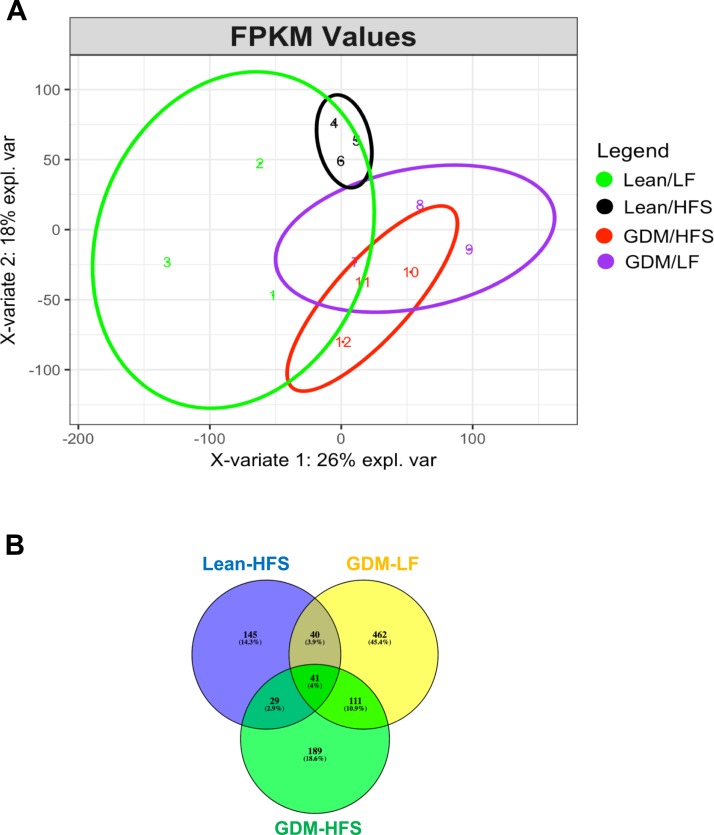
Next-generation sequencing of mRNA from pancreatic islets isolated from lean and GDM offspring detected a total of 17,407 different genes. We performed PLS-DA to understand whether different groups could be clustered based on the differential expression of the identified genes. A PLS-DA plot (Fig. 5A) shows that animals exposed to an HFS diet form a separate cluster from the control Lean-LF group, and GDM animals formed separate clusters from the lean animals. However, within the GDM animal group, the HFS diet did not completely separate the clusters from GDM animals exposed to the LF diet. To further narrow down and understand the clustering observed in PLS-DA, using Lean-LF islets as our baseline/comparison group, we determined the number of genes in each experimental group that showed a more than 2-fold change in gene expression. Postnatal HFS diet alone (Lean-HFS) significantly altered the expression of 255 genes (165 upregulated, 90 downregulated). Exposure to GDM alone without postnatal diet stress (GDM-LF) affected 654 genes (590 upregulated, 64 downregulated). GDM exposure plus a postnatal high-fat diet (GDM-HFS) altered the expression levels of 370 genes (277 upregulated, 93 downregulated). The Venn diagram in Fig. 5B shows alterations in the mRNA expression of 41 genes common to all three experimental conditions. These genes are listed in Table 4 and include regulators of cellular communication such as receptors (e.g., Folr1) and receptor ligands (e.g., Ccl9, Ccl22) as well as membrane trafficking (e.g., Rab17, Clhc1).
Figure 5.
(A) The PLS-DA plot of the RNA-seq samples using the fragments per kilobase of transcript per million mapped reads (FPKM) values. This plot shows that the Lean-HFS group is closely related to the Lean-LF group, and the GDM-LF group is closely related to the GDM-HFS group. Overall, the GDM animals are separate from the lean animals, demonstrating the influence of the maternal environment on the data. (B) Venn diagram showing genes that are significantly different (more than 2-fold changes or fewer than −2-fold changes) in the three groups of offspring compared with the Lean-LF offspring.
Table 4.
Forty-One Genes Differentially Expressed Commonly in Islets of All 15-Wk-Old Offspring Groups
| Gene Symbol | Gene Name | Lean-HFS | GDM-LF | GDM-HFS |
|---|---|---|---|---|
| Birc5 | Baculoviral IAP repeat containing 5 | 3.0 | 3.3 | 2.3 |
| Ccl9 | Chemokine ligand 9 | 2.8 | 4.1 | 2.2 |
| Ccl19 | Chemokine ligand 19 | 2.7 | 3.4 | 2.2 |
| Ccl22 | Chemokine ligand 22 | 4.0 | 7.7 | 3.6 |
| Cd2 | Cluster of differentiation 2 | 2.5 | 3.2 | 2.9 |
| Cenpw | Centromere protein W | 2.7 | 2.9 | 2.9 |
| Cldn10 | Claudin 10 | 2.8 | 2.6 | 2.9 |
| Clhc1 | Clathrin heavy chain linker 1 | 2.2 | 2.6 | 2.5 |
| Clpsl2 | Colipase-like 2 | 3.2 | 4.1 | 2.2 |
| Ctse | Cathepsin E | 2.9 | 3.4 | 2.4 |
| Dnai2 | Dynein, axonemal, intermediate chain 2 | 2.7 | 3.9 | 2.3 |
| Ebf3 | Early B-cell factor 3 | 2.6 | 2.2 | 2.4 |
| Erbb4 | Erb-b2 erythroblastic leukemia viral oncogene 4 | 2.6 | 2.5 | 3.0 |
| Fgl1 | Fibrinogen-like 1 | 2.2 | 2.9 | 2.6 |
| Folr1 | Folate receptor 1 (adult) | 2.7 | 3.0 | 2.5 |
| Haus1 | HAUS augmin-like complex, subunit 1 | 2.8 | 3.8 | 3.0 |
| Hyal4 | Hyaluronoglucosaminidase 4 | 2.7 | 3.6 | 4.2 |
| Iqcg | IQ motif containing G | −2.8 | −2.5 | −2.5 |
| Itga10 | Integrin, alpha 10 | 2.8 | 2.6 | 3.6 |
| Lin7a | Lin-7 homolog A (C. elegans) | 2.3 | 2.1 | 2.7 |
| LOC302022 | Similar to nidogen 2 protein | 2.1 | 2.6 | 2.2 |
