Patients with advanced dedifferentiated liposarcomas (DDLPS), a soft‐tissue sarcoma characterized by genomic amplification of CDK4 and MDM2, have universally poor outcomes following the limited therapies available. This article reports that the extent of MDM2 amplification, not simply the presence of MDM2 amplification, may be biologically important to the actions of DDLPS.
Keywords: Dedifferentiated liposarcomas, MDM2, Doxorubicin, p53, Biomarker
Abstract
Background.
Dedifferentiated liposarcomas (DDLPS) are mesenchymal tumors associated with universally poor response to treatment. Genomic amplification of murine double minute 2 (MDM2) is used as a diagnostic biomarker; however, no established biomarkers exist to guide DDLPS treatment. In the largest study of its kind, we report that the extent of MDM2 amplification, not simply the presence of MDM2 amplification, may be biologically important to the actions of DDLPS.
Patients and Methods.
The distribution of MDM2 amplification in DDLPS was assessed using data from a commercial sequencing laboratory (n = 642) and The Cancer Genome Atlas (n = 57). Data from two retrospective clinical trials (n = 15, n = 16) and one prospective clinical trial (n = 25) were used to test MDM2’s utility as a clinical biomarker. in vitro and in vivo assessments were conducted in DDLPS cell lines.
Results.
Genomic MDM2 amplification follows a highly reproducible log‐normal distribution. In patients with DDLPS treated with complete tumor resection, elevated MDM2 was associated with shortened time to recurrence as measured by genomic amplification (p = .003) and mRNA expression (p = .04). In patients requiring systemic therapy, higher MDM2 amplification was associated with reduced overall survival (p = .04). Doxorubicin treatment of DDLPS cells in vitro demonstrated variable sensitivity based on baseline MDM2 levels, and doxorubicin treatment elevated MDM2 expression. In vivo, treatment with doxorubicin followed by an MDM2 inhibitor improved doxorubicin sensitivity.
Conclusion.
MDM2 amplification levels in DDLPS follow a reproducible distribution and are associated with clinical outcomes and drug sensitivity. These results suggest that a prospective study of MDM2 as a predictive biomarker in DDLPS is warranted.
Implications for Practice.
No validated biomarkers exist for treatment selection in dedifferentiated liposarcoma (DDLPS). Although murine double minute 2 (MDM2) is currently used for diagnosis, the clinical relevance of MDM2 amplification has yet to be fully assessed. This study found that MDM2 amplification follows a predictable distribution in DDLPS and correlates with clinical and biological outcomes. These data suggests that MDM2 amplification may be a useful biomarker in DDLPS.
Introduction
Dedifferentiated liposarcomas (DDLPS) are aggressive mesenchymal tumors characterized by genomic amplification of the murine double minute 2 (MDM2) oncogene [1]. In the advanced setting, patients with DDLPS have universally poor outcomes with limited available therapies and a lack of validated biomarkers either for prognosis or for chemotherapy selection [2]. MDM2 amplifications represent a unique phenomenon in cancer biology [3] with its resultant product inhibiting the tumor‐suppressor functions of p53 [4]. Although amplification of MDM2 in DDLPS is well established as a diagnostic tool, the variability and clinical ramifications of the degree of MDM2 amplification is yet to be thoroughly understood. In preclinical models of DDLPS, MDM2‐p53 binding inhibitors (MDM2i) are active in DDLPS and have been shown to restore p53 function, halt tumor growth, and induce apoptosis [5], [6]. The clinical activity of MDM2 inhibitors as single agents has not been promising [7].
The action of MDM2 may also be important to the response of DDLPS to chemotherapy. Doxorubicin, a standard systemic treatment in DDLPS, induces DNA damage and leads to p53‐mediated apoptosis [8], [9]. Previous research suggests that p53 activity is critical for doxorubicin‐induced DNA damage response and apoptosis in multiple malignancies [5], [10], [11]. Better understanding of the MDM2:p53 axis in DDLPS may lead to better treatment selection for these patients.
In this article we report the largest study of MDM2 amplification in DDLPS and demonstrate that MDM2 genomic amplification in DDLPS is not randomly distributed. Furthermore, MDM2 status correlated with clinical outcomes from three separate clinical cohorts of patients with DDLPS. We also present preclinical data confirming the importance of MDM2 activity in DDLPS, how MDM2 is modulated by standard therapy, and potential agents to enhance sensitivity to this standard chemotherapy.
Subjects, Materials, and Methods
Tumor Sequencing Data from Foundation Medicine Inc.
The Foundation Medicine Inc. (FMI) data set contained 642 unique patients with MDM2‐amplified DDLPS assayed as previously described [12]. Copy number alteration calls were made using FMI's proprietary variant calling method.
Tumor Sequencing Data from The Cancer Genome Atlas
Data from The Cancer Genome Atlas (TCGA) [13], including 57 patients with DDLPS who were part of the soft tissue sarcoma cohort, were downloaded from the Genomics Data Commons (https://gdc.cancer.gov/). Sequencing data overlapping the MDM2 or GAPDH regions of the genome were selected as previously described [14]. MDM2 amplification was determined by quantifying the ratio of the uniquely mapped reads for MDM2 region (tumor tissue) to the uniquely mapped reads of GAPDH region (tumor tissue) per patient.
