Intrahepatic cholangiocarcinoma (ICC) patients who present fever as the initial symptom are rare, and the cases reported showed poor outcomes. This article compares the clinical features and prognoses of ICC patients presenting with and without fever as the first symptom and evaluates the efficiency of surgical treatment and prognostic factors influencing disease recurrence and survival.
Keywords: Intrahepatic cholangiocarcinoma, Fever, Prognosis, Hepatectomy
Abstract
Background.
Patients with intrahepatic cholangiocarcinoma (ICC) rarely present fever as the initial symptom. We aimed to identify clinical characteristics and prognostic factors for these feverish patients.
Subjects, Materials, and Methods.
This study retrospectively reviewed 31 patients with ICC with fever (≥38.0°C) treated at our hospital between January 2002 and December 2014. A propensity score was used to match patients with and without fever at a ratio of 1:2.
Results.
Patients with ICC with fever had higher serum γ‐glutamyl transferase and carcinoembryonic antigen levels, larger tumors, poorer tumor differentiation, and worse prognosis (all p < .05) than those without fever. This was supported by propensity score matching (PSM) analysis. Univariate and multivariate analyses indicated that microvascular invasion, hilar lymph node metastasis, and temperature ≥ 38.6°C were related to prognosis. Patients with ICC with fever had higher levels of leucocytes, neutrophils, neutrophil‐to‐lymphocyte ratio (NLR), and platelet‐to‐lymphocyte ratio (PLR) in peripheral blood before and after PSM analysis. Body temperature positively correlated with leucocytes (r = 0.599, p < .001), neutrophils (r = 0.644, p < .001), NLR (r = 0.681, p < .001), and PLR (r = 0.457, p = .010).
Conclusion.
Patients with ICC with fever ≥38.0°C and ≥38.6°C had poor and extremely poor prognosis, respectively. Radical surgical treatment may improve the prognosis of patients with ICC with fever <38.6°C. However, systemic therapy (e.g., anti‐inflammatory and immune therapy) may be preferable to surgery for these patients with fever ≥38.6°C.
Implications for Practice.
Patients with intrahepatic cholangiocarcinoma (ICC) with fever (≥38.0°C) as the initial symptom are extremely rare. Because their symptoms are similar to those of liver abscess, diagnosis is challenging, and most of these patients are already at an advanced stage at the time of diagnosis. Patients with ICC with fever had different clinical characteristics and worse prognosis than those without fever. The prognosis of those with temperature <38.6°C would be improved by timely surgical intervention. Those with fever ≥38.6°C had an extremely dismal outcome, although they all received radical surgical treatment. New therapeutic strategies are needed to improve survival for patients with ICC with temperature ≥38.6°C.
摘要
背景。肝内胆管癌 (ICC) 患者很少将发热作为首诊症状。我们的目的是确定这些发热患者的临床特征和预后因素。
受试者、材料和方法。本研究回顾性地分析了 2002 年 1 月至 2014 年 12 月期间在我院接受治疗的 31 名伴发热 (≥38.0°C) ICC患者。我们采用倾向评分以 1:2 的比例匹配伴发热与未伴发热的患者。
结果。与未伴发热的ICC患者相比,伴发热ICC患者的血清 γ‐谷氨酰转移酶和癌胚抗原水平较高,肿瘤较大,肿瘤分化较差,预后较差(全部的 p < 0.05)。我们通过倾向评分匹配 (PSM) 分析证实了这一点。单变量和多变量分析表明,微血管浸润、肝门淋巴结转移和体温 ≥38.6°C 与预后有关。伴发热ICC患者在PSM分析之前和之后的白细胞水平、中性粒细胞、中性粒细胞与淋巴细胞的比率 (NLR) 以及外周血中血小板与淋巴细胞的比率 (PLR) 均较高。体温与白细胞(r = 0.599,p < 0.001)、中性粒细胞(r = 0.644,p < 0.001)、NLR(r = 0.681,p < 0.001)以及PLR(r = 0.457,p = 0.010)呈正相关。
结论。体温 ≥38.0°C 和 ≥38.6°C 的伴发热ICC患者的预后效果分别为差和极差。根治性手术治疗可改善体温 <38.6°C 的伴发热ICC患者的预后。但是,对于体温 ≥38.6°C 的伴发热ICC患者,全身治疗(例如抗炎和免疫治疗)可能优于手术治疗。
实践意义:以发热 (≥38.0°C) 为首诊症状的肝内胆管癌(ICC)患者极为少见。由于其症状与肝脓肿相似,给诊断带来了很大的挑战,并且大多数患者在诊断时已处于晚期。伴发热ICC患者的临床特征与未伴发热ICC患者不同,并且预后较差。通过及时的手术干预可以改善体温 <38.6°C 的患者的预后。体温 ≥38.6°C 的发热患者虽然接受了根治性手术治疗,但预后极差。需要新的治疗策略来改善体温 ≥38.6°C 的ICC患者的生存期。
