Using data from the specialized Côte d'Or Gynecological Cancers Registry, this study was performed in conjunction with quality‐of‐life specialists and sociologists to investigate the clinical and socio‐economic determinants of long‐term health‐related quality of life (HRQoL) among cervical, endometrial, and ovarian cancer survivors. Long‐term HRQoL of gynecological cancer survivors is not impacted by stage of disease. The main determinants of poor HRQoL were comorbidities, deprivation, lack of availability and satisfaction with social support, and psychological outcomes.
Keywords: Health‐related quality of life, Cervical cancer, Ovarian cancer, Endometrial cancer
Abstract
Background.
The likelihood that health‐related quality of life (HRQoL) could depend on factors other than clinical data increases with the duration of follow‐up since diagnosis. The aim of this study was to identify determinants of long‐term HRQoL in women with cervical, endometrial, and ovarian cancer. Secondary objectives were to describe their living conditions (sexual function, psychological distress, social and professional reinsertion).
Materials and Methods.
In a cross‐sectional survey, women diagnosed with cervical, endometrial, and ovarian cancers from 2006 to 2013 were selected through the French gynecological cancers registry of Côte d'Or. Validated questionnaires exploring HRQoL (short‐form health survey; SF‐12), anxiety and depression (Hospital Anxiety and Depression Scale), social support (Sarason's Social Support Questionnaire), sexual function (Female Sexual Function Index), and living conditions (EPICES questionnaire) were used to assess HRQoL and its determinants. Social and professional reinsertion were also investigated using study‐specific questionnaires. Determinants of HRQoL were identified using a multivariable mixed‐regression model for each composite score of the SF‐12.
Results.
In total, 195 gynecological cancer survivors participated in the survey. HRQoL was deteriorated for almost all the SF‐12 dimensions. The main determinants of poor HRQoL were comorbidities, deprivation, lack of availability and satisfaction with social support, and psychological outcomes. Thirty‐four percent of survivors of gynecological cancer reported a negative impact of cancer on their work, and 73% reported an impaired ability to work after treatment.
Conclusions.
Long‐term HRQoL of survivors of gynecological cancer is not impacted by stage of disease. Specific interventions should focus on issues that promote social and professional reintegration and improve HRQoL.
Implications for Practice.
This study shows that women with gynecological cancer have problems related to work and sexual dysfunction, even 5 years after diagnosis. The results of this study will help improve clinicians’ awareness of the factors affecting the lives of gynecological cancer survivors, even long after diagnosis and treatment. They will also highlight for clinicians the areas that are of importance to gynecological cancer survivors, making it possible to guide management of these patients with a view to preventing deteriorated health‐related quality of life after treatment. For the health authorities, the results of this study underline that more than 5 years after gynecological cancer, the initial stage of disease no longer affects quality of life, but there is a clear need for actions targeting socio‐professional reintegration of survivors.
摘要
背景。健康相关的生活质量 (HRQoL) 取决于除临床数据之外因素的可能性会随着诊断后的随访持续时间而增加。本研究旨在确定患有宫颈癌、子宫内膜癌和卵巢癌的女性的长期 HRQoL 的决定因素。次要目标为描述他们的生活条件(性功能、心理压力、社会和职业重新融入)。
材料和方法。在横断面调查中,通过 Côte d'Or 的法国妇科癌症注册挑选自 2006 年至 2013 年期间被诊断为宫颈癌、子宫内膜癌和卵巢癌的女性。旨在探索 HRQoL(简明健康调查;SF‐12)、焦虑和抑郁(医院焦虑和抑郁量表)、社会支持(Sarason社会支持问卷)、性功能(女性性功能指数)以及生活条件(EPICES 问卷)的各种经验证的问卷被用于评估 HRQoL 及其决定因素。还利用特定于研究的问卷调查了社会和职业重新融入情况。对于 SF‐12 的每个综合评分,使用多变量混合回归模型确定了 HRQoL 的决定因素。
结果。一共有 195 名妇科癌症幸存者参与调查。HRQoL 在几乎所有的 SF‐12 方面均有下降。HRQoL 不佳的主要决定因素为合并症、社会支持供应不足和剥夺、对社会支持的不满意以及心理结果。34% 的妇科癌症幸存者报告了癌症对其工作的负面影响,73% 的妇科癌症幸存者报告了治疗之后的工作能力减损。
结论。妇科癌症幸存者的长期 HRQoL 不受疾病分期的影响。特定的干预措施应该重点关注促进社会和职业重新融入以及改进 HRQoL 的问题。
实践意义:本研究显示,患有妇科癌症的女性即使在确诊后 5 年会仍会有与工作和性功能障碍相关的问题。本研究的结果将有助于提高临床医生对影响妇科癌症幸存者生活的各种因素的认识,即使在诊断和治疗之后的很长时间。它们还将向临床医生强调对妇科癌症幸存者而言十分重要的方面,从而有可能指导此类患者的管理,以防止治疗后与健康相关的生活质量下降。对于卫生机构而言,本研究的结果强调,在妇科癌症超过 5 年之后,疾病的初始分期不再影响生活质量,但是,显然需要以幸存者的社会职业重新整合为目标的措施。
Introduction
In France, gynecological cancers (GC) represent 10% of new cancer cases among women [1]. The main types are cervical, endometrial, and ovarian cancers. Each of these is unique in terms of prognosis, treatment, and age at onset. Early detection and improvement in treatment of these cancers has led to improvements in survival and, consequently, an increase in the number of survivors [2]. However, survival is accompanied by several negative aspects, such as fatigue, physical changes, sexual dysfunction, anxiety, and/or depression [3], [4]. In addition to the physical and psychological disorders, survivors of gynecological cancer can experience economic problems related to work, or access to loans and insurance [5]. Although cancer occurs mainly in older adults, some people, particularly survivors of cervical and ovarian cancer, may experience cancer at an age where work is still of major importance [6]. For these women, a return to work represents a return to a normal social life and helps them to regain their self‐esteem. Furthermore, work is a source of emotional and financial support and has been shown to enhance health‐related quality of life (HRQoL) by its positive effect on self‐esteem [7]. Therefore, special attention must be paid to the well‐being of survivors of gynecological cancer, as well as to their social and professional reintegration.