Clinical DDLPS Sample Collection
Samples from patients with DDLPS were obtained in three different manners.
The Ohio State University James Comprehensive Cancer Center (OSUCCC). Patients with DDLPS were identified from the Sarcoma Registry (institutional review board [IRB] number: OSU‐14242). Clinical factors and MDM2 amplification as measured by FMI were extracted.
OSUCCC patients with DDLPS. Specimens from 16 patients at OSUCCC were profiled for MDM2 mRNA expression following standard of care surgical tumor resection (IRB: OSU 2014E0450). Tumor MDM2 levels were evaluated by reverse transcription polymerase chain reaction (RT‐PCR) and normalized to β‐actin.
Patients with DDLPS from the NCT01574716 trial. DDLPS specimens were collected from 25 patients with metastatic DDLPS whose tumors were sequenced using an Illumina Hiseq 4000 (Illumina, San Diego, CA). Tumors were normalized to an unrelated normal sample. Patients in this study were treated with gemcitabine (900 mg/m2 on days 1 and 8) and docetaxel (75 mg/m2 on day 8) with or without MORAB‐004 as a 21‐day cycle until progression or unacceptable toxicity.
Cell Culture
Culture of human liposarcoma (LPS) cell lines (LPS246, LPS863, LPS815) has been previously reported [6]. Dr. Jonathan Fletcher (Boston, MA) generously provided us with the LPS141 cell line. All cells were cultured in Dulbecco's modified Eagle's medium and supplemented with 10% fetal bovine serum and 10 U/mL penicillin‐streptomycin in a humidified chamber delivering 5% CO2 at 37°C.
Chemical Reagents
Doxorubicin was purchased from Cayman Chemical (Ann Arbor, MI). SAR405838 and Nutlin‐3 were purchased from Selleckchem (Houston, TX). All drugs were prepared per the manufacturers’ instructions. Serial dilutions were made to obtain final concentrations for cellular assays of dimethylsulfoxide not exceeding 0.01%.
Cell Proliferation via MTT Assay and Cooperativity Evaluation
Exponentially growing DDLPS cell lines were seeded into 96‐well plates and treated with the indicated compounds. Cell viability was determined by adding 20 μL of MTT reagent (Promega, Madison, WI) as per the manufacturer's instructions. Doxorubicin treatments ranged from 0.03 to 10 μM. Combination experiments used a constant dose of 300nM of SAR405838 with 0.03 to 0.3 μM of doxorubicin, and Nutlin‐3 and doxorubicin were combined at a constant 1:1 ratio and a range of 0.1 to 1 μM.
Western Blotting
Western blots were performed using Odyssey CLx (Li‐Cor Biosciences, Lincoln, NE) and enhanced chemiluminescence (PerkinElmer, Waltham, MA). The antibodies were used as indicated per experiment: p53, p21, β‐actin (Santa Cruz Biotechnology, Dallas, TX), MDM2 (Abcam, Cambridge, MA), and cleaved capsase‐3 (Cell Signaling Technology, Danvers, MA, USA).
Mouse Xenograft Models
Xenograft models were generated utilizing bilateral flank injections of the LPS863 cell line in 8‐week‐old athymic nude female mice. Bilateral flanks were injected with 1.5 × 106 cells mixed in Matrigel. Once tumors were palpable, mice were randomly divided into treatment arms: vehicle control, doxorubicin, SAR405838, or combination doxorubicin (dosed in the morning) and SAR405838 (dosed in the evening). SAR405838 (25 mg/kg) was administered twice weekly by oral gavage. Doxorubicin (0.7 mg/kg) was administered twice weekly by i.p. injection. Tumor and body weight measurements were performed every 3–4 days until animals were harvested at day 15 of therapy as planned prior to study initiation. At harvest, tumors were weighed and measured. All animal experiments were carried out under protocols approved by the Ohio State University Institutional Animal Care and Use Committee.
Statistical Analysis
All data was analyzed in R version 3.4.3 or GraphPad Prism version 8.0.0 (GraphPad Software, La Jolla, CA). Distribution of MDM2 amplification in the FMI and TCGA data sets were analyzed in R using the fitdistrplus [15] and MASS [16] packages. Hellinger distance was used to compare the concordance between MDM2 amplification distributions [17]. The Hellinger distance was reported as single numeral between 0 (perfectly concordant distributions) and 1 (perfectly discordant distributions). For clinical data, time to recurrence was defined as time of resection to time of relapse using RECIST version 1.1 criteria. Survival analysis was performed using the log‐rank (Mantel‐Cox) test for dichotomous cohorts, the Cox proportional hazard model when MDM2 was analyzed as a continuous variable, and the Gehan‐Breslow‐Wilcoxon survival test to account for late crossover of curves. Student's t test and one‐way analysis of variance with Turkey's multiple comparison test were used as appropriate. Drug synergy was evaluated using the Chou‐Talalay combination index method using CompuSyn (Biosoft Inc., Palo Alto, CA) [18]. Receiver‐operator curves (ROCs) for MDM2 status and time to tumor recurrence were calculated in R using the survivalROC package nearest neighbor estimation [19]. All data are reported as means ± SEM unless otherwise noted; p values <.05 were considered significant. All in vitro data were replicated in at least three independent experiments.