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy after hepatocellular carcinoma, and its incidence is increasing worldwide [1], [2]. Early detection of ICC enables curative resection, although the long‐term survival of patients with ICC remains unsatisfactory because of high recurrence rates and early metastasis after surgery [3], [4]. Patients with ICC who present fever as the initial symptom are extremely rare, and the few case reports have extremely poor outcomes (mean 3.5 months) [5], [6]. Patients with ICC with fever present clinical symptoms and imaging features similar to those with nonresolving liver abscess, so they are easily misdiagnosed until the tumor reaches an advanced stage [7]. Most patients are diagnosed after the best time for treatment has passed. Therefore, it is necessary to clarify the clinical features, outcomes, and prognostic factors of patients with ICC with fever.
Because they are rare in clinical practice, it is challenging to conduct a prospective study of patients with ICC with fever to explore their clinical characteristics and prognosis. Propensity score matching (PSM) analysis can overcome selection bias, balance differences in clinical features among groups, and increase the evidence level of a retrospective observational study [8], [9]. PSM analysis provides a useful method to explore the prognosis of these feverish patients with ICC, who share similar clinical features as those without fever.
In this study, we retrospectively analyzed 331 patients with ICC who underwent resection at our institute over a 13‐year period, including a group of 31 consecutive patients with ICC presenting fever as the first symptom and a group of 300 patients with ICC without fever as control. We compared the clinical features and prognosis of these two groups after surgery. Then, we used PSM analysis to investigate prognostic differences between the two groups. The efficiency of surgical treatment of patients with ICC with fever and prognostic factors influencing disease recurrence and survival were evaluated.
Subjects, Materials, and Methods
Patients
From January 2002 to December 2014, 31 patients with ICC presenting fever (≥38.0°C) as the first symptom underwent curative hepatic resection at the Department of Surgery, Zhongshan Hospital, Fudan University, Shanghai, China. The enrollment criteria for all patients in this study were as follows: patients with ICC with fever were selected as follows: (a) temperature ≥38.0°C; (b) less chills before fever; (c) blood and sputum smears and urine cultures were negative; (d) lack of response of fever to antibiotic therapy for at least 7 days; (e) absence of allergic mechanisms; (f) no cancer‐related treatments before surgery; (g) availability of complete clinicopathological data. The exclusion criteria were extrahepatic infection, septicemia, hepatolithiasis, biliary stricture, biliary infection, and febrile drug reaction. Histopathological diagnosis of ICC was determined based on the World Health Organization criteria. Tumor differentiation was defined according to the Edmondson grading system [10]. Liver function was assessed using the Child‐Pugh classification. Serum biochemistry data were collected at admission. Ethical approval was obtained from the Zhongshan Hospital Research Ethics Committee consistent with ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from each patient. To investigate differences in clinical outcomes for patients with ICC with or without fever, 300 patients with ICC without fever and with complete clinical and laboratory data within the same observational period were randomly selected and matched for age, gender, and year of diagnosis. All patients underwent tumor resection in this study. Tumor resection was defined as complete removal of tumor and dissection of the hilar and hepatoduodenal ligament lymph nodes, without macroscopic tumor thrombus. In general, resectable intrahepatic tumors were treated with hepatectomy with 1–2 cm of surrounding liver tissue [11].
Follow‐Up and Recurrence Treatment
Survival data, including overall survival (OS) and time to recurrence (TTR), were collected until December 30, 2016. Data were censored at the last follow‐up for surviving patients. OS and TTR were defined as the intervals between the date of surgery and death (or the last observation point taken) or any diagnosed relapse (intrahepatic recurrence and extrahepatic metastasis), respectively [12].
