This article reports the results of a retrospective analysis of the prognostic and/or predictive value of HER2‐enriched and basal‐like subtypes in patients with advanced, non‐steroidal aromatase inhibitor‐refractory, HR‐positive/HER2‐negative breast cancer from the phase III BOLERO‐2 study.
Keywords: Everolimus, Exemestane, Mammalian target of rapamycin, Advanced breast cancer, Intrinsic subtype
Abstract
Background.
The prognostic and predictive value of the two nonluminal (human epidermal growth factor receptor 2 [HER2]‐enriched and basal‐like) subtypes within advanced hormone receptor‐positive (HR+) breast cancer is currently unknown.
Materials and Methods.
This study retrospectively analyzed 261 tumors (80.7% primary; 19.3% metastatic) from the BOLERO‐2 study; BOLERO‐2 randomized 724 patients with advanced HR+/HER2‐negative breast cancer to everolimus plus exemestane or placebo plus exemestane. Tumors were classified using a PAM50 subtype predictor. Multivariable Cox regression analyses tested the independent prognostic significance of PAM50, and associations between PAM50 subtypes and treatment upon progression‐free survival (PFS) were evaluated.
Results.
Subtype distribution was 46.7% luminal A (n = 122), 21.5% HER2‐enriched (n = 56), 15.7% luminal B (n = 41), 14.2% normal‐like (n = 37), and 1.9% basal‐like (n = 5); HER2‐enriched subtypes were more common in metastatic versus primary tumors (32.0% vs. 18.7%; p = .038). Median PFS differences between luminal and nonluminal (6.7 vs. 5.2 months; adjusted hazard ratio, 0.66; 95% confidence interval [CI], 0.47–0.94; p = .020) and HER2‐enriched and non‐HER2‐enriched subtypes (5.2 vs. 6.2 months; adjusted hazard ratio, 1.53; 95% CI, 1.07–2.19; p = .019) were significant. Everolimus plus exemestane significantly improved median PFS versus placebo plus exemestane among patients with HER2‐enriched tumors (5.8 vs. 4.1 months; adjusted hazard ratio, 0.49; 95% CI, 0.26–0.90; p = .034); however, the association between HER2‐enriched tumors and everolimus benefit was nonsignificant (p = .433).
Conclusion.
The HER2‐enriched subtype was identified in a substantial proportion of advanced HR+/HER2‐negative breast tumors, and was a consistent biomarker of poor prognosis. Tailored therapies are therefore needed for HER2‐enriched tumors in the advanced HR+/HER2‐negative breast cancer setting.
Implications for Practice.
Using 261 tumor samples from the BOLERO‐2 phase III clinical trial, this study shows that a substantial proportion (20%–30%) of hormone receptor‐positive (HR+)/human epidermal growth factor receptor 2 (HER2)‐negative advanced breast cancers do not have a luminal A or B gene expression profile. This group of patients with nonluminal disease has a poor survival outcome regardless of the addition of everolimus to exemestane. This is the second study that confirms the prognostic value of this biomarker. Overall, these findings indicate a necessity to design novel clinical trials targeting nonluminal disease within HR+/HER2‐negative breast cancer.
摘要
背景。目前尚不清楚激素受体阳性 (HR+) 的晚期乳腺癌中两种非管腔 [人表皮生长因子受体 2 (HER2) ‐ 过表达和基底细胞样]亚型的预后和预测价值。
材料和方法。本研究回顾性分析了 BOLERO‐2 研究中的 261 例肿瘤(80.7% 原发;19.3% 转移);BOLERO‐2 将 724 例晚期 HR +/HER2 阴性的晚期乳腺癌患者随机分配至依维莫司加依西美坦或安慰剂加依西美坦。使用 PAM50 亚型预测因子对肿瘤进行分类。多变量 Cox 回归分析检验了 PAM50 的独立预后意义,并评估了 PAM50 亚型与治疗无进展生存期 (PFS) 之间的关联。
结果。亚型分布为 46.7% 管腔 A 型(n = 122),21.5% HER2 过表达(n = 56),15.7% 管腔 B 型(n = 41),14.2% 正常样型(n = 37),以及 1.9% 基底细胞样型(n = 5);HER2 过表达的亚型在转移性肿瘤中较原发性肿瘤更常见(32.0% vs. 18.7%;p = 0.038)。中位 PFS方面,管腔型和非管腔型之间[6.7 vs. 5.2 个月;校正风险比,0.66;95% 置信区间 (CI),0.47‐0.94;p = 0.020]以及 HER2 过表达和非 HER2 过表达亚型之间(5.2 vs. 6.2个月;校正风险比,1.53;95%CI,1.07‐2.19;p = 0.019)的差异明显。在 HER2 过表达的肿瘤患者中,依维莫司加依西美坦与安慰剂加依西美坦相比显著改善了中位 PFS(5.8 vs. 4.1 个月;校正风险比,0.49;95%CI,0.26‐0.90;p = 0.034);然而,HER2 过表达肿瘤与依维莫司治疗受益之间的关联并不显著(p = 0.433)。
结论。 HER2 过表达亚型在晚期 HR +/HER2 阴性乳腺肿瘤中占有相当大比例,并且是预后不良的一致性生物标志物。因此,对于晚期 HR +/HER2 阴性乳腺癌患者中 HER2 过表达的肿瘤需要个体化定制疗法。
实践意义:使用来自 BOLERO‐2 III 期临床试验的 261 份肿瘤样本,本研究显示相当大部分(20% ‐ 30%)激素受体阳性 (HR+)/人表皮生长因子受体 2 (HER2)‐阴性的晚期乳腺癌不具有管腔 A 型或 B 型基因表达谱。无论在依西美坦治疗中是否加入依维莫司,这组患有非管腔型肿瘤的患者的生存结果都很差。这是第二项证实了该生物标志物的预后价值的研究。总体而言,这些研究结果表明有必要设计针对 HR +/HER2 阴性非管腔型乳腺癌的新型临床试验。