Results
DDLPS MDM2 Amplification Levels Follow a Log‐Normal Distribution
MDM2 DNA copy number data from 642 subjects with MDM2‐amplified DDLPS from FMI's internal database demonstrated a right‐skewed distribution (Fig. 1A). Based on Akaike's information criterion (AIC), the log‐normal distribution had a near perfect fit as corroborated by the plot between empirical and theoretical cumulative distribution function (Fig. 1B). The distribution had a mean‐log of 3.70 and a standard deviation‐log of 0.82. Data from the 57 patients with MDM2‐amplified DDLPS in the TCGA data set also demonstrated a right‐skewed distribution of MDM2 DNA copy number, with AIC again identifying log‐normal distribution as the best fit (Fig. 1C and 1D). This distribution had a mean‐log of 3.38 and a standard deviation‐log of 0.81.
Figure 1.
MDM2 copy number alterations in MDM2‐amplified DDLPS. Distribution of MDM2 copy number status from 642 patients with DDLPS provided by FMI. MDM2 copy number demonstrated a right‐skewed distribution (A) and best fit a log‐normal distribution as corroborated by the overlap between the empirical and theoretical CDF (B). Distribution of MDM2 copy number status from 57 patients with DDLPS as part of TCGA sarcoma data set. MDM2 copy number demonstrated a right‐skewed distribution (C) and best fit a log‐normal distribution as corroborated by the overlap between the empirical and theoretical CDF (D). (E): MDM2 copy numbers from two independent data sets, FMI and TCGA, followed a similar distribution demonstrating a Hellinger distance of 0.14.
Abbreviations: CDF, cumulative distribution function; FMI, Foundation Medicine Inc.; MDM2, murine double minute 2; TCGA, The Cancer Genome Atlas.
To assess the similarity of these MDM2 DNA amplification level distributions, we calculated the Hellinger distance (Fig. 1E) to measure concordance between two probability distributions ranging from 0 (perfect fit) to 1 (disjoint). Despite the independent nature of these data sets, the two fitted distributions for the FMI and TCGA data sets had a Hellinger distance of 0.14, suggesting that MDM2 DNA copy number fits a well‐defined, log‐normal distribution in MDM2‐amplified DDLPS.
MDM2 Amplification Is Correlated with Tumor Recurrence After Resection in DDLPS
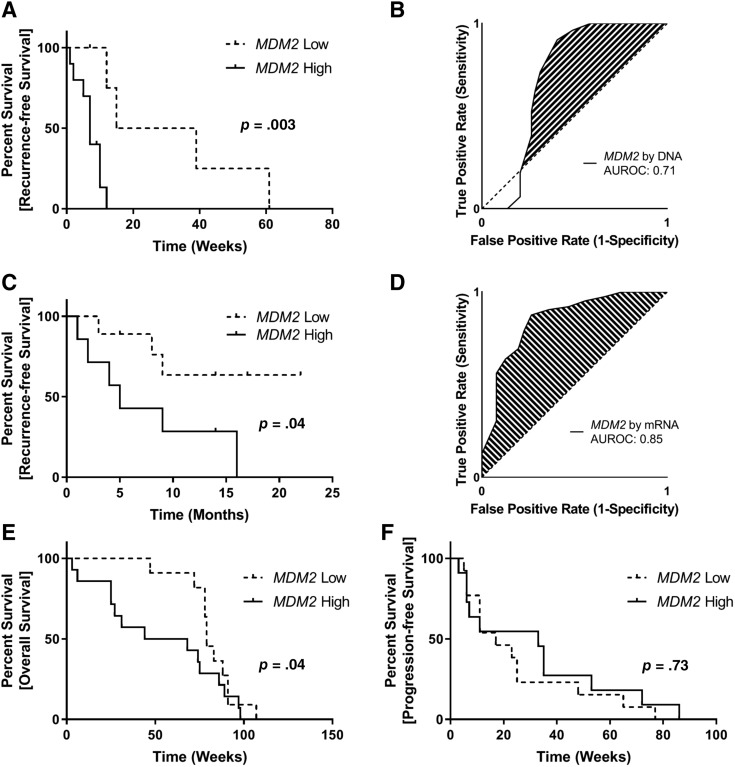
Of 642 patients with MDM2‐amplified DDLPS genomically profiled using the FoundationOne Heme platform (Foundation Medicine Inc., Cambridge, MA), 15 were treated by complete surgical resection at Ohio State University. Patients were categorized as MDM2 high (≥38 DNA copies; n = 10) and MDM2 low (<38 DNA copies; n = 5) utilizing a cut point described previously by Ricciotti et al. (demographics in supplemental online Table 1) [20]. Patients in the MDM2‐high cohort experienced median time to recurrence of 7 months compared with 27 months in patients in the MDM2‐low cohort (hazard ratio [HR], 8.2; 95% confidence interval [CI], 2.0–33.6; p = .003; Fig. 2A). As a continuous variable, MDM2 amplification was associated with HR for tumor recurrence of 1.03 (95% CI, 0.99–1.05; p = .06) with an area under the ROC (AUROC) of 0.71 (Fig. 2B).