| Mal | Mal, T-cell differentiation protein | 3.0 | 3.1 | 2.8 |
| Mbnl3 | Muscleblind-like splicing regulator 3 | 4.7 | 2.7 | 4.0 |
| Melk | Maternal embryonic leucine zipper kinase | 2.3 | 2.2 | 2.2 |
| Mir141 | MicroRNA mir-141 | 2.2 | 2.5 | 2.4 |
| Nphs1 | Nephrin | 3.5 | 4.6 | 3.8 |
| Npr3 | Natriuretic peptide receptor 3 | −2.5 | −2.8 | −2.0 |
| Plac8 | Placenta-specific 8 | 2.4 | 4.2 | 2.5 |
| Prnd | Prion protein 2 (dublet) | 2.9 | 2.2 | 2.2 |
| Psd2 | Pleckstrin and Sec7 domain containing 2 | 2.7 | 2.6 | 3.7 |
| Rab17 | RAB17, member RAS oncogene family | 2.1 | 3.2 | 2.6 |
| RGD1309350 | Similar to transthyretin (4L369) | 2.4 | 3.1 | 4.5 |
| Rn45s | 45S preribosomal RNA | 2.4 | −2.4 | −3.3 |
| RT1-Db2 | RT1 class II, locus Db2 | 2.3 | 2.7 | 2.7 |
| S100a5 | S100 calcium binding protein A5 | 3.9 | 3.4 | 3.1 |
| Serpinb5 | Serpin peptidase inhibitor, clade B (ovalbumin), member 5 | 3.4 | 2.4 | 2.7 |
| Slc30a2 | Solute carrier family 30 (zinc transporter), member 2 | 2.2 | 3.9 | 2.3 |
| Slc52a3 | Solute carrier family 52, riboflavin transporter, member 3 | 5.1 | 3.5 | 3.4 |
| Spert | Spermatid associated | −2.1 | −2.7 | −3.6 |
| Sprr1a | Small proline-rich protein 1A | 2.9 | 2.5 | 2.4 |
| Syt8 | Synaptotagmin VIII | 3.8 | 3.3 | 2.9 |
Data are log2 fold change. Fold cutoff (−2-fold or fewer changes or 2-fold or more changes), FDR of ≤0.05.
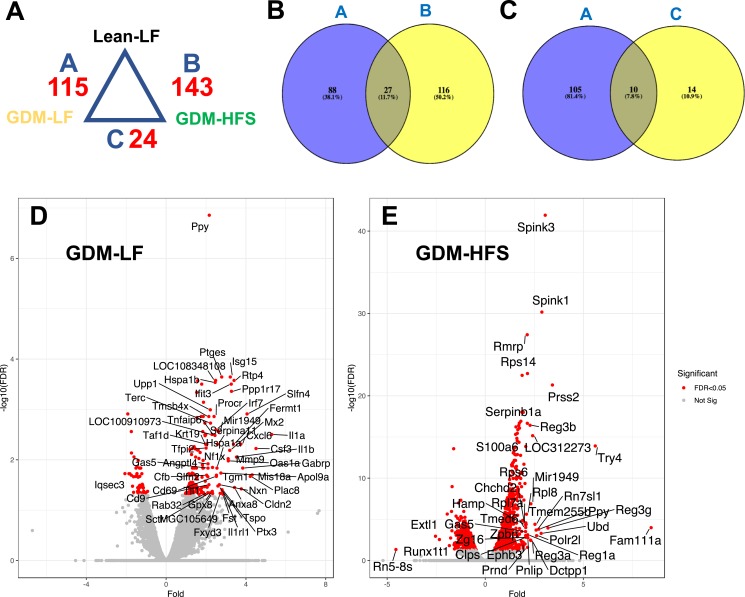
For a more stringent evaluation of the genes differentially affected by GDM exposure, we performed differential analysis using R package DESeq2. To determine the impact of GDM exposure, without the postnatal diet stress, we first compared the Lean-LF group with the GDM-LF group. This approach yielded 115 differentially expressed genes in response to GDM exposure (Fig. 6A). Of these, 102 genes were upregulated and 13 were downregulated. Among the upregulated set of genes, Il1-1a, Csf3, Slfn4, Cxcl6, Gabrp, Cldn2, and Mx2 showed the largest fold increases (Fig. 6D). Next, we determined the cumulative impact of GDM exposure plus postnatal HFS diet (GDM-HFS) on islet gene expression at 15 weeks of age using genes obtained by DESeq2 analysis. Comparison of the Lean-LF control group to the GDM-HFS group revealed 143 significantly differentially expressed genes (Fig. 6A), 126 of which were upregulated and 17 were downregulated. Among the upregulated genes, Fam111a, Prss2, Spink1/3, and Tyr4 showed the largest changes in gene expression (Fig. 6E). Among the downregulated genes, Rn5-8S, which is a noncoding RNA component of the large subunit of the ribosome, shows the largest suppression of expression (−4.6-fold).
Figure 6.
Transcriptomic profiling of islets from 15-wk-old lean and GDM offspring. (A) Differential analysis of gene expression comparing lean vs GDM groups. (B) Venn diagram showing overlapping genes that are significantly different (>1.5-fold and FDR of <0.05) between the GDM-LF and Lean-LF offspring compared with Lean-LF and GDM-HFS (e.g., A vs B). (C) Venn diagram showing overlapping genes that are significantly different (>1.5-fold and FDR of <0.05) between the GDM-LF and Lean-LF offspring compared with GDM-LF and GDM-HFS (e.g., A vs C). (D) Islet genes from GDM-LF offspring vs Lean-LF offspring, plotted as fold change against −log10(FDR) in a volcano plot. Significantly regulated genes (>1.5-fold) are marked in red. (E) Islet genes from GDM-HFS offspring vs Lean-LF offspring, plotted as fold change against −log10(FDR) in a volcano plot. Significantly regulated genes (>1.5-fold) are marked in red.