Introduction
The standard of care for advanced hormone receptor‐positive (HR+)/human epidermal growth factor receptor 2 (HER2)‐negative breast cancer is currently endocrine therapy alone or combined with inhibitors of cyclin‐dependent kinase 4/6 (CDK4/6) or mammalian target of rapamycin (mTOR) [1], [2]. However, HR+/HER2‐negative disease is clinically and biologically heterogeneous, and identification of subgroups of patients with differing prognoses and anticipated treatment responses is needed.
Studies of global gene expression patterns have identified four intrinsic HR+/HER2‐negative breast cancer subtypes (luminal A, luminal B, HER2‐enriched, and basal‐like) [3], [4], [5]. Compared with luminal A or B subtypes, nonluminal HER2‐enriched and basal‐like subtypes are less differentiated, and express higher levels of proliferation markers and lower levels of estrogen‐regulated genes [3]. However, these nonluminal subtypes are also clearly distinct. The HER2‐enriched/HER2‐negative subtype is highly similar to the classical HER2‐enriched/HER2‐positive subtype but lacks amplification/overexpression of the HER2 amplicon, whereas the basal‐like/HR+ subtype is highly similar to the classical basal‐like/triple‐negative subtype [6]. Thus, nonluminal HR+/HER2‐negative subtypes are more similar to nonluminal HER2‐positive or triple‐negative breast cancer subtypes than they are to luminal A or B subtypes.
Identification of the HER2‐enriched or basal‐like subtypes within HR+/HER2‐negative disease may be of clinical value. In early HR+/HER2‐negative breast cancer, both nonluminal subtypes are associated with estrogen independence [7], [8], chemosensitivity [9], and poor survival outcome [10]. Intrinsic subtype might also predict treatment outcome and benefit in the advanced or metastatic settings [11]. In a retrospective analysis of 644 tumor samples from the phase III EGF30008 study of first‐line letrozole with or without lapatinib for advanced HR+/HER2‐negative breast cancer, both nonluminal subtypes were associated with poor progression‐free survival (PFS) and overall survival (OS) [11]. Patients with the HER2‐enriched subtype benefited from lapatinib addition, concordant with the genomic profile of the tumor. These data support exploring new therapeutic approaches for patients with nonluminal subtypes of advanced HR+/HER2‐negative breast cancer [12].
Herein, we report the results of a retrospective analysis of the prognostic and/or predictive value of HER2‐enriched and basal‐like subtypes in patients with advanced, non‐steroidal aromatase inhibitor‐refractory, HR+/HER2‐negative breast cancer from the phase III BOLERO‐2 study.
Materials and Methods
Patient Data
BOLERO‐2 was an international, double‐blind phase III study, in which patients were randomized (2:1) to receive oral everolimus (10 mg daily) or matching placebo, plus oral exemestane (25 mg daily) until disease progression, unacceptable toxicity, or withdrawal of consent [13]. Randomization was stratified according to the presence of visceral metastases, and prior sensitivity to endocrine therapy. All patients provided written informed consent before enrollment. Eligibility criteria have been previously reported [13]. Patients were postmenopausal women with advanced, non‐steroidal aromatase inhibitor‐refractory, estrogen receptor‐positive/HER2‐negative breast cancer, measurable disease (or mainly lytic bone lesions), and Eastern Cooperative Oncology Group (ECOG) performance status 0–2. Key exclusion criteria included a history of brain metastases and prior exposure to exemestane or mTOR inhibitors.
The study was conducted per Good Clinical Practice guidelines, the Declaration of Helsinki, and local regulations, and was approved by the institutional review board at each site. A steering committee supervised the study. An independent data and safety monitoring committee performed semiannual reviews of safety and interim efficacy. The study is registered with ClinicalTrials.gov (NCT00863655).
