In this retrospective analysis, a large, detailed, and curated single center database was used to compare clinicopathologic features and outcomes between invasive lobular carcinoma and mixed invasive ductal and lobular carcinomas, focusing on the prognostic implications of histological grade and taking into consideration the differences in systemic therapies.
Keywords: Breast cancer, early; Carcinoma, lobular; Invasive ductal carcinoma, breast; Tumor grading; Outcomes research
Abstract
Background.
The diagnosis of mixed invasive ductal and lobular carcinoma (IDC‐L) in clinical practice is often associated with uncertainty related to its prognosis and response to systemic therapies. With the increasing recognition of invasive lobular carcinoma (ILC) as a distinct disease subtype, questions surrounding IDC‐L become even more relevant. In this study, we took advantage of a detailed clinical database to compare IDC‐L and ILC regarding clinicopathologic and treatment characteristics, prognostic power of histologic grade, and survival outcomes.
Materials and Methods.
In this retrospective cohort study, we identified 811 patients diagnosed with early‐stage breast cancer with IDC‐L or ILC. Descriptive statistics were performed to compare baseline clinicopathologic characteristics and treatments. Survival rates were subsequently analyzed using the Kaplan–Meier method and compared using the Cox proportional hazards model.
Results.
Patients with ILC had more commonly multifocal disease, low to intermediate histologic grade, and HER2‐negative disease. Histologic grade was prognostic for patients with IDC‐L but had no significant discriminatory power in patients with ILC. Among postmenopausal women, those with IDC‐L had significantly better outcomes when compared with those with ILC: disease‐free survival (DFS) and overall survival (OS; adjusted hazard ratio [HR], 0.54; 95% confidence interval [CI] 0.31–0.95). Finally, postmenopausal women treated with an aromatase inhibitor had more favorable DFS and OS than those treated with tamoxifen only (OS adjusted HR, 0.50; 95% CI, 0.29–0.87), which was similar for both histologic types (p = .212).
Conclusion.
IDC‐L tumors have a better prognosis than ILC tumors, particularly among postmenopausal women. Histologic grade is an important prognostic factor in IDC‐L but not in ILC.
Implications for Practice.
This study compared mixed invasive ductal and lobular carcinoma (IDC‐L) with invasive lobular carcinomas (ILCs) to assess the overall prognosis, the prognostic role of histologic grade, and response to systemic therapy. It was found that patients with IDC‐L tumors have a better prognosis than ILC, particularly among postmenopausal women, which may impact follow‐up strategies. Moreover, although histologic grade failed to stratify the risk of ILC, it showed an important prognostic power in IDC‐L, thus highlighting its clinical utility to guide treatment decisions of IDC‐L. Finally, the disease‐free survival advantage of adjuvant aromatase inhibitors over tamoxifen in ILC was consistent in IDC‐L.
摘要
背景。临床实践中混合浸润性导管和小叶癌 (IDC‐L) 的诊断通常与其预后和系统治疗反应相关的不确定性有关。随着人们日益认识到浸润性小叶癌 (ILC) 是一种独特的疾病亚型,围绕 IDC‐L 而产生的问题变得更加相关。在本次研究中,我们利用详细的临床数据库来比较 IDC‐L 和 ILC 的临床病理和治疗特征、组织学分级的预后能力以及生存预后。
材料和方法。在本次回顾性队列研究中,我们找到了 811 名被诊断为患有 IDC‐L 或 ILC 的早期乳腺癌患者。我们执行了描述性统计,以比较基线临床病理特征和治疗。随后,我们使用Kaplan–Meier分析方法对生存率进行了分析,并使用Cox比例风险模型进行了比较。
结果。ILC 患者患有更常见的多灶性疾病,组织学分级介于低级至中级之间,且为 HER2 阴性。组织学分级对 IDC‐L 患者而言是预后因素,但在 ILC 患者中没有显著的区分能力。在绝经后女性中,IDC‐L 患者的预后明显好于 ILC 患者:无病生存期 (DFS) 和总生存期 [OS;校正风险比 (HR),0.54;95% 置信区间 (CI) 0.31–0.95]。最后,采用芳香化酶抑制剂治疗的绝经后女性在 DFS 和 OS 方面比仅采用他莫昔芬治疗的绝经后女性更好(OS 校正 HR,0.50;95% CI,0.29–0.87),后一种治疗方式对这两种组织学类型而言效果相似 (p = 0.212)。
结论。IDC‐L 肿瘤的预后要好于 ILC 肿瘤,尤其在绝经后女性中更是如此。在 IDC‐L(而非 ILC)中,组织学分级是一个重要的预后因素。
实践意义:本研究将混合浸润性导管和小叶癌 (IDC‐L) 与浸润性小叶癌(ILC)进行对比,以评估总体预后、组织学分级的预后作用以及系统治疗的反应。研究发现,IDC‐L 肿瘤患者的预后要好于 ILC 患者,尤其在绝经后女性中更是如此,这可能会影响随访策略。此外,尽管组织学分级未能对 ILC 的风险进行分层,但是,它在 IDC‐L 中显示出重要的预后能力,因而突显其指导 IDC‐L 治疗决策的临床实用性。最后,辅助芳香化酶抑制剂在 ILC 中相对于他莫昔芬的无病生存期优势与在 IDC‐L 中的优势是一致的。
Introduction
Breast cancer is morphologically classified as either invasive breast carcinoma of no special type (NST), also known as invasive ductal carcinoma (IDC), or as a “special subtype” of breast cancer [1]. Special subtypes account for an array of different histological features, with invasive lobular carcinoma (ILC) being the most common subtype [2]. In addition, certain breast carcinomas present with varying proportions of NST and other types of breast cancers and are classified as carcinomas of mixed type. This category is defined as tumors in which at least 50% of the tumor has a specialized pattern and 10%–49% of the tumor has a nonspecialized pattern [1]. Mixed invasive ductal and lobular carcinomas (IDC‐Ls) account for approximately 5% of all breast cancers and, together with ILC, present a growing incidence [2], [3], [4].