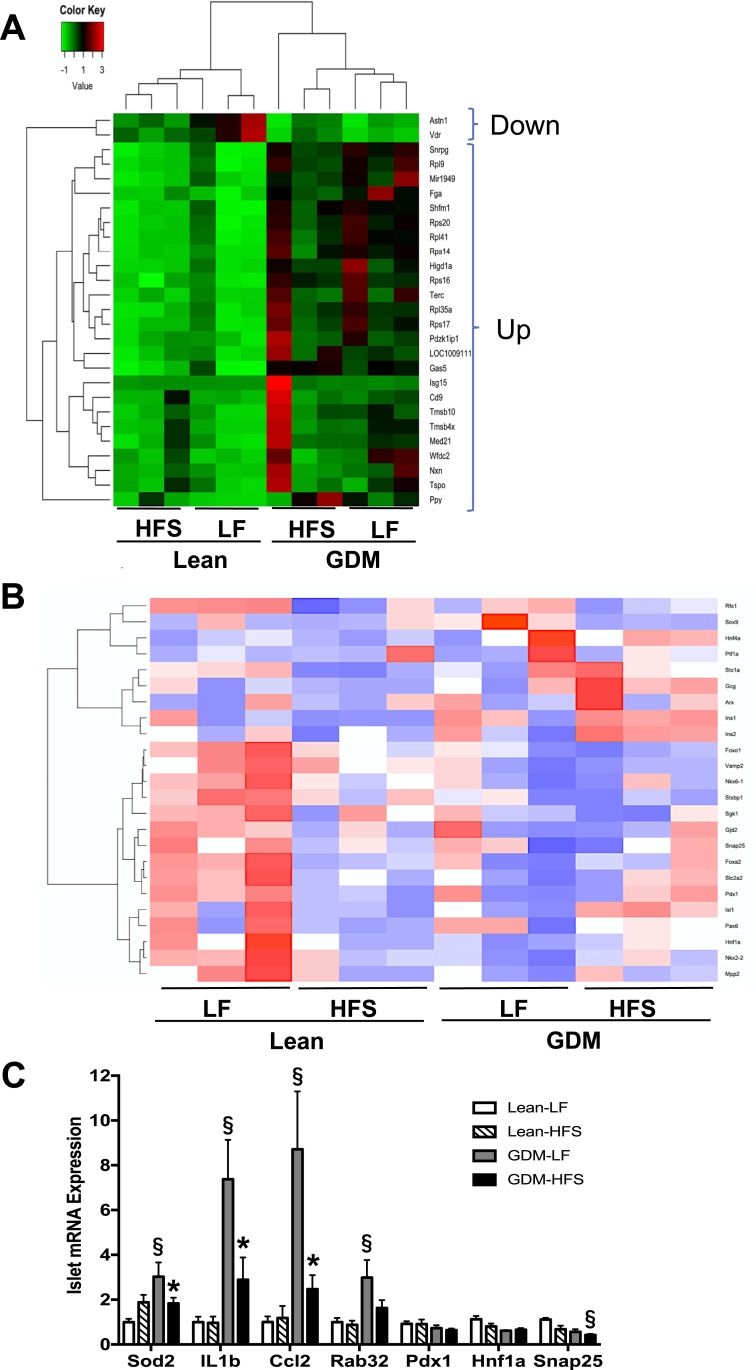
Comparison of the differentially expressed gene data sets in the GDM-LF and GDM-HFS groups revealed 27 common genes (Fig. 6B). Hierarchical clustering analysis of these 27 genes (Fig. 7A) across all four experimental groups showed two major clusters, which are formed based on GDM status and are independent of postnatal diet. Two genes were downregulated in both data sets (Astn1 and Vdr) and 25 were upregulated. Among the upregulated genes were Cd9, Gas5, Higd1a, Snrpg, and genes encoding ribosomal proteins.
Figure 7.
Enriched genes and pathways in islets from 15-wk-old lean and GDM offspring. (A) Heat map of 27 genes changed (>1.5-fold and FDR of <0.05) in the islets of GDM offspring compared with lean offspring. (B) Heat map of 24 genes involved in endocrine pancreas development and function. (C) Islet gene qPCR validation of RNA-seq expression profiles. qPCR data are mean ± SEM. The HFS diet included 45% fat (4.73 kcal/g); the LF diet included 10% fat (3.85 kcal/g). *P < 0.05, LF vs HFS; §P < 0.05, lean vs GDM using a two-way ANOVA followed by a Bonferroni posttest (n = 6 by group).
To determine how GDM exposure influenced gene expression after a postnatal HFS diet stress, we directly compared the GDM-LF group to the GDM-HFS group using DESeq2 analysis and found 24 differentially expressed genes (Fig. 6A), all of which were significantly downregulated in the GDM-HFS group (Table 5). When we compared this data set of 24 genes with the 115 genes found to be differentially expressed between the Lean-LF and GDM-LF groups, we found 10 common genes (Fig. 6C). All of these genes were upregulated in the GDM-LF offspring and downregulated in the GDM-HFS group (Table 6). Interestingly, most genes on this list are involved in inflammatory responses (Table 6).
Table 5.
Twenty-Four Genes Downregulated Represented in Islets From 15-Wk-Old GDM Offspring Compared With Lean Offspring
| Gene Symbol | Gene Name | GDM-HFS |
|---|---|---|
| Cd69 | CD69 molecule | −2.7 |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 | −2.3 |
| Tf | Transferrin | −2.1 |
| Ccr7 | C-C motif chemokine receptor 7 | −4.0 |
| Itm2a | Integral membrane protein 2A | −2.8 |
| Cp | Ceruloplasmin | −2.0 |
| Cxcl2 | C-X-C motif chemokine | −2.6 |
| Csf1 | Colony stimulating factor 1 | −1.9 |
| Igfbp5 | Insulin-like growth factor binding protein 5 | −1.9 |
| Gja1 | Gap junction protein, alpha 1 | −2.0 |
| Ptx3 | Pentraxin 3 | −4.4 |
| C3 | Complement C3 | −2.9 |
| Cfb | Complement factor B | −2.6 |
| Fmod | Fibromodulin | −6.1 |
| Il6 | Interleukin 6 | −2.3 |
| Lcn2 | Lipocalin 2 | −2.5 |
| Ifit3 | Interferon-induced protein with tetratricopeptide repeats 3 | −2.4 |
| Slfn4 | Schlafen 4 | −3.6 |
| Ngf | Nerve growth factor | −1.5 |
| Tnfaip2 | TNF alpha induced protein 2 | −2.5 |
| Ifitm3 | Interferon induced transmembrane protein 3 | −1.7 |
| Il1a | Interleukin 1 alpha | −3.9 |
| Icam1 | Intercellular adhesion molecule 1 | −1.7 |
| C1s | Complement C1s | −1.7 |
Data are log2 fold change. Fold cutoff (−1.5-fold or fewer changes or 1.5-fold or more changes), FDR of ≤0.05.