Gene Expression Analysis
A section of hematoxylin and eosin‐stained, formalin‐fixed paraffin‐embedded (FFPE) breast tissue was examined to confirm the presence of invasive tumor cells and determine the tumor area. RNA purification (High Pure FFPET RNA Isolation Kit; Roche, Basel, Switzerland) used up to three 10‐μm FFPE slides per tumor specimen, and macrodissection was performed where necessary to avoid contamination with normal breast tissue. A minimum of approximately 150 ng total RNA was used to measure expression of 50 PAM50 genes and five housekeeping genes (ACTB, MRPL19, PSMC4, RPLP0, and SF3A1) using the nCounter platform (NanoString Technologies, Seattle, WA). Data were log base 2 transformed and normalized using the five housekeeping genes.
Sample Data and PAM50 Intrinsic Subtyping
Of 724 tumor samples, 363 tumor blocks were sent to the central lab. A total of 300 samples had sufficient tumor tissue in hematoxylin and eosin. Minimum RNA quantity and quality was obtained from 261 samples (Fig. 1), and gene expression was successful in all samples. Intrinsic subtyping was performed using the previously described research‐based PAM50 intrinsic subtype predictor [11], [14]. PAM50 subtyping was performed by the Translational Genomic and Targeted Therapeutics in Solid Tumors group (IDIBAPS) by investigators blinded to clinical data.
Figure 1.
Consolidated Standards of Reporting Trials‐style diagram of the BOLERO‐2 PAM50 study.
Abbreviations: FFPE, formalin‐fixed paraffin‐embedded; HER2, human epidermal growth factor receptor 2.
Statistical Analysis
PFS outcomes were estimated using the Kaplan‐Meier methods and compared using log‐rank tests. Univariable and multivariable Cox regression analyses adjusted for treatment (everolimus vs. placebo) were used to test the independent prognostic significance of each variable. To test the predictive value of the PAM50 nonluminal subtypes, interaction tests between PAM50 subtypes and treatment for PFS were evaluated in univariable and multivariable models. The proportional‐hazards assumption was tested based on Schoenfeld residuals. A two‐sided p < .05 was used as the threshold for statistical significance.
Results
Patient Characteristics
Baseline characteristics were broadly similar between the 724 patients in the overall population and the 261 patients in the PAM50 subpopulation, although the PAM50 subpopulation comprised larger numbers of patients who were white (87.7% vs. 75.6%) or had primary tumors (80.1% vs. 53.6%; Table 1). In the PAM50 subpopulation, median age was 61 years, 225 (86.2%) patients had prior sensitivity to endocrine therapy, 156 (59.8%) had ECOG performance status 0, and 84 (32.2%) had three or more metastatic sites. Everolimus plus exemestane was associated with a significant PFS benefit versus placebo plus exemestane (median: 8.0 vs. 4.1 months; hazard ratio, 0.42; 95% confidence interval [CI], 0.31–0.56; p < .0001) in the PAM50 subpopulation; a similar observation was recorded in the overall population [15].
Table 1. Baseline patient demographics and disease characteristics.
Unless specified otherwise.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; PFS, progression‐free survival.
Intrinsic Subtype Distribution
In the PAM50 subpopulation, 46.7% of patients carried the luminal A subtype (n = 122), 21.5% the HER2‐enriched subtype (n = 56), 15.7% the luminal B subtype (n = 41), 14.2% the normal‐like subtype (n = 37), and 1.9% the basal‐like subtype (n = 5; Fig. 2A). Among the 56 patients carrying the HER2‐enriched subtype, 36 were recipients of everolimus plus exemestane and 20 were recipients of placebo plus exemestane. Among the 205 patients carrying non‐HER2‐enriched subtypes, 134 were recipients of everolimus plus exemestane and 71 were recipients of placebo plus exemestane. Of note, 59.5% and 79.5% of tumor samples within the non‐HER2‐enriched subtypes were luminal A and luminal A/B, respectively.
Figure 2.
Intrinsic subtype distribution in BOLERO‐2 tumor samples. (A): All patients (n = 261). (B): Primary tumors (n = 209). (C): Metastatic tumors (n = 50)*. *, Biopsy source unknown for two patients.
Abbreviation: HER2, human epidermal growth factor receptor 2.
Most of the tumor samples analyzed were derived from primary tumors (n = 209 [80.1%]) rather than metastatic tumors (n = 50 [19.2%]; Table 1); the biopsy source was unknown for two patients. Interestingly, 39 of 209 (18.7%) samples from primary tumors were HER2‐enriched (Fig. 2B), compared with 16 of 50 (32.0%) samples from metastatic tumors (p = .038; Fig. 2C).
Prognosis
Median PFS was 6.2 months (95% CI, 4.37–8.31) and 5.4 months (95% CI, 4.04–8.05) among patients with luminal A and B tumors, respectively, 5.2 months (95% CI, 3.91–6.70) among those with HER2‐enriched tumors, 3.2 months (95% CI, 1.51 to no result) among those with basal‐like tumors, and 6.8 months (95% CI, 4.07–11.07) among those with normal‐like tumors.
