Many patients with advanced cancer develop hypoactive delirium. This article describes current practice and overall effectiveness and adverse effects of commonly used pharmacotherapy for hypoactive delirium in advanced cancer patients.
Keywords: Delirium, Neoplasms, Antipsychotic agents, Palliative care, Pharmacovigilance
Abstract
Background.
Pharmacotherapy is generally recommended to treat patients with delirium. We sought to describe the current practice, effectiveness, and adverse effects of pharmacotherapy for hypoactive delirium in patients with advanced cancer, and to explore predictors of the deterioration of delirium symptoms after starting pharmacotherapy.
Subjects, Materials, and Methods.
We included data of patients with advanced cancer who were diagnosed with hypoactive delirium and received pharmacotherapy for treatment of delirium. This was a pharmacovigilance study characterized by prospective registries and systematic data‐recording using internet technology, conducted among 38 palliative care teams and/or units. The severity of delirium and other outcomes were assessed using established measures at days 0 (T0), 3 (T1), and 7 (T2).
Results.
Available data were obtained from 218 patients. The most frequently used agent was haloperidol (37%). A total of 67 and 42 patients (31% and 19%) had died or discontinued pharmacotherapy by T1 and T2, respectively. Delirium symptoms deteriorated between T0 and T1, but this trend did not reach statistical significance. The most prevalent adverse event was sedation (9%). Delirium severity worsened after starting pharmacotherapy in 121 patients (56%) at T1. In patients whose death was expected within a few days and those with delirium caused by organ failure, symptoms of delirium were significantly more likely to deteriorate after starting pharmacotherapy.
Conclusion.
Current pharmacotherapy for hypoactive delirium in patients with advanced cancer is not recommended, especially in those whose death is expected within a few days and in those with delirium caused by organ failure.
Implications for Practice.
Delirium is common among patients with advanced cancer, and hypoactive delirium is the dominant motor subtype in the palliative care setting. Pharmacotherapy is recommended and regularly used to treat delirium. This article describes the effectiveness and adverse effects of pharmacotherapy for hypoactive delirium in patients with advanced cancer. The findings of this study do not support the use of pharmacotherapy for treatment of hypoactive delirium in the palliative care setting. Pharmacotherapy should especially be avoided in patients whose death is expected within a few days and in those with delirium caused by organ failure.
Introduction
Delirium is a common neuropsychiatric condition characterized by acute onset of change in attention or awareness, accompanied by change in cognition [1]. Typically, these disturbances develop within hours or a few days, with fluctuating levels of severity during the course of a day. The prevalence of delirium among patients with advanced cancer in palliative care units ranges from 30% to 40% at admission, and up to 90% at the terminal stage [2], [3], [4], [5]. Delirium is mainly classified into three motor subtypes based on level of alertness or arousal: hyperactive, hypoactive, and mixed subtype. Hypoactive delirium, characterized by reduced psychomotor activity [1], is the most common subtype in palliative care settings [6], [7]. Hypoactive delirium is associated with profound distress in patients and families, as well as with other motor subtypes [8], [9].
Standard pharmacotherapy for hypoactive delirium has not yet been established. In general, treatment for delirium consists of seeking and correcting the underlying causes, nonpharmacological intervention, and pharmacological strategies [10]. Regarding pharmacological treatment, antipsychotics are most often recommended and regularly used [10], [11]. However, evidence to support this practice has usually been from trials in patients with hyperactive and mixed type delirium. To our best knowledge, only a few small studies and case reports suggest the effectiveness of antipsychotics, including haloperidol, chlorpromazine, and aripiprazole, in patients with hypoactive delirium [12], [13].
Identifying patients with hypoactive delirium in whom pharmacotherapy should be avoided is clinically important. Recently, a randomized controlled trail raised questions about the effectiveness and safety of haloperidol and risperidone for treating delirium in terminally ill cancer patients in palliative care settings [14]. However, these authors did not explore the effectiveness of those medications by motor subtype. There have been inconsistent findings in nonpalliative care settings regarding whether hypoactive delirium is less likely to respond to pharmacotherapy than other motor subtypes [15], [16], [17]. We anticipated that pharmacotherapy may deteriorate delirium symptoms, especially in patients with hypoactive delirium, because antipsychotic agents cause sedation to a greater or lesser degree regardless of the antipsychotic used. Consistent with our concerns, in their clinical practice guideline for postoperative delirium in older adults, the American Geriatric Society recommends not to prescribe antipsychotics for treating older adults with hypoactive delirium [18].
Considering that many advanced cancer patients develop hypoactive delirium, more information regarding pharmacotherapy for hypoactive delirium is required. We aimed to describe current practice, and the overall effectiveness and adverse effects of commonly used pharmacotherapy for hypoactive delirium in a consecutive, prospective cohort of patients with advanced cancer using data of the Japan Pharmacological Audit study of Safety and Efficacy in Real World (Phase‐R). We also sought to determine the predictors of deterioration in delirium symptoms after starting pharmacotherapy.
Subjects, Materials, and Methods
Phase‐R was a pharmacovigilance study characterized by prospective registries and systematic data‐recording using internet technology, to investigate the effectiveness and adverse effects of pharmacotherapies in patients with advanced cancer. The Phase‐R study was designed to preserve external validity, focusing on palliative care, where it is difficult to conduct controlled trials because of feasibility and ethical aspects. The importance of pharmacovigilance in the palliative setting has been described elsewhere [19]. The Phase‐R Delirium Study Group focused on pharmacotherapy for delirium among advanced cancer patients. This study was conducted with specialized palliative care teams working in acute wards and palliative care units at 38 hospitals across Japan between September 2015 and May 2016.