Table 6.
Ten Genes Common Between Condition A and Condition C Represented in Islets From 15-Wk-Old GDM Offspring
| Gene Symbol | Gene Name | Lean-LF | GDM-HFS |
|---|---|---|---|
| Cd69 | CD69 molecule | 2.5 | −2.7 |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 | 2.0 | −2.3 |
| Gja1 | Gap junction protein, alpha 1 | 1.9 | −2.0 |
| Ptx3 | Pentraxin 3 | 2.8 | −4.4 |
| Cfb | Complement factor B | 2.0 | −2.6 |
| Lcn2 | Lipocalin 2 | 2.0 | −2.5 |
| Ifit3 | Interferon-induced protein with tetratricopeptide repeats 3 | 3.3 | −2.4 |
| Slfn4 | Schlafen 4 | 4.1 | −3.6 |
| Ngf | Nerve growth factor | 1.8 | −1.5 |
| Il1a | Interleukin 1 alpha | 5.3 | −3.9 |
Data are log2 fold change. Fold cutoff (−1.5-fold or fewer changes or 1.5-fold or more changes), FDR of ≤0.05.
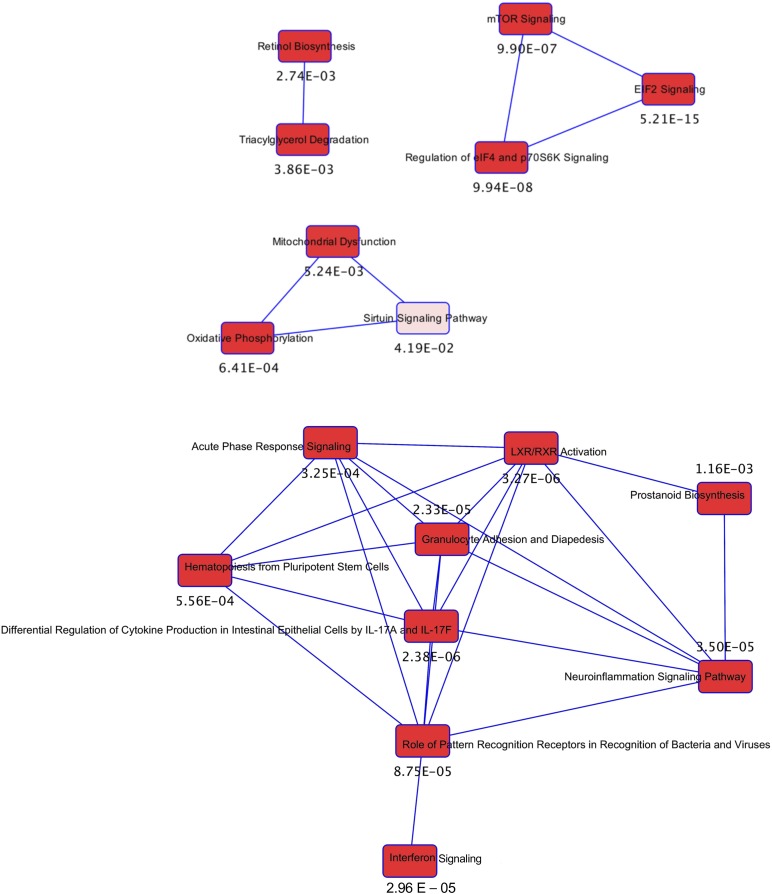
To identify novel pathways disrupted by GDM exposure, Ingenuity Pathway Analysis was used to functionally annotate the differentially expressed genes. This approach revealed an enrichment of genes involved in inflammatory pathways and cytokine production, suggesting that exposure to GDM triggers an inflammatory response in islets that persists into young adulthood. The genes ascribed to these cellular functions are listed in Table 7 and primarily include upregulated genes (e.g., Il1a, Il1b, Ptges). Additionally, a network of genes regulating protein translation and cell size, linking mTOR, EIF2, eIF4, and p70S6K signaling, was also highly represented (Fig. 8; Table 8). Finally, pathways involving retinol biosynthesis and triacylglycerol degradation were overrepresented and were linked to mitochondrial dysfunction, oxidative phosphorylation, and sirtuin signaling (Fig. 8; Table 9). Although the unbiased analyses of the RNA-seq data set revealed significant changes in the expression of many genes, we also performed a targeted assessment of a subset of 24 genes having known roles in endocrine pancreas development and function. Among these 24 genes, hierarchical clustering analysis across all four experimental groups showed a distinct cluster of 15 genes that were downregulated in Lean-HFS, GDM-LF, and GDM-HFS groups compared with Lean-LF controls (Fig. 7B). Among the downregulated genes were transcriptional regulators (e.g., Foxo1, Foxa2, Hnf1a, Isl1, Mpp2, Nkx2.2, Nkx6.1, Pax6, Pdx1, Sgk1), transporters (e.g., Slc2a2), and insulin exocytosis (e.g., Vamp2, Snap25, Stxbp1, Gjd2). We confirmed by qPCR five genes (Sod2, Il1b, Ccl2, Rab32, and Snap25) that were differentially expressed and had significant changes that were consistent with fold changes in our RNA-seq data set (Fig. 7C).
Table 7.
