First‐line treatment for metastatic colorectal cancer is usually a combination of a biologic, such as bevacizumab, with FOLFIRI or FOLFOX; however, these treatments are associated with increased toxicity. This article reports the results of the phase II STEAM trial, which was the largest study of FOLFOXIRI‐BEV in patients in the U.S., comparing the clinical efficacy and tolerability of FOLFOXIRI‐BEV vs FOLFOX‐BEV.
Keywords: Metastatic colorectal cancer, Concurrent FOLFOXIRI, Sequential FOLFOXIRI, FOLFOX, Bevacizumab
Abstract
Background.
First‐line treatment for metastatic colorectal cancer (mCRC) typically entails a biologic such as bevacizumab (BEV) with 5‐fluorouracil/leucovorin/oxaliplatin (FOLFOX) or 5‐fluorouracil/leucovorin/irinotecan (FOLFIRI). STEAM (NCT01765582) assessed the efficacy of BEV plus FOLFOX/FOLFIRI (FOLFOXIRI), administered concurrently (cFOLFOXIRI‐BEV) or sequentially (sFOLFOXIRI‐BEV, FOLFOX‐BEV alternating with FOLFIRI‐BEV), versus FOLFOX‐BEV for mCRC.
Patients and Methods.
Patients with previously untreated mCRC (n = 280) were randomized 1:1:1 to cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, or FOLFOX‐BEV and treated with 4–6‐month induction followed by maintenance. Coprimary objectives were overall response rate (ORR; first‐line cFOLFOXIRI‐BEV vs. FOLFOX‐BEV) and progression‐free survival (PFS; pooled first‐line cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV vs. FOLFOX‐BEV). Secondary/exploratory objectives included overall survival (OS), liver resection rates, biomarker analyses, and safety.
Results.
ORR was 72.0%, 72.8%, and 62.1% and median PFS was 11.9, 11.4, and 9.5 months with cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV, respectively. OS was similar between arms. ORR between cFOLFOXIRI‐BEV and FOLFOX‐BEV did not significantly differ (p = .132); thus, the primary ORR endpoint was not met. cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV numerically improved ORR and PFS, regardless of RAS status. Median PFS was higher with pooled concurrent and sequential FOLFOXIRI‐BEV versus FOLFOX‐BEV (11.7 vs. 9.5 months; hazard ratio, 0.7; 90% confidence interval, 0.5–0.9; p < .01). Liver resection rates were 17.2% (cFOLFOXIRI‐BEV), 9.8% (sFOLFOXIRI‐BEV), and 8.4% (FOLFOX‐BEV). Grade ≥ 3 treatment‐emergent adverse events (TEAEs) were observed in 91.2% (cFOLFOXIRI‐BEV), 86.7% (sFOLFOXIRI‐BEV), and 85.6% (FOLFOX‐BEV) of patients, with no increase in serious chemotherapy‐associated TEAEs.
Conclusion.
cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV were well tolerated with numerically improved ORR, PFS, and liver resection rates versus FOLFOX‐BEV, supporting triplet chemotherapy plus BEV as a first‐line treatment option for mCRC.
Implications for Practice.
The combination of first‐line FOLFIRI with FOLFOX and bevacizumab (concurrent FOLFOXIRI‐BEV) improves clinical outcomes in patients with metastatic colorectal cancer (mCRC) relative to FOLFIRI‐BEV or FOLFOX‐BEV, but it is thought to be associated with increased toxicity. Alternating treatment of FOLFOX and FOLFIRI (sequential FOLFOXIRI‐BEV) could improve tolerability. In the phase II STEAM trial, which is the largest study of FOLFOXIRI‐BEV in patients in the U.S., it was found that both concurrent and sequential FOLFOXIRI‐BEV are active and well tolerated in patients with previously untreated mCRC, supporting the use of these regimens as potential first‐line treatment options for this population.
摘要
背景。转移性结直肠癌 (mCRC) 的一线治疗通常需要生物制剂,如贝伐单抗 (BEV),以及 5‐氟尿嘧啶/亚叶酸钙/奥沙利铂 (FOLFOX) 或 5‐氟尿嘧啶/亚叶酸钙/伊立替康 (FOLFIRI)。STEAM (NCT01765582) 评估了BEV 和 FOLFOX/FOLFIRI (FOLFOXIRI) 在同步用药 (cFOLFOXIRI‐BEV) 或序贯用药(sFOLFOXIRI‐BEV,FOLFOX‐BEV 与 FOLFIRI‐BEV 交替使用)的情况下对比 FOLFOX‐BEV 治疗 mCRC 的有效性。
患者和方法。既往未经治疗的 mCRC 患者 (n = 280) 按照 1:1:1 的比例被随机分配使用 cFOLFOXIRI‐BEV、sFOLFOXIRI‐BEV 或 FOLFOX‐BEV,并在进行 4~6 个月的诱导治疗之后接受维持治疗。共同的主要终点为总缓解率(ORR;一线 cFOLFOXIRI‐BEV vs. FOLFOX‐BEV)以及无进展生存期(PFS;联合一线 cFOLFOXIRI‐BEV 和 sFOLFOXIRI‐BEV vs. FOLFOX‐BEV)。次要/探索性终点包括总生存期 (OS)、肝脏切除率、生物标志物分析和安全性。
结果。cFOLFOXIRI‐BEV、sFOLFOXIRI‐BEV 和 FOLFOX‐BEV 的 ORR 分别为 72.0%、72.8% 和 62.1%,中位 PFS 分别为 11.9 个月、11.4 个月和 9.5 个月。组间的 OS 很相似。cFOLFOXIRI‐BEV 和 FOLFOX‐BEV 之间的 ORR 没有显著差异 (p = 0.132);因此,未达到主要 ORR 终点。无论 RAS 状态如何,cFOLFOXIRI‐BEV 和 sFOLFOXIRI‐BEV 均在数字方面改进了 ORR 和 PFS。联合同步和序贯 FOLFOXIRI‐BEV 对比 FOLFOX‐BEV 的中位 PFS 更高(11.7 个月vs. 9.5 个月;风险比,0.7;90% 置信区间,0.5–0.9;p < 0.01)。肝脏切除率为 17.2% (cFOLFOXIRI‐BEV)、9.8% (sFOLFOXIRI‐BEV) 和 8.4% (FOLFOX‐BEV)。在 91.2% (cFOLFOXIRI‐BEV)、86.7% (sFOLFOXIRI‐BEV) 和 85.6% (FOLFOX‐BEV) 的患者中观察到治疗相关的 ≥ 3 级不良反应 (TEAE),与严重化疗相关的 TEAE 没有增加。
结论。 与 FOLFOX‐BEV 对比而言,cFOLFOXIRI‐BEV 和 sFOLFOXIRI‐BEV 具有良好的耐受性,在数字方面改进了 ORR、PFS 和肝脏切除率,因而支持将三联化疗加 BEV 作为 mCRC 的一线治疗方案。
实践意义:相对于 FOLFIRI‐BEV 或 FOLFOX‐BEV,一线 FOLFIRI 与 FOLFOX 和贝伐单抗(同步 FOLFOXIRI‐BEV)的组合可以为转移性结直肠癌 (mCRC) 患者改进临床结果,但是,该组合方案被认为与毒性增加有关。FOLFOX 和 FOLFIRI(序贯 FOLFOXIRI‐BEV)交替治疗可以提高耐受性。在 II 期 STEAM 试验(即美国患者中最大型的 FOLFOXIRI‐BEV 研究)中,我们发现同步和序贯 FOLFOXIRI‐BEV 对既往未经治疗的 mCRC 患者具有活性和良好的耐受性,因而支持使用此类疗法作为针对此类人群的潜在一线治疗方案。
Introduction
Colorectal cancer is the fourth most common cancer in the U.S., with more than 135,000 new cases and 50,000 deaths estimated for 2017 [1]. Patients with metastatic colorectal cancer (mCRC) have a 5‐year survival rate of less than 14% [1]. Prior to the widespread availability of targeted therapies, management of mCRC commonly employed an infusion of 5‐fluorouracil/leucovorin/irinotecan (FOLFIRI) [2], [3] or 5‐fluorouracil/leucovorin/oxaliplatin (FOLFOX) chemotherapeutic regimens [4]. A triplet approach combining FOLFOX and FOLFIRI, 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan (FOLFOXIRI), demonstrated improved response rates and surgical resection rates compared with FOLFIRI alone, with increased but manageable toxicity [5], [6].