Figure 2.
Clinical outcomes in patients with DDLPS stratified By MDM2 amplification status. (A): MDM2 DNA amplification is predictive for recurrence‐free survival in 15 patients with DDLPS treated with complete tumor resection. Patients were split into groups based on DNA amplification of MDM2 (≥38 copies). When MDM2 was used as a continuous variable, amplification was borderline significantly associated with tumor recurrence (hazard ratio [HR], 1.03; 95% confidence interval [CI], 0.99–1.05; p = .06). (B): Receiver‐operator curve (ROC) for the prediction of tumor recurrence by MDM2 as measured by DNA amplification level. An AUROC of 0.71 suggests that MDM2 as measured by DNA amplification level may be an effective predictor of recurrence‐free survival in DDLPS. (C): MDM2 mRNA expression is predictive for recurrence‐free survival in 16 patients with DDLPS treated with complete tumor resection. Patients were split into groups based on median MDM2 mRNA expression. When MDM2 was used as a continuous variable, amplification was significantly associated with tumor recurrence (HR, 1.08; 95% CI, 1.01–1.17; p = .02). (D): ROC for the prediction of tumor recurrence by MDM2 as measured by mRNA expression level. An AUROC of 0.85 suggests that MDM2 as measured by mRNA expression level may be an effective predictor of recurrence‐free survival in DDLPS. MDM2 amplification was prognostic for overall survival in patients with DDLPS treated with a combination of gemcitabine (900 mg/m2 on days 1 and 8) and docetaxel (75 mg/m2 on day 8) with or without MORAB‐004 (E), but it was not predictive for progression‐free survival (F). Absolute copy number was not available for these patients; therefore, patients were split into groups based on median MDM2 DNA amplification.
Abbreviations: AUROC, area under the receiver‐operator curve; MDM2, murine double minute 2.
MDM2 Expression Levels Are Correlated with Tumor Recurrence After Resection in DDLPS
To assess the clinical correlation between MDM2 mRNA expression and clinical outcomes in DDLPS, we queried a series of 16 consecutive patients with DDLPS enrolled on Ohio State University trial 2014E0450 who were treated with surgical resection and had sufficient samples available for MDM2 mRNA expression analysis by RT‐PCR (demographics in supplemental online Table 2). These patients predated our institutional usage of the tumor sequencing, and thus these data were not available for comparison. Patients were stratified into MDM2‐high and MDM2‐low cohorts based on the group median MDM2 expression. Patients in the MDM2‐high cohort experienced a reduced median time to recurrence of 5 months, whereas patients in the MDM2‐low cohort did not reach median time to recurrence (HR, 4.04; 95% CI, 1.0–16.2; p = .04; Fig. 2C). When MDM2 expression was used as a continuous variable, elevated MDM2 expression was significantly associated with tumor recurrence (HR, 1.09; 95% CI, 1.01–1.17; p = .02) with an AUROC of 0.85 (Fig. 2D).
MDM2 Amplification Is Correlated with Overall Survival Following Gemcitabine/Docetaxel Treatment in DDLPS
Data from 25 patients with DDLPS who were treated as part of NCT01574716 were generously provided by Morphotek (Exton, PA). These patients were treated with a combination of gemcitabine (900 mg/m2 on days 1 and 8) and docetaxel (75 mg/m2 on day 8) with or without MORAB‐004. The MORAB‐004 compound failed to yield any improved progression‐free survival (PFS) or overall survival (OS) compared with gemcitabine and docetaxel alone. To account for any potential interaction between study arm and MDM2 status in this analysis, stratified Cox proportional hazards models were constructed, identifying no statistical interaction between these two variables in regard to PFS (p = .49) or OS (p = .70). Tumors were profiled for MDM2 DNA copy numbers, and patients were split into high and low MDM2 cohorts at the group median for this analysis. Patients in the MDM2‐low cohort experienced significantly longer OS (median, 79 months vs. 56 months; p = .04 [Gehan‐Breslow‐Wilcoxon survival test]; Fig. 2E). In contrast, stratifying patients by MDM2 amplification status did not result in a difference in PFS (p = .73; Fig. 2F).
