Personalized medicine has greatly affected medical research and innovation. This article provides an overview of personalized medicine, looking back over the years since the term was first publicized and describing some of the achievements in relation to the treatment of malignant diseases.
Abstract
On April 16, 1999, a short article appeared in The Wall Street Journal entitled “New Era of Personalized Medicine: Targeting Drugs for Each Unique Genetic Profile,” and here, the public was introduced to the term “personalized medicine” for the first time. A few months after publication of the article, it was reprinted in The Oncologist. The article describes the formation of the Single Nucleotide Polymorphisms Consortium, which was established as a collaboration between a number of major pharmaceutical companies and several academic research institutions, with support from the Wellcome Trust Foundation. Reading the article today, one will find that several of the important arguments for an individualized therapy are described in a similar way as we have known it from the past 20 years of discussion. The article mentioned the poor efficacy of the current pharmacotherapy, disease heterogeneity, and genetic variability, a showdown with the “one‐size‐fits‐all” approach, and the use of predictive safety and efficacy biomarkers. Today, personal medicine is in competition with other terms such as “precision medicine” and “stratified medicine” and is no longer the preferred term for describing the individualized health care approach. Even though personalized medicine arose from the idea of improving and individualizing pharmacotherapy, the concept has influenced most other areas of our health care system. No matter if we use the term precision medicine or personalized medicine, the ideas that originated 20 years ago have greatly impacted the way we develop and implement new initiatives in relation to diagnosis, prevention, and treatment today.
Implications for Practice.
Since the publication of the ideas behind personalized medicine in The Wall Street Journal and The Oncologist 20 year ago, they have permeated medical research and innovation. This review will provide an overview of the background, definitions, and terminology and will describe some of the achievements in relation to the treatment of malignant diseases.
Introduction
On April 16, 1999, a short article appeared in The Wall Street Journal entitled “New Era of Personalized Medicine: Targeting Drugs for Each Unique Genetic Profile,” and here, the public was introduced to the term “personalized medicine” for the first time [1]. Two staff reporters, Robert Langreth and Michael Waldholz, wrote the article that described the formation of the Single Nucleotide Polymorphisms (SNP) Consortium. This consortium was established as a collaboration between a number of major pharmaceutical companies and several academic research institutions in the U.S. and U.K. with the support from the Wellcome Trust Foundation [2]. The goal was to provide a public resource on SNPs in the human genome, and the plan was to identify at least 300,000 [3]. The motivation for the pharmaceutical companies to join the consortium was the possibility of developing drugs designed to target the individual patients’ molecular and genetic makeups and thereby individualize pharmacotherapy. A few months after the article first appeared in The Wall Street Journal, it reappeared in The Oncologist [1]. If you make a PubMed search today using the search‐term personalized medicine, The Oncologist version of the article from 1999 will show up as the first discussing personalized medicine as we perceive it today. By republishing The Wall Street Journal article 20 years ago, The Oncologist became the first journal to introduce the idea of personalized medicine to the medical community, a topic that they have covered and promoted excellently ever since.
The article by Langreth and Waldholz contained several of the arguments for individualizing pharmacotherapy, as we know them, from the past 20 years of discussions [1]. They stated that the current pharmacotherapy was a “one‐size‐fits‐all” approach in which even the best drugs work in only 50%–70% of the patients. This aspect was also addressed a couple of years later by Spear et al. in an article published in Trends in Molecular Medicine in 2001, which has been quoted frequently in relation to the discussion about efficacy of pharmacotherapy and personalized medicine [4], [5]. Langreth and Waldholz also mentioned disease heterogeneity and the genetic variability as a factor that may impact the treatment outcome negatively. Furthermore, it was emphasized that an understanding of this variability might be able to improve the treatment outcome for the single patient.
The concept of companion diagnostics was also described but not mentioned in general terms as such; however, the authors discussed a simple diagnostic test that could inform the treating physicians of who would benefit from certain drugs and who was at risk of developing serious side effects. The description given in the article is very close to the definitions of companion diagnostics, recently defined in different guidance documents issued by the regulators in the U.S., the European Union (EU), and other countries worldwide [6], [7]. The article gave several examples of when a diagnostic test could be potentially useful as a treatment decision tool. One example was in relation to treatment of women with breast cancer. Just a few months before the article was published in The Wall Street Journal, the first treatment based on a monoclonal antibody guided by a diagnostic test was approved by the Food and Drug Administration (FDA). At the end of September 1998, trastuzumab (Herceptin; Genentech, South San Francisco, CA) obtained regulatory approval for treatment of patients with metastatic breast cancer whose tumors overexpress the HER2 protein [8]. An immunohistochemical assay (HercepTest; Dako, Glostrup, Denmark) for detecting HER2 overexpression in the tumor tissue was approved simultaneously with the drug [9]. This assay aimed at selecting patients likely to respond to treatment with trastuzumab. The development of trastuzumab was the first drug to use the drug‐diagnostic codevelopment model, in which a companion diagnostic assay is developed in parallel to the drug based on a thorough molecular understanding of the pathophysiology and the mechanism of action of the drug. Since the turn of the century this model has proven successful numerous times, especially within oncology and hematology [10].