Bevacizumab, a humanized monoclonal antibody that blocks angiogenesis by inhibiting vascular endothelial growth factor A, has been shown to improve progression‐free survival (PFS) and overall survival (OS) in patients with mCRC when used in combination with chemotherapy [7], [8], [9]. As such, the current standard of care for patients with previously untreated mCRC entails the addition of a biologic agent such as bevacizumab to either FOLFOX or FOLFIRI [10], with FOLFOX being more commonly used in the U.S. However, further improvement in the efficacy of frontline regimens is an ongoing objective. One possibility for improving outcomes is intensification of the chemotherapy backbone by replacing FOLFIRI or FOLFOX with FOLFOXIRI, which demonstrated acceptable safety in combination with bevacizumab in patients with mCRC [11].
The phase III TRIBE study comparing FOLFOXIRI plus bevacizumab (FOLFOXIRI‐BEV) versus FOLFIRI plus bevacizumab (FOLFIRI‐BEV) in patients in Italy demonstrated that FOLFOXIRI‐BEV was associated with significant improvements in the overall response rate (ORR; 65% vs. 53%; p = .006), PFS (hazard ratio [HR], 0.77; 95% confidence interval [CI] 0.65–0.93; p = .006), and OS (HR, 0.80; 95% CI 0.65–0.98; p = .03) [12, 13]. The European phase II OLIVIA study of FOLFOXIRI‐BEV compared with a modified FOLFOX regimen plus bevacizumab demonstrated a higher tumor response rate (81%; 95% CI 65–91 vs. 62%; 95% CI 45–77), as well as a higher liver resection rate (61% vs. 49%) and improved median PFS (18.6 months; 95% CI 12.9–22.3 vs. 11.5 months; 95% CI 9.6–13.6) for the triplet regimen [14].
Despite the results from TRIBE and OLIVIA, a number of questions remain, including the efficacy and safety of FOLFOXIRI‐BEV in patients in the U.S. Additionally, although the overall safety profile of FOLFOXIRI‐BEV is generally acceptable, the triplet regimen is associated with increased toxicity compared with the respective control groups [12], [14]. Alternating treatment with FOLFOX and FOLFIRI (i.e., sequential FOLFOXIRI) has been hypothesized to improve the tolerability of the regimen without negatively affecting efficacy [15]. We undertook this study to define the activity and tolerability of concurrent and sequential FOLFOXIRI‐BEV in patients with mCRC.
The Sequencing Triplet with Avastin and Maintenance (STEAM; NCT01765582) trial is a randomized, multicenter, open‐label, U.S.‐based, phase II trial investigating FOLFOXIRI‐BEV administered concurrently (cFOLFOXIRI‐BEV) or sequentially (sFOLFOXIRI‐BEV, i.e., FOLFOX‐BEV alternating with FOLFIRI‐BEV) versus FOLFOX‐BEV in patients with previously untreated mCRC. The primary efficacy endpoints were (a) the overall response rate of cFOLFOXIRI‐BEV versus FOLFOX‐BEV and (b) PFS of pooled cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV versus FOLFOX‐BEV. Here, we describe the final efficacy and safety results, as well as selected exploratory biomarker analyses, from the STEAM study.
Materials and Methods
Study Design
STEAM is a randomized, open‐label, multicenter, phase II study of patients in the U.S. with previously untreated mCRC. After a screening period of up to 21 days, patients were randomized 1:1:1 using a dynamic hierarchal randomization algorithm to a treatment induction phase consisting of cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, or FOLFOX‐BEV, followed by a maintenance phase and a planned second‐line phase (supplemental online Fig. 1). Patients were stratified by extent of metastatic disease (liver‐limited disease vs. non‐liver‐limited disease), primary tumor location (right vs. left) [16], and study center.
The study protocol was approved by the institutional review boards at each participating study site, and the trial conformed to the principles outlined in the Declaration of Helsinki, the International Conference on Harmonisation E6 guideline for Good Clinical Practice, and applicable local laws. Patients provided written informed consent per institutional guidelines.
The preplanned coprimary efficacy endpoints were investigator‐assessed ORR for first‐line treatment with cFOLFOXIRI‐BEV versus FOLFOX‐BEV, and PFS from first‐line treatment with pooled FOLFOXIRI‐BEV (i.e., concurrent plus sequential FOLFOXIRI‐BEV) versus FOLFOX‐BEV. Secondary endpoints included safety, OS, and liver resection rate. Assessment of biomarkers associated with treatment outcomes was an exploratory objective.
Patients
Key patient inclusion criteria were histologically confirmed mCRC with at least one measurable lesion considered unresectable at baseline; age of 18–75 years; Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 if aged <71 years or ECOG performance status of 0 if aged 71–75 years; adequate hematologic, liver, and renal function; and a willingness to comply with the study protocol, including tissue and blood sampling.
Key exclusion criteria included prior systemic treatment for mCRC, except for use of palliative radiosensitizers; adjuvant chemotherapy completed <12 months prior to study enrollment; sensory peripheral neuropathy of grade ≥ 2; surgery within 28 days prior to randomization; minor surgery <7 days before or after bevacizumab infusion; current or recent (≤10 days prior to the first dose of bevacizumab) use of aspirin >325 mg/day (full‐dose anticoagulants were permitted if dose was stable and clinical coagulation parameters were within therapeutic range); and inadequately controlled hypertension, clinically significant cardiovascular disease, serious nonhealing wound, active peptic ulcer, or untreated bone fracture.
Procedures
The induction phase consisted of FOLFOX‐BEV, cFOLFOXIRI‐BEV (concurrent FOLFOX‐BEV and FOLFIRI‐BEV), or sFOLFOXIRI‐BEV (alternating 4‐week administrations of FOLFOX‐BEV and FOLFIRI‐BEV) by intravenous infusion every 2 weeks for 4 months (supplemental online Fig. 1, supplemental online Table 1). Patients could continue the assigned treatment for up to 2 additional months, at the investigator's discretion, if the patient exhibited a good response and the treatment was well tolerated. Induction treatment was followed by a maintenance phase with intravenous 5‐fluorouracil, leucovorin, and bevacizumab every 2 weeks or oral capecitabine plus intravenous bevacizumab every 3 weeks until disease progression. Upon progression, patients were to enter a planned second‐line phase consisting of treatment with bevacizumab and a fluoropyrimidine‐based chemotherapy of the investigator's choice.
Treatment continued until progression, death, withdrawal of consent, or unacceptable toxicity. If any of the individual components of the originally assigned regimen were discontinued early, the remaining drugs in the regimen were continued. Two safety follow‐up visits occurred 28 (±3) days and 3 months (±7 days) after the last dose of study drugs. Patients then entered a survival follow‐up period with survival status assessed every 3 months.