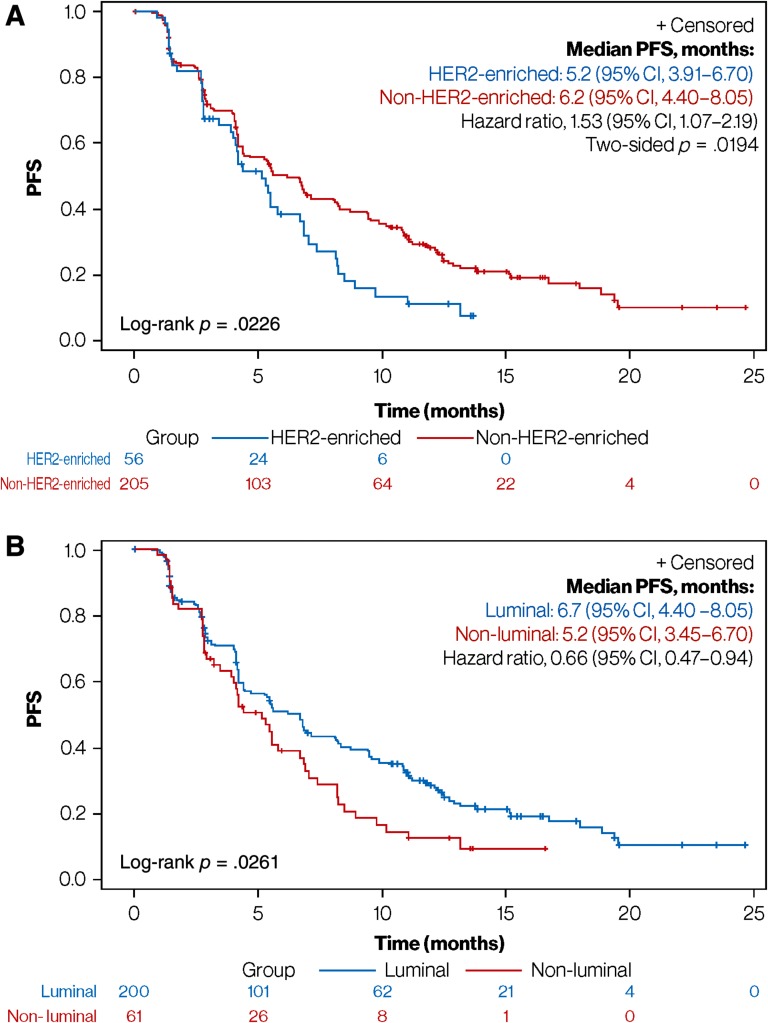
To analyze the association between HER2‐enriched subtype and survival, PFS was compared between the HER2‐enriched (median PFS, 5.2 months; 95% CI, 3.91–6.70) and combined non‐HER2‐enriched subtypes (median PFS, 6.2 months; 95% CI, 4.40–8.05). The 1‐month median PFS difference in favor of the non‐HER2‐enriched subtypes was statistically significant (adjusted hazard ratio, 1.53; 95% CI, 1.07–2.19; p = .019), with pronounced separation of the survival curves observed after more than 50% of patients had progressed (Fig. 3A). Overall, 80% of patients with a HER2‐enriched or non‐HER2‐enriched subtype had progressed at 8 and 15 months, respectively.
Figure 3.
Prognosis based on subtype classification. (A): PFS in HER2‐enriched versus non‐HER2‐enriched subgroups. (B): PFS in luminal (including normal‐like) versus nonluminal subgroups.
Abbreviations: CI, confidence interval; HER2, human epidermal growth factor receptor 2; PFS, progression‐free survival.
PFS was also compared between the combined luminal (median PFS, 6.7 months; 95% CI, 4.40–8.05) and nonluminal subgroups (median PFS, 5.2 months; 95% CI, 3.45–6.70). The 1.5‐month median PFS difference was statistically significant (adjusted hazard ratio, 0.66; 95% CI, 0.47–0.94; p = .020; Fig. 3B). A limited number of basal‐like tumors (n = 5) prevented the independent evaluation of this subgroup. An exploratory analysis comparing PFS between the luminal A and B subtypes showed no statistically significant differences.