HRQoL is a multidimensional concept which encompasses physical and mental health as well as social well‐being. Although in recent decades, several studies [8], [9], [10] have focused on HRQoL and its determinants in survivors of gynecological cancer, HRQoL has mainly been studied as it pertains to clinical data or with short follow‐up durations. Our previous studies in long‐term survivors of breast cancer [11], [12] have shown that the likelihood that HRQoL will depend on other factors increases in line with the length of follow‐up since diagnosis. On this basis, using data from the specialized Côte d'Or GC registry, we performed this study in conjunction with HRQoL specialists and sociologists to investigate the clinical and socio‐economic determinants of long‐term HRQoL among survivors of cervical, endometrial, and ovarian cancer. Secondary objectives were to describe their living conditions (namely, in terms of sexual function, psychological distress, and social and professional reinsertion).
Materials and Methods
Patients
A cross‐sectional study was carried out in survivors of gynecological cancer using data from the Côte d'Or (France) specialized registry. This is the only registry in France to focus on breast cancer and GC and has been collecting data on all cases of breast cancer and GC occurring in residents of Côte d'Or since 1982. The registry catchment area has approximately 500,000 inhabitants, including 270,000 women. This population is predominantly rural with low migration. Information about clinical characteristics, tumors, treatments, and vital status for patients recorded in the registry was obtained from various sources (medical records, letters to general practitioners, data of the National Institute of Statistics and Economic Studies). All women living in Côte d'Or and newly diagnosed with primary invasive nonmetastatic cervical, endometrial, or ovarian cancer from 1 January, 2006, to 31 December, 2013, were identified through the Côte d'Or registry. Women who died before January 2017 were excluded.
In January 2017, participants were mailed a study information pack that included the study questionnaires and an information letter. The letter presented the aims of the study and the legal information and invited them to participate in the study. In the absence of any response from patients within 1 month, a reminder was sent. The study was approved by the French national data protection authority (Commission nationale de l'informatique et des libertés MR003 N°1989764 v. 0).
Study Variables and Endpoints
HRQoL, sexual function, social support, socio‐economic status, anxiety, and depression were assessed using validated self‐administered questionnaires.
The Medical Outcomes Study 12‐item Short Form health survey (SF‐12) is a validated tool used to assess general HRQoL [13]. It comprises eight scales, namely physical functioning, role physical, bodily pain, role emotional, vitality, social functioning, mental health, and general health. All scales were scored according to the standard scoring method described in the SF‐12 scoring manual [14]. Each score ranges from 0 to 100 with higher scores representing a better HRQoL. Two summary scales, namely the Physical Component Summary (PCS) and the Mental Component Summary (MCS), were computed from the eight scales.
The Female Sexual Function Index (FSFI) is a self‐reported measurement of sexual functioning developed by Rosen [15]. A French version has been validated [16]. This 19‐item questionnaire explores the six scales of sexual function, namely: desire, arousal, lubrication, orgasm, sexual satisfaction, and pain of intercourse. The global score ranges from 2 to 36 with a score <26.5 corresponding to sexual dysfunction. For each scale, a score <3.9 is considered as an alteration on that scale.
Anxiety and depression were assessed with the Hospital Anxiety and Depression Scale [17], a 14‐item questionnaire that explores both anxiety and depression. To obtain a score for each dimension, the scores of the items on each scale are summed. Both the anxiety and depression subscores range from 0 to 21, with a score of 11 or higher indicating the probable presence of the mood disorder.
Social support was assessed using the Sarason Social Questionnaire [18]. This six‐item tool measures the availability of social support and the respondent's perceived satisfaction with that support. Each item presents a situation in which the patient may need social support; in the first part of the response, the patient is asked to list a number of persons who could provide support in that situation, and in the second part, to evaluate their satisfaction with the support provided. Satisfaction scores range from 6 to 36, and availability scores range from 0 to 54. A higher social support score represents better social support. These scores were categorized into two classes according to the median.
Socio‐economic deprivation was assessed with the French EPICES questionnaire [19]. This questionnaire, developed specifically for the French context, contains 11 items that take into account the overall living conditions. It explores deprivation and social health. Scores vary from 0 to 100 and enables classification of patients as deprived or not (>30 and ≤30, respectively).