ILC has long been distinguished from other types of breast cancer for its unique clinicopathologic features and, more recently, genomic landscape [5], [6], [7]. When compared with IDC, ILC tends to lack the cell adhesion molecule e‐cadherin, is more frequently multifocal, is hormone receptor‐positive/HER2‐negative, is lower grade (I or II), presents reduced response rates to preoperative chemotherapy, and may benefit differently from adjuvant endocrine therapies [8], [9], [10], [11]. In contrast, studies characterizing IDC‐L are currently scarce and limited by cohort size, lack of granular clinicopathological/treatment data, or short follow‐up [2], [12], [13], [14], [15], [16]. It is thus unclear how patients with these tumors perform in terms of survival outcomes and whether known classic prognostic features of IDC, such as histologic grade, apply to IDC‐L.
In this retrospective analysis, we took advantage of a large, detailed, and curated single center database to compare clinicopathologic features and outcomes between ILC and IDC‐L. We further focused on the prognostic implications of histological grade, taking into consideration differences in systemic therapies.
Subjects, Materials, and Methods
Study Design and Data Source
This is a retrospective cohort study using prospectively collected data from the Dana‐Farber Cancer Institute (DFCI) and stored in the National Comprehensive Cancer Network Oncology Outcomes Database. The current study was approved by the DFCI Institutional Review Board and complies with all national regulations. We applied the STROBE statement in reports of cohort studies (http://www.strobe-statement.org/).
Patient Selection and Extracted Information
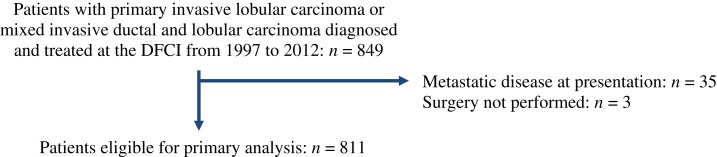
We identified all patients who were older than 18 years of age and were diagnosed and treated at DFCI for stage I–III breast cancer of ILC or IDC‐L histology from 1997 to 2007. IDC‐L was defined as tumors in which at least 50% of the tumor is of lobular pattern and 10%–49% is of nonspecialized pattern. Follow‐up details (disease recurrence, new primaries, and death) were available up to January 2012 and analyzed as per registry specifications. Dates of study entry were balanced between groups. We excluded patients with metastatic disease at presentation, patients who received neoadjuvant therapy, patients who did not have surgery, and patients with other concurrent primary tumors. A cohort of 811 patients was identified for the analysis (Fig. 1).
Figure 1.
Study Consort diagram.
Abbreviation: DFCI, Dana‐Farber Cancer Institute.
Overall survival (OS), disease‐free survival (DFS), and time to specific relapse were defined as time from diagnosis to death, time from diagnosis to any relapse or death, and time from diagnosis to local, regional, or distant relapse, respectively.
Statistical Analysis
Descriptive statistics of baseline demographic, clinicopathologic, and treatment characteristics were performed. Differences between groups were tested using chi‐squared test or t test where applicable. Time‐to‐event data were analyzed using the Kaplan–Meier method and compared using Cox proportional hazards models. All patients with missing data in relevant variables were excluded from the multivariate analysis. All the presented analyses successfully met proportional hazards assumption as assessed by the Schoenfeld residuals. Missing information was considered as missing at random, as per study design. The analyses were completed using Stata 12.3 (StataCorp LP; College Station, TX).