Biomarker Assessments
Biomarker‐evaluable patients were defined as those who provided informed consent to participate in the tissue biomarker program and had at least one viable sample for analysis. Single‐nucleotide variants for RAS (KRAS and NRAS) and BRAF were identified by next‐generation sequencing using the AVENIO Expanded Kit (Roche, Basel, Switzerland). Secondary sequence analysis was performed through an established Cancer Personalized Profiling by Deep Sequencing (CAPP‐Seq) bioinformatics pipeline, which included molecular barcoding and integrated digital error suppression methods [17], [18].
Liver Resection and Assessments
Patients with planned resections stopped bevacizumab treatment at least 4–6 weeks prior to surgery and were permitted to restart treatment when ≥4 weeks had elapsed since the surgery. The choice of therapy after resection was at the investigator's discretion from the following options: bevacizumab, 5‐fluorouracil, capecitabine, oxaliplatin, irinotecan, or any combination thereof.
After surgery, liver metastasis resections were classified as R0 (complete resection with clear margins ≥1 mm), R1 (presence of exposed tumor along the line of transection, presence of tumor cells at the line of transection detected by histologic examination, or < 1 mm microscopic margin), or R2 (macroscopic positive margins or incomplete resection at time of surgery). Liver resection rates were calculated by classification and overall.
To distinguish the effect of treatment from the effect of surgery in the calculation of ORR, Response Evaluation Criteria in Solid Tumors (RECIST) assessments were censored after curative liver resection.
Statistical Analysis
Efficacy analyses were conducted in the intent‐to‐treat (ITT) population, including all randomized patients regardless of whether treatment was received. Safety analyses were conducted on all patients who received at least one dose of study medication; safety data were summarized by treatment arm based on actual treatment received (i.e., the safety population).
The sample size of 280 patients was based on assumed ORRs of 70%, 65%, and 50% in the cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV arms, respectively, and a 43% improvement in median PFS (from 10 months in FOLFOX‐BEV to 14.3 months in pooled FOLFOXIRI‐BEV). In this scenario and under constant hazard assumptions, the study would have 89% power to detect a difference in ORR between the cFOLFOXIRI‐BEV and FOLFOX‐BEV arms, with 5% type 1 error (one‐sided) and 82% power to detect a difference between the pooled cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV and FOLFOX‐BEV arms in PFS, with 5% type 1 error (one‐sided).
ORR was defined as the percentage of patients assessed by site investigators as having a complete or partial response per RECIST version 1.1 (unconfirmed) in first‐line treatment. Logistic regression was used to calculate 90% CI for the odds ratio of the cFOLFOXIRI‐BEV or sFOLFOXIRI‐BEV arm versus the FOLFOX‐BEV arm, stratified by extent of metastatic disease and primary tumor location. P‐values were determined by a one‐sided Cochran‐Mantel‐Haenszel test, stratified by extent of metastatic disease and primary tumor location.
PFS was defined as the time from randomization to first occurrence of disease progression or death from any cause during first‐line treatment, whichever occurred earlier. Patients without an event were censored at their last tumor assessment. The Kaplan‐Meier approach was used to estimate median PFS and corresponding CIs for each treatment arm and for combined arms. Cox regression (with proportional hazards), stratified by extent of metastatic disease and primary tumor location, was used to estimate the HR and to calculate its 90% CIs. A one‐sided log‐rank test between FOLFOXIRI/bevacizumab regimen arms (concurrent and sequential) and the FOLFOX/bevacizumab arm, stratified by extent of metastatic disease and primary tumor location, provided p values.
To adjust for multiplicity due to having two primary endpoints, a fixed‐sequence hypothesis testing procedure was to be implemented; however, the study was terminated early. The hypothesis test for ORR was conducted at a one‐sided α of 5%. If ORR was significantly higher in the cFOLFOXIRI‐BEV arm than in the FOLFOX‐BEV arm, PFS was to be tested at a one‐sided α of 5% for the pooled cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV arms versus the FOLFOX‐BEV arm. Except for the primary analysis of ORR and PFS, all p values were considered nominal and were not adjusted for multiple comparisons.
On November 10, 2015, Genentech conducted the final analysis of ORR and a planned interim analysis of PFS. Based on the results of these efficacy analyses, Genentech elected to terminate the trial early. This report describes the final efficacy and safety analyses, as well as selected exploratory biomarker analyses, with data based on follow‐up until March 14, 2016.
Results
Patients
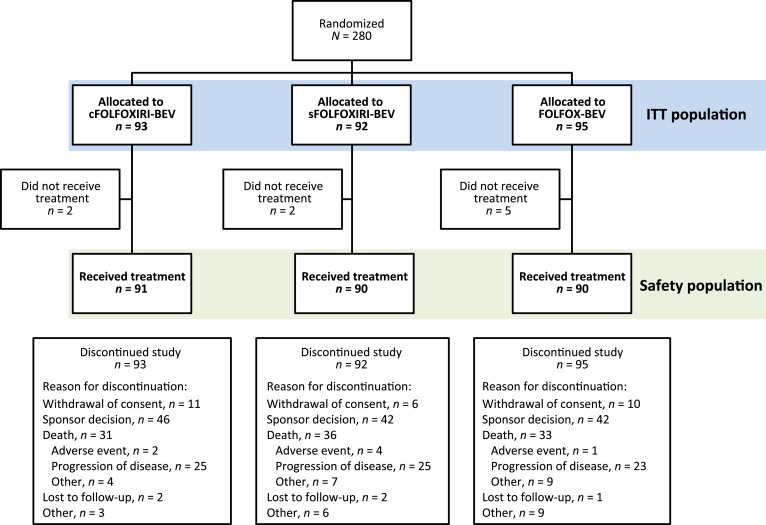
Between January 23, 2013, and December 26, 2014, 280 patients were randomized in STEAM to the cFOLFOXIRI‐BEV (n = 93), sFOLFOXIRI‐BEV (n = 92), or FOLFOX‐BEV (n = 95) treatment arms (Fig. 1). Within each respective arm, 72 (77.4%), 74 (80.4%) and 72 (75.8%) patients completed cycle 8 (4 months of treatment). Median duration of follow‐up was 85.1 weeks (range, 19–148), 84.7 weeks (range, 33–151), and 83.4 weeks (range, 9–132), respectively. Cumulative dose for each drug within each treatment arm are shown in supplemental online Table 2.
Figure 1.
Patient disposition.
Abbreviations: cFOLFOXIRI‐BEV, concurrent 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; FOLFOX‐BEV, 5‐fluorouracil/leucovorin/oxaliplatin plus bevacizumab; ITT, intent‐to‐treat; sFOLFOXIRI‐BEV, sequential 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab.