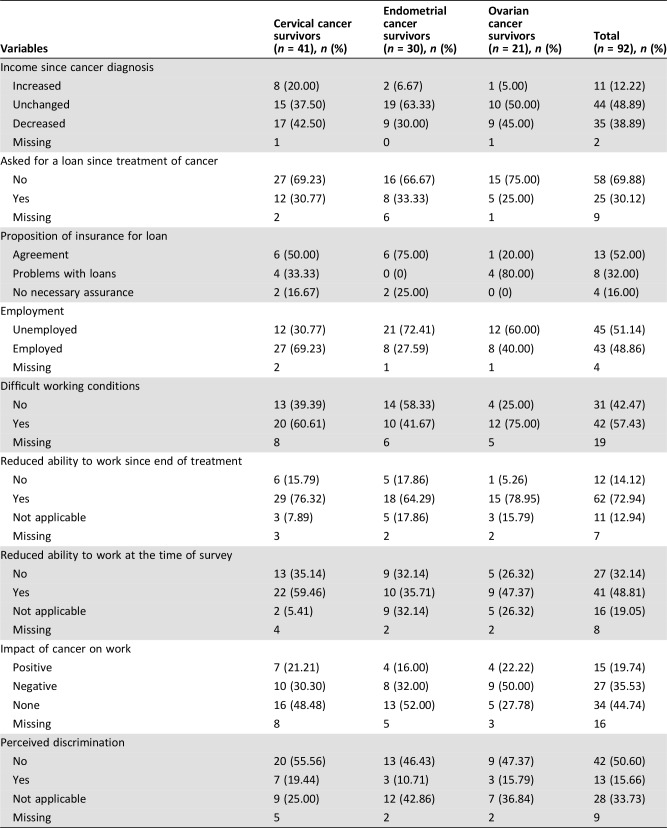
Social and occupational reintegration was assessed using a specific study questionnaire developed in conjunction with sociologists and psychologists. Data collected were problems relating to loans, income since diagnosis, ability to work (after treatment and at the time of assessment), impact of cancer, and perceived discrimination in their professional life.
Additional information, such as disease recurrence and patient's weight and height, was collected through a complementary questionnaire. Patient and tumor characteristics, including age at diagnosis, Charlson's comorbidity score, tumor stage, hormonal status, and treatments, were extracted from the Côte d'Or GC registry database. Age at diagnosis was classed as <70 and ≥70 years. Time since diagnosis was categorized as <5 and ≥5 years. Body mass index (BMI) was classified as underweight and normal weight (BMI ≤25 kg/m2) and overweight (BMI > 25 kg/m2). Tumor stage was categorized according to the International Federation of Gynecology and Obstetrics.
Statistical Analysis
We compared clinical characteristics and treatment between respondents and nonrespondents using the chi‐square or Fisher's exact test for categorical variables and the Mann‐Whitney test for continuous variables. For variables with more than two classes, we performed the Freeman‐Halton or Kruskal‐Wallis test depending on whether the variable was qualitative or continuous. A descriptive analysis of clinical and social characteristics and treatment was performed for each cancer type and for the whole population. Qualitative variables are presented as number and percentage, and quantitative variables are presented as mean ± SD or median and range as appropriate. The numbers of missing scores are also provided. HRQoL scores were described and compared across tumor sites. We assessed and described social and professional reinsertion in patients aged less than 60 years at the time of diagnosis. To identify the determinants of HRQoL, we performed a mixed regression model. Variables with a p value <.20 by univariate analysis were eligible for inclusion in the multivariate analysis. Analyses were adjusted for age at the time of the survey, tumor site, menopausal status, treatment by radiotherapy, and time since diagnosis to account for a response bias because respondents and nonrespondents differed on these factors. Significant determinants of HRQoL were determined with a backward stepwise selection procedure. The results are reported as multivariable analysis coefficients, SDs, and p values.
Two‐sided tests were used when reporting the results. As SF‐12 HRQoL scores cannot be considered independent of each other, Bonferroni's correction was used to adjust the α‐risk in the two multivariable models. The significance limit was thus set at 0.025 for multivariable models.
Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
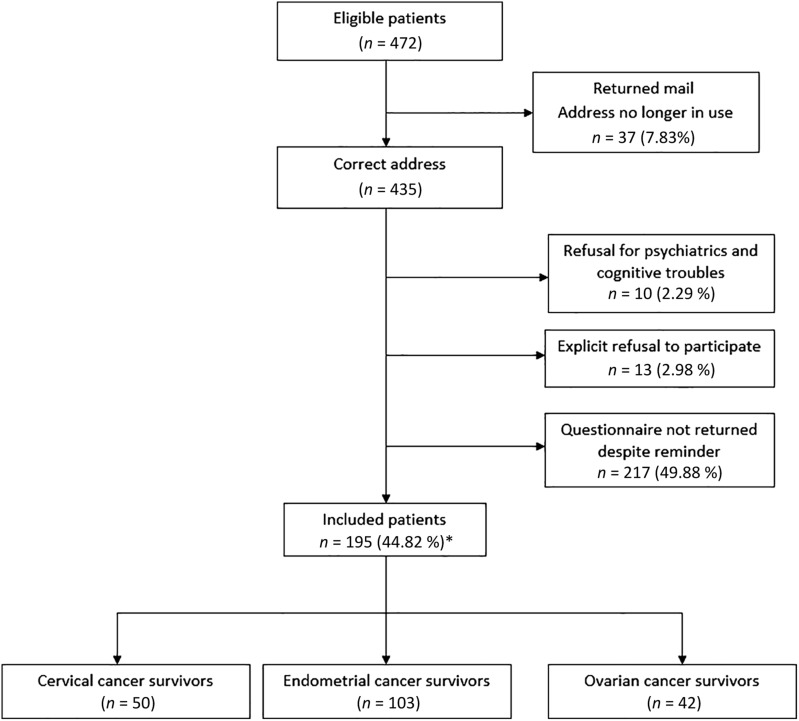
Four hundred and seventy‐two patients with cervical, endometrial, and ovarian cancers were eligible for this study. Among these, 37 were lost to follow‐up because of an invalid address and the questionnaire was mailed to 435 participants. Among these, 195 completed the questionnaire (Fig. 1).
Figure 1.