Benefit of Everolimus Therapy
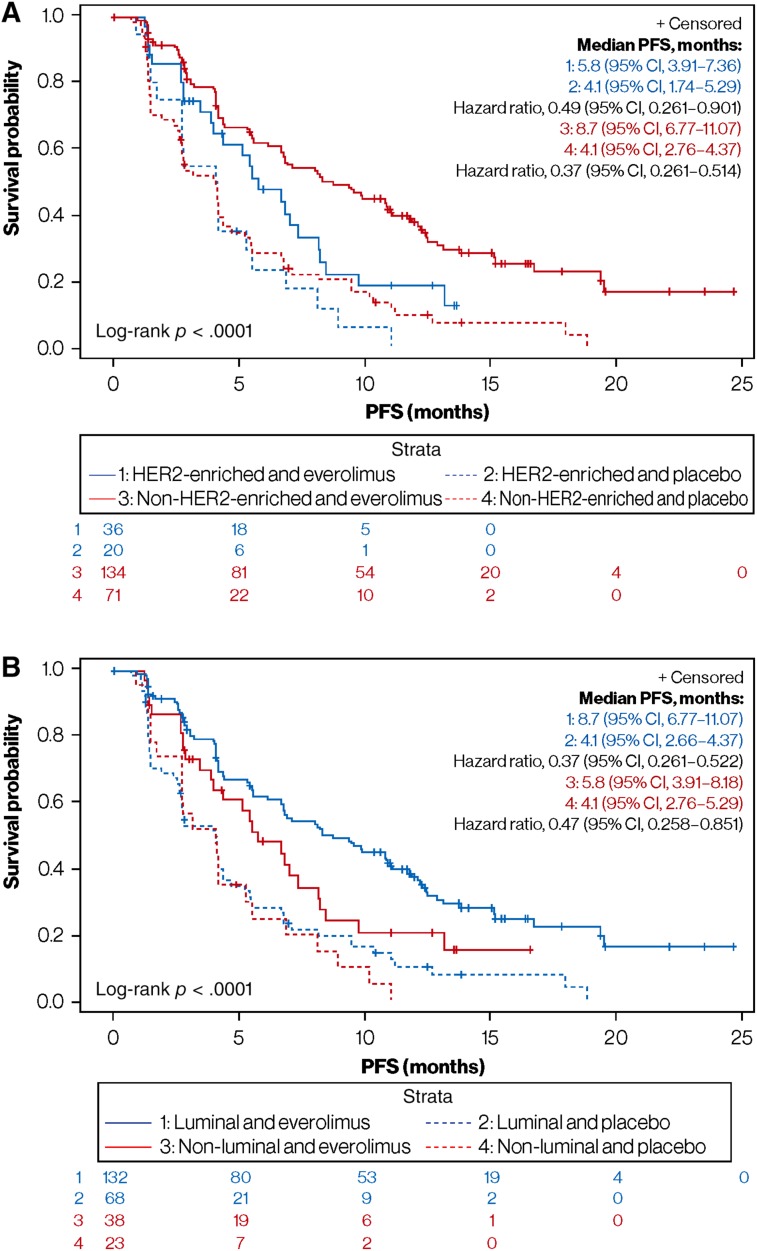
Among patients with HER2‐enriched disease, the addition of everolimus to exemestane had a small but statistically significant impact on median PFS compared with the placebo arm (5.8 vs. 4.1 months; adjusted hazard ratio, 0.49; 95% CI, 0.26–0.90; p = .034; Fig. 4A). In the non‐HER2‐enriched subgroup, the median PFS difference between the everolimus and placebo arms was more pronounced (8.7 vs. 4.1 months; adjusted hazard ratio, 0.37; 95% CI, 0.26–0.51; p < .0001; Fig. 4A). The interaction between HER2‐enriched tumors and everolimus benefit with respect to PFS was not statistically significant (p = .433).
Figure 4.
Everolimus PFS benefit based on subtype classification. (A): HER2‐enriched versus non‐HER2‐enriched subgroups. (B): Luminal (including normal‐like) versus nonluminal subgroups.
Abbreviations: CI, confidence interval; HER2, human epidermal growth factor receptor 2; PFS, progression‐free survival.
Among patients with nonluminal disease, the addition of everolimus to exemestane also had a small but statistically significant impact on median PFS compared with the placebo arm (5.8 vs. 4.1 months; adjusted hazard ratio, 0.47; 95% CI, 0.26–0.85; p = .027; Fig. 4B). In the luminal subgroup, the median PFS difference between the everolimus and placebo arms was also more pronounced (8.7 vs. 4.1 months; adjusted hazard ratio, 0.37; 95% CI, 0.26–0.52; p < .0001; Fig. 4B). As expected, the interaction between nonluminal and everolimus benefit with respect to PFS was not statistically significant (p = .534).
Among patients with the luminal A subtype, median PFS was 8.3 months (95% CI, 5.59–11.10) in the everolimus arm versus 4.1 months (95% CI, 2.63–5.26) in the placebo arm (adjusted hazard ratio, 0.39; 95% CI, 0.25–0.61; p < .0001). Among patients with the luminal B subtype, median PFS was 6.8 months (95% CI, 4.11–11.70) in the everolimus arm versus 2.8 months (95% CI, 1.48–7.13) in the placebo arm (adjusted hazard ratio, 0.69; 95% CI, 0.34–1.39; p = .349).