Although trastuzumab was the first targeted cancer drug to use the drug‐diagnostic codevelopment model successfully, the first steps to combine drugs and diagnostics were taken 2 decades earlier. Here, the selective estrogen receptor modulator tamoxifen (Nolvadex; AstraZeneca, Cambridge, U.K.) was developed for treatment of metastatic breast cancer, and data on the estrogen receptor (ER) status was correlated with treatment outcome. Based on the results from a phase II trial, published in 1976, the investigators concluded that “the high degree of correlation between response and positive ER suggests the value of this test as a means to select patients for tamoxifen treatment” [11]. However, in this phase II trial, testing for ER status was only performed in 17 out of 76 enrolled patients, and the test result was not used as a selection criterion as we know it from today's enrichment trial design.
Just after the turn of the century, another important milestone for targeted therapy was passed, namely the development and regulatory approval of the small molecule tyrosine kinase inhibitor imatinib (Gleevec; Novartis, Basel, Switzerland) [12]. This drug inhibits the BCR‐ABL protein tyrosine kinase, a constitutively activated tyrosine kinase, which is present in virtually all patients with chronic myeloid leukemia (CML). The cause of CML is the translocation of regions of the BCR and ABL genes to form a BCR‐ABL fusion gene, and the product of this gene is the BCR‐ABL protein. Clinical studies with imatinib induced impressive high rates of both cytogenetic and hematologic responses in patients with CML [13], [14].
Individualized Pharmacotherapy
The goal of individualized pharmacotherapy has been on the agenda of the health care providers for decades, and one of the key elements in this effort has been the principles of rational use of drugs or rational pharmacotherapy. The essence of these principles was that the individual patients should receive medications appropriate to their clinical needs in order to optimize the benefit and minimize the harm. Already in the 1960s and 70s, these principles were translated into, for example, “the right drug for the right patient in the right dose at the right time.” [15], [16]. When we discuss personalized medicine today, we still use the same different “rights” to describe the concept. However, there is one major difference when we compare then with now, and that is the increase in our molecular understanding of the pathophysiology and the mechanisms of action of drugs, which is essential to the implementation of individualized pharmacotherapy and personalized medicine.
In the 1970s, the importance of a molecular understanding was already recognized, and when we look in a textbook on basic pharmacology from the 70s, we find the following comment regarding the concept of rational pharmacotherapy: “However, our knowledge about the pathophysiology, including the biochemical lesions for the underlying disease, is incomplete, which also applies to the kinetics and dynamics of a number of drugs. Consequently, the objectives of rational pharmacotherapy are rarely achieved in practice” [17]. During the past decades, we have experienced substantial advances in molecular medicine, which have slowly enabled us to start to practice a more individualized pharmacotherapy and develop drugs for defined subsets of patients. We have improved our molecular understanding of the drug mechanism of action as well as the exposure‐response relationship [18]. Furthermore, we have recognized that most diseases are heterogeneous and in order to achieve a more effective and safer pharmacotherapy drugs must be developed accordingly [19].
Definitions
Despite being 20 years since Langreth and Waldholz launched the idea on the “New Era of Personalized Medicine,” there is still no clear general consensus on the definition of personalized medicine. Especially within one disease area, namely oncology and hematology, the idea of individualized therapy has had great impact and here, the National Cancer Institute (NCI) describes personalized medicine as follows:
“A form of medicine that uses information about a person's genes, proteins, and environment to prevent, diagnose, and treat disease. In cancer, personalized medicine uses specific information about a person's tumor to help diagnose, plan treatment, find out how well treatment is working, or make a prognosis. Examples of personalized medicine include using targeted therapies to treat specific types of cancer cells, such as HER2‐positive breast cancer cells, or using tumor marker testing to help diagnose cancer. Also called precision medicine” [20].
Likewise, at the webpage of the U.S. FDA, we also find a definition or description of personalized medicine/precision medicine, which says that “precision medicine, sometimes known as ‘personalized medicine’ is an innovative approach to tailoring disease prevention and treatment that takes into account differences in people's genes, environments, and lifestyles. The goal of precision medicine is to target the right treatments to the right patients at the right time” [21].
For some years now, there have been lively discussions on whether to use the term personalized medicine or precision medicine and on whether they have different meanings [22], [23], [24], [25]. In the recently published European Society for Medical Oncology (ESMO) Precision Medicine Glossary, the use of the two terms was also discussed, and the conclusion was that they were interchangeable [26]. Furthermore, as it appears from the descriptions given by NCI and the U.S. FDA, the two organizations do not seem to distinguish between the two terms and thus regard them as interchangeable, or at least very similar.