Flow chart of the study population. Number of women who responded to the questionnaires, number who did not respond, and number of women with cervical, endometrial, and ovarian cancers, respectively. *Participation rate was calculated using number of correct addresses as denominator.
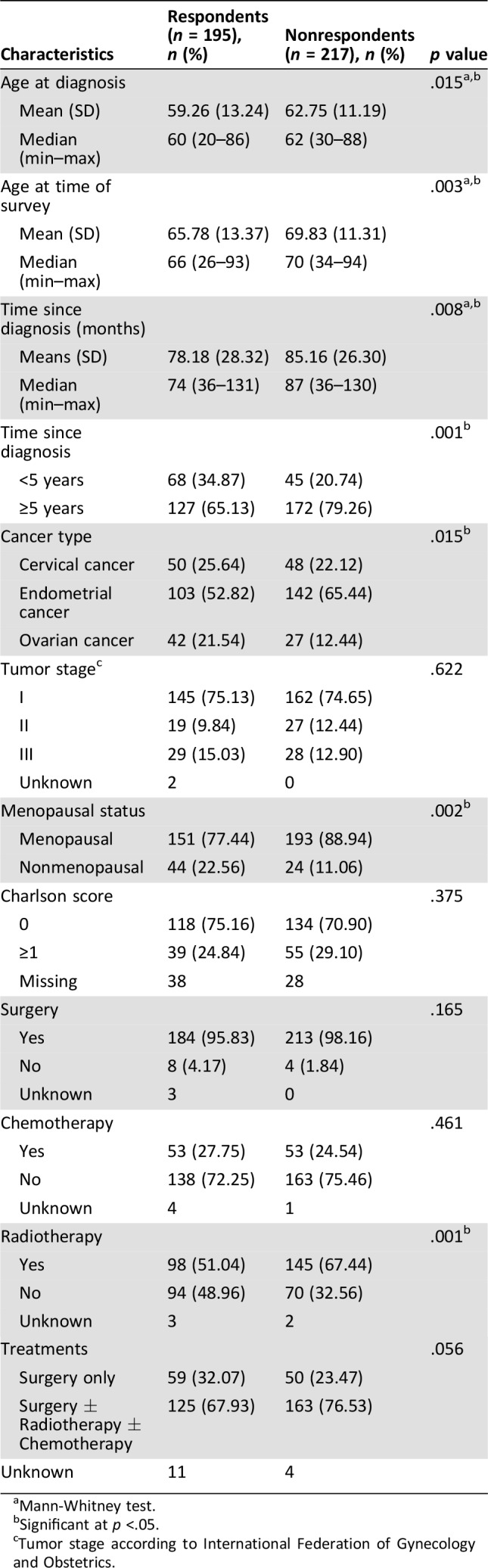
Respondents and nonrespondents differed in terms of age at diagnosis, age at the time of the survey, tumor site, hormonal status, radiotherapy treatment, and time since diagnosis (Table 1).
Table 1. Comparison of clinical and pathological characteristics between respondents and nonrespondents.
Mann‐Whitney test.
Significant at p <.05.
Tumor stage according to International Federation of Gynecology and Obstetrics.
Description of Clinical and Pathological Features of Participants
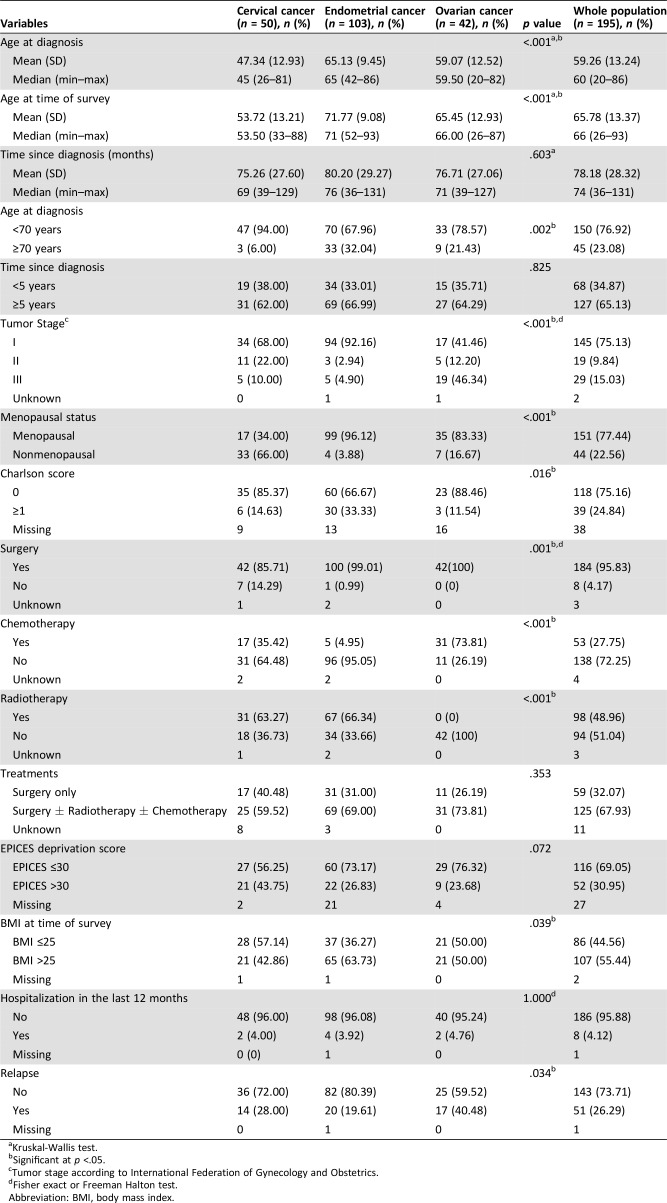
The clinical, socio‐demographic, and pathological characteristics of the participants by cancer type are shown in Table 2. Among participants, 103 (53%), 50 (26%), and 42 (22%) had endometrial, cervical, and ovarian cancer, respectively (Fig. 1). The median time since diagnosis was 74 months (range, 36–131) for the whole population. The mains characteristics of the population were a time since diagnosis ≥5 years in 65%, BMI >25 in 55%, no comorbidities in 75%, no relapse in 74%, and presence of deprivation in lower 69%. More than 95% of patients underwent surgery and more than half were treated with a combination of therapies.