Doxorubicin Sensitivity Is Associated with MDM2 Levels, and Doxorubicin Raises MDM2 Levels
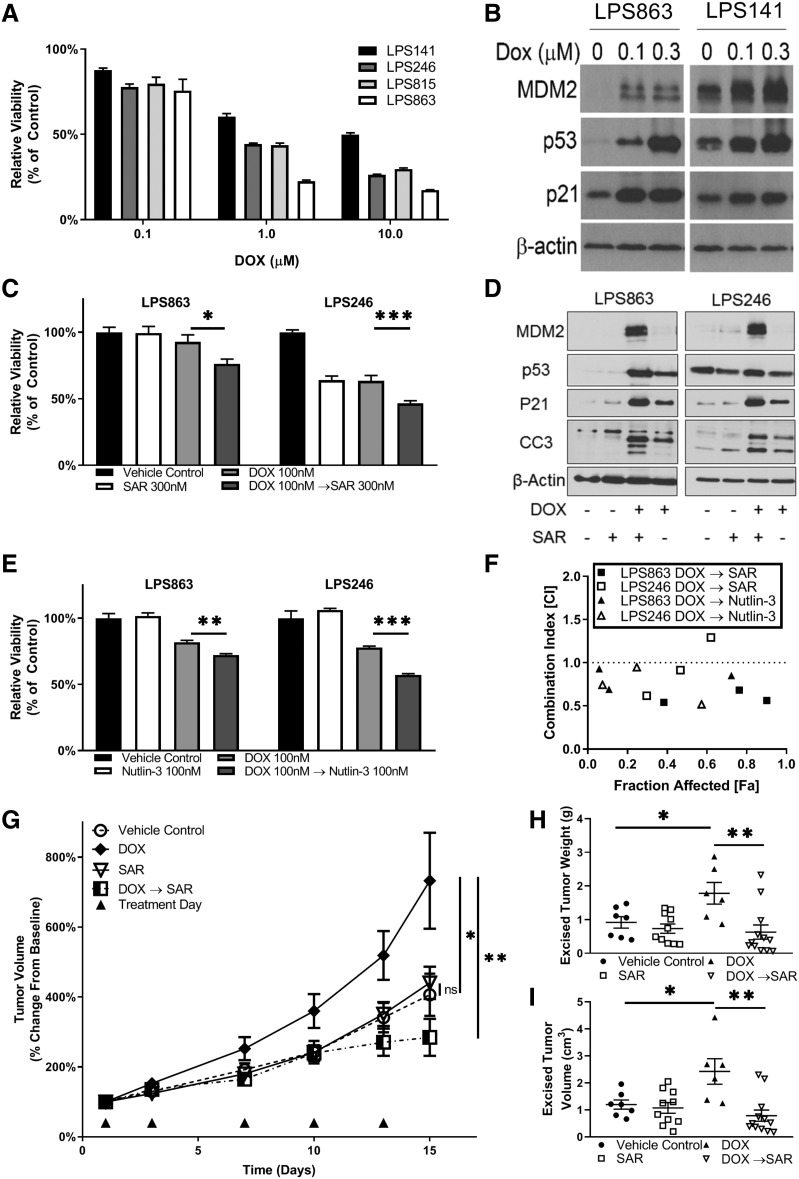
Our previous study of DDLPS cell lines, including full characterization of MDM2, identified variability in MDM2 status between cell lines [6]. In brief, MDM2 mRNA expression can be described as follows from lowest to highest: LPS863, LPS815, LPS141, and LPS246. To test the importance of MDM2 status with regard to chemosensitivity in DDLPS, cell lines were treated with increasing concentrations of doxorubicin for 96 hours and evaluated for relative viability compared with vehicle controls. LPS141 (MDM2 high) was the least sensitive to doxorubicin, whereas the LPS863 cell line (MDM2 low) was the most sensitive (Fig. 3A) The LPS863 and LPS141 cell lines were then treated for 24 hours before being evaluated for protein expression. Doxorubicin treatment increased MDM2 and p53 expression in both cell lines compared with baseline (Fig. 3B).
Figure 3.
MDM2 status is correlated with doxorubicin response in vitro and in vivo. (A): DDLPS cell lines were treated with doxorubicin at varying concentrations. MDM2‐low cell lines tended to be more sensitive than MDM2‐high cell lines. (B): LPS863 (MDM2‐low) and LPS246 (MDM2‐high) DDLPS cell lines were treated with increasing doses of doxorubicin for 24 hours. MDM2 levels increased as a function of doxorubicin treatment with corresponding p53 feedback upregulation. (C): DDLPS cell lines were treated sequentially with 100 nM doxorubicin for 48 hours followed by 300 nM SAR405838 for 48 hours. Controls for single agent used vehicle instead of the second agent in the commensurate time frame. Cell proliferation was evaluated by MTT in both scenarios at the end of treatment. Bar graphs demonstrate that doxorubicin followed by SAR405838 reduced cellular viability compared with doxorubicin alone. (D): DDLPS cell lines were treated with 300 nM of doxorubicin and 300 nM SAR405838 either alone or in sequence for 24 hours. The sequence of doxorubicin followed by SAR405838 resulted in the greatest degree of apoptosis. MDM2 expression was highest in the cell lines treated with doxorubicin followed by SAR405838. This expression was so great that it overwhelmed the expression of MDM2 in other treatments requiring a longer exposure to identify MDM2 expression with other treatment conditions. Demonstration of MDM2 expression on this blot at different exposures is noted in supplemental online Figure 2. (E): LPS863 and LPS246 cells were treated with 100 nM doxorubicin for 48 hours followed by 100 nM Nutlin‐3 for 48 hours. Doxorubicin followed by Nutlin‐3 treatment greatly reduced viability compared with doxorubicin as a single agent. (F): Combination index for doxorubicin followed by either SAR405838 or Nutlin‐3. The addition of MDM2 inhibitors following doxorubicin treatment resulted in strong synergy in both LPS863 and LPS246 cell lines. Additional combination data is noted in supplemental online Figure 1. Xenograft models with bilateral flank injections of the LPS863 cell line were randomly divided into four treatment arms: vehicle control, doxorubicin, SAR405838, or doxorubicin (dosed in the morning) followed by SAR405838 (dosed in the evening). Doses were administered twice weekly. Tumors were measured with calipers every 3–4 days (G). Mice were sacrificed at day 15, and the tumors were weighed (H) and measured (I).