Participants
Eligibility criteria for inclusion in the study were (a) adult patients with advanced cancer referred to a specialized palliative care team and/or units and (b) those who had delirium diagnosed by trained psychiatrists and/or palliative care physicians. Exclusion criteria were patients with (a) postoperative delirium and (b) alcohol or other drug withdrawal delirium. This study was conducted by extracting the data of 223 patients diagnosed with hypoactive delirium, who received first‐line pharmacotherapy for delirium.
This study was conducted in accordance with the principles of the Declaration of Helsinki. Institutional Review Boards at each participating site approved the study. According to the Osaka University Research Ethics Committee (the lead site) and Institutional Review Boards at all participating sites, informed consent was not needed (ethical waivers) because all data were obtained thorough only routine clinical practice and there was minimal risk to patients who participated in the study. We used an opt‐out method for patients and families to refuse participation in this study.
Procedure
Patients were consecutively sampled at each trial site. When eligible patients were referred to a palliative care team and/or unit and diagnosed with delirium, their medical information was consecutively recorded using an electronic data capturing system. If pharmacotherapy was administered by psychiatrists and/or palliative care physicians as part of clinical practice, the information described below was recorded. In addition to the antipsychotics, trazodone (an antidepressant having antagonistic effects on the 5‐hydroxytryptamine (5‐HT) 2 receptor) was included, because trazodone is often prescribed for delirium in Japan, targeting delirium symptoms, in particular sleep quality, or in consideration of minimizing adverse effects of pharmacotherapy [20], [21].
Measurements
Patient assessments were conducted by trained psychiatrists and/or palliative care physicians at days 0 (T0), 3 (T1), and 7 (T2) after starting pharmacotherapy. At T0, the diagnosis of delirium was ascertained according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Delirium subtypes were assessed using the Delirium Motor Subtype Scale [22], [23]. Delirium Rating Scale, Revised‐98 (DRS‐R98) [24]; Nursing Delirium Screening Scale (Nu‐DESC) [25]; Richmond Agitation‐Sedation Scale‐Palliative version (RASS‐PAL); Communication Capacity Scale (CCS); and Agitation Distress Scale (ADS) was performed at both T0 and T1. The Common Terminology Criteria for Adverse Events (CTCAE) was administered at T0, T1, and T2. The Drug‐Induced Extrapyramidal Symptoms Scale (DIEPSS) was used at the worst point of extrapyramidal symptoms during the 7 days after starting medication [26]. If patients died or dropped out of the study before T1 or T2, information from the last observation was retrieved.
DRS‐R98.
The DRS‐R98 is a 16‐item, clinician‐rated scale with anchored item‐descriptions corresponding to both symptoms and temporal aspects of delirium [24]. The severity scale includes a total 13 items, each rated 0–3, with scores ranging from 0 to 39, where higher scores indicate greater severity of delirium. The Japanese version of the DRS‐R98 has demonstrated excellent reliability and validity [27].
Nu‐DESC.
The Nu‐DESC was developed as a fast and simple screening instrument for clinical use [25]. The Nu‐DESC consists of a 5‐item scale with items rated 0–2 and total scores ranging from 0 to 10, where a higher score indicates high probability of a diagnosis of delirium. This scale can be used to evaluate the severity of delirium symptoms [25]. In this study, the composite subscore of items 2 (inappropriate behavior), 3 (inappropriate communication), and 4 (illusions and hallucinations) was used to indicate delirium severity because these three were the target symptoms of delirium associated with distress, according to the previous study [14].
RASS‐PAL.
This is a simple observational instrument that assesses levels of sedation and agitation in palliative care populations [28]. RASS‐PAL has scores ranging from +4 (an overtly combative patient) to −5 (patient cannot be aroused), per responses to either voice or physical stimulation.
CCS and ADS.
These are observer‐rated instruments to quantify levels of communication capacity and agitation distress in terminally ill cancer patients with delirium [29]. Originally, the CCS was conceptualized to assess patients’ ability to comprehend their circumstances and to appropriately express their intentions, and included a 5‐item scale. We used item 4, voluntary communication, with scores ranging from 0 to 3; a higher score indicates that the patient is more severely impaired. The ADS includes a 6‐item scale. In this study, we used item 2, extent of motor anxiety, with scores ranging from 0 to 3; a higher score indicates more severe motor anxiety.
CTCAE, version 3.0.
The CTCAE is a descriptive terminology that can be used for adverse event (AE) reporting [30]. The items included were eight events, and grade 3 or more severe adverse events. A grading (severity) scale is provided for each AE term (grade 1, mild AE to grade 5, death related to AE). Grade 3 (severe AE) indicates a “possible” or stronger causal relationship according to the Clinical Safety Data Management Guideline defined by the Japanese Clinical Oncology Group [31].
DIEPSS.
Extrapyramidal symptoms after pharmacotherapy were assessed using the DIEPSS [26]. This scale consists of nine items rated on a 5‐point scale (0, none to 4, severe), based on objective observation. A higher score indicates more severe symptoms. The total score, obtained by summing all items except item 9 (global domain), was used to measure severity.
Patient Characteristics and Biomedical Information.
Patient data also included demographic factors, medical factors such as primary cancer site, performance status (PS) as defined by Eastern Cooperative Oncology Group (ECOG) criteria, palliative performance status [32], and palliative prognostic index [33]. In addition, past and present history of organic brain disease (e.g., brain metastasis, dementia), ability of oral intake, prognosis estimation (within a few days, weeks, or months), and potentially underlying etiology and precipitating factors of delirium [34], [35] as evaluated by the researchers were also recorded. Detailed information regarding pharmacotherapy was also collected.