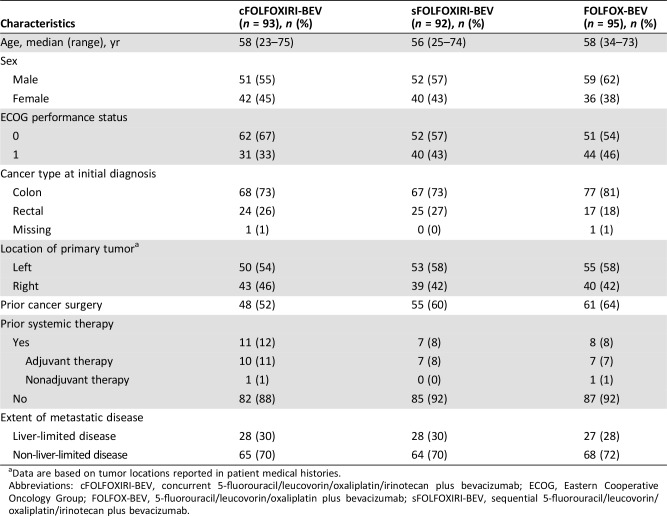
Patient demographics and characteristics were generally balanced between treatment arms, although the cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV arms had lower proportions of patients with prior surgery for colorectal cancer compared with FOLFOX‐BEV (51.6% and 59.8% vs. 64.2%; Table 1). Additionally, more patients in the cFOLFOXIRI‐BEV arm had an ECOG status of 0 than those in the sFOLFOXIRI‐BEV or FOLFOX‐BEV arms (66.7% vs. 56.5% and 53.7%).
Table 1. Baseline characteristics (intent‐to‐treat population).
Data are based on tumor locations reported in patient medical histories.
Abbreviations: cFOLFOXIRI‐BEV, concurrent 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; ECOG, Eastern Cooperative Oncology Group; FOLFOX‐BEV, 5‐fluorouracil/leucovorin/oxaliplatin plus bevacizumab; sFOLFOXIRI‐BEV, sequential 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab.
Efficacy Outcomes
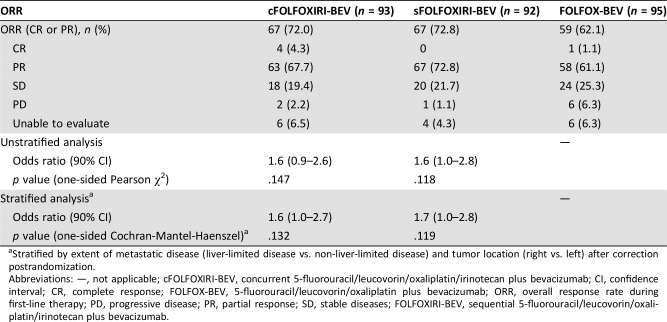
ORR was 72.0% (67/93, four complete responses [CRs]) in the cFOLFOXIRI‐BEV treatment arm, 72.8% (67/92, no CR) in the sFOLFOXIRI‐BEV arm, and 62.1% (59/95, 1 CR) in the FOLFOX‐BEV arm (Table 2). The stratified odds ratios numerically favored the cFOLFOXIRI‐BEV (1.6; 90% CI, 1.0–2.7) and sFOLFOXIRI‐BEV (1.7; 90% CI, 1.0–2.8) treatment arms compared with FOLFOX‐BEV, and significantly favored pooled concurrent and sequential FOLFOXIRI‐BEV versus FOLFOX‐BEV (1.6; 90% CI, 1.1–2.6). However, as the coprimary endpoint of ORR for cFOLFOXIRI‐BEV versus FOLFOX‐BEV treatment did not show a statistically significant difference (p = .132), the sponsor elected for early termination of the STEAM study.
Table 2. Overall response rate during first‐line treatment (intent‐to‐treat population).
Stratified by extent of metastatic disease (liver‐limited disease vs. non‐liver‐limited disease) and tumor location (right vs. left) after correction postrandomization.
Abbreviations: —, not applicable; cFOLFOXIRI‐BEV, concurrent 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; CI, confidence interval; CR, complete response; FOLFOX‐BEV, 5‐fluorouracil/leucovorin/oxaliplatin plus bevacizumab; ORR, overall response rate during first‐line therapy; PD, progressive disease; PR, partial response; SD, stable diseases; FOLFOXIRI‐BEV, sequential 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab.
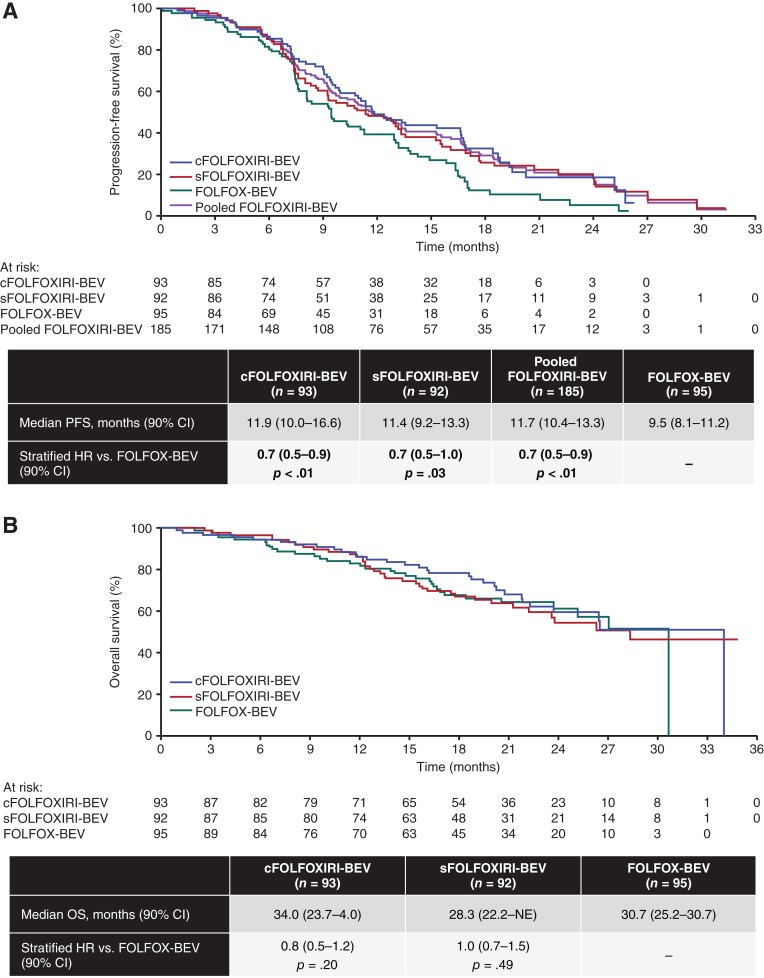
Median PFS was 11.9 months in the cFOLFOXIRI‐BEV arm, 11.4 months in the sFOLFOXIRI‐BEV arm, and 9.5 months in the FOLFOX‐BEV arm. Compared with FOLFOX‐BEV, median PFS was higher with pooled concurrent and sequential FOLFOXIRI‐BEV (HR, 0.7; 90% CI, 0.5–0.9; Fig. 2A). Estimates for median OS were 34.0, 28.3, and 30.7 months in the cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV treatment arms, respectively. The differences in OS between FOLFOX‐BEV versus cFOLFOXIRI‐BEV (HR, 0.8; 90% CI, 0.5–1.2) or sFOLFOXIRI‐BEV (HR, 1.0; 90% CI, 0.7–1.5) were not statistically significant (Fig. 2B).
Figure 2.
Outcomes by treatment arm (intent‐to‐treat population). (A): PFS. (B): OS. A total of 61, 69, and 72 PFS events and 31, 36, and 33 OS events occurred in the cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV arms, respectively. The data were calculated by Cox proportional hazards model that also included the extent of metastatic disease (liver‐limited vs. not) and tumor location (left vs. right) after correction postrandomization.
Abbreviations: cFOLFOXIRI‐BEV, concurrent 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; CI, confidence interval; FOLFOX‐BEV, 5‐fluorouracil/leucovorin/oxaliplatin plus bevacizumab; FOLFOXIRI‐BEV, 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; HR, hazard ratio; NE, not evaluable; OS, overall survival; PFS, progression‐free survival; sFOLFOXIRI‐BEV, sequential 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab.