Results
Study Population and Baseline Characteristics
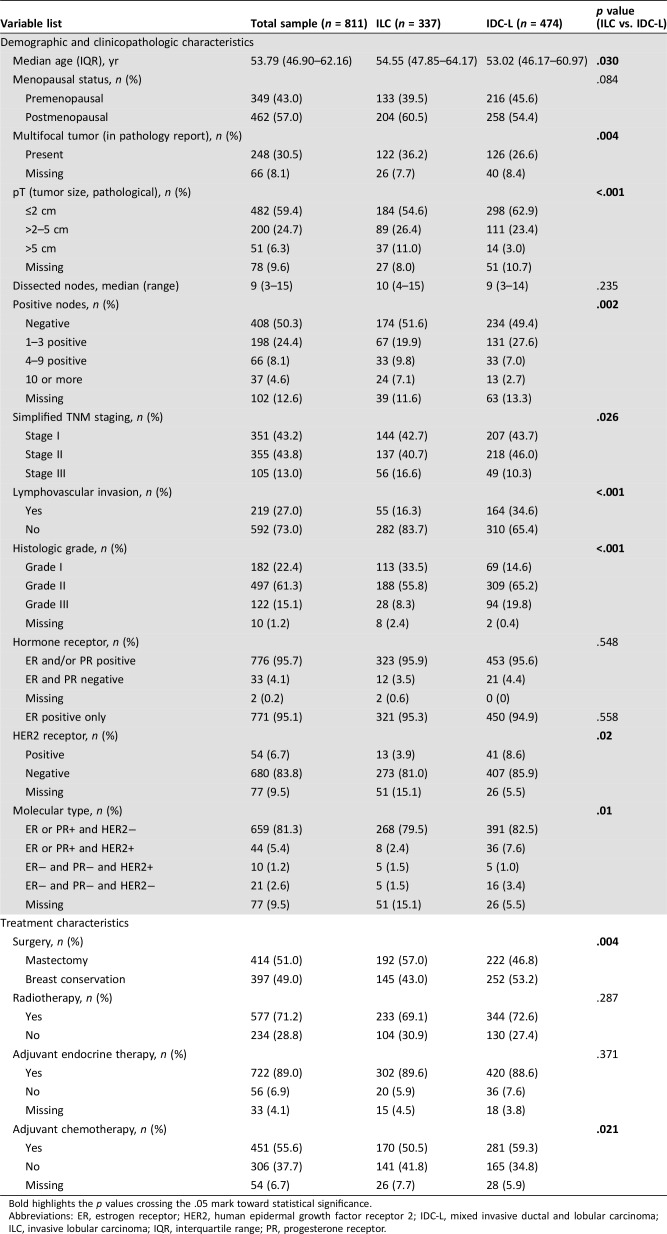
The study population included 811 patients, 337 (41.6%) with ILC and 474 (58.4%) with IDC‐L (Table 1). When compared with patients with IDC‐L, patients with ILC were slightly older and had larger tumors (11.0% had tumors >5 cm vs. 3.0% for IDC‐L; p < .001), more positive nodes (16.9% had ≥4 nodes vs. 9.7% for IDC‐L; p = .002), and less frequent poorly differentiated tumors (8.3% vs. 19.8% for IDC‐L; p < .001). In addition, ILC was less likely to be HER2 positive (3.9% vs. 8.6%; p = .02). Finally, multifocal disease was also more common in patients with ILC (36.2% vs. 26.6%; p = .004).
Table 1. Patient demographics, clinicopathologic characteristics, and treatments overall and by histologic type.
Bold highlights the p values crossing the .05 mark toward statistical significance.
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IDC‐L, mixed invasive ductal and lobular carcinoma; ILC, invasive lobular carcinoma; IQR, interquartile range; PR, progesterone receptor.
Treatment
Patients with ILC underwent mastectomy more frequently than those with IDC‐L (57.0% vs. 46.8%; p = .004; Table 1). Yet no significant differences were found in the frequency of radiotherapy (69.1% vs. 72.6% for IDC‐L; p = .287). Nevertheless, despite the higher tumor burden at diagnosis, patients with ILC received chemotherapy less frequently (50.5% vs. 59.3% for IDC‐L; p = .021).
Outcomes
The median follow‐up for the entire cohort was 7.9 years and was similar for both histologic types (p = .190). Among 337 patients with ILC, 73 (21.7%) developed a DFS event and 62 (18.4%) developed an OS event, whereas among 474 patients with IDC‐L, 70 (14.8%) developed a DFS event and 59 (12.5%) developed an OS event. For patients with ILC, the 5‐ and 10‐year proportion of patients free of a DFS event was 89.3% (95% confidence interval [CI], 85.3–92.2) and 74.2% (95% CI, 67.8–79.6), respectively, whereas for IDC‐L, the 5‐ and 10‐year rates were 90.4% (95% CI, 87.3–92.8) and 81.0% (95% CI, 75.7–85.3), respectively.