Statistical Analysis
Paired t test and Wilcoxon signed‐rank tests were used to investigate change in the continuous and ordinal outcome measures before and after pharmacotherapy, respectively. Multiple logistic regression analysis was performed to predict deterioration of delirium after starting pharmacotherapy [36]. We defined deterioration as a one point or greater increase in the total score of the DRS‐R98. Independent variables were age, etiologies of delirium, setting, ECOG PS, and prognostic estimation. We conducted forced entry multiple logistic regression analysis using these variables. We further calculated likelihood ratios (LRs) or stratum‐specific likelihood ratios (SSLRs) to express by how much a given status of each predictor would raise or lower the odds of having deterioration of delirium for an individual respondent, compared with the total population.
A p value <.05 was adopted as the significance level in all statistical analyses; all reported p values were two‐tailed. All statistical procedures were conducted using IBM SPSS Statistics version 25 for Mac OS (IBM Corp., Armonk, NY).
Results
Patients’ Baseline Characteristics and Flow Diagram
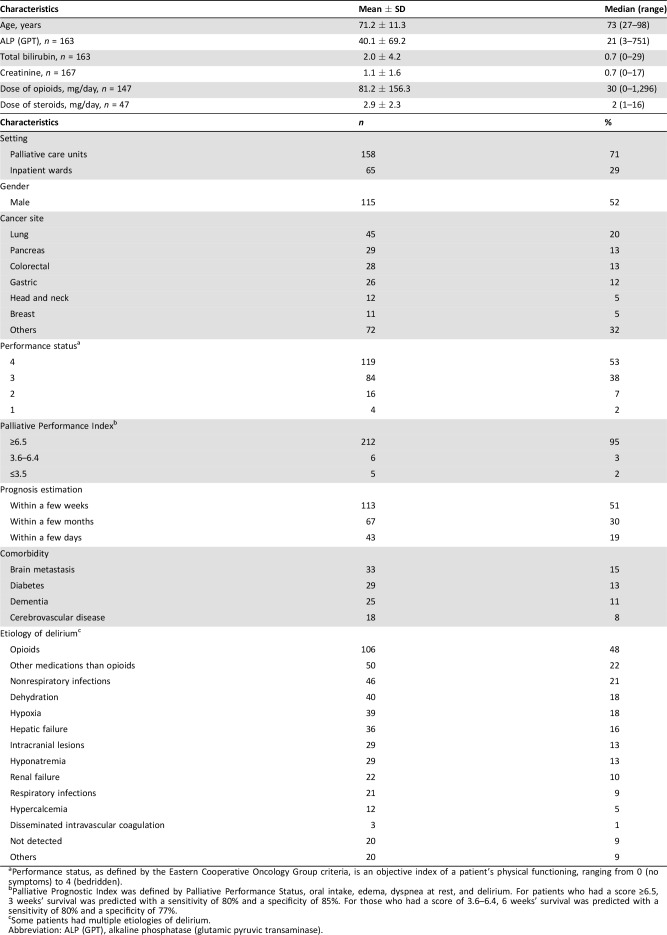
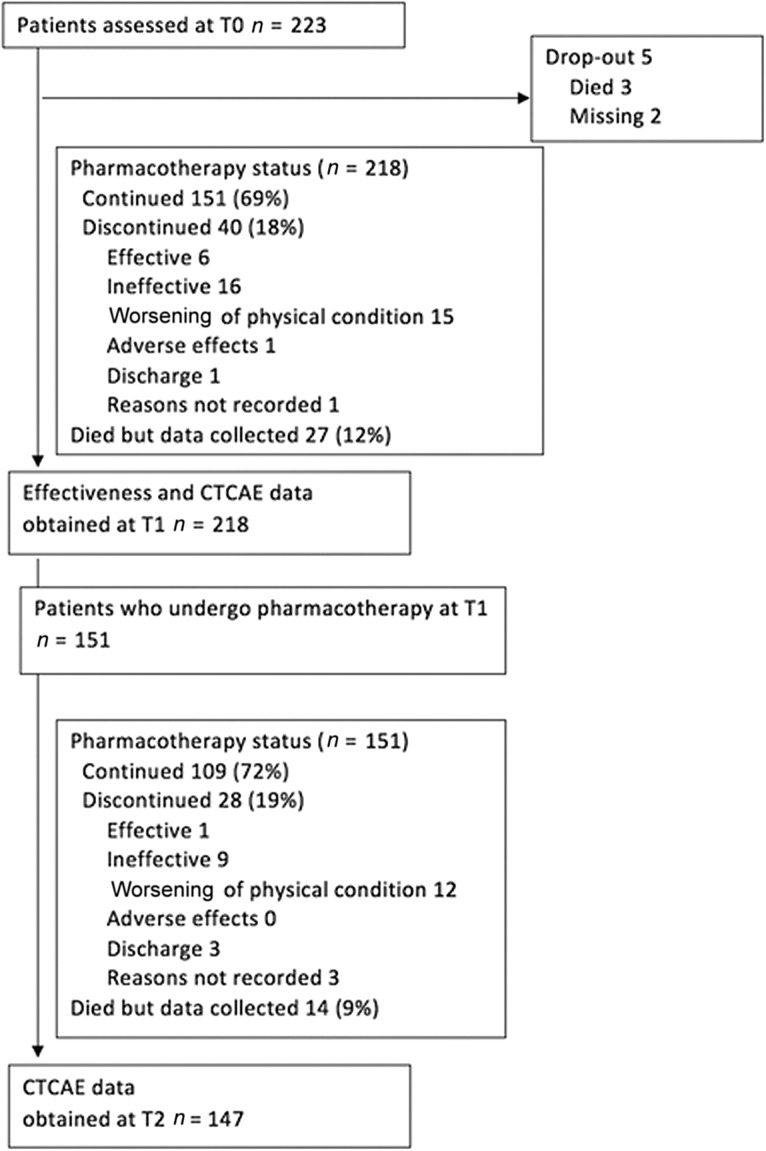
The data of a total 223 patients who had hypoactive delirium were included in this study. Participants’ demographic and clinical data are shown in Table 1. About 70% of participants were in palliative care units, and 53% had ECOG PS 4. A patient flow diagram is shown in Figure 1.