Liver Resection Rates
Liver resection rates during first‐line treatment in the ITT population in the cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV arms were 17.2% (16/93), 9.8% (9/92), and 8.4% (8/95), respectively; R0 resection rates were 16.1% (15/93), 8.7% (8/92), and 6.3% (6/95). Median first‐line treatment cycles prior to liver resection were eight (range, 1–25), eight (range, 4–17), and nine (range, 6–13) cycles in each arm, respectively. In ITT population patients with liver‐limited disease, resection rates were 28.6% (8/28), 21.4% (6/28), and 18.5% (5/27) in the cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV arms, respectively, and R0 resection rates were 25.0% (7/28), 17.9% (5/28), and 11.1% (3/27). The differences in overall resection rates between the FOLFOXIRI‐BEV arms and FOLFOX‐BEV did not reach statistical significance.
In addition to the higher first‐line resection rate reported with cFOLFOXIRI‐BEV, numerically higher rates of overall conversion from unresectable to resectable disease (i.e., the proportion of patients considered by the investigator to be unresectable at study enrollment who subsequently underwent attempted curative resections of metastases) were also observed with cFOLFOXIRI‐BEV (24% [22/93]) and sFOLFOXIRI‐BEV (17% [16/92]) versus FOLFOX‐BEV (14% [13/95]), with 20% (19/93), 13% (12/92), and 11% (10/95) of patients receiving R0 resections, respectively.
To assess the impact of liver resection on outcomes, sensitivity analyses were conducted for ORR (in which all liver resections were considered to be CRs) and PFS (in which patients with liver metastasectomy were excluded). The results for both measures remained consistent with the primary analyses of ORR and PFS in the first line.
Subgroup Analyses by Molecular Biomarkers and Tumor Location
The biomarker‐evaluable populations from the cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV treatment arms included 57/93 (61.3%), 67/92 (72.8%), and 58/95 (61.1%) patients, respectively. Baseline characteristics in the biomarker‐evaluable population were generally similar between treatment arms with the exception of cancer type, for which patients in the FOLFOX‐BEV arm had a higher incidence of colon (vs. rectal) cancer than those in the FOLFOXIRI‐BEV arms (supplemental online Table 3). Clinical outcomes in this population were comparable to those in the ITT population (supplemental online Table 4).
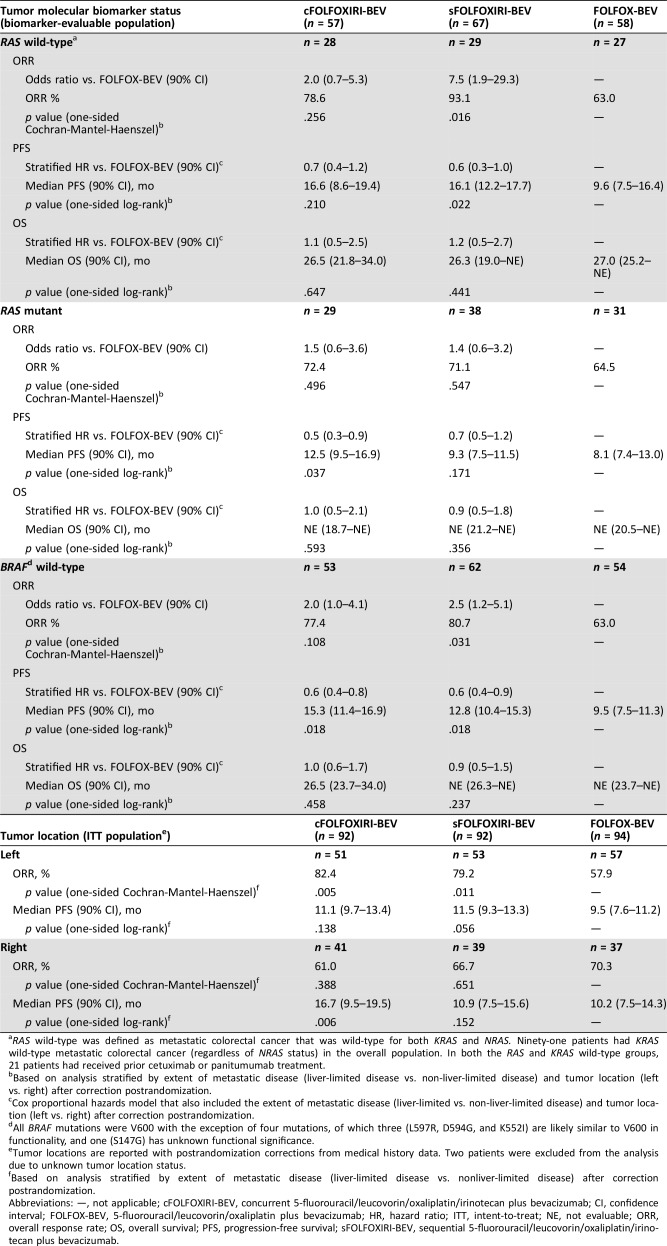
ORR, PFS, and OS were evaluated by RAS (KRAS or NRAS) and BRAF tumor status (Table 3) in each treatment arm. Although numbers were limited, patients with RAS wild‐type or BRAF wild‐type tumor status generally had higher ORR and PFS relative to those with RAS‐mutated or BRAF‐mutated tumors, respectively. In patients with RAS wild‐type tumors, those treated with cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV had ORRs of 78.6%, 93.1 %, and 63.0%, respectively, with the difference between sFOLFOXIRI‐BEV and FOLFOX‐BEV reaching statistical significance (odds ratio, 7.5; 90% CI, 1.9–29.3; Table 3). Median PFS in the three treatment arms was 16.6, 16.1, and 9.6 months, respectively (Table 3, supplemental online Fig. 2A). cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV also produced numerically higher ORR and median PFS compared with FOLFOX‐BEV in patients with RAS‐mutated tumors (ORR, 72.4%, 71.1%, and 64.5%, respectively; median PFS, 12.5, 9.3, and 8.1 months), with statistical significance observed for PFS in the cFOLFOXIRI‐BEV versus FOLFOX‐BEV arms (HR, 0.5; 90% CI, 0.3–0.9; Table 3, supplemental online Fig. 2B).
Table 3. ORR, PFS, and OS for cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV versus FOLFOX‐BEV by tumor molecular biomarker status and tumor location.
RAS wild‐type was defined as metastatic colorectal cancer that was wild‐type for both KRAS and NRAS. Ninety‐one patients had KRAS wild‐type metastatic colorectal cancer (regardless of NRAS status) in the overall population. In both the RAS and KRAS wild‐type groups, 21 patients had received prior cetuximab or panitumumab treatment.
Based on analysis stratified by extent of metastatic disease (liver‐limited disease vs. non‐liver‐limited disease) and tumor location (left vs. right) after correction postrandomization.
Cox proportional hazards model that also included the extent of metastatic disease (liver‐limited vs. non‐liver‐limited disease) and tumor location (left vs. right) after correction postrandomization.
All BRAF mutations were V600 with the exception of four mutations, of which three (L597R, D594G, and K552I) are likely similar to V600 in functionality, and one (S147G) has unknown functional significance.
Tumor locations are reported with postrandomization corrections from medical history data. Two patients were excluded from the analysis due to unknown tumor location status.
Based on analysis stratified by extent of metastatic disease (liver‐limited disease vs. nonliver‐limited disease) after correction postrandomization.