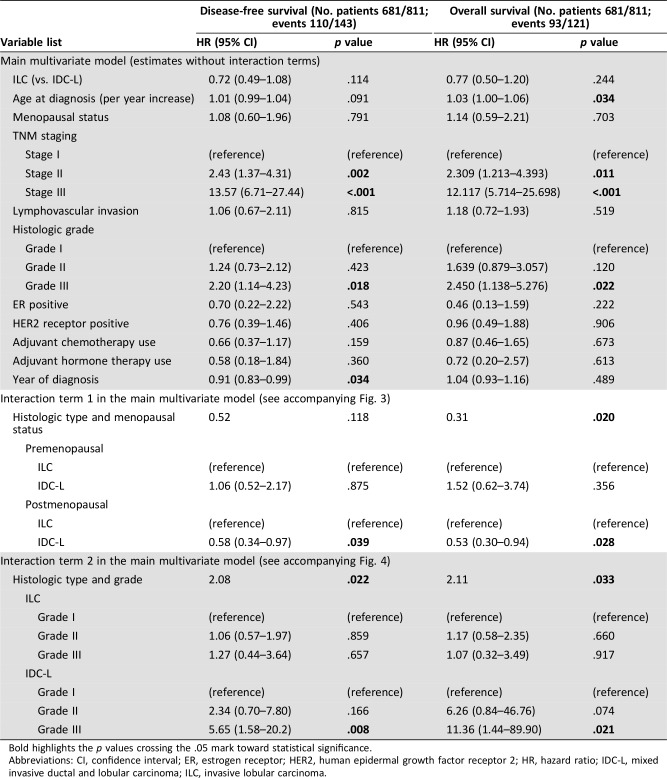
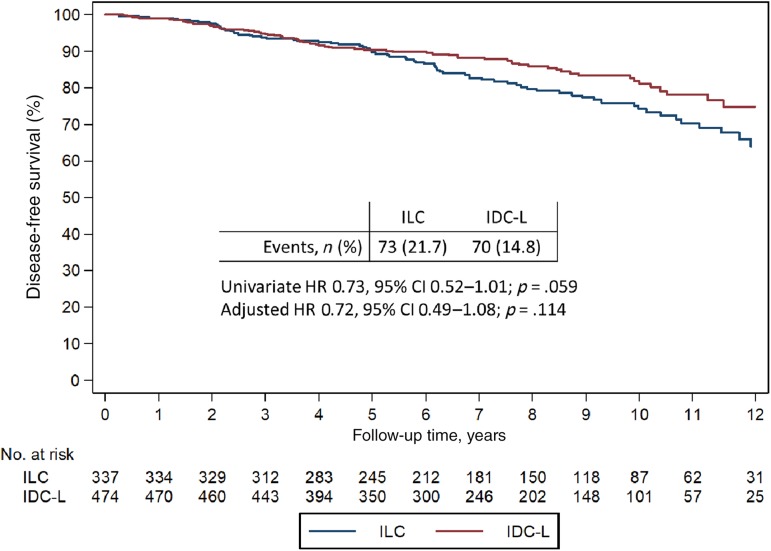
In a multivariate model, variables associated with DFS included year of diagnosis, TNM stage, and histologic grade, whereas variables associated with OS included age at diagnosis, TNM staging, and histologic grade (Table 2). Overall, the differences in DFS and OS outcomes by histologic type were not statistically significant, despite a trend towards an improved outcome for IDC‐L when compared with ILC. Specifically, the hazard ratio for DFS was 0.72 (95% CI, 0.49–1.08; p = .114; Table 2; Fig. 2) and the hazard ratio for OS was 0.77 (95% CI, 0.50–1.20; p = .244; Table 2; supplemental online Fig. 1).
Table 2. Multivariate Cox proportional hazards model for overall survival and disease‐free survival.
Bold highlights the p values crossing the .05 mark toward statistical significance.
Abbreviations: CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; IDC‐L, mixed invasive ductal and lobular carcinoma; ILC, invasive lobular carcinoma.
Figure 2.
Disease‐free survival in ILC and IDC‐L.
Abbreviations: CI, confidence interval; IDC‐L, mixed invasive ducal and lobular carcinoma; ILC, invasive lobular carcinoma; HR, hazard ratio.
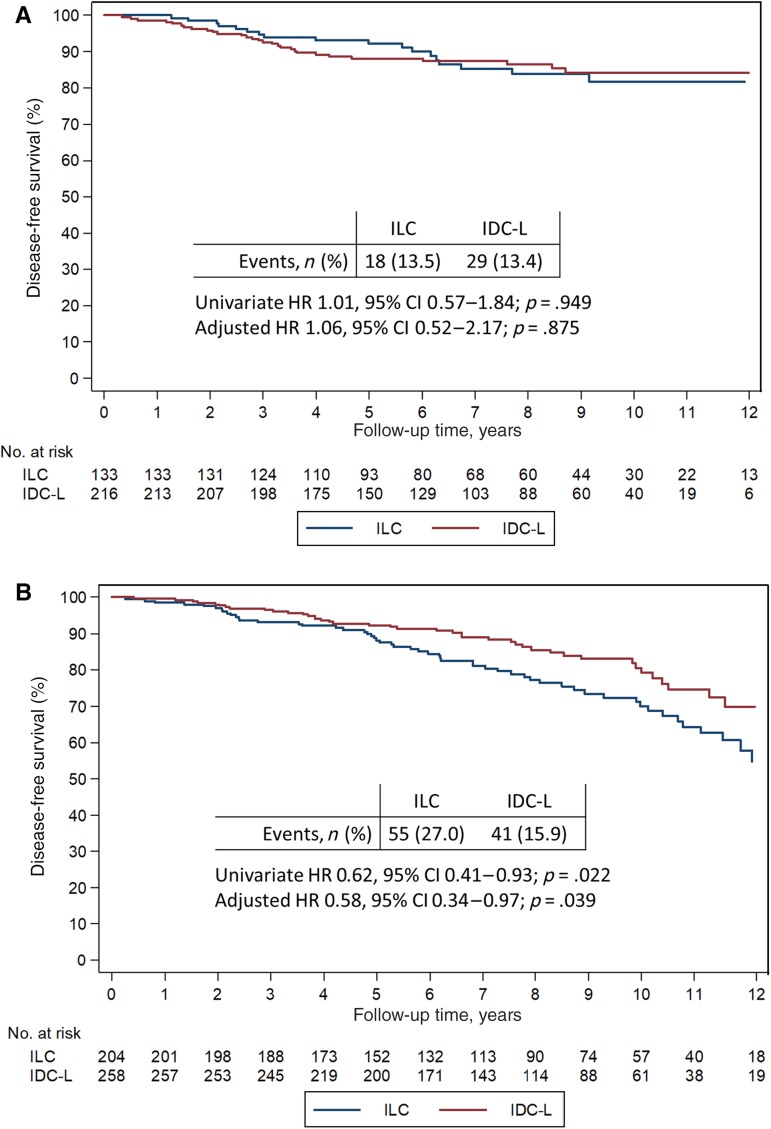
Given the hormone dependent nature of lobular carcinomas, we tested whether menopausal status modified outcomes according to histologic type. The interaction between histology and menopausal status was statistically significant for OS, and a trend in the same direction was noted for DFS (Table 2). When stratifying the analysis by menopausal status, no difference in DFS or OS was seen in premenopausal patients (adjusted hazard ratio [HR] for DFS, 1.06; 95% CI, 0.52–2.17; p = .875), but superior outcome is evident for postmenopausal patients with IDC‐L compared with ILC (adjusted HR for DFS, 0.58; 95% CI, 0.34–0.97; p = .039; Fig. 3; supplemental online Fig. 2).