Significance level symbols: ns, not significant; *, p < .05; **, p < .01; ***, p < .001.
Abbreviations: CC3, cleaved capsase‐3; DOX, doxorubicin; LPS, liposarcoma; MDM2, murine double minute 2; SAR, SAR405838.
Inhibition of MDM2 Potentiates Doxorubicin Chemosensitivity
To evaluate the effect of MDM2 antagonism on doxorubicin treatment, we used the MDM2i SAR405838 and Nutlin‐3 to restore p53 action [6]. LPS863 and LPS246 cells were treated with doxorubicin, SAR405838, or doxorubicin followed by SAR405838 for 96 hours before being evaluated for viability relative to vehicle control. SAR405838 was added following doxorubicin to address elevated MDM2 expression induced by doxorubicin. Treatment with doxorubicin followed by either SAR405838 or Nutlin‐3 demonstrated potent synergy in both LPS863 and LPS246 cell lines (Fig. 3C, 3E–F; supplemental online Fig. 1A–C). The combination of doxorubicin followed by SAR405838 induced the greatest expression of cleaved capsase‐3 (Fig. 3D; supplemental online Fig. 2).
To further study this effect, LPS863 tumor bearing mice were treated with vehicle control, doxorubicin (0.7 mg/kg i.p.), SAR405838 (25 mg/kg p.o.), or doxorubicin followed by SAR405838 (doxorubicin in the morning, SAR405838 in the afternoon) twice weekly. After 2 weeks of treatment, doxorubicin‐treated tumors significantly outgrew control tumors (control, 406.14 ± 160.57 mm3; doxorubicin, 732.5 ± 336.76 mm3; p = .04; Fig. 3G), whereas SAR405838 had no effect on tumor growth (control, 406.14 ± 160.57 mm3; SAR405838m 440.30 ± 145.93 mm3; p = .41). Tumors treated with doxorubicin followed by SAR405838 were significantly smaller compared with doxorubicin alone (doxorubicin, 732.5 ± 336.76 mm3; doxorubicin followed by SAR405838, 284.25 ± 183.92 mm3; p = .002). At excision, tumor volume (Fig. 3H) and weight (Fig. 3I) followed similar trends.
Discussion
DDLPS is characterized by genomic amplification of MDM2; however, the clinical actionability of the extent of MDM2 amplification within DDLPS has not been fully evaluated. Previous consensus regarding MDM2‐amplified DDLPS was largely silent on the extent of MDM2 amplification or expression in this disease. This is likely because of the rarity of DDLPS, which has prevented the accumulation of a large‐enough database to properly query the landscape of MDM2 amplification until this time.
In this article, we first present the largest genomic analysis of DDLPS MDM2 amplification levels and detail its distribution and variability. Data regarding MDM2 amplification in DDLPS was limited to date, with previous studies limited by small patient cohorts and a lack of access to validation data sets [20]. Here, we present two independent data sets of patients with DDLPS, demonstrating that the distribution of MDM2 amplification follows a log‐normal distribution. This assessment in 699 unique patient samples provides the most robust assessment of MDM2 amplification in DDLPS to date. The predictable log‐normal distribution, as demonstrated here, allows further clinical evaluation of MDM2 amplification.
To assess the clinical importance of MDM2 status within DDLPS, we collected data from two internal clinical studies as well as one multicenter randomized clinical trial. In the first data set, including patients whose tumors were sequenced by FMI, a MDM2 amplification cutoff previously shown to affect clinical outcomes in this disease (≥38 MDM2 copies) was found to be predictive of time to tumor recurrence in patients with DDLPS treated with complete surgical resection [20]. Interestingly, utilizing MDM2 genomic amplification as a continuous variable trended toward statistically significant correlation with recurrence‐free survival. This suggests that in this data set MDM2 is significantly correlated with the clinical outcomes of patients. In the second data set, including patients whose tumors were tested for MDM2 mRNA expression by RT‐PCR, patients with MDM2 expression above the cohort median experienced drastically reduced recurrence‐free survival. As a continuous variable, MDM2 mRNA expression was highly correlated with recurrence‐free survival. This suggests that in this data set, MDM2 mRNA expression is significantly correlated to the clinical outcomes of patients. In a third clinical data set from a multicenter clinical trial of patients with DDLPS treated with gemcitabine and docetaxel, MDM2 DNA amplification was predictive of OS but not of PFS. Although this highlights the potential importance of MDM2 amplification and clinical outcomes in DDLPS, it also is indicative of the complex nature of this disease. This would imply that other biomarkers may be necessary to stratify patients, depending on treatment modality.