GDM-Induced Enrichment of Inflammatory Response Genes in 15-Wk-Old Offspring Islets
| Gene Symbol | Gene Name | GDM-LF | GDM-HFS |
|---|---|---|---|
| Birc3 | Baculoviral IAP repeat containing 3 | 1.9 | 0.7 |
| Cfb | Complement factor B | 2.0 | −0.4 |
| Cldn2 | Claudin 2 | 3.8 | 0.5 |
| Csf3 | Colony stimulating factor 3 | 4.5 | 2.0 |
| Cxcl6 | C-X-C motif chemokine ligand 6 | 3.7 | 1.7 |
| Fga | Fibrinogen alpha chain | 1.8 | 2.0 |
| Gabrp | Gamma-aminobutyric acid type A receptor pi subunit | 3.8 | 0.6 |
| Grin1 | Glutamate ionotropic receptor NMDA type subunit 1 | −1.7 | −1.4 |
| Hpx | Hemopexin | 1.6 | 0.5 |
| Ifit3 | Interferon induced protein with tetratricopeptide repeats 3 | 3.3 | 1.0 |
| Il1a | Interleukin 1 alpha | 5.3 | 2.1 |
| Il1b | Interleukin 1 beta | 3.5 | 1.2 |
| Il1rl1 | Interleukin 1 receptor like 1 | 2.8 | 1.7 |
| Il11 | Interleukin 11 | 1.5 | 0.8 |
| Irf7 | Interferon regulatory factor 7 | 2.5 | 0.6 |
| Isg15 | ISG15 ubiquitin-like modifier | 3.2 | 1.6 |
| Lcn2 | Lipocalin 2 | 2.0 | −0.5 |
| Mmp9 | Matrix metallopeptidase 9 | 3.1 | 0.7 |
| Mx2 | MX dynamin like GTPase 1 | 3.2 | 1.1 |
| Ngf | Nerve growth factor | 1.8 | 0.3 |
| Oas1a | 2′-5′-Oligoadenylate synthetase 1 | 3.1 | 1.3 |
| Ptges | Prostaglandin E synthase | 2.8 | 1.1 |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 | 2.0 | −0.2 |
| Ptx3 | Pentraxin 3 | 2.8 | −1.6 |
| Sod2 | Superoxide dismutase 2 | 1.5 | 0.6 |
Data are log2 fold change. Fold cutoff (−1.5-fold or fewer changes or 1.5-fold or more changes), FDR of ≤0.05.
Figure 8.
The interaction between differentially regulated transcriptional networks from the Ingenuity Pathway Analysis of rat islet mRNA from the offspring of GDM dams. Each pathway as a single node is colored proportionally to the Fisher exact test P value (numeric value adjacent to the node) where darker red indicates greater significance. A line connects two pathways when at least one data-set molecule is common between pathways.
Table 8.
GDM-Induced Enrichment of Protein Translation and Cell Size Genes in Islets from 15-Wk-Old Offspring
| Gene Symbol | Gene Name | GDM-LF | GDM-HFS |
|---|---|---|---|
| Rps6 | Ribosomal protein S6 | 1.7 | 2.0 |
| Rps12 | Ribosomal protein S12 | 1.4 | 1.5 |
| Rps14 | Ribosomal protein S14 | 2.0 | 2.1 |
| Rps16 | Ribosomal protein S16 | 1.5 | 1.6 |
| Rps18 | Ribosomal protein S18 | 1.2 | 1.6 |
| Rps20 | Ribosomal protein S20 | 1.6 | 1.7 |
| Rps21 | Ribosomal protein S21 | 1.4 | 1.7 |
| Rps23 | Ribosomal protein S23 | 1.3 | 1.7 |
| Rps28 | Ribosomal protein S28 | 1.4 | 1.8 |
| Rps15a | Ribosomal protein S15a | 1.5 | 1.5 |
| Rpl9 | Ribosomal protein L9 | 1.8 | 1.6 |
| Rpl27 | Ribosomal protein L27 | 1.2 | 1.5 |
| Rpl37 | Ribosomal protein L37 | 1.1 | 1.6 |
| Rpl41 | Ribosomal protein L41 | 1.6 | 1.9 |
| Rpl35a | Ribosomal protein L35a | 1.5 | 1.6 |
| Rpl36a | Ribosomal protein L36a | 1.3 | 1.5 |
| Rpl37a | Ribosomal protein L37a | 1.3 | 1.7 |
| Rpl7a | Ribosomal protein L7a | 1.5 | 2.1 |
Data are log2 fold change. Fold cutoff (−1.5-fold or fewer changes or 1.5-fold or more changes), FDR of ≤0.05.
Table 9.
GDM-Induced Enrichment of Triacylglycerol Degradation/Mitochondrial Function Genes in Islets From 15-Wk-Old Offspring
| Gene Symbol | Gene Name | GDM-LF | GDM-HFS |
|---|---|---|---|
| Atp5f1 | ATP synthase, mitochondrial Fo complex subunit B1 | 1.3 | 1.9 |
| Atp5j2 | ATP synthase, mitochondrial Fo complex subunit F2 | 1.2 | 1.6 |
| Cox4i2 | Cytochrome c oxidase subunit 4I2 | 1.1 | 1.6 |
| Cox7a2 | Cytochrome c oxidase subunit VIIa polypeptide 2-like 2 | 1.1 | 1.5 |
| Ndufa1 | NADH:ubiquinone oxidoreductase subunit A1 | 1.2 | 1.7 |
| Pnlip | Pancreatic lipase | 0.9 | 2.2 |
| Pnliprp1 | Pancreatic lipase related protein 1 | 0.8 | 1.7 |
| Pnliprp2 | Pancreatic lipase related protein 2 | 0.9 | 1.6 |
| Tomm7 | Translocase of outer mitochondrial membrane 7 | 1.1 | 1.8 |
| Tspo | Translocator protein | 2.6 | 1.7 |
Data are log2 fold change. Fold cutoff (−1.5-fold or fewer changes or 1.5-fold or more changes), FDR of ≤0.05.
Discussion
Evidence from human cohort studies indicated that exposure to diabetes during pregnancy impaired β-cell function in the offspring and correlated with early-onset type 2 diabetes [reviewed in (2)]; however, the underlying mechanisms responsible for these findings remain poorly defined. Fetal exposure to diabetes during pregnancy may alter gene expression, which could lead to defective insulin secretion and/or aberrant islet structure. We used a rat model of HFS diet–induced GDM (8) to determine how GDM programs islet structure and function in both infancy (1-day-old neonate) and in young adulthood (15 weeks of age) as well as to examine how the maternal environment interacts with the postnatal diet to influence the islet. GDM exposure markedly reduced insulin and glucagon secretion in islets isolated from male offspring and prevented compensatory increases in islet mass and number in response to postnatal HFS-diet feeding. Correspondingly, GDM induced marked changes in islet gene expression.