Figure 3.
Disease‐free survival in ILC and IDC‐L. Disease‐free survival in premenopausal (A) and postmenopausal (B) patients.
Abbreviations: CI, confidence interval; IDC‐L, mixed invasive ducal and lobular carcinoma; ILC, invasive lobular carcinoma; HR, hazard ratio.
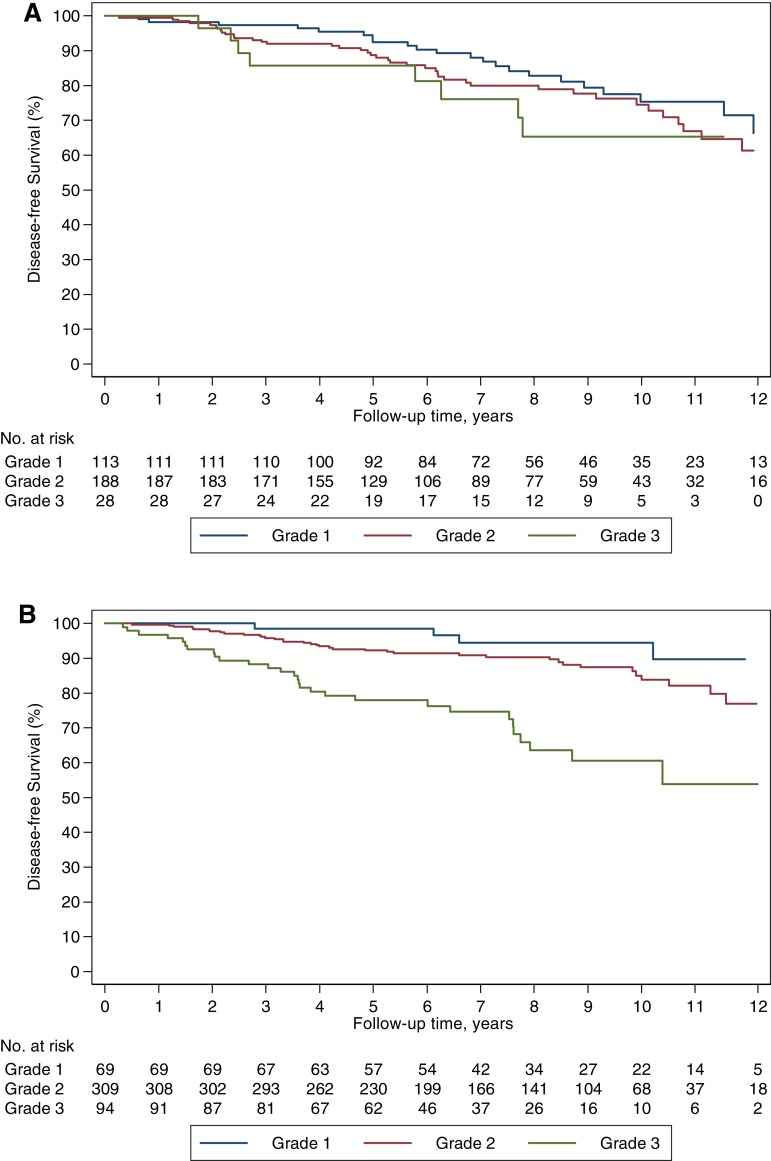
To explore the prognostic role of histologic grade, we performed an interaction analysis between histology and grade, which was statistically significant (Table 2). Although histologic grade was unable to discriminate the prognosis of patients with ILC, it was an effective tool to discriminate the prognosis of those with IDC‐L (Fig. 4; supplemental online Fig. 3).
Figure 4.
Disease‐free survival based on tumor grade. Disease‐free survival in ILC (A) and IDC‐L (B).