Table 2. Clinical, socio‐demographic, and pathological characteristics of the participants by tumor site.
Kruskal‐Wallis test.
Significant at p <.05.
Tumor stage according to International Federation of Gynecology and Obstetrics.
Fisher exact or Freeman Halton test.
Abbreviation: BMI, body mass index.
Survivors of endometrial cancer were older (65.13 ± 9.45), had a higher BMI, and had comorbidities more often than patients with other gynecological cancers. Survivors of cervical cancer were younger (47.34 ± 12.93) and more often premenopausal (66%). Survivors of ovarian cancer were mostly initially diagnosed at stage III (46%) and had not undergone radiotherapy (Table 2). Deprivation was present in respectively 44%, 27%, and 24% of women with cervical, endometrial, and ovarian cancer.
HRQoL, Sexual Function, Social Support, Anxiety, and Depression
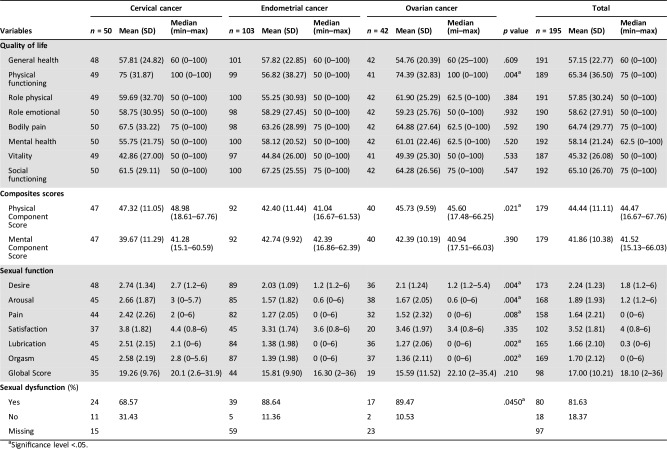
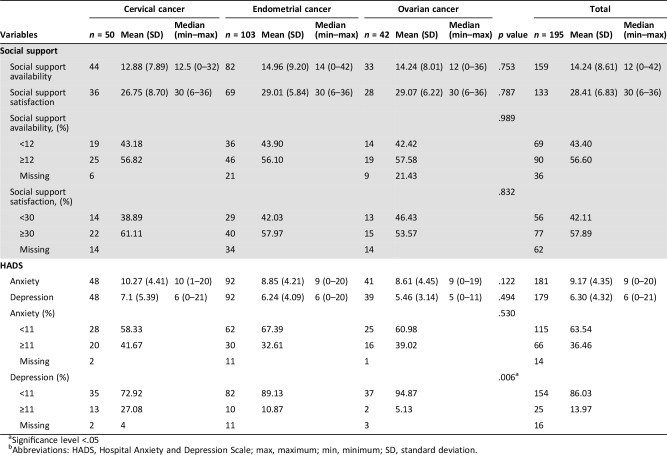
The scores of HRQoL, anxiety, depression, sexual function, and social support assessments are presented in Tables 3 and 4.
Table 3. Sexual function and HRQoL scores of the studied population by tumor site.
Significance level <.05.
Table 4. Social support, anxiety, and depression scores by tumor site.
Significance level <.05
Abbreviations: HADS, Hospital Anxiety and Depression Scale; max, maximum; min, minimum; SD, standard deviation.
HRQoL Scores.
Means scores for the SF‐12 dimensions were mainly under 60, except physical functioning, bodily pain, and social functioning. There were less than 10% of missing values in the summary scores of the SF‐12. Mean SF‐12 scores were not statistically different between CS, except for physical functioning, which was higher in cervical and ovarian CS than in endometrial CS (p = .004).
Sexual Function.
The mean score of FSFI was 17 (SD ± 10.21) for all diagnoses. Eighty out of the 98 (82%) women for whom it was possible to generate a global score reported a sexual dysfunction. Among the women who had global score, 69% of survivors of cervical cancer and 89% of survivors of both endometrial and ovarian cancer reported sexual dysfunction. Means scores for each subscale were < 3.9 regardless of location. Meanwhile, the scores were better in survivors of cervical cancer (Table 3).
Social Support, Anxiety, and Depression.
The median social support availability score was 12 and the median social support satisfaction score was 30.
Using the threshold of 11 to define the presence of mood disorders, there were 66 cases (37%) of anxiety and 25 cases (14%) of depression in the whole population. According to tumor site, there were 20 (42%), 30 (32%), and 16 (39%) cases of anxiety in survivors of cervical, endometrial, and ovarian cancer, respectively. Survivors of cervical cancer were more depressed than survivors of other gynecological cancer (n = 13, 27%).
Determinants of HRQoL
Whole Population.
Table 5 shows the significant determinants of HRQoL in the overall population and among survivors of cervical, ovarian, and endometrial cancer.