Follow‐up procedures were described previously [13]. Patients were followed up every 2–4 months as appropriate, and tests included serum carcinoembryonic antigen (CEA), CA19‐9, liver ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and bone scan. If ICC recurrence was confirmed, a second hepatectomy, radiofrequency ablation, percutaneous ethanol injection, transcatheter arterial chemoembolization, external radiotherapy, or symptomatic treatment was given according to the numbers, sizes, and sites of recurrent tumors.
Propensity Score Matching Analysis
To investigate the associations between different groups and long‐term outcomes in an observational, nonrandomized study, we conducted PSM analysis to overcome possible bias in selecting patients. The propensity score for an individual was calculated using a logistic regression model in which the dependent variable was fever or not, and the covariates were the basic patient characteristics (age, sex), and other important prognostic factors for patients with ICC (including γ‐glutamyl transferase [GGT], CEA, CA19‐9, tumor size, tumor encapsulation, microvascular invasion, tumor differentiation, number of tumors and hilar lymph node metastasis, and tumor node metastasis (TNM) stage) [1], [14]. A one‐to‐two matching requirement was applied via the nearest‐neighbor matching algorithm without replacement to select matched pairs of patients.
Histopathology and Immunohistochemistry
Paraffin‐embedded tissue blocks of 31 patients with ICC with fever were obtained from the Pathology Department, Zhongshan Hospital. The samples were stained with hematoxylin and eosin (H&E) and immunostained for tumor markers as described previously [15]. Cytokeratin 7 (CK7), cytokeratin 19 (CK19), hepatocyte paraffin 1 (HepPar‐1), and Ki‐67 were considered positive if moderate or intense staining was observed in ≥5%, 5%, 10%, and 20% of the tumor cells, respectively [16], [17], [18].
Statistical Analysis