Abbreviations: —, not applicable; cFOLFOXIRI‐BEV, concurrent 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; CI, confidence interval; FOLFOX‐BEV, 5‐fluorouracil/leucovorin/oxaliplatin plus bevacizumab; HR, hazard ratio; ITT, intent‐to‐treat; NE, not evaluable; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival; sFOLFOXIRI‐BEV, sequential 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab.
In patients with BRAF wild‐type tumors, treatment with sFOLFOXIRI‐BEV versus FOLFOX‐BEV improved ORR (80.7% vs. 63.0%; odds ratio, 2.5; 90% CI, 1.2–5.1) and median PFS (12.8 vs. 9.5 months; HR, 0.6; 90% CI, 0.4–0.9; Table 3). PFS was also higher in the cFOLFOXIRI‐BEV arm (15.3 vs. 9.5 months; HR, 0.6; 90% CI, 0.4–0.8) compared with FOLFOX‐BEV. The number of patients in the BRAF‐mutated subgroup was small, but in this subset, ORRs were 50.0%, 80.0%, and 75.0% in the cFOLFOXIRI‐BEV (n = 4), sFOLFOXIRI‐BEV (n = 5), and FOLFOX‐BEV (n = 4) treatment arms, and median PFS durations were 7.1, 7.4, and 12.4 months, respectively.
cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV conferred numerically higher ORR and median PFS in left‐sided tumors compared with FOLFOX‐BEV (ORR, 82.4% and 79.2% vs. 57.9%; PFS, 11.1 and 11.5 vs. 9.5 months; Table 3, supplemental online Fig. 2C). In right‐sided tumors, numerical improvements for median PFS, but not ORR, were observed for cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV versus FOLFOX‐BEV (ORR, 61.0% and 66.7% vs. 70.3%; PFS, 16.7 and 10.9 vs. 10.2 months; Table 3, supplemental online Fig. 2D). A multivariate analysis by molecular biomarkers and tumor location found no significant differences in PFS, with the exception of significant improvements with sFOLFOXIRI‐BEV versus FOLFOX‐BEV treatment in patients with left‐sided tumors (supplemental online Table 5). Adjusted HRs for PFS in right‐ versus left‐sided tumors in the cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV arms were 0.7 (90% CI, 0.4–1.2), 1.3 (90% CI, 0.8–2.0), and 0.6 (90% CI, 0.3–1.0), respectively.
Safety
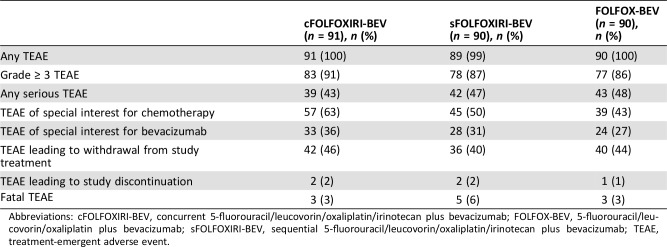
All patients except for one in the sFOLFOXIRI‐BEV arm experienced at least one treatment‐emergent adverse event (TEAE). In the cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV arms, 91.2%, 86.7%, and 85.6% of patients, respectively, had at least one grade ≥ 3 TEAE. Fatal TEAEs were experienced by three, five, and three patients in the three arms, respectively. Small intestine obstruction, sepsis, and death (attributed to disease while on study) each resulted in a fatal adverse event in more than one patient. Serious TEAEs were experienced by 42.9%, 46.7%, and 47.8% of patients in each treatment arm, respectively (Table 4). No new safety signals were observed in patients treated with cFOLFOXIRI‐BEV or sFOLFOXIRI‐BEV, and incidences of grade 4 and 5 TEAEs were similar between the two arms. There was no increase in serious chemotherapy‐associated TEAEs.
Table 4. Overview of treatment‐emergent adverse events (safety population).
Abbreviations: cFOLFOXIRI‐BEV, concurrent 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; FOLFOX‐BEV, 5‐fluorouracil/leucovorin/oxaliplatin plus bevacizumab; sFOLFOXIRI‐BEV, sequential 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; TEAE, treatment‐emergent adverse event.
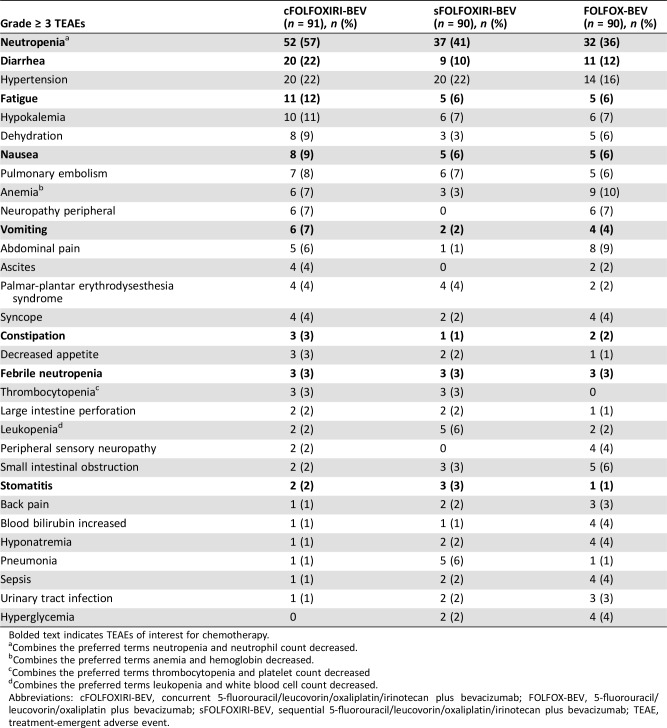
The most common grade ≥ 3 TEAEs (occurring in ≥10% of patients in any treatment arm) were neutropenia (57.1%, 41.1%, and 35.6% with cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV, respectively), hypertension (22.0%, 22.2%, 15.6%), diarrhea (22.0%, 10.0%, 12.2%), fatigue (12.1%, 5.6%, 5.6%), hypokalemia (11.0%, 6.7%, 6.7%), and anemia (6.6%, 3.3%, 10.0%) (Table 5). Grade ≥ 3 chemotherapy toxicities were more common in the cFOLFOXIRI‐BEV arm than in the other two arms, with the exception of febrile neutropenia, constipation, and stomatitis, which were similar across all three treatment arms (Table 5). Granulocyte colony stimulating factor was administered to 45/91 (49.5%), 31/90 (34.4%), and 23/90 (25.6%) patients in the cFOLFOXIRI‐BEV, sFOLFOXIRI‐BEV, and FOLFOX‐BEV arms, respectively.
Table 5. Grade ≥ 3 TEAEs occurring in at least five patients (safety population).
Bolded text indicates TEAEs of interest for chemotherapy.
Combines the preferred terms neutropenia and neutrophil count decreased.
Combines the preferred terms anemia and hemoglobin decreased.
Combines the preferred terms thrombocytopenia and platelet count decreased
Combines the preferred terms leukopenia and white blood cell count decreased.
Abbreviations: cFOLFOXIRI‐BEV, concurrent 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; FOLFOX‐BEV, 5‐fluorouracil/leucovorin/oxaliplatin plus bevacizumab; sFOLFOXIRI‐BEV, sequential 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; TEAE, treatment‐emergent adverse event.