Table 1. Baseline demographical and clinical characteristics of participants (n = 223).
Performance status, as defined by the Eastern Cooperative Oncology Group criteria, is an objective index of a patient's physical functioning, ranging from 0 (no symptoms) to 4 (bedridden).
Palliative Prognostic Index was defined by Palliative Performance Status, oral intake, edema, dyspnea at rest, and delirium. For patients who had a score ≥6.5, 3 weeks' survival was predicted with a sensitivity of 80% and a specificity of 85%. For those who had a score of 3.6–6.4, 6 weeks' survival was predicted with a sensitivity of 80% and a specificity of 77%.
Some patients had multiple etiologies of delirium.
Abbreviation: ALP (GPT), alkaline phosphatase (glutamic pyruvic transaminase).
Figure 1.
Patient flow diagram.
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events.
Current Practice of Pharmacotherapy for Hypoactive Delirium
Medications started and the routes of administration at T0 are shown in Table 2. The most commonly prescribed drug was haloperidol (37%), followed by quetiapine (23%). Change in antipsychotic treatment status is also shown in Figure 1. Among 218 patients started on pharmacotherapy, 67 and 42 patients (31% and 19%) had died or discontinued pharmacotherapy at T1 and T2, respectively.
Table 2. Pharmacotherapy used for the treatment of hypoactive delirium.
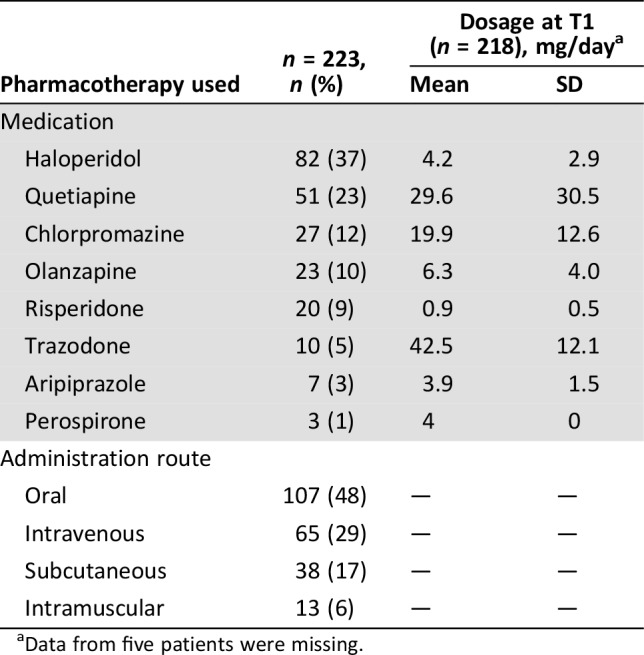
Data from five patients were missing.
Sixty‐four and 16 patients (29%, 7%) concurrently were administered hypnotics and other antipsychotics in addition to the agents started at T0. Palliative sedation was started in six patients between T0 and T1.
Effectiveness of Pharmacotherapy
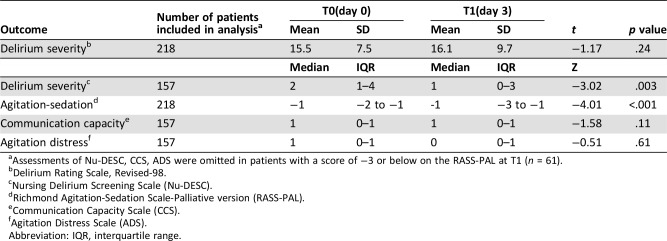
Preintervention and postintervention outcome scores are described in Table 3. Overall delirium severity assessed using the DRS‐R98 deteriorated between T0 and T1, but this trend did not reach statistical significance. We did not find significant changes in the CCS and ADS; however, Nu‐DESC and RASS‐PAL scores decreased significantly. Additionally, we investigated the effectiveness of pharmacotherapy on the DRS‐R‐98 score in the subgroup of patients who received the antipsychotics (n = 208, excluding 10 patients who received trazodone) and found nonsignificant changes, consistent with the results obtained in the whole sample.
Table 3. Pre‐ and postintervention scores for the study outcomes.
Assessments of Nu‐DESC, CCS, ADS were omitted in patients with a score of −3 or below on the RASS‐PAL at T1 (n = 61).
Delirium Rating Scale, Revised‐98.
Nursing Delirium Screening Scale (Nu‐DESC).
Richmond Agitation‐Sedation Scale‐Palliative version (RASS‐PAL).
Communication Capacity Scale (CCS).
Agitation Distress Scale (ADS).
Abbreviation: IQR, interquartile range.
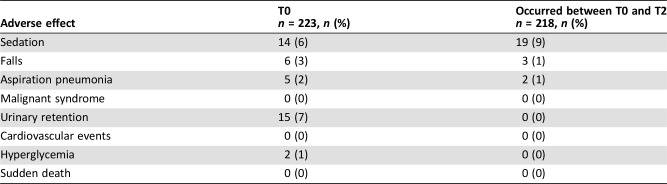
Adverse Effects of Pharmacotherapy
The most frequently reported adverse effect with a “possible” or stronger causal relationship was sedation (9%; Table 4). Mean DIEPSS score at the worst point during the observation period (n = 213) was 1.15 (SD 2.51, median 0, range 0–9). No deaths were considered adverse effects that had a “possible” or stronger causal relationship with pharmacotherapy.