We further explored the relative effectiveness of tamoxifen versus aromatase inhibitor (AI) among postmenopausal patients with hormone receptor‐positive tumors. Patients treated with an AI (as monotherapy or sequentially with tamoxifen) had more favorable outcomes than those treated with tamoxifen only, both in terms of DFS (HR, 0.36; 95% CI, 0.21–0.61; p < .001) and OS (HR, 0.50; 95% CI, 0.29–0.87; p = .015; supplemental online Table 1). The magnitude of benefit was similar for both histologic types (p = .212). Similar results for both analyses were obtained after the introduction of a landmark analysis including only patients free of recurrence at 24 months (which would be a reasonable date of endocrine therapy switch from tamoxifen to an AI in clinical practice).
Disease Recurrence
A total of 91 patients had a disease recurrence: 44 (48.35%) patients with ILC and 47 (51.65%) patients with IDC‐L (p = .163; supplemental online Table 2). When considering the specific site of disease recurrence, bone was the most frequent site in both histologic types (14 [37.84%] vs. 17 cases [53.12%] for ILC and IDC‐L, respectively). Nevertheless, intra‐abdominal recurrences (excluding liver) were only identified in ILC (7 [18.9%]).
Finally, using a multivariate analysis model (controlling for the same variables detailed in Table 2), we found no significant differences according to histologic type for other outcomes, namely locoregional recurrence (HR, 0.97; 95% CI, 0.36–2.60; p = .944), distant recurrence (HR, 0.69; 95% CI, 0.41–1.18; p = .174), bone recurrence (HR, 0.80; 95% CI, 0.35–1.80; p = .584), and second breast cancers (HR, 1.81; 95% CI, 0.65–5.05; p = .258).
Discussion
In this retrospective analysis, we took advantage of a clinical database including 811 patients to compare clinicopathologic features, management, and survival outcomes between IDC‐L and ILC. Patients with ILC were older, had more multifocal disease, larger tumors, more positive nodes, and more HER2‐negative tumors, and received less frequent adjuvant chemotherapy than patients with IDC‐L. When compared with ILC, IDC‐L had superior survival outcomes, particularly for women in the postmenopausal setting. Histologic grade was an important prognostic factor for IDC‐L but not for ILC. These observations resemble differences between hormone receptor‐positive IDC and ILC [12], [17], [18], [19].
Previous retrospective studies have failed to identify meaningful differences in survival outcomes in patients with ILC compared with patients with IDC‐L [13], [14], [15], [16], [20], [21]. By contrast, in a retrospective series including 140 patients with IDC‐L, Rakha et al. reported worse outcomes for patients with IDC‐L than for those with ILC (n = 380) [12]. The interpretation of previous results is impaired by cohort size, limited multivariate adjustment, or short follow‐up. In this study, the overall results suggested similar survival outcomes between patients with ILC and IDC‐L, but when stratifying by menopausal status, we noticed superior survival outcomes for patients with IDC‐L. These observations were corroborated by a large analysis of the Surveillance, Epidemiology, and End Results (SEER) database, including a total of 209,109 patients [22]. In the SEER analysis, Xiao et al. compared survival outcomes based on histology, including 172,379 patients with IDC, 17,503 patients with ILC, and 19,227 patients with IDC‐L. The survival analysis performed pointed to better breast cancer‐specific survival for patients with IDC‐L than with IDC and ILC. The evaluation of HR over time using scaled Schoenfeld residual plots revealed interesting findings; the HR of IDC‐L versus IDC increased over time, indicating a continuous long‐term risk of relapse, which could be attributed to the lobular component of mixed tumors. By contrast, the HR for the comparison of IDC‐L versus ILC decreased over time, indicating better long‐term prognosis for IDC‐L versus ILC. When evaluating the differences in outcomes between IDC‐L and ILC, patients aged >50 years diagnosed with IDC‐L had superior outcomes [22]. Although the larger sample size from the SEER analysis provided robust prognostic information, the lack of detailed clinicopathologic information (e.g. HER2 status) and treatment information is an important limitation.
Our results complement the findings from the SEER analysis, given that we were able to interpret survival outcomes correcting for important clinicopathologic variables (e.g., adjuvant systemic therapy). Taken together, available data suggests that patients diagnosed with IDC‐L have a better survival outcome when compared with patients with ILC, which is probably explained by the continuous long‐term risk of relapse associated with ILC. Furthermore, patients with IDC‐L generally did not develop intra‐abdominal relapses that characterize ILC. The Cancer Genome Atlas (TCGA) research network recently published results of genomic characterization of 490 IDC, 127 ILC, 88 IDC‐L, and 112 other breast cancer cases [23]. As expected, ILC‐like tumors were enriched for luminal A subtype, CDH1 mutations, and loss of e‐cadherin by mRNA expression. Among the 88 cases of IDC‐L, there did not appear to be a distinct genomic profile; rather, the IDC‐L cases segregated into IDC‐like (n = 64) or ILC‐like (n = 24) tumors. The overrepresentation of molecular IDC‐like tumors in the clinical IDC‐L cases in the TCGA is consistent with our findings—IDC‐L (as assessed by pathological evaluation) diverged from ILC in histologic grade, frequency of HER2 status, and survival outcomes, among other differences, which would be expected if most clinical IDC‐Ls are molecular IDC‐like. Further research is needed to investigate whether there is any clinical utility of molecularly classifying IDC‐L for the purpose of prognostic evaluation and/or treatment planning.