By the end of 2015, the Council of the European Union issued a note on the conclusion on personalized medicine for patients, and here, they also underlined that there is no commonly agreed definition of the term personalized medicine [27]. However, they stated that it is widely understood that personalized medicine refers to a medical model using characterization of individuals’ phenotypes and genotypes (e.g., molecular profiling, medical imaging, lifestyle data) for tailoring the right therapeutic strategy for the right person at the right time, and/or to determine the predisposition to disease and/or to deliver timely and targeted prevention. The Council also state that personalized medicine relates to the broader concept of patient‐centered care, which takes into account that the health care system should respond better to patient needs. The note underlined the potential of using different “omics” technologies in the treatment of patients and clearly stated that the development and implementation of personalized medicine goes hand‐in‐hand with the development of relevant diagnostics. Furthermore, in relation to the implementation of personalized medicine it was emphasized that it is cross‐disciplinary, thus including disciplines such as bio‐informatics, epidemiology, genetics, molecular medicine, pharmacology, and statistics.
Precision Medicine, Personalized Medicine, or Stratified Medicine
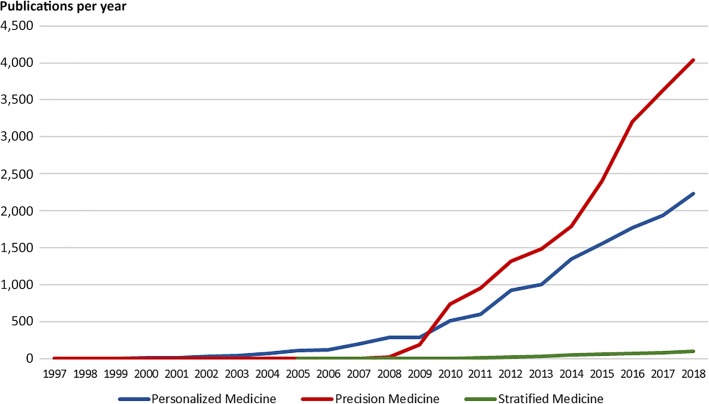
Over the past 20 years, different terms have been used to describe individualization of therapy, and in the last 8–10 years, the preferred term has shifted from personalized medicine to precision medicine, as shown in Figure 1. In fact, the term precision medicine was suggested by Prawase Wasi already back in 1997 in relation to the discussion of the perspectives of the future genomic medicine [28]. In an article discussing human genome research he wrote:
Figure 1.
Number of publications per year. The figures are based on a PubMed search using the terms of “personalized medicine,” “precision medicine,” and “stratified medicine.” This figure was updated January 2019.
“Genomics will bring about a revolution in biology and health, because it is equivalent to having a “Biological Periodic Table” which is a foundation for understanding life, health, disease and for deriving of new tools for diagnosis, treatment, prognosis and prevention. Human genomics will give rise to Predictive‐Preventive Medicine and Precision Medicine.”
In relation to precision medicine, Wasi envision that genomic medicine would have an impact on several aspects of clinical medicine, such as diagnosis, prognosis, and treatment. Although the article by Wasi was published in 1997, it was not until 2009 and 2010 that the term precision medicine started to be used with the same frequency as personalized medicine and with the announcement of the “Precision Medicine Initiative” in January 2015, by former U.S. president Barack Obama in his State of the Union Address, it became the absolute preferred term [29].
For clinicians and scientists working with assay development, the term precision medicine might give rise to some odd associations. Precision is a term used frequently in relation to the analytical validation of a diagnostic assay, which expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under specified conditions [30]. If an assay or a method is said to have a high precision, it indicates that it can reliably be reproduced again and again, but it does not say anything about accuracy; in fact, a method with a high precision can very well be inaccurate. Imagine a picture with a bull's‐eye target with all darts clustered together, but not in the center, and you see what a precise but inaccurate approach that is. If this should be translated to a therapeutic intervention, it could be a drug that interacts with the same target over and over again, but this target may not be the one intended, and thus will hardly lead to the desired outcome.
Another term that has been used much less frequent is stratified medicine, which to some extent reflects how individualized pharmacotherapy is currently practiced. According to ESMO Precision Medicine Glossary, it is defined as “the use of a molecular assay to define subpopulations, rather than individuals, who are likely to benefit from a treatment intervention” [26]. The term stratified medicine was suggested by Sean Xinghua Hu and colleagues in 2005 in an article discussing pharmacogenomics and personalized medicine [31]. In a report issued in 2013 by the World Health Organization on the “Priority Medicines for Europe and the World,” personalized medicine was discussed, and the conclusion was that “stratified medicine” was currently the most appropriate term to use [32]. The reason for this preference was that it reflects the realistic effects of medicines at population level, whereas the term personalized medicine more reflects the promise of individualized unique drug targeting and development. In the U.K., the term stratified medicine has been used relatively frequently thus far, and its use has also been endorsed by several researchers in the past [25], [33], [34].