Discussion
In this study, PAM50 intrinsic subtyping was performed on tumor samples from 261 patients whose advanced HR+/HER2‐negative breast cancer received treatment in the BOLERO‐2 study. Of note, all the intrinsic molecular subtypes were identified. The HER2‐enriched subtype was present in 21.5% of cases and was enriched in metastatic tumor samples rather than primary tumor tissues. Nonluminal subtypes, including the HER2‐enriched subtype, were associated with a poorer prognosis compared with luminal subtypes. Although intrinsic subtyping did not predict which subgroup would derive the greatest benefit from everolimus, the PFS gain among patients with the HER2‐enriched subtype was small (1.7 months). To our knowledge, this is the first study to report the intrinsic disease subtypes of a population of patients with non‐steroidal aromatase inhibitor‐refractory, HR+/HER2‐negative advanced breast cancer.
Everolimus, an mTOR inhibitor, has shown clinical activity in patients with advanced HR+/HER2‐negative breast cancer as a monotherapy [16], and in combination with endocrine therapy [17], [18], [19]. In the phase III BOLERO‐2 study, the combination of everolimus and exemestane significantly improved median PFS versus placebo plus exemestane (7.8 vs. 3.2 months; hazard ratio, 0.45; 95% CI, 0.38–0.54; p < .0001) in patients with non‐steroidal aromatase inhibitor‐refractory, HR+/HER2‐negative advanced breast cancer, leading to the approval of this combination in this setting [15]. However, HR+/HER2‐negative disease is clinically and biologically heterogeneous, and identification of subgroups of patients with different prognoses and anticipated treatment responses is needed.
Two prior studies of tumor or blood samples from BOLERO‐2 have evaluated the prognostic and/or predictive value of single biomarkers [20], [21]. In one study, next‐generation sequencing of samples from 302 patients unexpectedly revealed that PFS benefit with everolimus was maintained regardless of PIK3CA, FGFR1, and CCND1 mutations or mutations to the corresponding pathways; however, quantitative differences in everolimus benefit were observed between patients with PIK3CA mutations in exon 20 and those with PIK3CA mutations in exon 9, and between patients with different degrees of chromosomal instability in their tumor tissues [21]. In the second study, ESR1 mutations Y537S and D538G in cell‐free DNA were evaluated in samples from 541 patients. Interestingly, detection of one or both mutations was associated with a more aggressive tumor biology [20]. Although promising, further analyses and clinical validation of these potential biomarkers are needed.
Evidence for the ability of the HER2‐enriched subtype to predict prognosis and/or treatment benefit is mostly confined to the early breast cancer setting [7], [8], [9], [10]. Similar evidence in the advanced or metastatic setting was previously limited to a single retrospective PAM50 analysis of 821 tumor samples from the phase III EGF30008 study [11]; the HER2‐enriched subtype was significantly associated with poor PFS in patients treated with first‐line letrozole with or without lapatinib. This result, together with that observed in the PAM50 subpopulation of this study, confirms the HER2‐enriched subtype to be a consistent and poor prognostic biomarker for advanced or metastatic HR+/HER2‐negative breast cancer.
In early breast cancer, the HER2‐enriched subtype represents 3%–10% of HR+/HER2‐negative disease [3], [4], [5]. However, a higher proportion of 15%–20% is being observed in patients with advanced or metastatic breast cancer [22]. This increase may be attributed to patient selection and/or a shift in tumor biology due to inherent tumor evolution or treatment effects. Indeed, patients with HER2‐enriched, early HR+/HER2‐negative breast cancer may have a higher likelihood of relapsing than those with luminal disease [10]. Thus, a given population of relapsed patients, such as that enrolled in BOLERO‐2, is more likely to carry the HER2‐enriched subtype than a population of patients with early breast cancer. However, 10%–15% of primary luminal A or B tumors acquire a HER2‐enriched profile in the metastatic tumor sample at first recurrence [22]. This increase in the HER2‐enriched profile in metastatic tumor tissue compared with primary tumor tissue was also observed here, in BOLERO‐2 (32.0% vs. 18.7%). Overall, the acquisition of a HER2‐enriched profile might reflect the appearance of estrogen independence in a previously estrogen‐dependent tumor (i.e., luminal).
The identification of HER2‐enriched tumors without HER2 overexpression/amplification might seem counterintuitive. However, intrinsic subtypes are defined based on global expression patterns of 50 genes, rather than a single gene. Thus, how different is a HER2‐negative/HER2‐enriched tumor from a classical HER2‐positive/HER2‐enriched tumor? A previous study compared thousands of molecular features of DNA, mRNA, miRNA, and proteins [6]. The results revealed that a very small number of molecular features (<2%) were significantly different between the two groups, most of which were in the HER2 amplicon (17q12‐21) itself. Thus, from a biological perspective, HER2‐enriched/HER2‐negative tumors resemble classical HER2‐enriched/HER2‐positive tumors except for a lack of HER2 amplification/overexpression. Elucidating the driver(s) of the HER2‐enriched phenotype is a current research priority. One potential candidate is epidermal growth factor receptor (EGFR), because patients with the HER2‐enriched subtype within HR+/HER2‐negative disease seem to benefit from lapatinib, an orally available dual EGFR/HER2 tyrosine kinase inhibitor [11].