Table 4. Adverse effects with a “possible” or stronger causal relationship.
Predictors of Deterioration of Delirium Symptoms After Starting Pharmacotherapy
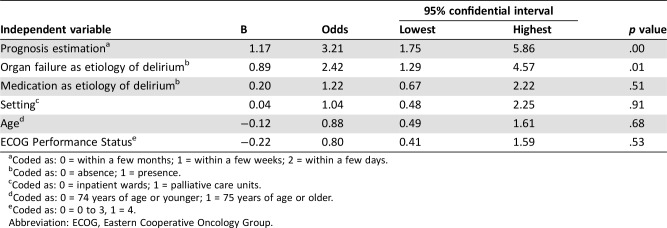
Delirium symptoms deteriorated after starting pharmacotherapy in 121 patients at T1 (56%; 95% confidence interval [CI], 49%–62%). Prognosis estimation and presence of organ failure as the etiology of delirium were found to be significant predictors (Table 5). The SSLR for prognosis estimation was 4.8 (95% CI, 1.8–13.1), 1.02 (95% CI, 0.7–1.4), and 0.58 (95% CI, 0.4–0.9) for the strata within a few days, within a few weeks, and within a few months, respectively. The LR for presence of organ failure was 1.6 (95% CI, 1.1–2.4).
Table 5. Predictors of deterioration of delirium symptoms after starting pharmacotherapy (n = 218).
Coded as: 0 = within a few months; 1 = within a few weeks; 2 = within a few days.
Coded as: 0 = absence; 1 = presence.
Coded as: 0 = inpatient wards; 1 = palliative care units.
Coded as: 0 = 74 years of age or younger; 1 = 75 years of age or older.
Coded as: 0 = 0 to 3, 1 = 4.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Discussion
This multicenter observational study revealed several new aspects regarding pharmacotherapy for hypoactive delirium in patients with advanced cancer. We found that (a) the most frequently used agent was haloperidol (37%), and about one third and one fifth of patients had died or discontinued pharmacotherapy by day 3 and day 7, respectively; (b) effectiveness of pharmacotherapy for reducing the delirium symptoms was not demonstrated; (c) the most frequently reported adverse effect was sedation; and (d) prognosis estimation and the presence of organ failure as the etiology of delirium were significant predictors of deterioration of hypoactive delirium symptoms after starting pharmacological treatment.
Pharmacological treatment of hypoactive delirium in advanced cancer patients has not yet been established. Severely ill cancer patients form a heterogeneous group with respect to comorbidity; decreased physical, psychological, and cognitive function; limited prognosis; and so on. Although several phase III studies have been published recently [14], [37], generalizability of the evidence in the real‐world settings is limited. Thus, integration of phase III evidence and pharmacovigilance data is essential to establish the treatment [19]. Ours is the first pharmacovigilance study to investigate the effectiveness and adverse effects of current pharmacological treatment for hypoactive delirium among advanced cancer patients. Overall delirium severity, communication capacity, and agitation distress were not significantly improved by current pharmacological treatment. We could not determine how pharmacotherapy affects the course of delirium because this study lacked controlled conditions. But to our knowledge, this study offers some of the best evidence for the effectiveness of pharmacotherapy in hypoactive delirium among advanced cancer patients, as rigorous controlled trials with no loss in the quality of external validity cannot be conducted. Based on these considerations, we recommend not to use pharmacotherapy for hypoactive delirium in advanced cancer patients, especially in those whose death is expected within a few days. The negative association between survival estimation and effectiveness of pharmacotherapy indicates that advanced cancer patients were heterogeneous according to prognostic estimation and that future empirical trials to investigate the effectiveness of pharmacotherapy in this population should be conducted among specific patient groups defined by survival estimation.
Delirium symptoms deteriorated somewhat after starting pharmacotherapy in about half of patients. This may partly be explained by the progressive nature of their disease. This explanation is supported by the finding that the presence of organ failure as the delirium etiology was a significant predictor of deterioration in this study, consistent with existing findings among patients with delirium (not limited to hypoactive type) in palliative care settings [35]. More importantly, pharmacotherapy itself may deteriorate delirium symptoms. Our results revealed that prognostic estimation of patients’ remaining life had a unique role in detecting patients who should not receive pharmacotherapy for the treatment of hypoactive delirium. The clinical application of our findings can be greatly facilitated by using stratum‐specific likelihood ratios in the Bayesian framework, in which the pretest probability will be converted into the post‐test probability via the likelihood ratio [38]. For example, for a patient with pretest probability of delirium deterioration after starting pharmacotherapy of 50% (clinically uncertain: the pretest probably estimate can be based on some group characteristics such as performance status or age; or on the available individual patient information, except life prognosis estimation) and estimated life prognosis of within a few days, application of the pretest probability with the SSLR for this particular life prognosis estimate (in this case 4.8) provides an approximately 83% post‐test probability of delirium deterioration. The clinician may then be justified in not starting pharmacotherapy. Translation of pretest probability into post‐test probability via the SSLR can be done manually using the useful nomogram proposed by Fagan (1975). There is a spreadsheet (available at: http://ebmh.med.kyoto-u.ac.jp/toolbox.html at Department of Health Promotion and Human Behavior, Kyoto University Graduate School of Medicine) or a smartphone app (DocNomo: available at: https://itunes.apple.com/jp/app/docnomo/id901279945?mt=8) that allow easy calculation at beside.