SPSS 18.0 (IBM, Armonk, NY) and R (version 3.2.1) software programs were used for data analysis. OS and cumulative recurrence rate (CRR) were analyzed using Kaplan‐Meier survival curves and a log‐rank test. A chi‐square test, Fisher's exact test, and Student's t test were used for comparisons between groups as appropriate. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) was calculated as follows: NLR = absolute neutrophil count/absolute lymphocyte count [19]; PLR = absolute platelet count/absolute lymphocyte count [20]. Cox proportional hazard regression model was used to determine prognostic factors. Two‐tailed p values <.05 were considered statistically significant.
Results
Baseline Characteristics of Patients with ICC Presenting with Fever
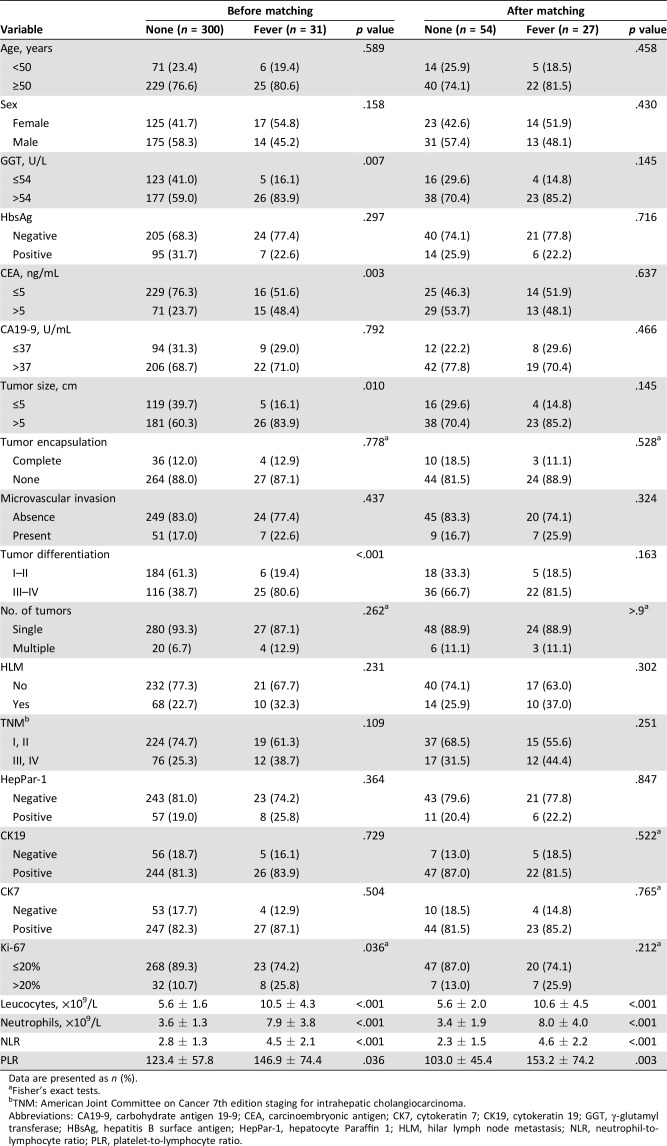
Thirty‐one patients with fever diagnosed with ICC were consecutively enrolled in this study, including 14 men and 17 women. Compared with those without fever (n = 300), patients with ICC with fever (n = 31) had higher serum GGT levels (26/31, 83.9% vs. 177/300, 59.0%; p = .007), higher serum CEA levels (15/31, 48.4% vs. 71/300, 23.7%; p = .003), larger tumor size (>5 cm, 26/31, 83.9% vs. 181/300, 60.3%; p = .010), and poorer tumor differentiation (25/31, 80.6% vs. 116/300, 38.7%; p < .001; Table 1).
Table 1. Baseline characteristics of patients with intrahepatic cholangiocarcinoma with/without fever before and after propensity score matching.
Data are presented as n (%).
Fisher's exact tests.
TNM: American Joint Committee on Cancer 7th edition staging for intrahepatic cholangiocarcinoma.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CK7, cytokeratin 7; CK19, cytokeratin 19; GGT, γ‐glutamyl transferase; HBsAg, hepatitis B surface antigen; HepPar‐1, hepatocyte Paraffin 1; HLM, hilar lymph node metastasis; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio.
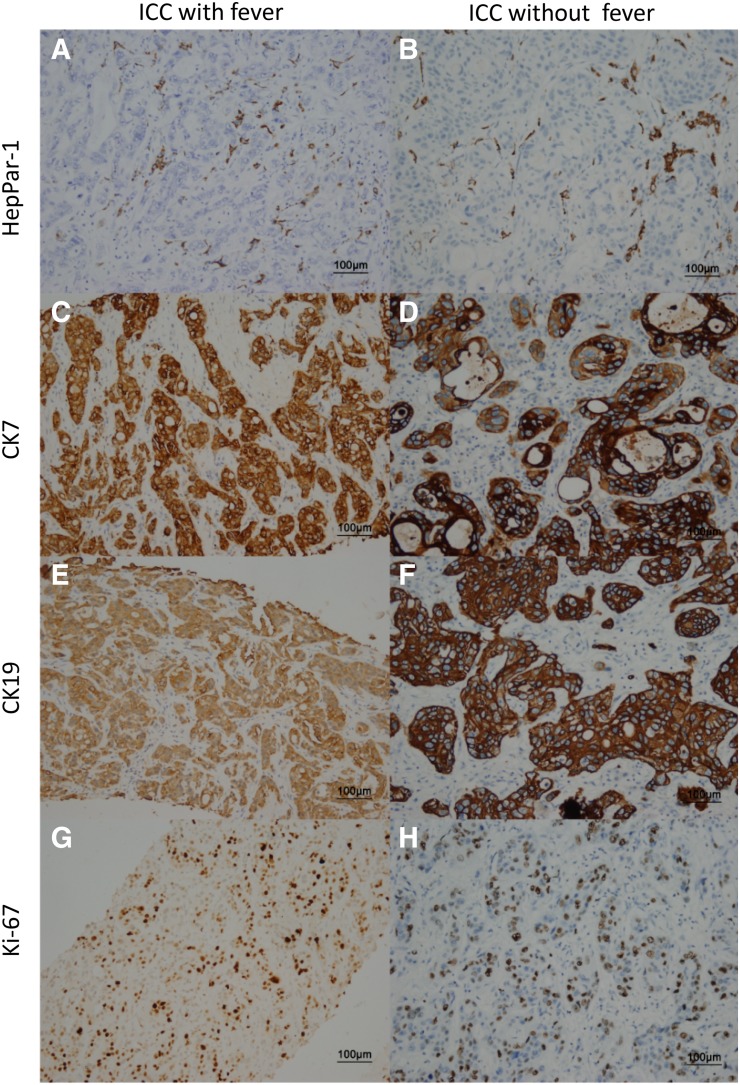
Four immunohistochemical markers were evaluated for patients with ICC with fever, including HepPar‐1, Ki‐67, CK7, and CK19. Positive expression of HepPar‐1, CK19, and CK7 in ICC with fever were 25.8%, 83.9%, and 87.1%, respectively, similar to ICC without fever (19.0%, 81.3%, and 82.3%, respectively; p > .05). However, the percentage of Ki‐67‐positive (>20%) was higher in specimens from patients with ICC with fever than in ICC without fever (8/31, 25.8% vs. 32/300, 10.7%; p = .036; Fig. 1; Table 1).