An interesting observation was that there were no significant PFS or OS differences between luminal A and B subtypes. These results are inconsistent with those of the phase III EGF30008 study, in which PFS and OS were better among patients with the luminal A subtype versus those with the luminal B subtype [11]. One important aspect is that the vast majority of tumor samples analyzed (80.1%–85.7%) in this study and in the EGF30008 study were derived from the primary tumor, and were collected years before the patient relapsed. This might be important, as intrinsic subtype may evolve over time. In a recent study, in which 123 primary versus metastatic paired samples were PAM50 profiled, ∼55% of luminal A primary tumors were either luminal B or HER2‐enriched at relapse; by contrast, ∼80% of luminal B primary tumors remained luminal B at relapse [22]. Thus, it is likely that, compared with the EGF30008 trial, in which no patients received prior aromatase inhibitors, a large proportion of luminal A tumors identified in BOLERO‐2 are no longer luminal A.
The present study has several limitations. First, only 36.0% (261 of 724 patients) of the BOLERO‐2 study population was evaluated. Although the PAM50 subpopulation was well balanced and achieved a similarly significant PFS benefit with everolimus plus exemestane versus placebo plus exemestane compared with the original population, the study was underpowered to detect more significant differences between variables. Second, 80.1% of the samples profiled were from primary, rather than metastatic, tumor tissues. Whether the prognostic and/or predictive associations would have improved if metastatic tumor tissue had been profiled is currently unknown. Third, this is an unplanned retrospective analysis of a prospective clinical trial. However, to minimize bias, a statistical analysis plan was prepared before initiating the project and the laboratory that performed and reported the gene expression results for each sample was blinded to clinical data. Finally, we could not evaluate the prognostic and/or predictive ability of the basal‐like subtype, because only five cases (1.9%) were identified. As such, this analysis focuses on patients with HER2‐enriched versus non‐HER2‐enriched tumors. This study therefore adds valuable data describing the prognosis for patients with both HER2‐enriched and non‐HER2‐enriched disease.
Conclusion
The HER2‐enriched subtype is present in a substantial proportion of advanced HR+/HER2‐negative breast tumors and is a consistent biomarker of poor prognosis. Future trials in the advanced or metastatic setting should take this heterogeneity into account, because one patient out of five is likely to have nonluminal disease. Finally, the identification of tailored therapies for HER2‐enriched tumors within HER2‐negative disease is urgently needed.
Acknowledgments
We acknowledge Parul Patel (Novartis Pharmaceuticals Corporation) for bio‐sample management. This trial was sponsored by Novartis Pharmaceuticals Corporation, who also provided financial support for medical editorial assistance provided by Matthew Young, DPhil. This work was also supported by funds from the Spanish Society of Medical Oncology (to A.P.), Instituto de Salud Carlos III ‐ PI13/01718 (to A.P.), Pas a Pas (to A.P.), Save the Mama (to A.P.), Instituto de Salud Carlos III ‐ PI16/00904 (to A.P.), a Career Catalyst Grant (CCR13261208) from the Susan Komen Foundation (to A.P.), and Premio Jóven Investigador de la Fundación AstraZeneca.
Author Contributions
Conception/design: Aleix Prat, Jan Christoph Brase, Yuan Cheng, Paolo Nuciforo, Laia Paré, Tomás Pascual, Débora Martínez, Patricia Galván, Maria Vidal, Barbara Adamo, Gabriel N. Hortobagyi, José Baselga, Eva Ciruelos
Provision of study material or patients: Aleix Prat, Jan Christoph Brase, Yuan Cheng, Paolo Nuciforo, Laia Paré, Tomás Pascual, Débora Martínez, Patricia Galván, Maria Vidal, Barbara Adamo, Gabriel N. Hortobagyi, José Baselga, Eva Ciruelos
Collection and/or assembly of data: Aleix Prat, Jan Christoph Brase, Yuan Cheng, Paolo Nuciforo, Laia Paré, Tomás Pascual, Débora Martínez, Patricia Galván, Maria Vidal, Barbara Adamo, Gabriel N. Hortobagyi, José Baselga, Eva Ciruelos
Data analysis and interpretation: Aleix Prat, Jan Christoph Brase, Yuan Cheng, Paolo Nuciforo, Laia Paré, Tomás Pascual, Débora Martínez, Patricia Galván, Maria Vidal, Barbara Adamo, Gabriel N. Hortobagyi, José Baselga, Eva Ciruelos
Manuscript writing: Aleix Prat, Jan Christoph Brase, Yuan Cheng, Paolo Nuciforo, Laia Paré, Tomás Pascual, Débora Martínez, Patricia Galván, Maria Vidal, Barbara Adamo, Gabriel N. Hortobagyi, José Baselga, Eva Ciruelos
Final approval of manuscript: Aleix Prat, Jan Christoph Brase, Yuan Cheng, Paolo Nuciforo, Laia Paré, Tomás Pascual, Débora Martínez, Patricia Galván, Maria Vidal, Barbara Adamo, Gabriel N. Hortobagyi, José Baselga, Eva Ciruelos
Disclosures