In our cohort, histologic grade was prognostic for IDC‐L but not for ILC. In a previous retrospective series pooling outcomes from 707 classic ILCs, 102 special subtypes of ILC, and 44 mixed tumors, Talman et al. found a significant difference in OS and DFS between grade II and III tumors but not between grade I and II tumors [24]. In addition, a subsequent study including 517 patients with ILC [25] reported a significant prognostic value for histologic grade. However, approximately one third of cases in the series were special subtypes of ILC often characterized by tubule formation, and when tubule formation was removed from the analysis, the remaining histologic grade variables (i.e., mitotic count and nuclear pleomorphism) were no longer associated with outcome. Collectively, our findings and those of others suggest that the current grading system may be limited for ILC but useful for IDC‐L.
In an exploratory analysis from our cohort, postmenopausal patients receiving adjuvant AI, either as monotherapy or sequentially after tamoxifen, had better outcomes when compared with patients treated with tamoxifen monotherapy independently of the histologic subtype. These results are in agreement with the updated AI overview meta‐analysis [26]. Of interest, two retrospective studies have compared the effectiveness of AI versus tamoxifen among patients diagnosed with ILC or IDC: the BIG1‐98 study and the ABCSG‐8 study [10], [11]. In the BIG1‐98 study, patients with ILC derived a greater benefit with letrozole when compared with tamoxifen [10], and in the ABCSG‐8 study, patients diagnosed with ILC had better survival outcomes when treated with a sequential regimen (tamoxifen‐AI) than with tamoxifen monotherapy [11] Our current study is limited in its ability to determine a difference between AI and tamoxifen for patients diagnosed with ILC and IDC‐L, and further investigation into this topic is needed.
We acknowledge a number of limitations to study. Despite the methodological rigor, as a retrospective observational study, it is amenable to residual confounding. Although pathologic review was, in most cases, performed by an academic pathologist at Brigham and Women's Hospital, central pathology review and additional immunohistochemical studies, such as e‐cadherin/p120 [27] to further characterize these tumors, were not performed. Tumor classifications were taken from the diagnostic pathology reports and likely reflect individual pathologist preferences as well as changing tumor classification practices over the period of this study. Finally, the relative effectiveness of tamoxifen versus AI results is based on observational data and not a randomized trial.
Conclusion
In this study, we report several important findings: (a) patients diagnosed with IDC‐L have a better prognosis than patients with ILC, particularly for postmenopausal women; (b) histologic grade is an imperfect tool for patients with ILC but provides relevant information for patients with IDC‐L; and (c) consistent with data from phase III studies, where AIs have shown a DFS advantage over tamoxifen that appeared greatest in the ILC subset, these improvements also held true for patients with IDC‐L. Taken together, our work adds to the literature pointing to significant differences in survival outcomes for patients with IDC‐L when compared with patients with ILC. Patients with IDC‐L have more favorable outcomes, particularly for those in the postmenopausal setting; the unfavorable outcomes associated with ILC are likely to be explained by its continuous pattern of relapse beyond year 5.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by the Susan G. Komen Foundation for the Cure (Grant PDF14302599), Fundação para a Ciência e a Tecnologia (Grant HMSP‐ICJ/0007/2013 under the Harvard Medical School Portugal program), and the Maor Foundation. The funding sources did not interfere in any step of the study.
Contributed equally.
Author Contributions
Conception/design: Otto Metzger‐Filho, Arlindo R. Ferreira
Provision of study material or patients: Otto Metzger‐Filho, Arlindo R. Ferreira
Collection and/or assembly of data: Otto Metzger‐Filho, Arlindo R. Ferreira
Data analysis and interpretation: Otto Metzger‐Filho, Arlindo R. Ferreira, Rinath Jeselsohn, William T. Barry, Deborah A. Dillon, Jane E. Brock, Ines Vaz‐Luis, Melissa E. Hughes, Eric P. Winer, Nancy U. Lin
Manuscript writing: Otto Metzger‐Filho, Arlindo R. Ferreira, Rinath Jeselsohn, William T. Barry, Deborah A. Dillon, Jane E. Brock, Ines Vaz‐Luis, Melissa E. Hughes, Eric P. Winer, Nancy U. Lin
Final approval of manuscript: Otto Metzger‐Filho, Arlindo R. Ferreira, Rinath Jeselsohn, William T. Barry, Deborah A. Dillon, Jane E. Brock, Ines Vaz‐Luis, Melissa E. Hughes, Eric P. Winer, Nancy U. Lin
Disclosures
William T. Barry: Pfizer (RF); Deborah A. Dillon: Oncology Analytics, Inc. (C/A, SAB). Eric P. Winer: Genentech, Leap, Glaxo‐Smith Kline, Eli Lilly & Co., Infinite MD, Carrick Therapeutics (H), Verastem (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Sinn HP, Kreipe H. A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel) 2013;8:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer 2005;93:1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li CI, Andreson BO, Daling JR et al. Trends in Incidence Rates of Invasive Lobular and Ductal Breast Carcinoma. JAMA 2003;289:1421–1424. [DOI] [PubMed] [Google Scholar]