Figure 1.
Immunohistochemistry of liver tumor marker. Expression levels of four tumor markers are shown in samples of ICC liver tissues with and without fever by immunohistochemistry. Staining of HepPar‐1 (A, B), CK7 (C, D), CK19 (E, F), and Ki‐67 (G, H).
Abbreviations: CK7, cytokeratin 7; CK19, cytokeratin 19; HepPar‐1, hepatocyte paraffin 1; ICC, intrahepatic cholangiocarcinoma.
Median body temperature of patients with ICC with fever was 38.6°C (range 38.0°C–40.0°C), which we used as a cutoff for further analysis. These patients always presented with intermittent fever. Median days of the delay between the appearance of fever and surgery was 28 days (range 7–91 days). These patients did not experience chills before fever. Blood and sputum smears and urine cultures were always negative. All patients with fever received surgical resection, and fever always disappeared after surgical resection.
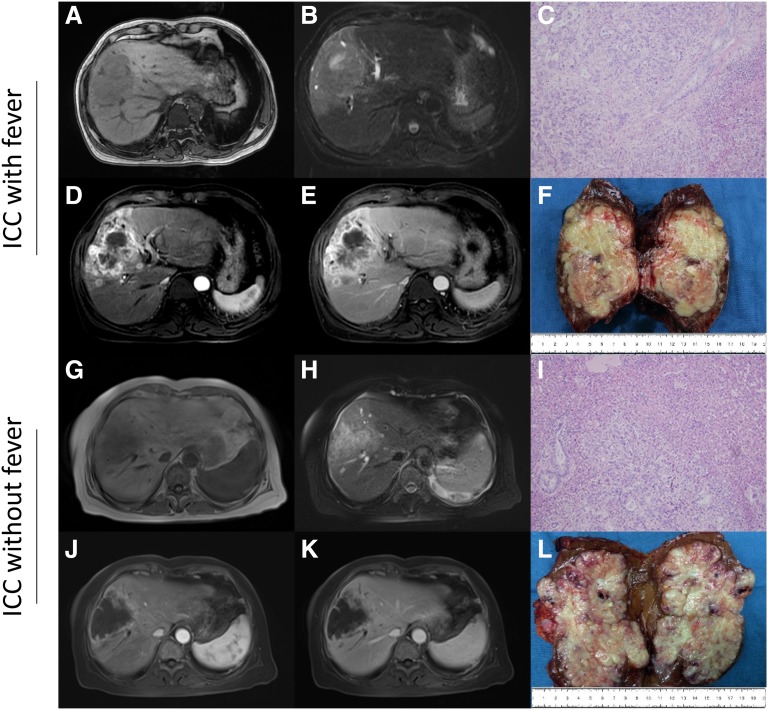
Many patients with cancer can be diagnosed by contrast CT or MRI, which can shorten the time to diagnosis. Abdominal ultrasonography and normal CT can detect liver lobe hypodense lesion with irregular shaggy margins, which appears similar to liver abscess. ICC is differentiated from liver abscess in patients with fever by performing interventional imaging such as contrast CT or MRI. Most patients with ICC with fever display irregular rim‐like hyperenhancement in the tumor periphery, which differentiates it from liver abscess. We present contrast MRI images of typical cases that displayed right lobe hypodense multiseptate lesion (8 × 9 cm) with irregular rim‐like hyperenhancement consistent with ICC without fever (Fig. 2G, 2H, 2J, 2K) and ICC with fever (Fig. 2A, 2B, 2D, 2E). No obvious abscess lesion was observed in the resected tumor specimen from patients with ICC with fever. Resected tumor specimens from ICC with and without fever had similar histological characteristics after H&E staining (Fig. 2C, 2F, 2I, 2L, respectively).