Aleix Prat: NanoString Technologies, GlaxoSmithKline, Susan G. Komen Foundation (RF), NanoString Technologies, Novartis Pharmaceuticals Corporation (C/A), Novartis Pharmaceuticals Corporation, Pfizer, Roche (SAB), Novartis Pharma AG (E); Jan Christoph Brase: Novartis (E); Yuan Cheng: Novartis (E); Gabriel N. Hortobagyi: Novartis (RF); José Baselga: Roche (H), Grail, PMV Pharma, Juno, Northern Biologics, Varian Medical Systems, Bristol‐Myers Squibb, Foghorn (SAB), Tango, Aura (OI).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Cardoso F, Costa A, Senkus E et al. 3rd ESO‐ESMO international consensus guidelines for advanced breast cancer (ABC 3). Breast 2017;31:244–259. [DOI] [PubMed] [Google Scholar]
- 2.Rugo HS, Rumble BR, Macrae E et al. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 2016;34:3069–3103. [DOI] [PubMed] [Google Scholar]
- 3.Prat A, Pineda E, Adamo B et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015;24(suppl 2):S26–S35. [DOI] [PubMed] [Google Scholar]
- 4.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011;5:5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker JS, Mullins M, Cheang MC et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prat A, Carey LA, Adamo B et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2‐positive breast cancer. J Natl Cancer Inst 2014;106. pii: dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunbier AK, Anderson H, Ghazoui Z et al. Association between breast cancer subtypes and response to neoadjuvant anastrozole. Steroids 2011;76:736–740. [DOI] [PubMed] [Google Scholar]
- 8.Ellis MJ, Suman VJ, Hoog J et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor‐rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50‐based intrinsic subtype‐‐ACOSOG Z1031. J Clin Oncol 2011;29:2342–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prat A, Fan C, Fernández A et al. Response and survival of breast cancer intrinsic subtypes following multi‐agent neoadjuvant chemotherapy. BMC Med 2015;13:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prat A, Parker JS, Fan C et al. Concordance among gene expression‐based predictors for ER‐positive breast cancer treated with adjuvant tamoxifen. Ann Oncol 2012;23:2866–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prat A, Cheang MC, Galván P et al. Prognostic value of intrinsic subtypes in hormone receptor‐positive metastatic breast cancer treated with letrozole with or without lapatinib. JAMA Oncol 2016;2:1287–1294. [DOI] [PubMed] [Google Scholar]
- 12.Cejalvo JM, Pascual T, Fernández‐Martínez A et al. Clinical implications of the nonluminal intrinsic subtypes in hormone receptor‐positive breast cancer. Cancer Treat Rev 2018;67:63–70. [DOI] [PubMed] [Google Scholar]
- 13.Baselga J, Campone M, Piccart M et al. Everolimus in postmenopausal hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2012;366:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidal M, Peg V, Galván P et al. Gene expression‐based classifications of fibroadenomas and phyllodes tumours of the breast. Mol Oncol 2015;9:1081–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yardley DA, Noguchi S, Pritchard KI et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO‐2 final progression‐free survival analysis. Adv Ther 2013;30:870–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellard SL, Clemons M, Gelmon KA et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol 2009;27:4536–4541. [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Semiglazov V, van Dam P et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor‐positive breast cancer. J Clin Oncol 2009;27:2630–2637. [DOI] [PubMed] [Google Scholar]
- 18.Bachelot T, Bourgier C, Cropet C et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor‐positive, human epidermal growth factor receptor 2‐negative metastatic breast cancer with prior exposure to aromatase inhibitors: A GINECO study. J Clin Oncol 2012;30:2718–2724. [DOI] [PubMed] [Google Scholar]
- 19.Kornblum N, Manola J, Klein P et al. PrECOG 0102: A randomized, double‐blind, phase II trial of fulvestrant plus everolimus or placebo in post‐menopausal women with hormone receptor (HR)‐positive, HER2‐negative metastatic breast cancer (MBC) resistant to aromatase inhibitor (AI) therapy. Abstract S1‐02 presented at: San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX.
- 20.Chandarlapaty S, Chen D, He W et al. Prevalence of ESR1 mutations in cell‐free DNA and outcomes in metastatic breast cancer: A secondary analysis of the BOLERO‐2 clinical trial. JAMA Oncol 2016;2:1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hortobagyi GN, Chen D, Piccart M et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor‐positive, human epidermal growth factor receptor 2‐negative advanced breast cancer: Results from BOLERO‐2. J Clin Oncol 2016;34:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cejalvo JM, Martínez de Dueñas E, Galván P et al. Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Cancer Res 2017;77:2213–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]