- 4.Li CI, Anderson BO, Porter P et al. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer 2000;88:2561–2569. [PubMed] [Google Scholar]
- 5.Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol 2010;27:49–61. [DOI] [PubMed] [Google Scholar]
- 6.McCart Reed AE, Kutasovic JR, Lakhani SR et al. Invasive lobular carcinoma of the breast: Morphology, biomarkers and ’omics. Breast Cancer Res 2015;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desmedt C, Zoppoli G, Gundem G et al. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol 2016;34:1872–1881. [DOI] [PubMed] [Google Scholar]
- 8.Katz A, Saad ED, Porter P et al. Primary systemic chemotherapy of invasive lobular carcinoma of the breast. Lancet Oncol 2007;8:55–62. [DOI] [PubMed] [Google Scholar]
- 9.Truin W, Voogd AC, Vreugdenhil G et al. Effect of adjuvant chemotherapy in postmenopausal patients with invasive ductal versus lobular breast cancer. Ann Oncol 2012;23:2859–2865. [DOI] [PubMed] [Google Scholar]
- 10.Metzger Filho O, Giobbie‐Hurder A, Mallon E et al. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1‐98 trial. J Clin Oncol 2015;33:2772–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knauer M, Gruber C, Dietze O et al. Survival advantage of anastrozol compared to tamoxifen for lobular breast cancer in the ABCSG‐8 study. Cancer Res 2015;75(suppl 9):S2‐06a. [Google Scholar]
- 12.Rakha EA, Gill MS, El‐Sayed ME et al. The biological and clinical characteristics of breast carcinoma with mixed ductal and lobular morphology. Breast Cancer Res Treat 2009;114:243–250. [DOI] [PubMed] [Google Scholar]
- 13.Bharat A, Gao F, Margenthaler JA. Tumor characteristics and patient outcomes are similar between invasive lobular and mixed invasive ductal/lobular breast cancers but differ from pure invasive ductal breast cancers. Am J Surg 2009;198:516–519. [DOI] [PubMed] [Google Scholar]
- 14.Suryadevara A, Paruchuri LP, Banisaeed N et al. The clinical behavior of mixed ductal/lobular carcinoma of the breast: A clinicopathologic analysis. World J Surg Oncol 2010;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zengel B, Yararbas U, Duran A et al. Comparison of the clinicopathological features of invasive ductal, invasive lobular, and mixed (invasive ductal + invasive lobular) carcinoma of the breast. Breast Cancer 2015;22:374–381. [DOI] [PubMed] [Google Scholar]
- 16.Arps DP, Healy P, Zhao L et al. Invasive ductal carcinoma with lobular features: A comparison study to invasive ductal and invasive lobular carcinomas of the breast. Breast Cancer Res Treat 2013;138:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pestalozzi BC, Zahrieh D, Mallon E et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: Combined results of 15 international breast cancer study group clinical trials. J Clin Oncol 2008;26:3006–3014. [DOI] [PubMed] [Google Scholar]
- 18.Wasif N, Maggard MA, Ko CY et al. Invasive lobular vs. ductal breast cancer: A stage‐matched comparison of outcomes. Ann Surg Oncol 2010;17:1862–1869. [DOI] [PubMed] [Google Scholar]
- 19.Cristofanilli M, Gonzalez‐Angulo A, Sneige N et al. Invasive lobular carcinoma classic type: Response to primary chemotherapy and survival outcomes. J Clin Oncol 2005;23:41–48. [DOI] [PubMed] [Google Scholar]
- 20.Sastre‐Garau X, Jouve M, Asselain B et al. Infiltrating lobular carcinoma of the breast: Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer 1996;77:113–120. [DOI] [PubMed] [Google Scholar]
- 21.Colleoni M, Rotmensz N, Maisonneuve P et al. Outcome of special types of luminal breast cancer. Ann Oncol 2012;23:1428–1436. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Y, Ma D, Ruan M et al. Mixed invasive ductal and lobular carcinoma has distinct clinical features and predicts worse prognosis when stratified by estrogen receptor status. Sci Rep 2017;7:10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciriello G, Gatza ML, Beck AH et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 2015;163:506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talman ML, Jensen MB, Rank F. Invasive lobular breast cancer. Prognostic significance of histological malignancy grading. Acta Oncol 2007;46:803–809. [DOI] [PubMed] [Google Scholar]
- 25.Rakha EA, El‐Sayed ME, Menon S et al. Histologic grading is an independent prognostic factor in invasive lobular carcinoma of the breast. Breast Cancer Res Treat 2008;111:121–127. [DOI] [PubMed] [Google Scholar]
- 26.Early Breast Cancer Trialists, Collaborative Group . Aromatase inhibitors versus tamoxifen in early breast cancer: Patient‐level meta‐analysis of the randomised trials. Lancet 2015;386:1341–1352. [DOI] [PubMed] [Google Scholar]
- 27.Dabbs DJ, Bhargava R, Chivukula M. Lobular versus ductal breast neoplasms: The diagnostic utility of P120 catenin. Am J Surg Pathol 2007;31:427–437. [DOI] [PubMed] [Google Scholar]