Previous studies using a rodent model of 60% high-fat diet–induced maternal obesity reported greater β-cell mass in the pancreas of chow-fed male offspring (19, 20). In a streptozotocin-induced model of diabetes during pregnancy, the chow-fed male offspring also had larger islets (21, 22). Consistent with these findings, our data showed that islet size was elevated in the newborn pups of GDM dams. However, we did not observe increased islet size in young adult rats. Interestingly, we show that 15-week-old offspring of GDM dams failed to increase their islet size in response to postnatal HFS feeding in a similar manner as did the Lean-HFS offspring, suggesting that GDM impaired the ability of the endocrine pancreas to adapt to the insulin resistance of HFS feeding through increasing β-cells. We also acknowledge the composition of the LF diet used in our study could impact the interpretation of findings, because the lean dams and LF diet–fed offspring consume more sucrose than did their GDM and HFS diet–fed counterparts (LF 33% vs HFS 17%). Given these differences in sucrose content, future studies should match the sucrose content of the diets. Nonetheless, the HFS diet we used contains more sucrose than do other equivalent high-fat diets, and we observed in this study as well as others (8, 23–25) that both the dams and their offspring that consumed an HFS diet developed metabolic dysfunction compared with groups fed the LF control diet. Compared with the programming effects of severe maternal hyperglycemia on the offspring pancreas in the streptozotocin-induced diabetes during pregnancy model (8), we show that the combination of mild hyperglycemia in pregnancy and high levels of fat in the offspring diet impaired β-cell expansion in the male offspring.
Despite an apparent increase in β-cell abundance reported in the aforementioned models, impaired GSIS was observed in the islets isolated from offspring exposed to maternal diabetes and high-fat diet–induced maternal obesity (20, 21). We also observed impaired GSIS in the GDM-exposed offspring. Additionally, we observed a synergistic effect of GDM exposure and postnatal HFS diet on GSIS impairment. This could be due to a combination of GDM-induced effects on islet function and an inability of the GDM offspring to compensate for HFS diet–induced insulin resistance. Mechanistically, the GDM-induced impairment of insulin secretion could result from altered gene expression. Consistent with this notion, the unbiased analysis of the RNA-seq data set revealed significant (less than 2-fold) changes in genes in several categories, including inflammation, mitochondrial function/oxidative stress resistance, and ribosomal proteins. Although we may not have detected alterations in the expression of genes that did not reach statistical significance due to the limited sample size we used for this analysis, we did observe significant changes in the expression of 654 genes in the GDM-LF group and 370 genes in the GDM-HFS group compared with lean controls. The targeted assessment of genes involved in pancreatic islet growth, differentiation, or hormone secretion capacity revealed downregulated expression of 15 genes in the Lean-HFS, GDM-LF, and GDM-HFS groups compared with the Lean-LF controls (Fig. 7B). Interestingly, these genes included several involved in insulin exocytosis such as Snap25 (Fig. 7C). Because the islet insulin content remained consistent between all four groups (Fig. 4I), the significant downregulation of known insulin exocytosis genes and impairments in maximal insulin secretion suggest that GDM exposure may impair insulin exocytosis. Dysregulation of these genes could contribute to impaired glucose-induced hormone secretion by the islets.
In addition to these genes, GDM exposure also induced marked changes in several other classes of genes in categories of inflammation, mitochondrial function/oxidative stress resistance, and protein translation. The involvement of the immune system in the regulation of metabolism may be an adaptation to changes in nutrient availability (26). The endocrine pancreas adapts to conditions of increased insulin demand by increasing its functional mass, which may be triggered by mild hyperglycemia that provokes IL-1β production from the islet (27). Additionally, IL-6 secretion is necessary for the expansion of α-cell mass and the maintenance of functional β-cells (28). Conversely, the immune system may have maladaptive effects on the endocrine pancreas when the duration and degree of metabolic stress is sustained (29). We recently reported that spleen cells isolated from the offspring of GDM dams are conditioned to increase and sustain cytokine production in response to Toll-like receptor 4 activation (23). In this study, we observed elevated chemokine expression (e.g., CCL2) in the islets of GDM offspring that could enhance recruitment of immune cells to the islet. Notably, IL-1β expression, the master regulator of other cytokines and chemokines, as well as the IL-1 receptor are upregulated in the islets of GDM offspring, consistent with its upregulation in the islets from patients with type 2 diabetes (30–32). Hou et al. (33) also observed increased expression of inflammation genes in the islets of Goto-Kakizaki rats. Using a combination of transcriptomics and proteomics, this study reported apparent inflammation priming that occurred prior to diabetes onset (4 to 6 weeks of age), whereas later stages of islet deterioration (characterized by overt diabetes) revealed amplification of inflammatory genes (33). Because we also observed the elevation of inflammatory genes in the islets of the GDM offspring, it is possible that the priming of inflammatory responses could contribute to islet dysfunction potentially leading to a more substantial hyperglycemia as the GDM offspring age. This needs to be examined in future studies.
GDM also induced changes in genes involved in mitochondrial function and oxidative stress. The ability of the β-cell to secrete insulin in response to changing blood glucose levels is tightly coupled to mitochondrial function. Thus, elevated ATP synthase mitochondrial Fo complex subunit expression in islets from GDM-exposed offspring could be an adaptive response to elevated glucose levels experienced in utero that persists into young adulthood. Concurrent with the upregulation of genes involved in oxidative phosphorylation, GDM exposure also elevated the expression of antioxidant enzymes (e.g., Sod2), suggesting that GDM exposure alters the redox balance of islets and triggers a state of oxidative stress. Recent studies have reported altered mRNA levels of genes involved in oxidative phosphorylation and mitochondrial dysfunction in islets from patients with type 2 diabetes (32, 34).