Table 5. Significant determinants of health‐related quality of life.
Mixed regression models.
Adjusted for tumor site, time since diagnosis, hormonal status, treatment by radiotherapy, age.
Time since diagnosis, hormonal status, treatment by radiotherapy, age.
Time since diagnosis, hormonal status, age.
Abbreviations: BMI, body mass index; MCS, Mental Component Summary; PCS, Physical Component Summary; SF‐12, 12‐item Short Form health survey.
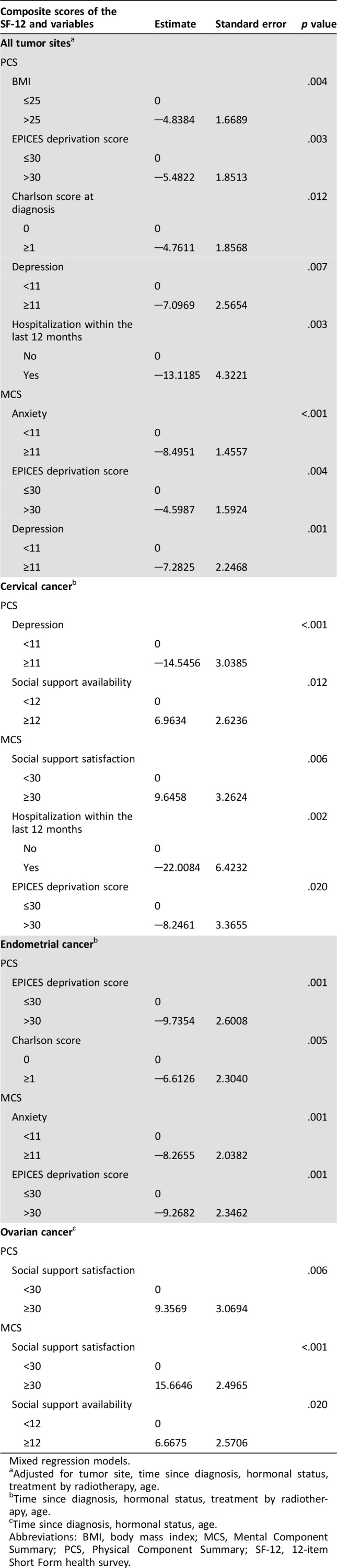
By multivariate analysis, significant determinants of physical component of HRQoL were BMI (p = .004), EPICES deprivation score (p = .005), Charlson's comorbidity score (p = .012), depression (p = .007), and hospitalization within the last 12 months (p = .003). Survivors of gynecological cancer who were overweight, had comorbidities, were deprived, or had been hospitalized within the last 12 months had worse HRQoL. Anxiety (p < .001), depression (p = .004), and EPICES deprivation score (p = .001) were significantly associated with MCS.
By Tumor Site.
In survivors of cervical cancer, depression (p < .001) and social support availability (p = .012) were linked to PCS, whereas women who were satisfied with their social support, were not deprived, and were not hospitalized within the last 12 months were more likely to have good mental HRQoL. EPICES deprivation score (p = .001) and Charlson's comorbidity score (p = .005) were determinants of worse physical HRQoL in survivors of endometrial cancer. Patients with anxiety (p = .001) and deprivation (p = .001) were more likely to have worse mental HRQoL. Social support was a determinant of physical and mental HRQoL among survivors of ovarian cancer.
Social and Occupational Reinsertion.
Ninety‐two patients (47%) were aged <60 years at diagnosis. Among these, 35 (39%) specified that their income had decreased since diagnosis. Twenty‐five (30%) women had sought a bank loan since diagnosis, and eight (32%) reported problems obtaining it (refusal, higher premiums, or exclusions in the contract). Thirty‐five percent of women reported that cancer had a negative impact on their professional life, with half of these survivors of ovarian cancer. Survivors of gynecological cancer also reported a decrease in their ability to work after treatment (73%) and at the time of assessment (49%). The full details of the social and occupational reintegration are given in Table 6.
Table 6. Social and professional outcomes in women aged <60 years at diagnosis.
Discussion
This study analyzed the medical and socioeconomic determinants of HRQoL among women identified through the French regional registry of GC of Côte d'Or and treated for the three main GC subtypes.
One hundred and ninety‐five women (44.82%) participated in this study. Although the participation rate was low, it is similar to expected rates in a population‐based study. Our response rate was similar to that reported by Le Borgne et al. [20]. This may be because of the length of the questionnaires and the age of patients. Indeed, nonrespondents were older than respondents. In addition, some patients declared that they did not feel concerned by this study because they felt cured.
A surprising finding was that nearly half the women with ovarian cancer had stage III disease at the time of diagnosis, and nearly 65% had a time since diagnosis ≥5 years, in line with a previous report by Cress et al. [21]. One possible explanation for this is the improvement in surgical techniques, which have largely contributed to minimizing residual disease [22], as well as the emergence of new targeted treatments, for example PARP inhibitors, and the fact that these patients can be considered as “cured” [10].
These results may be useful for clinical practice in terms of counseling about the prognosis of this type of cancer. Our study showed that unlike survivors of breast cancer, women with GC had a deteriorated HRQoL about 5 years after diagnosis. Korfage et al. [8] and Le Borgne et al. [20] have also shown an impairment of HRQoL in survivors of gynecological cancer, although their population consisted of survivors of cervical cancer only. Physical functioning was the only subscale of the SF‐12 that differed across tumor sites. Indeed, survivors of endometrial cancer have impaired physical functioning compared to other types of GC. One potential explanation for this result is that endometrial cancer was treated by surgery in the majority, in association with radiotherapy, which is known to affect HRQoL even in the long term [23]. Meanwhile, we cannot exclude the fact that survivors of endometrial cancer were older than the other patient groups.