The incidence of patients with TEAEs of special interest for bevacizumab was somewhat higher in the cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV arms compared with FOLFOX‐BEV (36.3% and 31.1% vs. 26.7%). Those occurring with ≥1% greater frequency in the pooled FOLFOXIRI‐BEV group versus FOLFOX‐BEV included hypertension (19.9% vs. 13.3%), arterial thromboembolic events (3.3% vs. 0%), and proteinuria (1.1% vs. 0%). Bleeding/hemorrhage (1.7% vs. 5.6%) and fistula/abscess (0.6% vs. 2.2%) occurred with lower frequency in the pooled FOLFOXIRI‐BEV group versus FOLFOX‐BEV.
Discussion
STEAM was the largest study of FOLFOXIRI‐BEV in patients in the U.S. and the first to compare a sequential FOLFOXIRI‐BEV regimen with FOLFOX‐BEV. In this analysis, we observed both numerically higher ORR for cFOLFOXIRI‐BEV versus FOLFOX‐BEV and an increase in median PFS for pooled concurrent and sequential FOLFOXIRI‐BEV versus FOLFOX‐BEV. Biomarker analyses indicated that cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV produced numerically higher ORR and median PFS compared with FOLFOX‐BEV in patients with wild‐type RAS or BRAF tumors. Consistent with these differences in efficacy, cFOLFOXIRI‐BEV treatment also resulted in numerically higher complete liver resection rates compared with FOLFOX‐BEV. Because the primary ORR endpoint for STEAM was not met, the study was closed early, and the efficacy data presented in this analysis are final.
These data support the results of the phase III TRIBE study conducted by the Gruppo Oncologico del Nord Ovest, which demonstrated an ORR of 65% for cFOLFOXIRI‐BEV compared with 53% for FOLFIRI‐BEV [12, 13], and the phase II OLIVIA trial, a multinational European study, which found a tumor response rate of 81% for FOLFOXIRI‐BEV compared with 62% for a modified FOLFOX‐BEV regimen [14]. All three studies yielded prolonged PFS for FOLFOXIRI‐BEV versus the control arms [12], [13], [14]. Intriguingly, results from both STEAM and OLIVIA also suggest that FOLFOXIRI‐BEV may have the potential to increase curative liver resection rates compared with FOLFOX‐BEV [14]. The overall results from STEAM, TRIBE, and OLIVIA support the application of the triplet regimen in combination with bevacizumab in improving clinical outcomes compared with either the FOLFOX or FOLFIRI doublet regimens with bevacizumab. Additionally, this study demonstrates that sequential FOLFOXIRI‐BEV has activity in colorectal tumors, supporting it as a potential treatment option in patients with mCRC.
Compared with FOLFOX‐BEV and FOLFIRI‐BEV, FOLFOXIRI‐BEV was associated with a manageable increase in toxicity in TRIBE and OLIVIA [12], [14]. No new safety signals were observed with cFOLFOXIRI‐BEV or sFOLFOXIRI‐BEV in the STEAM analysis, confirming the safety and feasibility of FOLFOXIRI‐BEV treatment in patients in the U.S. Although the incidence of some TEAEs (e.g., grade ≥ 3 neutropenia and diarrhea) were numerically lower in the sFOLFOXIRI‐BEV arm compared with cFOLFOXIRI‐BEV, the incidence of grade 4 and 5 TEAEs was similar, and we observed no overall differences in safety between the concurrent and sequential regimens.
Tumor location and molecular biomarkers, such as RAS and BRAF, have been shown to influence clinical outcomes and/or treatment efficacy in colorectal tumors [16], [19]. Although therapies targeting epidermal growth factor receptor are commonly used in the treatment of mCRC, tumors with activating KRAS and NRAS mutations are typically refractory to these agents. The TRIBE study found that RAS and BRAF status did not affect the treatment effect of FOLFOXIRI‐BEV compared with FOLFIRI‐BEV [13]. In the current analysis, cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV conferred numerically higher ORR and PFS compared with FOLFOX‐BEV independent of RAS mutational status. Too few patients in STEAM had BRAF‐mutated disease to allow correlation with outcomes.
Right‐sided tumors are associated with a markedly poorer prognosis compared with left‐sided tumors [16]. Interestingly, in this analysis, cFOLFOXIRI‐BEV imparted a higher PFS in patients with right‐sided tumors than in those with left‐sided tumors. cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV were associated with high median PFS regardless of tumor sidedness, and improved PFS compared with FOLFOX‐BEV in both right‐ and left‐sided tumors. ORR was notably higher with cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV treatment in left‐sided (but not right‐sided) tumors compared with FOLFOX‐BEV.
The small numbers of patients in some biomarker subgroups, such as the BRAF‐mutated group, and the use of 90% CI for ORR, PFS, and OS limited the interpretation of these analyses. Furthermore, because of early closure of the trial, results for OS are immature, and data collected for the second‐line treatment phase of the study were insufficient for analyses of efficacy, toxicity, or treatments after protocol. Additional biomarker analyses are ongoing to identify subgroups of patients who might derive the greatest benefits from treatment with FOLFOXIRI‐BEV.
The concept of aggressive induction for the treatment of mCRC is supported by the TRIBE study [12], [13]. The use of multiple anticancer agents with distinct mechanisms of action may help prevent the development of resistant clones and provide longer disease control [20]. Our findings suggest that cFOLFOXIRI‐BEV and sFOLFOXIRI‐BEV are active and well tolerated in patients with mCRC, consistent with prior data from TRIBE and OLIVIA, as well as several recent meta‐analyses [21], [22]. cFOLFOXIRI‐BEV conferred either statistically significant or strong trends for improvements in both ORR and PFS compared with FOLFOX‐BEV, benefits that appeared largely independent of the molecular characteristics or sidedness of the tumor. To further optimize the first‐line FOLFOXIRI‐BEV treatment strategy, the randomized, phase III TRIBE‐2 trial will directly assess whether the use of concurrent FOLFOXIRI‐BEV improves survival relative to sequential FOLFOXIRI‐BEV [23]. Taken together, these data support the use of concurrent or sequential FOLFOXIRI‐BEV as potential first‐line treatment options for patients with mCRC who can tolerate these treatment regimens.
Conclusion
The phase II randomized STEAM trial was the largest study of FOLFOXIRI‐BEV in patients in the U.S. In this study, data showed that both concurrent and sequential FOLFOXIRI‐BEV numerically improved clinical outcomes compared with FOLFOX‐BEV in patients with mCRC in the first line, with acceptable tolerability. These data confirm the feasibility of these regimens as potential first‐line treatments for mCRC; however, the patient population most likely to benefit from triplet chemotherapy with BEV remains to be addressed.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This study was funded by F. Hoffmann‐La Roche, Ltd. Support for third‐party writing assistance was provided by Sabrina Hom, Ph.D., of CodonMedical, an Ashfield Company, part of UDG Healthcare Plc, and was funded by F. Hoffmann‐La Roche, Ltd./Genentech, Inc. H.I.H. is currently affiliated with Genentech, Inc., South San Francisco, CA. These data were presented in part as the following abstracts: Bendell JC, Tan BR, Reeves JA et al. Overall response rate (ORR) in STEAM, a randomized, open‐label, phase 2 trial of sequential and concurrent FOLFOXIRI‐bevacizumab (BEV) vs FOLFOX‐BEV for the first‐line (1 L) treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC). American Association of Clinical Oncology Gastrointestinal Cancers Symposium; January 21–23, 2016; San Francisco, CA; Abstract 492. Hurwitz H, Tan BR, Reeves JA et al. Updated efficacy, safety, and biomarker analyses of STEAM, a randomized, open‐label, phase II trial of sequential (s) and concurrent (c) FOLFOXIRI‐bevacizumab (BV) vs FOLFOX‐BV for first‐line (1 L) treatment (tx) of patients with metastatic colorectal cancer (mCRC). American Association of Clinical Oncology Gastrointestinal Cancers Symposium; January 19–21, 2017; San Francisco, CA; Abstract 657.