Drug‐Diagnostics Combinations
Predictive biomarkers are important elements in the realization of personalized medicine, and probably the single most important one. These biomarkers are used to identify patients who are likely to experience a favorable outcome of pharmacotherapy and thereby enable individualization and avoid inappropriate and often expensive treatments. The regulatory terms for these predictive biomarker assays are companion or complementary diagnostics, often developed in parallel to the drugs they are meant to guide using the drug‐diagnostic codevelopment model [35].
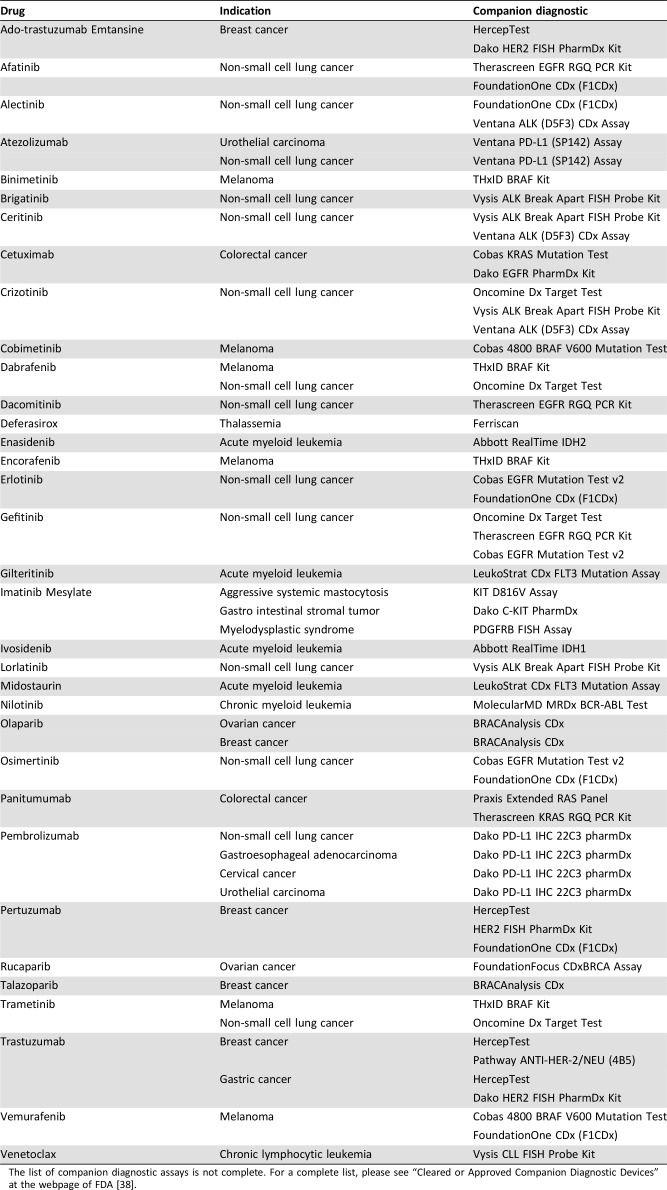
Because of the increasing role of companion diagnostics in drug development and in clinical practice, especially within oncology and hematology, new guidelines and legislation have been issued in recent years in a number of countries and regions, such as Australian, Canada, the EU, Japan, and the U.S. [35]. In 2014, an official definition of a companion diagnostic was published in a guideline issued by the FDA stating that it is an in vitro diagnostic assay that provides information that is essential for the safe and effective use of a corresponding therapeutic product [36]. Recently, the EU has also issued new legislation on in vitro diagnostic medical devices, which contain a definition similar to the one by the FDA [37]. Currently in the U.S., more than 30 different drugs have a companion diagnostic linked to their use. These drugs are listed in Table 1, together with their indications and one or more companion diagnostic assays [38]. For these drugs, information on testing is included in the labelling, as they are considered safe and effective only if they are used together with their companion diagnostic assay [36].
Table 1. Drugs that have an FDA approved companion diagnostic assay linked to their use.
The list of companion diagnostic assays is not complete. For a complete list, please see “Cleared or Approved Companion Diagnostic Devices” at the webpage of FDA [38].