Overweight, comorbidities, deprivation, less social support, and psychological distress were independent predictors of worse HRQoL among survivors of gynecological cancer in this study. Indeed, a high BMI has been linked to morbid‐mortality in cancer survivors, especially of endometrial [24] and ovarian cancer [25]. In our study, 55% of women were overweight, with the highest representation among survivors of endometrial (64%) and ovarian (50%) cancer. Anxiety and depression were also determinants of HRQoL in this study. Indeed, it is well known that they are associated with an increase in morbidity and mortality in women with gynecological cancer [26]. Fear of recurrence may be an explanation for this. Indeed, fear of recurrence persists over time in patients with gynecological cancer [26]. In our study, one third of survivors of gynecological cancer had a time since diagnosis <5 years. For these women, recurrence could still occur and might be a source of worry for them.
Concerning the determinants of HRQoL across tumor sites, we observed that depression and social support were predictors of HRQoL in survivors of cervical cancer. Indeed, survivors of cervical cancer had the highest depression scores in this study. Our results are similar to those reported by Osann et al. [27], who reported a high level of depression (26%) 9–30 months after diagnosis in women with cervical cancer. Two hypotheses can support this findings, namely the fear of recurrence and worry about reproductive ability. Indeed, most survivors of cervical cancer in this study were premenopausal, and thus, sexual function and childbirth may be of great importance to them. Women with cervical cancer also had less social support available. In fact, as with other survivors, their ability to share problems with others decreased with time, suggesting waning social support [28].
In this study, the only determinant of HRQoL in survivors of ovarian cancer was social support. Teng et al. [10] found similar results. This suggests that survivors of ovarian cancer must pay more attention to psychosocial factors than physical sequelae [25]. However, it should be noted that other disorders induced by chemotherapy, such as neurotoxic and digestive disorders that could also impact HRQoL in this population, were not evaluated in our study.
Sexual function is an important component of HRQoL among survivors of gynecological cancer. Indeed, sexual dysfunction is associated with negative psychological changes and has a major impact on HRQoL in survivors of gynecological cancer. Furthermore, because of the nature, localization, and treatments for gynecological cancers, they incur the greatest risk of sexual dysfunction [29]. In our study, sexual function was impaired, but it is important to consider that when women reported no sexual activity within the last 4 weeks, overall FSFI score was generated. Therefore, this may have led to some overestimation of sexual impairment in our population.
Women with GC reported a decrease in their ability to work after treatment and also difficulties in obtaining loans, with a greater impact observed in women with ovarian cancer. An explanation for this reduced ability to work in women with ovarian cancer might be the fact that 74% of these patients were treated with chemotherapy, which has previously been reported to negatively affect work ability [30].
The strengths of our study are the use of validated instruments to assess HRQoL features and psychological outcomes and the use of a specialized registry database, which had the twofold advantage of being representative of patients treated in the region and enabling us to assess long‐term HRQoL.
Conclusion
In conclusion, 6 years after diagnosis, clinical factors such as disease stage were not found to have an impact on HRQoL in survivors of gynecological cancer. The main determinants of HRQoL 6 years after diagnosis were overweight, comorbidities, deprivation, anxiety and depression, and less social support. Because these factors are also determinants of HRQoL in the general population, we can assume that 6 years after diagnosis, HRQoL of survivors of gynecological cancer is not impacted by the stage disease, and specific interventions in these populations should focus on the promotion of social and professional reintegration and improvement of HRQoL.
Acknowledgements
We thank Ludovic Bouzigues for data collection and Laurence Collet for correcting the manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Author Contributions
Conception/design: Ariane Mamguem Kamga, Agnès Dumas, Florence Joly, Charles Coutant, Pierre Fumoleau, Patrick Arveux, Tienhan Sandrine Dabakuyo‐Yonli
Provision of study material or patients: Agnès Dumas, Marie‐Laure Poillot, Ariane Darut‐Jouve, Patrick Arveux, Tienhan Sandrine Dabakuyo‐Yonli
Collection and/or assembly of data: Marie‐Laure Poillot, Ariane Darut‐Jouve
Data analysis and interpretation: Agnès Dumas, Florence Joly, Oumar Billa, Julien Simon, Ariane Mamguem Kamga, Charles Coutant, Pierre Fumoleau, Patrick Arveux, Tienhan Sandrine Dabakuyo‐Yonli
Manuscript writing: Ariane Mamguem Kamga, Agnès Dumas, Florence Joly, Oumar Billa, Charles Coutant, Pierre Fumoleau, Patrick Arveux, Tienhan Sandrine Dabakuyo‐Yonli
Final approval of manuscript: Ariane Mamguem Kamga, Agnès Dumas, Florence Joly, Oumar Billa, Julien Simon, Marie‐Laure Poillot, Ariane Darut‐Jouve, Charles Coutant, Pierre Fumoleau, Patrick Arveux, Tienhan Sandrine Dabakuyo‐Yonli
Disclosures
The authors indicated no financial relationships.