Author Contributions
Conception/design: Herbert I. Hurwitz, John F. Palma, John J. Lee, Alan Nicholas, Nicolas Sommer, Johanna Bendell
Provision of study material or patients: Herbert I. Hurwitz, Benjamin R. Tan, James A. Reeves, Henry Xiong, Brad Somer, Heinz‐Josef Lenz, Howard S. Hochster, John F. Palma, John J. Lee, Johanna Bendell
Collection and/or assembly of data: Herbert I. Hurwitz, Henry Xiong, Brad Somer, Heinz‐Josef Lenz, Howard S. Hochster, Alan Nicholas, Johanna Bendell
Data analysis and interpretation: Herbert I. Hurwitz, Brad Somer, Heinz‐Josef Lenz, Frank Scappaticci, John F. Palma, Richard Price, John J. Lee, Alan Nicholas, Nicolas Sommer, Johanna Bendell
Manuscript writing: Herbert I. Hurwitz, Benjamin R. Tan, James A. Reeves, Henry Xiong, Brad Somer, Heinz‐Josef Lenz, Howard S. Hochster, Frank Scappaticci, John F. Palma, Richard Price, John J. Lee, Alan Nicholas, Nicolas Sommer, Johanna Bendell
Final approval of manuscript: Herbert I. Hurwitz, Benjamin R. Tan, James A. Reeves, Henry Xiong, Brad Somer, Heinz‐Josef Lenz, Howard S. Hochster, Frank Scappaticci, John F. Palma, Richard Price, John J. Lee, Alan Nicholas, Nicolas Sommer, Johanna Bendell
Disclosures
Herbert I. Hurwitz: Roche/Genentech (RF, E, SAB); Benjamin R. Tan: Roche/Genentech (RF, C/A), Bayer, Boehringer Ingelheim, Eisai, Exelixis, Eli Lilly & Co., Merck Serono, Pfizer, Sillajen, Tyrogenex, Bristol‐Myers Squibb, Beigene (RF); James A. Reeves: Celgene (C/A); Heinz‐Josef Lenz: Bayer, Bristol‐Myers Squibb, Roche/Genentech (C/A, RF, H), Merck (H, C/A), EMD, Incyte, Taiho (RF); Howard S. Hochster: Bayer, Boehringer Ingelheim, Genentech, Amgen, Sirtex Medical, Bristol‐Myers Squibb (C/A); Frank Scappaticci: Genentech (E), Roche (OI); John F. Palma: Roche (E, OI); Richard Price: Roche/Genentech (E, OI); John J. Lee: Roche (E, OI); Alan Nicholas: Genentech (E, OI); Nicolas Sommer: Genentech (E), Roche (OI); Johanna Bendell: Bristol Myers Squibb, Roche, Merck, Taiho Oncology, Amgen, Genentech, Merrimack, Celgene, Medimmune, Daiichi Sankyo (C/A, RF), Seattle Genetics, Janssen, Translational Drug Development, Five Prime Therapeutics, Moderna Therapeutics, Tolero, Evelo Biosciences, Arrys Therapeutics, Forma Therapeutics, Tanabe Research Laboratories, BeiGene, Continuum Clinical, Cerulean (C/A), EMD Serono, Ipsen Biopharma, Incyte, Novartis, Eisai, Pfizer, Millenium, Imclone, Boston Biomedical, CALGB, Acerta Pharma, Lilly, Gilead Sciences, Leap Therapeutics, Macrogenics, OncoMed Pharmaceuticals, Takeda Pharmaceuticals, Rgenix, Novocure, Merus, N.V., Blueprint Medicine, Array Biopharma, ARMO Biosciences, Agios (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program. Cancer stat facts: Colon and rectum cancer. Available at https://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 11, 2018. [Google Scholar]
- 2.Douillard JY, Cunningham D, Roth AD et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first‐line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000;355:1041–1047. [DOI] [PubMed] [Google Scholar]
- 3.Saltz LB, Cox JV, Blanke C et al.; Irinotecan Study Group. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 2000;343:905–914. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M et al. Leucovorin and fluorouracil with or without oxaliplatin as first‐line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938–2947. [DOI] [PubMed] [Google Scholar]
- 5.Falcone A, Ricci S, Brunetti I et al.; Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first‐line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670–1676. [DOI] [PubMed] [Google Scholar]
- 6.Masi G, Vasile E, Loupakis F et al. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: An updated analysis. J Natl Cancer Inst 2011;103:21–30. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 8.Bir A, Tan W, Wilding GE et al. 5‐fluorouracil, leucovorin and oxaliplatin plus bevacizumab in the first‐line treatment of metastatic colorectal cancer: A single‐institute study. Oncology 2007;72:4–9. [DOI] [PubMed] [Google Scholar]
- 9.Saltz LB, Clarke S, Díaz‐Rubio E et al. Bevacizumab in combination with oxaliplatin‐based chemotherapy as first‐line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 2008;26:2013–2019. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network . Colon cancer. Version 2.2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed April 11, 2018.
- 11.Masi G, Loupakis F, Salvatore L et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first‐line treatment for metastatic colorectal cancer: A phase 2 trial. Lancet Oncol 2010;11:845–852. [DOI] [PubMed] [Google Scholar]
- 12.Loupakis F, Cremolini C, Masi G et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–1618. [DOI] [PubMed] [Google Scholar]
- 13.Cremolini C, Loupakis F, Antoniotti C et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first‐line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open‐label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306–1315. [DOI] [PubMed] [Google Scholar]
- 14.Gruenberger T, Bridgewater J, Chau I et al. Bevacizumab plus mFOLFOX‐6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: The OLIVIA multinational randomised phase II trial. Ann Oncol 2015;26:702–708. [DOI] [PubMed] [Google Scholar]
- 15.Hebbar M, Chibaudel B, André T et al. Randomized trial of simplified LV5FU2 versus FOLFOX7 followed by FOLFIRI (MIROX) in patients with initially resectable metastatic colorectal cancer: A GERCOR study. J Chemother 2013;25:104–111. [DOI] [PubMed] [Google Scholar]
- 16.Loupakis F, Yang D, Yau L et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 2015;107:dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman AM, Bratman SV, To J et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med 2014;20:548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman AM, Lovejoy AF, Klass DM et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol 2016;34:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham DM, Coyle VM, Kennedy RD et al. Molecular subtypes and personalized therapy in metastatic colorectal cancer. Curr Colorectal Cancer Rep 2016;12:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogelstein B, Papadopoulos N, Velculescu VE et al. Cancer genome landscapes. Science 2013;339:1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cremolini C, Loupakis F, Masi G et al. FOLFOXIRI or FOLFOXIRI plus bevacizumab as first‐line treatment of metastatic colorectal cancer: A propensity score‐adjusted analysis from two randomized clinical trials. Ann Oncol 2016;27:843–849. [DOI] [PubMed] [Google Scholar]
- 22.Tomasello G, Petrelli F, Ghidini M et al. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: A systematic review and pooled analysis. JAMA Oncol 2017;3:e170278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremolini C, Marmorino F, Loupakis F et al.; Gruppo Oncologico del Nord Ovest. TRIBE‐2: A phase III, randomized, open‐label, strategy trial in unresectable metastatic colorectal cancer patients by the GONO group. BMC Cancer 2017;17:408. [DOI] [PMC free article] [PubMed] [Google Scholar]