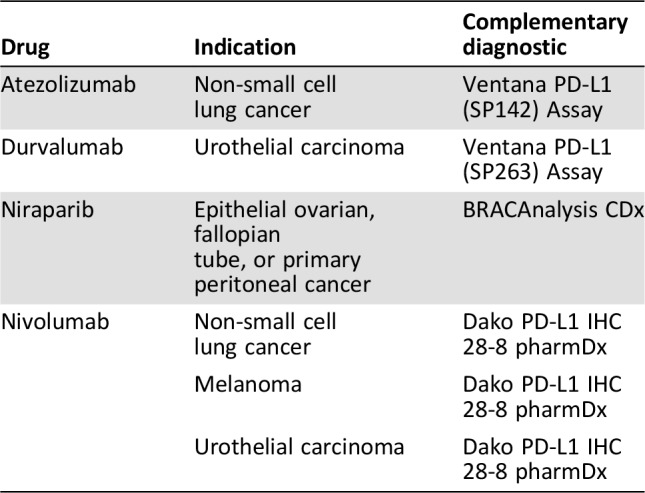
With the recent approval of the immune checkpoint inhibitors targeting programmed cell death 1 or programmed cell death ligand 1 a new regulatory class of biomarker assays has emerged, which is the complementary diagnostic [10]. This term is relatively new and introduced by the FDA when they approved nivolumab (Opdivo; Bristol‐Myers Squibb, New York City, NY) for second‐line treatment of nonsquamous non‐small cell lung cancer in the autumn of 2015. During the review of the clinical documentation for a new drug and its companion diagnostic assay, the FDA may determine that the assay is not essential for the safe and effective use of the corresponding therapeutic product. However, if the assay identifies a biomarker‐defined subset of patients that respond differentially to the drug and also aids the risk/benefit assessment for the individual patients simultaneously, the term complementary diagnostic can be used [39]. To date, there are four drugs that have been approved with a complementary diagnostic linked to their use. These drugs are listed in Table 2, together with their indications and assays. In contrast to the regulatory requirements for drugs that have a companion diagnostic assay linked to their use, testing with a complementary diagnostic is not mandatory before prescribing the drug, and testing information is not included in the labelling for the therapeutic product [10].
Table 2. U.S. Food and Drug Administration‐approved drug with a complementary diagnostic linked to their use.
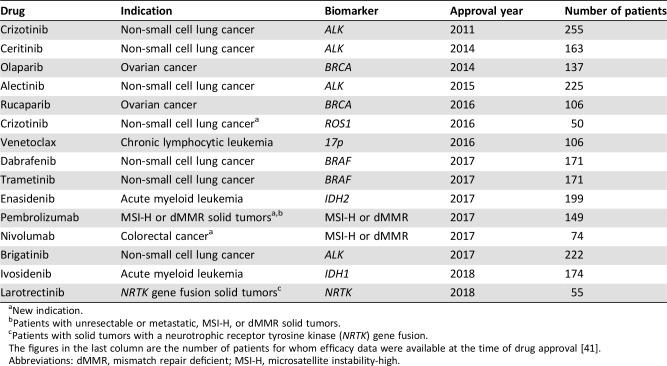
The drug‐diagnostic codevelopment model has had major impact on drug development in the past 20 years. Only a few years back, the traditional path in drug development would have involved phase I–III clinical trials including a large number of patients using an all‐comers design [35], [40]. In the drug‐diagnostic codevelopment model the assumption is that specific molecular characteristics need to be present for the drug to be effective. This means that molecular testing becomes an important part of the inclusion criteria when patients are enrolled in a clinical trial. For a number of targeted cancer drugs, this approach has shown that efficacy can be demonstrated in a surprisingly small number of patients because a large part of the nonresponders have been screened out by companion diagnostic testing. In Table 3 are listed examples of targeted cancer drugs that have obtained FDA approval, together with a companion diagnostic assay based on efficacy data from phase I/II studies involving relatively few patients in single‐arm, nonrandomized enrichment trials [27]. Just a few years ago, it would have been inconceivable that drugs or new indications would have obtained regulatory approval based on efficacy data from only 50–100 patients, as recently seen with drugs like crizotinib (Xalkori; Pfizer, New York City, NY), rucaparib (Rubraca; Clovis Oncology; Boulder, CO), Venetoclax (Venclexta; AbbVie/Genentech; North Chicago, IL), Larotrectinib (Vitrakvi; Loxo Oncology/Bayer; Stamford, CT), and nivolumab [41]. These examples are still few, but no doubt in the years to come they will be joined by other targeted drugs developed for molecularly defined subsets of patients. Therefore, the traditional drug development model, with its phase I–III clinical trials, seems to be replaced gradually by a more innovative and adaptive development process [42]. What we currently experience within oncology may be regarded as a kind of “disruption” of the traditional drug development model [35].
Table 3. Drug‐diagnostic combinations that have obtained FDA approval based on efficacy data from small phase I/II trials.
New indication.
Patients with unresectable or metastatic, MSI‐H, or dMMR solid tumors.
Patients with solid tumors with a neurotrophic receptor tyrosine kinase (NRTK) gene fusion.
The figures in the last column are the number of patients for whom efficacy data were available at the time of drug approval [41].
Abbreviations: dMMR, mismatch repair deficient; MSI‐H, microsatellite instability‐high.