Most terminally ill patients experience delirium before death [34]. Our findings indicate that pharmacotherapy for hypoactive delirium in this stage may be unfeasible, ineffective, and even exacerbate delirium symptoms. Further, taking the present study findings together with those of existing studies [14], [34], [39] and a review of guidelines for the management of delirium [40], we consider that hypoactive delirium occurring near the end of life may be reconceptualized as a part of the normal dying process rather than a psychiatric disorder requiring pharmacological intervention. Provision of care that prioritizes comfort including nursing intervention, discontinuation of diagnostic or treatment efforts, and comprehensive care for both patients and families by an interdisciplinary team, is of paramount importance [41]. Care strategies perceived as useful by bereaved family members of cancer patients diagnosed with delirium in terminal stage (e.g., respect for the patients’ subjective world, treating patients the same as before they reached a terminal stage) may also be worth consideration [9], [42]. Additionally, support for caregivers, including provision of appropriate information regarding delirium [43] and grief care, should be provided.
The most prevalent adverse event was sedation. Significant change in RSS‐PAL scores also indicated that pharmacotherapy causes sedation. Several bias risks may cause underestimation of adverse effects. First, the fact that one third of participants had dropped out by T2 indicates the risk of selection bias. Second, there might be a risk of information bias when assessing the causality between prescribed drug and observed events.
The present study has several limitations. First, this study lacks controlled conditions, and therefore, we did not discuss the benefits and harms of pharmacotherapy by comparing with treatment as usual. But this point is incompatible with the concept of a pharmacovigilance study. Second, the outcomes were assessed by physicians who use pharmacotherapy in their patients. This may have caused information bias—overestimation of the effectiveness and underestimation of the adverse effects of pharmacotherapy. Third, there was a risk of attention bias for some outcomes including Nu‐DESC. Fourth, caution must be used when interpreting the results of Nu‐DESC score, because Nu‐DESC itself or use of composite subscore has not been validated in Japan. Fifth, we did not explore the effectiveness and adverse effects of individual agents. Because each agent was used arbitrarily based on clinical judgment, the results of such analysis were thought difficult to interpret because of selection bias. Finally, caution must be used when applying the findings to patients in other settings, especially non‐terminally ill patients.
Conclusion
The present systematic pharmacovigilance data indicate that current pharmacotherapy for hypoactive delirium in patients with advanced cancer did not result in improvement of its severity. Current pharmacological treatment for hypoactive delirium in advanced cancer patients is not recommended, especially in those whose death is expected within a few days and those with delirium caused by organ failure. It is important to set appropriate goals of delirium care that consider the patient's prognosis.
Acknowledgments
We gratefully acknowledge Toshi A. Furukawa, M.D., Ph.D., Department of Health Promotion and Human Behavior, Kyoto University Graduate School of Medicine/School of Public Health, for his help in interpreting the significance of the results of this study and in the preparation of the manuscript. We thank Analisa Avila, ELS, of Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. Collaborators of the Phase‐R Delirium Study Group (in addition to the authors listed above) include the following (in alphabetical order): Hirofumi Abo, M.D. (Rokkou Hospital); Nobuya Akizuki, M.D., Ph.D. (Chiba Cancer Center); Koji Amano, M.D. (Osaka City General Hospital); Daisuke Fujisawa, M.D., Ph.D. (Keio University Hospital); Shingo Hagiwara, M.D. (Tsukuba Medical Center Hospital); Takeshi Hirohashi, M.D. (Eiju General Hospital); Takayuki Hisanaga, M.D. (Tsukuba Medical Center Hospital); Kengo Imai, M.D. (Seirei Mikatahara General Hospital); Shuji Inada, M.D., Ph.D. (The University of Tokyo); Satoshi Inoue, M.D. (Seirei Mikatahara General Hospital); Shinichiro Inoue, M.D. (Okayama University Hospital); Aio Iwata, M.D. (National Cancer Center Hospital East); Keisuke Kaneishi, M.D. (JCHO Tokyo Shinjuku Medical Center); Akifumi Kumano, M.D. (Rokkou Hospital); Isseki Maeda, M.D., Ph.D. (Garcia Hospital); Yoshinobu Matsuda, M.D. (National Hospital Organization Kinki‐Chuo Chest Medical Center); Takashi Matsui, M.D. (Tochigi Cancer Center); Yoshihisa Matsumoto, M.D., Ph.D. (National Cancer Center Hospital East); Naoki Matsuo, M.D. (Sotoasahikawa Hospital); Kaya Miyajima, M.D., Ph.D. (Keio University Hospital); Ichiro Mori, M.D., Ph.D. (Garcia Hospital); Sachiyo Morita, M.D., Ph.D. (Shiga University of Medical Science Hospital); Nobuhisa Nakajima, M.D., Ph.D. (Tohoku University Hospital); Hiroyuki Nobata, M.D. (National Cancer Center Hospital East); Takuya Odagiri, M.D. (Komaki City Hospital); Ken Shimizu, M.D. (National Cancer Center Hospital); Yuki Sumazaki Watanabe, M.D. (National Cancer Center Hospital East); Emi Takeuchi, M.A. (Keio University Hospital); Mari Takeuchi, M.D., Ph.D. (Keio University Hospital); Ryohei Tatara, M.D. (Osaka City General Hospital); Akihiro Tokoro, M.D., Ph.D. (National Hospital Organization Kinki‐Chuo Chest Medical Center); Megumi Uchida, M.D., Ph.D. (Nagoya City University Hospital); Hiroaki Watanabe, M.D. (Komaki City Hospital); Ritsuko Yabuki, M.D. (Tsukuba Medical Center Hospital); and Toshihiro Yamauchi, M.D. (Seirei Mikatahara General Hospital). This work was supported by a Grant‐in‐Aid for Scientific Research from the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED) [grant number 15ck0106059h0002].