Taken together, the data represented in these three independent clinical cohorts of MDM2‐amplified DDLPS suggest that although the extent of MDM2 amplification and expression may be important to clinical outcomes for patients with DDLPS, certain limitations inherent to the statistical methods utilized in the clinical analysis presented here must be taken into account. Dichotomizing a continuous variable, such as with the data presented in Figure 2, creates inherent limitations, including reduced power and concealed nonlinearity in the relationship between variables and outcome [21]; however, dichotomizing MDM2 amplification is necessary here to provide both exploratory and visual analysis. In both data sets including patients who were treated by tumor resection, the use of MDM2 as a dichotomous predictor (high vs. low) of clinical outcomes was bolstered by further analysis of MDM2 as a continuous predictor of clinical outcomes. In both cases, the interpretation was similar, in that higher MDM2 expression or amplification was associated with reduced time to tumor recurrence. This provides further preliminary evidence that MDM2 status may play a role in clinical outcomes in DDLPS. This method was not employed in data from the NCT01574716 trial, as these samples were normalized to an unrelated sample, thus limiting our confidence in using MDM2 as a continuous variable in this specific data set.
The clinical findings of this study should not be overstated and are both retrospective and exploratory in nature. The statistical limitation of the small clinical cohorts presented here, as is typical in rare cancers, reduces the external validity of these findings. Additionally, as demonstrated here, MDM2 status is complicated, warranting further prospective study. Although the evidence presented in this study suggests that variable amplification and expression of MDM2 is biologically important to the action of DDLPS, this should not be interpreted as being validated clinically. In addition to the statistical limitation of this study already mentioned,variable methods of calling somatic MDM2 copy numbers make comparison between data sets difficult [22]; therefore, consistent and robust calling methods must be established to unify genomic practice in patient care.
To further assess the effect of variable MDM2 status in DDLPS, we selected DDLPS cell lines previously characterized by our lab [6]. In addition to a correlation between MDM2 status and doxorubicin sensitivity, we noted that doxorubicin may induce its own resistance through the further elevation of MDM2. The upregulation of both MDM2 and p53 may be explained in part by a feedback loop in which doxorubicin‐induced DNA damage increases p53 expression which in turn induces transcription of MDM2 [4]. Our data suggest that, at low doses, doxorubicin may promote its own resistance, resulting in enhanced tumor growth through the upregulation of MDM2 and subsequent inhibition of p53. Moreover, in vitro and in vivo treatment with doxorubicin followed by MDM2i resulted in a potently synergistic relationship, suggesting that inhibition of MDM2 can induce doxorubicin sensitivity. These data, when taken together, may suggest that inhibition of doxorubicin‐induced MDM2 activity leads to reactivation of p53 and induction of apoptosis. Further study is necessary to identify the role of MDM2 in doxorubicin resistance.
Conclusion
In this comprehensive genomic assessment of MDM2 in DDLPS, we present data demonstrating that MDM2 amplification levels are heterogeneous and follow a reproducible distribution across multiple platforms. We further demonstrate that this distribution may be clinically meaningful, as the degree of MDM2 amplification and expression was predictive for overall survival and recurrence after surgery. Furthermore, our in vitro and in vivo work suggests that MDM2 levels may be predictive of doxorubicin sensitivity and that a potential mechanism of doxorubicin resistance could be mediated through the induction of MDM2 expression. Further prospective clinical and translational studies are required to identify and confirm appropriate statistical cutoffs to define actionable phenotypes.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by an NIH SARC Career Development Award (to J.L.C.), The Ohio State CTSA Davis Bremer Award (to J.L.C.), NIH K12 CA133250 award (to J.L.H.), and a grant from the NIH SARC sarcoma SPORE: U54168512 (to R.E.P.).
Contributed equally.