The recent remarkable approvals of larotrectinib (Vitrakvi; Loxo Oncology/Bayer) for patients with NTRK gene fusion and pembrolizumab (Keytruda; Merck Sharp & Dohme, Chalfont, PA) for microsatellite instability‐high (MSI‐H) and mismatch repair deficient (dMMR)‐positive patients marks a paradigm shift in biomarker guided drug development [43], [44]. In contrast to the previous drugs, they are not developed for a conventional cancer indication defined by tumor histology and origin but solely on their effect related to specific molecular characteristics. The clinical efficacy data that led to the approval of larotrectinib comprised data from 55 patients diagnosed with 12 different conventional cancer indications, but with one common denominator that their tumors harbored a NTRK gene fusion [45]. For pembrolizumab, the efficacy data originate from 149 MSI‐H/dMMR‐positive patients with 15 conventional cancer indications, as shown in Table 3 [46], [47]. For the NTRK gene fusion indication in relation to larotrectinib and the MSI‐H/dMMR indication for pembrolizumab, the “basket trial” approach seems to work, but this is not the case for all targeted cancer drugs. A couple of recent published basket trials, one with vemurafenib (Zelboraf; Roche/Genentech) in BRAF V600 mutation‐positive patients and one with neratinib (Nerlynx; Puma Biotechnology, Los Angeles, CA) in patients harboring HER2 or HER3 mutations, show that the drugs do not seem to show the same universal efficacy [48], [49]. In these trials only, a small part of the conventionally defined cancer indications responded to the treatment despite the presence of the specific mutations, so here, tumor histology and origin may play a role.
When discussing the development and regulatory approval of the increasing number of targeted cancer drugs, it is important to bear in mind the changes in approval policy. Based on new legislation, the FDA has implemented programs intended to facilitate and expedite development and review of new drugs, which address unmet medical need in the treatment of serious or life‐threatening conditions, such as Breakthrough Therapy Program [50]. These programs have facilitated faster access to new innovative drugs for a number of patients with cancer. Many new cancer drugs are now approved based on response‐rate and progression‐free survival endpoints, whereas in the past, the gold standard for receiving FDA approval was improved overall survival [51]. The way that most cancer drugs are developed through the drug‐diagnostic codevelopment model calls for an integration of the different medical and scientific disciplines. The FDA's innovative response to this was to form the Oncology Center of Excellence, where scientists and reviewers from different medical product centers have joined under one regulatory umbrella in order to strengthen the collaborative review of drug‐diagnostic combinations [35], [52].
Future Aspects and Concluding Remarks
Personalized medicine should be considered a continuum of the efforts that have been exerted for decades to try to individualize pharmacotherapy. Before the genomic era, discriminant analysis, based on phenotypical characteristics, was used to try to determine whether this type of information could predict outcome, although often with limited positive results. With the development of molecular medicine, our ability to understand the pathophysiology and the drug mechanism of action have increased considerably. Drugs work at the molecular level, and it is here we must seek solutions to a more effective and individualized pharmacotherapy. Within the past few decades, the advances in molecular diagnostics have enabled health care providers, especially within oncology and hematology, to match the patients with an optimal treatment and thus improve the outcome.
For the past decades, the increased knowledge of the human genome and the use of next‐generation sequencing (NGS) have had major impact on drug development activities. However, when it comes to the routine clinical patient care, DNA sequencing has played a minor role so far, which is also reflected in the relativity few FDA‐approved companion diagnostics based on NGS. This will change, and we have recently seen the first assays, such as the FoundationOne CDx assay (Foundation Medicine, Cambridge, MA) and the Oncomine Dx Target Test (Thermo Fisher Scientific, Waltham, MA), obtain FDA approval [38]. No doubt, NGS will play an increasing role in relation to personalized medicine not only in relation to specific gene panels, as with the current assays, but also with regard to both whole exome and whole genome sequencing. Another technology that has emerged rapidly within the past few years is the use of “liquid biopsies” in which mainly cell‐free DNA (cfDNA) derived from plasma is analyzed [53]. Although the technology needs to be further developed, cfDNA analysis has the potential to transform both drug development and patient care, especially within oncology and hematology.
As seen in Table 1, the majority of the drugs were developed based on the “one biomarker one drug” approach, which has dominated targeted cancer drug development for the past couple of decades. With the increased understanding of disease heterogeneity and molecular pathways, a different type of companion diagnostics assays will be developed in the years to come. These assays will have a much more complex signature and likely integrate different types of omics data, such as proteomics, genomics, microbiomics, metabolomics, and phenotypical data, into a kind of “composite biomarker” using different types of algorithms [39]. Compared with the single biomarker approach, these signatures should be able to integrate more elements of the pathophysiology and the mechanisms of action of the drugs. However, tests based on such a complex signature will possess a number of challenges both with respect to the development, validation, and interpretation as well as in the clinical implementation.
Personalized medicine arises out of the idea of improving and individualizing pharmacotherapy. This concept has spread to most areas of our health care system and today greatly influences the way we implement initiatives related to diagnosis, prevention, and treatment. When it comes to pharmacotherapy, we have learned that one size does not fit all, and 20 years ago, Langreth and Waldholz named the efforts of individualizing therapy, which they called personalized medicine. This term has acquired strong competition from individualized and precision medicine during the last 5–10 years, but the idea will survive, and the efforts in relation to reaching the proclaimed goal of “targeting drugs for each unique genetic profile” will continue with increased pace in decades ahead.