Contributor Information
Tatsuo Akechi, Email: takechi@med.nagoya-cu.ac.jp.
Collaborators: on behalf of Phase‐R Delirium Study Group, Hirofumi Abo, Nobuya Akizuki, Koji Amano, Daisuke Fujisawa, Shingo Hagiwara, Takeshi Hirohashi, Takayuki Hisanaga, Kengo Imai, Shuji Inada, Satoshi Inoue, Shinichiro Inoue, Aio Iwata, Keisuke Kaneishi, Akifumi Kumano, Isseki Maeda, Yoshinobu Matsuda, Takashi Matsui, Yoshihisa Matsumoto, Naoki Matsuo, Kaya Miyajima, Ichiro Mori, Sachiyo Morita, Nobuhisa Nakajima, Hiroyuki Nobata, Takuya Odagiri, Ken Shimizu, Yuki Sumazaki Watanabe, Emi Takeuchi, Mari Takeuchi, Ryohei Tatara, Akihiro Tokoro, Megumi Uchida, Hiroaki Watanabe, Ritsuko Yabuki, and and Toshihiro Yamauchi
Author Contributions
Conception/design: Kazuhiro Yoshiuchi, Asao Ogawa, Satoru Iwase, Tatsuo Akechi
Provision of study material or patients: Toru Okuyama, Naosuke Yokomichi, Akihiro Sakashita, Keita Tagami, Keiichi Uemura, Rika Nakahara
Collection and/or assembly of data: Toru Okuyama, Naosuke Yokomichi, Akihiro Sakashita, Keita Tagami, Keiichi Uemura, Rika Nakahara
Data analysis and interpretation: Toru Okuyama, Tatsuo Akechi
Manuscript writing: Toru Okuyama, Kazuhiro Yoshiuchi, Asao Ogawa, Satoru Iwase, Naosuke Yokomichi, Akihiro Sakashita, Keita Tagami, Keiichi Uemura, Rika Nakahara, Tatsuo Akechi
Final approval of manuscript: Toru Okuyama, Kazuhiro Yoshiuchi, Asao Ogawa, Satoru Iwase, Naosuke Yokomichi, Akihiro Sakashita, Keita Tagami, Keiichi Uemura, Rika Nakahara, Tatsuo Akechi
Disclosures
The authors indicated no financial relationships.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM‐5). Arlington, VA: American Psychiatric Press, 2013. [Google Scholar]
- 2.Inouye SK. Delirium in older persons. N Engl J Med 2006;354:1157–1165. [DOI] [PubMed] [Google Scholar]
- 3.Centeno C, Sanz A, Bruera E. Delirium in advanced cancer patients. Palliat Med 2004;18:184–194. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in‐patients: A systematic literature review. Age Ageing 2006;35:350–364. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association . Practice guideline for the treatment of patients with delirium. Am J Psychiatry 1999;156:1–20. [PubMed] [Google Scholar]
- 6.Stagno D, Gibson C, Breitbart W. The delirium subtypes: A review of prevalence, phenomenology, pathophysiology, and treatment response. Palliat Support Care 2004;2:171–179. [DOI] [PubMed] [Google Scholar]
- 7.Hosie A, Davidson PM, Agar M et al. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: A systematic review. Palliat Med 2013;27:486–498. [DOI] [PubMed] [Google Scholar]
- 8.Breitbart W, Gibson C, Tremblay A. The delirium experience: Delirium recall and delirium‐related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics 2002;43:183–194. [DOI] [PubMed] [Google Scholar]
- 9.Morita T, Akechi T, Ikenaga M et al. Terminal delirium: Recommendations from bereaved families' experiences. J Pain Symptom Manage 2007;34:579–589. [DOI] [PubMed] [Google Scholar]
- 10.Breitbart W, Alici Y. Evidence‐based treatment of delirium in patients with cancer. J Clin Oncol 2012;30:1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grassi L, Caraceni A, Mitchell AJ et al. Management of delirium in palliative care: A review. Curr Psychiatry Rep 2015;17:550. [DOI] [PubMed] [Google Scholar]
- 12.Boettger S, Breitbart W. An open trial of aripiprazole for the treatment of delirium in hospitalized cancer patients. Palliat Support Care 2011;9:351–357. [DOI] [PubMed] [Google Scholar]
- 13.Platt MM, Breitbart W, Smith M et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci 1994;6:66–67. [DOI] [PubMed] [Google Scholar]
- 14.Agar MR, Lawlor PG, Quinn S et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: A randomized clinical trial. JAMA Intern Med 2017;177:34–42. [DOI] [PubMed] [Google Scholar]
- 15.Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics 2002;43:175–182. [DOI] [PubMed] [Google Scholar]
- 16.Devlin JW, Roberts RJ, Fong JJ et al. Efficacy and safety of quetiapine in critically ill patients with delirium: A prospective, multicenter, randomized, double‐blind, placebo‐controlled pilot study. Crit Care Med 2010;38:419–427. [DOI] [PubMed] [Google Scholar]
- 17.Boettger S, Friedlander M, Breitbart W et al. Aripiprazole and haloperidol in the treatment of delirium. Aust N Z J Psychiatry 2011;45:477–482. [DOI] [PubMed] [Google Scholar]
- 18.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults . American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015;63:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Currow DC, Vella‐Brincat J, Fazekas B et al. Pharmacovigilance in hospice/palliative care: Rapid report of net clinical effect of metoclopramide. J Palliat Med 2012;15:1071–1075. [DOI] [PubMed] [Google Scholar]