In response to increased demand induced by insulin resistance, hyperglycemia, hyperlipidemia, and chronic overnutrition, β-cells translate large quantities of proinsulin. In healthy β-cells, up to 20% of newly synthesized proinsulin may misfold and fail to reach its native conformation (35, 36). Under conditions such as insulin resistance, this proportion rises as high as 50% (35). When the level of misfolded proteins exceeds the threshold to handle misfolded proteins, β-cell failure and diabetes can result. Interestingly, in GDM offspring, we observed increased expression of a range of 18 different genes encoding ribosomal proteins, and ribosome biogenesis has been reported to occur concurrently with endoplasmic reticulum stress (37). Our findings are also consistent with recent studies that used RNA-seq to analyze the transcriptome profile of human islets of patients with type 2 diabetes and reported expression changes in genes involved in protein synthesis that are regulated by mTOR, EIF2, eIF4, and p70S6K signaling (32, 34). It is possible that elevated ribosomal protein expression is a compensatory response to prime the islets of GDM offspring to translate large quantities of proinsulin. Collectively, our findings show that GDM exposure induced marked changes in islet mRNA expression in the offspring in gene categories, including inflammation, ribosomal proteins, mitochondrial function, and oxidative stress resistance. Although the changes in mRNA expression were validated in a separate group of samples, we did not validate changes in protein expression or activity in this study. Nonetheless, the hypotheses generated by this research warrants further investigation that will be a subject of our future research.
In conclusion, this study provides a comprehensive analysis of how GDM affects the islets of the offspring. GDM induced impaired glucose-regulated hormone secretion (e.g., insulin and glucagon). Although GDM did not affect islet size and numbers in LF diet–fed offspring, GDM did impair the ability of islets to increase their size and numbers in response to high-fat feeding, which is consistent with the two-hit hypothesis [reviewed in (2)]. Our data also provide a snapshot of GDM-induced mRNA changes in young adult offspring at 15 weeks of age and represent the transcriptomic changes in islet dysfunction, but much remains to be understood regarding dynamic changes in gene expression during and following GDM exposure that occur prior to the onset of islet dysfunction. Our findings are most relevant to offspring exposed to GDM that is associated with maternal obesity-driven GDM and may not extend to GDM in lean individuals and their offspring. Nonetheless, our approach provides a way to control both the maternal and postnatal environments. A strength of our study is that we characterized the effects of GDM on offspring islet structure, function, and gene expression and compared postnatal responses of islets to LF and HFS diets. Future studies are required to understand the function of these genes in islets to understand how they are involved in the development and progression of GDM-induced islet dysfunction.
Acknowledgments
We acknowledge the excellent technical work of Marilyne Vandel and Troy J. Pereira. We thank McGill University and the Genome Quebec Innovation Centre for performing the sequencing of the RNA libraries.
Financial Support: This work was supported by Environments, Genes, and Chronic Disease Canadian Institutes for Health Research Team Grant 144626 and Canadian Institutes for Health Research Grant MOP 136885 and by funding from Research Manitoba. P.A. is the recipient of a postdoctoral fellowship from Research Manitoba. T.S.M. is the recipient of a Canada Graduate Scholarship-M and a Vanier Scholarship Award. S.M.K. is the recipient of a Research Manitoba studentship. L.K.C. was the recipient of a Canadian Institutes for Health Research/Heart and Stroke Foundation of Canada IMPACT Fellowship. G.M.H. is a Canada Research Chair in Molecular Cardiolipin Metabolism. C.A.D. is the Dr. J. A. Moorhouse Fellow of the Diabetes Foundation of Manitoba. V.W.D. is the Allen Rouse–Manitoba Medical Services Foundation Basic Scientist. Funding sources were not involved in the study design or collection or interpretation of the data.
Author Contributions: P.A., N.B., T.S.M., S.M.K., M.A.F., L.K.C., B.X., K.L.H., N.S., and C.A.D. performed the experiments. P.A. and A.J. performed bioinformatics analyses. P.A., T.S.M., S.M.K., L.K.C., N.S., G.M.H., C.A.D., and V.W.D. designed the experiments and wrote the manuscript.
Glossary
Abbreviations:
- FDR, false discovery rate
GDM, gestational diabetes mellitus
- GSIS, glucose-stimulated insulin secretion
GTT, glucose tolerance test
- HFS, high-fat, high-sucrose, IF
immunofluorescence
- KRB
Krebs–Ringer bicarbonate
- LF, low-fat
PLS-DA, partial least squares discriminant analysis
- qPCR, quantitative PCR
RNA-seq, RNA sequencing
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176–185. [DOI] [PubMed] [Google Scholar]
- 2. Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, Dolinsky VW. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci. 2018;55(2):71–101. [DOI] [PubMed] [Google Scholar]
- 3. Tam WH, Ma RC, Ozaki R, Li AM, Chan MH, Yuen LY, Lao TT, Yang X, Ho CS, Tutino GE, Chan JC. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care. 2017;40(5):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kelstrup L, Damm P, Mathiesen ER, Hansen T, Vaag AA, Pedersen O, Clausen TD. Insulin resistance and impaired pancreatic β-cell function in adult offspring of women with diabetes in pregnancy. J Clin Endocrinol Metab. 2013;98(9):3793–3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh R, Pearson E, Avery PJ, McCarthy MI, Levy JC, Hitman GA, Sampson M, Walker M, Hattersley AT. Reduced beta cell function in offspring of mothers with young-onset type 2 diabetes. Diabetologia. 2006;49(8):1876–1880. [DOI] [PubMed] [Google Scholar]
- 6. Kaung HL. Growth dynamics of pancreatic islet cell populations during fetal and neonatal development of the rat. Dev Dyn. 1994;200(2):163–175. [DOI] [PubMed] [Google Scholar]
- 7. Portha B, Chavey A, Movassat J. Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res. 2011;2011:105076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pereira TJ, Fonseca MA, Campbell KE, Moyce BL, Cole LK, Hatch GM, Doucette CA, Klein J, Aliani M, Dolinsky VW. Maternal obesity characterized by gestational diabetes increases the susceptibility of rat offspring to hepatic steatosis via a disrupted liver metabolome. J Physiol. 2015;593(14):3181–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. RRID:AB_659831, https://scicrunch.org/resolver/AB_659831.
- 10. RRID:AB_659820, https://scicrunch.org/resolver/AB_659820.
- 11. RRID:AB_2313606, https://scicrunch.org/resolver/AB_2313606.