Disclosures
Jan Trøst Jørgensen: Agilent Technologies, Euro Diagnostica, Azanta, Oncology Venture (C/A), AstraZeneca, Roche, Merck Sharp & Dohme (H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Langreth R, Waldholz M. New era of personalized medicine: Targeting drugs for each unique genetic profile. The Oncologist 1999;4:426–427. [PubMed] [Google Scholar]
- 2.SNP Consortium Announced . BioProcess Online. Available at https://www.bioprocessonline.com/doc/snp‐consortium‐announced‐0001. April 19, 1999. Accessed November 28, 2018.
- 3.Sachidanandam R, Weissman D, Schmidt SC et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 2001;409:928–933. [DOI] [PubMed] [Google Scholar]
- 4.Spear BB, Heath‐Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med 2001;7:201–204. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen JT. Clinical application of companion diagnostics. Trends Mol Med 2015;21:405–407. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration . In Vitro Companion Diagnostic Devices. Guidance for Industry and Food and Drug Administration Staff. Available at http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM262327.pdf. August 6, 2014. Accessed November 29, 2018.
- 7.European Union . Regulation (EU) 2017/746 of the European Parliament and of the council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. Official Journal of the European Union. Available at http://eur‐lex.europa.eu/legal‐content/EN/TXT/PDF/?uri=CELEX:32017R0746&from=EN. April 5, 2017. Accessed November 29, 2018.
- 8.Slamon DJ, Leyland‐Jones B, Shak S at al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen JT, Winther H. The development of the HercepTest ‐ From bench to bedside In: Jørgensen JT, Winther H. eds. Molecular Diagnostics: The Key Driver of Personalized Cancer Medicine. Singapore: Pan Stanford Publishing, 2010:43–60. [Google Scholar]
- 10.Jørgensen JT. Hersom M. Companion diagnostics–a tool to improve pharmacotherapy. Ann Transl Med 2016;4:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerner HJ, Band PR, Israel L et al. Phase II study of tamoxifen: Report of 74 patients with stage IV breast cancer. Cancer Treat Rep 1976;60:1431–1435. [PubMed] [Google Scholar]
- 12.Chabner BA. The oncologic four‐minute mile. The Oncologist 2001;6:230–232. [PubMed] [Google Scholar]
- 13.Druker BJ, Talpaz M, Resta DJ et al. Efficacy and safety of a specific inhibitor of the BCR‐ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344:1031–1037. [DOI] [PubMed] [Google Scholar]
- 14.Kantarjian H, Sawyers C, Hochhaus A et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645652. [DOI] [PubMed] [Google Scholar]
- 15.Klett CJ, Moseley EC. The right drug for the right patient. J Consult Psychol 1965;29:546–551. [DOI] [PubMed] [Google Scholar]
- 16.Galbrecht CR, Klett CJ. Predicting response to phenothiazines: The right drug for the right patient. J Nerv Ment Dis 1968;147:173–183. [DOI] [PubMed] [Google Scholar]
- 17.Juul P. Basic Pharmacology, 2nd ed Copenhagen: Forlaget for Faglitteratur; 1978. [Google Scholar]
- 18.Lesko LJ, Schmidt S. Individualization of drug therapy: history, present state, and opportunities for the future. Clin Pharmacol Ther 2012;92:458–466. [DOI] [PubMed] [Google Scholar]
- 19.Jørgensen JT. A challenging drug development process in the era of personalized medicine. Drug Discov Today 2011;16:891–897. [DOI] [PubMed] [Google Scholar]
- 20.NCI Dictionary of Cancer Terms . Personalized Medicine. National Cancer Institute. Available at https://www.cancer.gov/publications/dictionaries/cancer‐terms/def/personalized‐medicine. Accessed December 1, 2018.
- 21.Precision Medicine. U.S. Food and Drug Administration. (https://www.fda.gov/medicaldevices/productsandmedicalprocedures/invitrodiagnostics/precisionmedicine‐medicaldevices/default.htm). Updated September 27, 2018. Accessed December 1, 2018.
- 22.Roden DM, Tyndale RF. Genomic medicine, precision medicine, personalized medicine: What's in a name? Clin Pharmacol Ther 2013;94:169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman AM. Bench‐to‐bedside; Clinical and translational research; Personalized medicine; Precision medicine‐what's in a name? Clin Transl Sci 2015;8:171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali‐Khan S, Kowal S, Westerly L et al. Terminology for personalized medicine: A systematic collection. PACEOMICS. Available at http://paceomics.org/wp‐content/uploads/2016/01/Terminology‐for‐PM‐methods_final1.pdf. January 2016. Accessed December 2, 2018. [Google Scholar]
- 25.March R, Schott C. Personalized/Precision Medicine/Personalised Healthcare: The art of giving different names to the same thing? Per Med 2017;14:463–466. [DOI] [PubMed] [Google Scholar]
- 26.Yates LR, Seoane J, Le Tourneau C et al. The European Society for Medical Oncology (ESMO) Precision Medicine Glossary. Ann Oncol 2018;29:30–35. [DOI] [PubMed] [Google Scholar]
- 27.Council of the European Union . Council conclusions on personalised medicine for patients. 15054/15. Brussels. Available at http://data.consilium.europa.eu/doc/document/ST‐15054‐2015‐INIT/en/pdf. December 7, 2015. Accessed December 3, 2018.