- 20.Wada K, Morita Y, Iwamoto T et al. First‐ and second‐line pharmacological treatment for delirium in general hospital setting‐Retrospective analysis. Asian J Psychiatr 2018;32:50–53. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto Y, Matsuoka Y, Sasaki T et al. Trazodone in the treatment of delirium. J Clin Psychopharmacol 1999;19:280–282. [DOI] [PubMed] [Google Scholar]
- 22.Uchida M, Okuyama T, Ito Y et al. Prevalence, course and factors associated with delirium in elderly patients with advanced cancer: A longitudinal observational study. Jpn J Clin Oncol 2015;45:934–940. [DOI] [PubMed] [Google Scholar]
- 23.Meagher D, Moran M, Raju B et al. A new data‐based motor subtype schema for delirium. J Neuropsychiatry Clin Neurosci 2008;20:185–193. [DOI] [PubMed] [Google Scholar]
- 24.Trzepacz PT, Mittal D, Torres R et al. Validation of the Delirium Rating Scale‐revised‐98: Comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci 2001;13:229–242. [DOI] [PubMed] [Google Scholar]
- 25.Gaudreau JD, Gagnon P, Harel F et al. Fast, systematic, and continuous delirium assessment in hospitalized patients: The nursing delirium screening scale. J Pain Symptom Manage 2005;29:368–375. [DOI] [PubMed] [Google Scholar]
- 26.Inada T, Beasley CM Jr., Tanaka Y et al. Extrapyramidal symptom profiles assessed with the Drug‐Induced Extrapyramidal Symptom Scale: Comparison with Western scales in the clinical double‐blind studies of schizophrenic patients treated with either olanzapine or haloperidol. Int Clin Psychopharmacol 2003;18:39–48. [DOI] [PubMed] [Google Scholar]
- 27.Kato M, Kishi Y, Okuyama T et al. Japanese version of the Delirium Rating Scale, Revised‐98 (DRS‐R98‐J): Reliability and validity. Psychosomatics 2010;51:425–431. [DOI] [PubMed] [Google Scholar]
- 28.Bush SH, Grassau PA, Yarmo MN et al. The Richmond Agitation‐Sedation Scale modified for palliative care inpatients (RASS‐PAL): A pilot study exploring validity and feasibility in clinical practice. BMC Palliat Care 2014;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita T, Tsunoda J, Inoue S et al. Communication Capacity Scale and Agitation Distress Scale to measure the severity of delirium in terminally ill cancer patients: A validation study. Palliat Med 2001;15:197–206. [DOI] [PubMed] [Google Scholar]
- 30.Japan Clinical Oncology Group . Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events v4.0 ‐ JCOG. Available at http://www.jcog.jp/doctor/tool/ctcaev4.html. Accessed May 29, 2013.
- 31.Japan Clinical Oncology Group . Clinical Safety Data Management Guideline. Available at http://www.jcog.jp/basic/policy/A_020_0010_16_1.pdf. Accessed June 25, 2013.
- 32.Anderson F, Downing GM, Hill J et al. Palliative performance scale (PPS): A new tool. J Palliat Care 1996;12:5–11. [PubMed] [Google Scholar]
- 33.Morita T, Tsunoda J, Inoue S et al. The Palliative Prognostic Index: A scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer 1999;7:128–133. [DOI] [PubMed] [Google Scholar]
- 34.Lawlor PG, Gagnon B, Mancini IL et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: A prospective study. Arch Intern Med 2000;160:786–794. [DOI] [PubMed] [Google Scholar]
- 35.Morita T, Tei Y, Tsunoda J et al. Underlying pathologies and their associations with clinical features in terminal delirium of cancer patients. J Pain Symptom Manage 2001;22:997–1006. [DOI] [PubMed] [Google Scholar]
- 36.Wolff R, Whiting P, Susan M et al. PROBAST: Prediction model risk of bias assessment tool. Presented at: Cochrane Colloquium; 2015; Vienna, Austria.
- 37.Hui D, Frisbee‐Hume S, Wilson A et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: A randomized clinical trial. JAMA 2017;318:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furukawa T, Strauss S, Bucher H et al. Diagnostic tests In: Guyatt G, Drummond R, Meade M, Cook D, eds. Users' Guides to the Medical Literature: A Manual for Evidence‐Based Practice. 3rd ed New York: The McGraw‐Hill Companies, Inc., 2014:345–358. [Google Scholar]
- 39.Bush SH, Lawlor PG, Ryan K et al. Delirium in adult cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2018;29(suppl 4):iv143–iv165. [DOI] [PubMed] [Google Scholar]
- 40.Bush SH, Leonard MM, Agar M et al. End‐of‐life delirium: Issues regarding recognition, optimal management and the role of sedation in the dying phase. J Pain Symptom Manage 2014;48:215–230. [DOI] [PubMed] [Google Scholar]
- 41.Blinderman CD, Billings JA. Comfort care for patients dying in the hospital. N Engl J Med 2015;373:2549–2561. [DOI] [PubMed] [Google Scholar]
- 42.Finucane AM, Lugton J, Kennedy C et al. The experiences of caregivers of patients with delirium, and their role in its management in palliative care settings: An integrative literature review. Psychooncology 2017;26:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otani H, Morita T, Uno S et al. Effect of leaflet‐based intervention on family members of terminally ill patients with cancer having delirium: Historical control study. Am J Hosp Palliat Care 2014;31:322–326. [DOI] [PubMed] [Google Scholar]