- 12. RRID:AB_2336881, https://scicrunch.org/resolver/AB_2336881.
- 13. Li DS, Yuan YH, Tu HJ, Liang QL, Dai LJ. A protocol for islet isolation from mouse pancreas. Nat Protoc. 2009;4(11):1649–1652. [DOI] [PubMed] [Google Scholar]
- 14. Allister EM, Robson-Doucette CA, Prentice KJ, Hardy AB, Sultan S, Gaisano HY, Kong D, Gilon P, Herrera PL, Lowell BB, Wheeler MB. UCP2 regulates the glucagon response to fasting and starvation. Diabetes. 2013;62(5):1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seshadri N, Jonasson ME, Hunt KL, Xiang B, Cooper S, Wheeler MB, Dolinsky VW, Doucette CA. Uncoupling protein 2 regulates daily rhythms of insulin secretion capacity in MIN6 cells and isolated islets from male mice. Mol Metab. 2017;6(7):760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graus-Nunes F, Dalla Corte Frantz E, Lannes WR, da Silva Menezes MC, Mandarim-de-Lacerda CA, Souza-Mello V. Pregestational maternal obesity impairs endocrine pancreas in male F1 and F2 progeny. Nutrition. 2015;31(2):380–387. [DOI] [PubMed] [Google Scholar]
- 20. Zambrano E, Sosa-Larios T, Calzada L, Ibáñez CA, Mendoza-Rodríguez CA, Morales A, Morimoto S. Decreased basal insulin secretion from pancreatic islets of pups in a rat model of maternal obesity. J Endocrinol. 2016;231(1):49–57. [DOI] [PubMed] [Google Scholar]
- 21. Han J, Xu J, Long YS, Epstein PN, Liu YQ. Rat maternal diabetes impairs pancreatic β-cell function in the offspring. Am J Physiol Endocrinol Metab. 2007;293(1):E228–E236. [DOI] [PubMed] [Google Scholar]
- 22. Aerts L, Vercruysse L, Van Assche FA. The endocrine pancreas in virgin and pregnant offspring of diabetic pregnant rats. Diabetes Res Clin Pract. 1997;38(1):9–19. [DOI] [PubMed] [Google Scholar]
- 23. Li Q, Pereira TJ, Moyce BL, Mahood TH, Doucette CA, Rempel J, Dolinsky VW. In utero exposure to gestational diabetes mellitus conditions TLR4 and TLR2 activated IL-1beta responses in spleen cells from rat offspring. Biochim Biophys Acta. 2016;1862(11):2137–2146. [DOI] [PubMed] [Google Scholar]
- 24. Mughal W, Nguyen L, Pustylnik S, da Silva Rosa SC, Piotrowski S, Chapman D, Du M, Alli NS, Grigull J, Halayko AJ, Aliani M, Topham MK, Epand RM, Hatch GM, Pereira TJ, Kereliuk S, McDermott JC, Rampitsch C, Dolinsky VW, Gordon JW. A conserved MADS-box phosphorylation motif regulates differentiation and mitochondrial function in skeletal, cardiac, and smooth muscle cells. Cell Death Dis. 2015;6(10):e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vuong B, Odero G, Rozbacher S, Stevenson M, Kereliuk SM, Pereira TJ, Dolinsky VW, Kauppinen TM. Exposure to gestational diabetes mellitus induces neuroinflammation, derangement of hippocampal neurons, and cognitive changes in rat offspring. J Neuroinflammation. 2017;14(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schumann DM, Maedler K, Franklin I, Konrad D, Størling J, Böni-Schnetzler M, Gjinovci A, Kurrer MO, Gauthier BR, Bosco D, Andres A, Berney T, Greter M, Becher B, Chervonsky AV, Halban PA, Mandrup-Poulsen T, Wollheim CB, Donath MY. The Fas pathway is involved in pancreatic beta cell secretory function. Proc Natl Acad Sci USA. 2007;104(8):2861–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R, Martens G, Kerr-Conte J, Pattou F, Berney T, Pipeleers D, Halban PA, Schuit FC, Donath MY. Interleukin-6 regulates pancreatic α-cell mass expansion. Proc Natl Acad Sci USA. 2008;105(35):13163–13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Böni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC, Donath MY. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150(12):5218–5229. [DOI] [PubMed] [Google Scholar]
- 30. Böni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY. Increased interleukin (IL)-1β messenger ribonucleic acid expression in β-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93(10):4065–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110(6):851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Segerstolpe Å, Palasantza A, Eliasson P, Andersson EM, Andréasson AC, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK, Smith DM, Kasper M, Ämmälä C, Sandberg R. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24(4):593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hou J, Li Z, Zhong W, Hao Q, Lei L, Wang L, Zhao D, Xu P, Zhou Y, Wang Y, Xu T. Temporal transcriptomic and proteomic landscapes of deteriorating pancreatic islets in type 2 diabetic rats. Diabetes. 2017;66(8):2188–2200. [DOI] [PubMed] [Google Scholar]
- 34. Xin Y, Kim J, Okamoto H, Ni M, Wei Y, Adler C, Murphy AJ, Yancopoulos GD, Lin C, Gromada J. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab. 2016;24(4):608–615. [DOI] [PubMed] [Google Scholar]
- 35. Liu M, Haataja L, Wright J, Wickramasinghe NP, Hua QX, Phillips NF, Barbetti F, Weiss MA, Arvan P. Mutant INS-gene induced diabetes of youth: proinsulin cysteine residues impose dominant-negative inhibition on wild-type proinsulin transport [published correction appears in PLoS One. 2010;5(10) doi: 10.1371/annotation/6d5e12f2-defc-48b5-84f6-43253f593a2a]. PLoS One. 2010;5(10):e13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu M, Li Y, Cavener D, Arvan P. Proinsulin disulfide maturation and misfolding in the endoplasmic reticulum. J Biol Chem. 2005;280(14):13209–13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asahara S, Matsuda T, Kido Y, Kasuga M. Increased ribosomal biogenesis induces pancreatic beta cell failure in mice model of type 2 diabetes. Biochem Biophys Res Commun. 2009;381(3):367–371. [DOI] [PubMed] [Google Scholar]