- 28.Wasi P. Human genomics: Implications for health. Southeast Asian J Trop Med Public Health. 1997;28(suppl 2):19–24. [PubMed] [Google Scholar]
- 29.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Food and Drug Administration . Guideline for Industry. Text on Validation of Analytical Procedures. ICH‐Q2A. Available at https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073381.pdf. March 1995. Accessed December 6, 2018.
- 31.Kaplan W, Wirtz VJ, Mantel‐Teeuwisse A et al. Priority Medicines for Europe and the World 2013 Update. Geneva, Switzerland: World Health Organization; July 9, 2013. Available at http://www.who.int/medicines/areas/priority_medicines/MasterDocJune28_FINAL_Web.pdf. Accessed December 8, 2018. [Google Scholar]
- 32.Hu SX, Foster T, Kieffaber A. Pharmacogenomics and personalized medicine: Mapping of future value creation. Biotechniques 2005;39(suppl):1–6. [DOI] [PubMed] [Google Scholar]
- 33.Jørgensen JT. Are we approaching the post‐blockbuster era? Pharmacodiagnostics and rational drug development. Expert Rev Mol Diagn 2008;8:689–695. [DOI] [PubMed] [Google Scholar]
- 34.Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: Strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov 2007;6:287–293. [DOI] [PubMed] [Google Scholar]
- 35.Jørgensen JT, Hersom M. Clinical and regulatory aspects of companion diagnostic development in oncology. Clin Pharmacol Ther 2018;103:999–1008. [DOI] [PubMed] [Google Scholar]
- 36.Food and Drug Administration . In Vitro Companion Diagnostic Devices. Guidance for Industry and Food and Drug Administration Staff. Available at http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM262327.pdf. August 6, 2014. Accessed December 7, 2018.
- 37.European Union . Regulation of the European Parliament and of the Council on in vitro diagnostic medical devices. 2012/0267 (COD). Available at http://eur‐lex.europa.eu/legal‐content/EN/TXT/PDF/?uri=CONSIL:PE_15_2017_INIT&from=EN. April 5 2017. Accessed December 8, 2017.
- 38.List of cleared or approved companion diagnostic devices (in vitro and imaging tools). U.S. Food and Drug Administration. (http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm). December 13, 2018. Accessed February 16, 2019.
- 39.Beaver JA, Tzou A, Blumenthal GM et al. An FDA perspective on the regulatory implications of complex signatures to predict response to targeted therapies. Clin Cancer Res 2017;23:1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jørgensen JT. The importance of predictive biomarkers in oncology drug development. Expert Rev Mol Diagn 2016;16:807–809. [DOI] [PubMed] [Google Scholar]
- 41.Drugs@FDA: FDA approved drug products. U.S. Food and Drug Administration . Available at https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed December 13, 2018.
- 42.Chabner BA. Approval after phase I: Ceritinib runs the three‐minute mile. The Oncologist 2014;19:577–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site ‐ when a biomarker defines the indication. N Engl J Med 2017;377:1409–1412. [DOI] [PubMed] [Google Scholar]
- 44.Drilon A, Laetsch TW, Kummar S et al. Efficacy of larotrectinib in TRK fusion‐positive cancers in adults and children. N Engl J Med 2018;378:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Food and Drug Administration . Prescribing information for Vitrakvi (larotrectinib). Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211710s000lbl.pdf. Updated November 2018. Accessed December 15, 2018.
- 46.Food and Drug Administration . Prescribing information for Keytruda (Pembrolizumab). Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125514s042lbl.pdf. November 9, 2018. Accessed December 15, 2018.
- 47.Jørgensen JT. When biomarkers define a drug indication. Expert Rev Mol Diagn 2018;18:315–317. [DOI] [PubMed] [Google Scholar]
- 48.Hyman DM, Puzanov I, Subbiah V et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015;373:726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hyman DM, Piha‐Paul SA, Won H et al. HER kinase inhibition in patients with HER2‐ and HER3‐mutant cancers. Nature 2018;554:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.U.S. Food and Drug Administration . Guidance for industry: Expedited programs for serious conditions – drugs and biologics. Available at https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM358301.pdf. May 2014. Accessed February 16, 2019.
- 51.Chabner BA. While we're at it, let's whack the FDA. The Oncologist 2016;21:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oncology center of excellence . U.S. Food and Drug Administration. Available at https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/OCE/default.htm). Updated December 14, 2018. Accessed February 16, 2019.
- 53.Corcoran RB, Chabner BA. Application of cell‐free DNA analysis to cancer treatment. N Engl J Med 2018;379:1754–1765. [DOI] [PubMed] [Google Scholar]