References
- 1.Grosclaude P, Remontet L, Belot A et al. Survival of people with cancer in France, 1989‐2007. Study based on Francim cancer registries [in French]. Saint‐Maurice: Institute for Public Health Surveillance 2013. [Google Scholar]
- 2.Walker AJ, Benrubi ID, Ward KK. Care of survivors of gynecologic cancers. World J Obstet Gynecol 2016;5:140–149. [Google Scholar]
- 3.Gonçalves V. Long‐term quality of life in gynecological cancer survivors. Curr Opin Obstet Gynecol 2010;22:30–35. [DOI] [PubMed] [Google Scholar]
- 4.Akalin A, Pinar G. Unmet needs of women diagnosed with gynecologic cancer: An overview of literature. Palliat Care Med 2016;1–6. [Google Scholar]
- 5.Mols F, Thong MS, Vissers P et al. Socio‐economic implications of cancer survivorship: Results from the PROFILES registry. Eur J Cancer 2012;48:2037–2042. [DOI] [PubMed] [Google Scholar]
- 6.Mehnert A. Employment and work‐related issues in cancer survivors. Crit Rev Oncol Hematol 2011;77:109–130. [DOI] [PubMed] [Google Scholar]
- 7.Islam T, Dahlui M, Majid HA et al. Factors associated with return to work of breast cancer survivors: A systematic review. BMC Public Health 2014;14(suppl 3):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korfage IJ, Essink‐Bot ML, Mols F et al. Health‐related quality of life in cervical cancer survivors: A population‐based survey. Int J Radiat Oncol Biol Phys 2009;73:1501–1509. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter KM, Fowler JM, Maxwell GL et al. Direct and buffering effects of social support among gynecologic cancer survivors. Ann Behav Med 2010;39:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng FF, Kalloger SE, Brotto L et al. Determinants of quality of life in ovarian cancer survivors: A pilot study. J Obstet Gynaecol Can 2014;36:708–715. [DOI] [PubMed] [Google Scholar]
- 11.Dialla PO, Chu WO, Roignot P et al. Impact of age‐related socio‐economic and clinical determinants of quality of life among long‐term breast cancer survivors. Maturitas 2015;81:362–370. [DOI] [PubMed] [Google Scholar]
- 12.Chu WO, Dialla PO, Roignot P et al. Determinants of quality of life among long‐term breast cancer survivors. Qual Life Res 2016;25:1981–1990. [DOI] [PubMed] [Google Scholar]
- 13.Gandek B, Ware JE, Aaronson NK et al. Cross‐Validation of Item Selection and Scoring for the SF‐12 health survey in Nine Countries: Results from the IQOLA Project. J Clin Epidemiol 1998;51:1171–1178. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE, Kosinski M, Turner‐Bowker DM et al. How to Score Version 2 of the SF‐12 Health Survey: With a Supplement Documenting Version 1. Lincoln, RI: Quality Metric, 2005.
- 15.Rosen R, Brown C, Heiman J et al. The Female Sexual Function Index (FSFI): A multidimensional self‐report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208. [DOI] [PubMed] [Google Scholar]
- 16.Wylomanski S, Bouquin R, Philippe HJ et al. Psychometric properties of the French Female Sexual Function Index (FSFI). Qual Life Res 2014;23:2079–2087. [DOI] [PubMed] [Google Scholar]
- 17.Lepine JP, Godchau M, Brun P. Anxiety and depression in inpatients. Lancet 1985;28:1425–1426. [DOI] [PubMed] [Google Scholar]
- 18.Rascle N, Bruchon‐Schweitzer M, Sarason IG. Short form of Sarason's social support questionnaire: French adaptation and validation. Psychol Rep 2005;97:195–202. [DOI] [PubMed] [Google Scholar]
- 19.Sass C, Moulin JJ, Guéguen R et al. The Epices score: An individual score of deprivation. Score construction and measurement of relationships with health data, in a population of 197389 [in French]. Bull Epidemiol Hebdomadaire 2006;14:93–96. [Google Scholar]
- 20.Le Borgne G, Mercier M, Woronoff AS et al. Quality of life in long‐term cervical cancer survivors: A population‐based study. Gynecol Oncol 2013;129:222–228. [DOI] [PubMed] [Google Scholar]
- 21.Cress RD, Chen YS, Morris CR et al. Characteristics of long‐term survivors of epithelial ovarian cancer. Obstet Gynecol 2015;126:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elattar A, Bryant A, Winter‐Roach BA et al. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev 2011;10:CD007565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foerster R, Schnetzke L, Bruckner T et al. Prognostic factors for long‐term quality of life after adjuvant radiotherapy in women with endometrial cancer. Strahlenther Onkol 2016;192:895–904. [DOI] [PubMed] [Google Scholar]
- 24.Fader AN, Frasure HE, Gil KM et al. Quality of life in endometrial cancer survivors: What does obesity have to do with it? Obstet Gynecol Int 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed‐Lecheheb D, Joly F. Ovarian cancer survivors’ quality of life: A systematic review. J Cancer Surviv 2016;10:789–801. [DOI] [PubMed] [Google Scholar]
- 26.Hodgkinson K, Butow P, Fuchs A et al. Long‐term survival from gynecologic cancer: Psychosocial outcomes, supportive care needs and positive outcomes. Gynecol Oncol 2007;104:381–389. [DOI] [PubMed] [Google Scholar]
- 27.Osann K, Hsieh S, Nelson EL et al. Factors associated with poor quality of life among cervical cancer survivors: Implications for clinical care and clinical trials. Gynecol Oncol 2014;135:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaendler KS, Wenzel L, Mechanic MB et al. Cervical cancer survivorship: Long‐term quality of life and social support. Clin Ther 2015;37:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin AO, Carpenter KM, Fowler JM et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torp S, Nielsen RA, Gudbergsson SB et al. Worksite adjustments and work ability among employed cancer survivors. Support Care Cancer 2012;20:2149–2156. [DOI] [PubMed] [Google Scholar]