Author Contributions
Conception/design: Kate Lynn J. Bill, Nathan D. Seligson, John L. Hays, James L. Chen
Provision of study material or patients: John L. Hays, Megan C. Duggan, Sherri Z. Millis, Reena Shakya, Cynthia D. Timmers, Paul E. Wakely, Jr., Raphael E. Pollock, James L. Chen
Collection and/or assembly of data: Kate Lynn J. Bill, Nathan D. Seligson, John L. Hays, Achal Awasthi, Bryce Demoret, Colin W. Stets, Megan C. Duggan, Manojkumar Bupathi, Guy N. Brock, Sherri Z. Millis, Reena Shakya, Cynthia D. Timmers, Paul E. Wakely, Jr., Raphael E. Pollock, James L. Chen
Data analysis and interpretation: Kate Lynn J. Bill, Nathan D. Seligson, John L. Hays, Achal Awasthi, Manojkumar Bupathi, Guy N. Brock, James L. Chen
Manuscript writing: Kate Lynn J. Bill, Nathan D. Seligson, John L. Hays, Achal Awasthi, James L. Chen
Final approval of manuscript: Kate Lynn J. Bill, Nathan D. Seligson, John L. Hays, Achal Awasthi, Bryce Demoret, Colin W. Stets, Megan C. Duggan, Manojkumar Bupathi, Guy N. Brock, Sherri Z. Millis, Reena Shakya, Cynthia D. Timmers, Paul E. Wakely, Jr., Raphael E. Pollock, James L. Chen
Disclosures
Nathan D. Seligson: SZM (E); Sherri Z. Millis: Foundation Medicine Inc. (E); James L. Chen: Novartis (H), Syapse (C/A), Foundation Medicine Inc. (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Fletcher CDM, World Health Organization. WHO Classification of Tumours of Soft Tissue and Bone . Lyon, France: IARC Press, 2013. [Google Scholar]
- 2.Italiano A, Toulmonde M, Cioffi A et al. Advanced well‐differentiated/dedifferentiated liposarcomas: Role of chemotherapy and survival. Ann Oncol 2012;23:1601–1607. [DOI] [PubMed] [Google Scholar]
- 3.Dembla V, Somaiah N, Barata P et al. Prevalence of MDM2 amplification and coalterations in 523 advanced cancer patients in the MD Anderson phase 1 clinic. Oncotarget 2018;9:33232–33243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manfredi JJ. The MDM2‐p53 relationship evolves: MDM2 swings both ways as an oncogene and a tumor suppressor. Genes Dev 2010;24:1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kojima K, Konopleva M, Samudio IJ et al. MDM2 antagonists induce p53‐dependent apoptosis in AML: Implications for leukemia therapy. Blood 2005;106:3150–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bill KL, Garnett J, Meaux I et al. SAR405838: A novel and potent inhibitor of the mDM2:P53 axis for the treatment of dedifferentiated liposarcoma. Clin Cancer Res 2016;22:1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer TM, Gounder MM, Weise AM et al. A phase 1 study of MDM2 inhibitor DS‐3032b in patients with well/de‐differentiated liposarcoma (WD/DD LPS), solid tumors (ST) and lymphomas (L). J Clin Oncol 2018;36:11514–11514. [Google Scholar]
- 8.Anthoney DA, McIlwrath AJ, Gallagher WM et al. Microsatellite instability, apoptosis, and loss of p53 function in drug‐resistant tumor cells. Cancer Res 1996;56:1374–1381. [PubMed] [Google Scholar]
- 9.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene 2003;22:9030–9040. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Rousseau RF, Middleton SA et al. Pre‐clinical evaluation of the MDM2‐p53 antagonist RG7388 alone and in combination with chemotherapy in neuroblastoma. Oncotarget 2015;6:10207–10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senturk JC, Bohlman S, Manfredi JJ. MDM2 selectively suppresses DNA damage arising from inhibition of topoisomerase II independent of p53. Oncogene 2017;36:6085–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frampton GM, Fichtenholtz A, Otto GA et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature Biotechnol 2013;31:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network. Electronic address EDSC and Cancer Genome Atlas Research N . Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell 2017;171:950–965 e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seligson ND, Kautto EA, Passen EN et al. BRCA1/2 functional loss defines a targetable subset in leiomyosarcoma. The Oncologist 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delignette‐Muller M, Dutang C. Fitdistrplus: An R package for fitting distributions. J Stat Softw 2015;64:1–34. [Google Scholar]
- 16.Venables WN, Ripley BD. Modern applied statistics with S. New York, NY: Springer; 2002. [Google Scholar]
- 17.Hellinger E. Neue begründung der theorie quadratischer formen von unendlichvielen veränderlichen. Journal für die reine und angewandte Mathematik 1909;136:210–271. [Google Scholar]
- 18.Chou TC. Drug combination studies and their synergy quantification using the Chou‐Talalay method. Cancer Res 2010;70:440–446. [DOI] [PubMed] [Google Scholar]
- 19.Heagerty PJ, Lumley T, Pepe MS. Time‐dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–344. [DOI] [PubMed] [Google Scholar]
- 20.Ricciotti RW, Baraff AJ, Jour G et al. High amplification levels of MDM2 and CDK4 correlate with poor outcome in patients with dedifferentiated liposarcoma: A cytogenomic microarray analysis of 47 cases. Cancer Genet 2017;218–219:69–80. [DOI] [PubMed] [Google Scholar]
- 21.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Rawe J, Jiang T, Sun G et al. Low concordance of multiple variant‐calling pipelines: Practical implications for exome and genome sequencing. Genome Medicine 2013;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]