Figure 2.
Magnetic resonance imaging and histopathology. A 55‐year‐old male with typical ICC with fever in the right lobe of the liver (A–F), and a 63‐year‐old male with typical ICC without fever in the right lobe of the liver (G–L). T1‐weighted image (A, G), T2‐weighted image (B, H), arterial‐phase dynamic image (D, J), venous‐phase dynamic image (E, K), tumor specimen (F, L), and hematoxylin and eosin staining (C, I) are shown.
Abbreviation: ICC, intrahepatic cholangiocarcinoma.
Clinical Outcomes of Patients with ICC Presenting with Fever After Surgery Before PSM Analysis
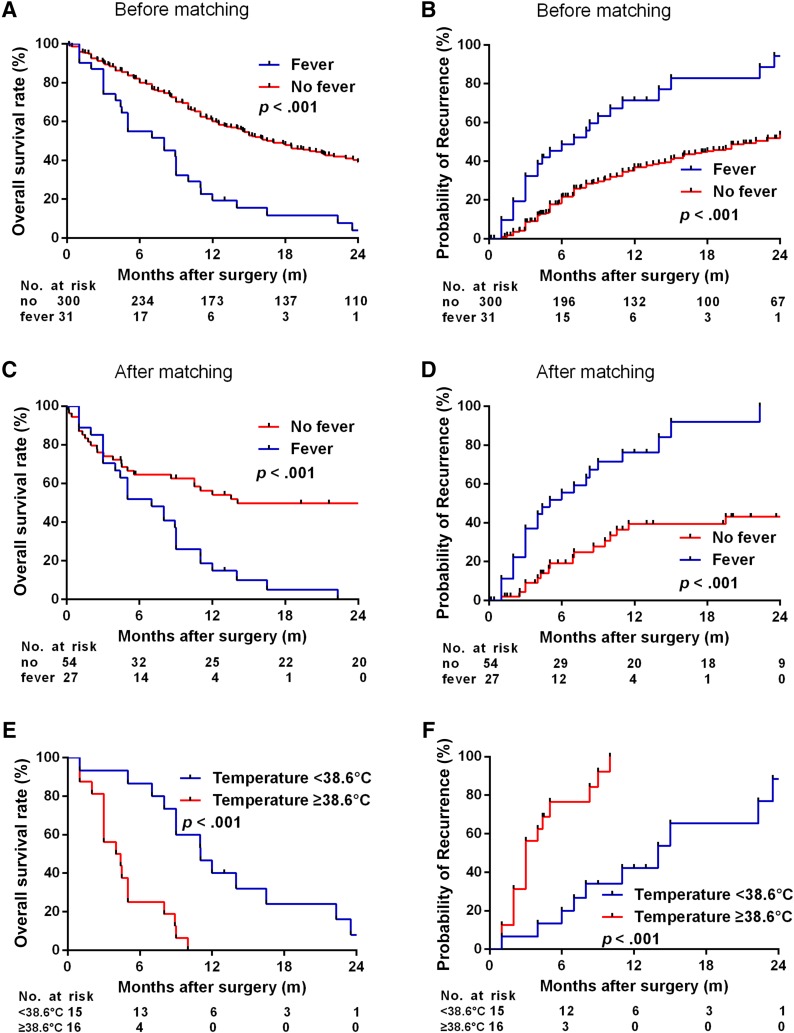
In this study, median follow‐up was 45 months. At the last follow‐up, 80.6% (25/31) of patients with ICC with fever had recurrence and 93.5% (29/31) had died. Most of these patients (25/31, 80.6%) died within 1 year after surgery, and 21 of 25 cases died from tumor recurrence. The remaining 6 of 31 patients survived more than 1 year; of these, 4 died from recurrence and only 1 patient survived more than 2 years after surgery without tumor recurrence at the last follow‐up. The 1‐, 2‐, 3‐, and 5‐year CRRs in patients with ICC with and without fever were 71.4%, 94.3%, 94.3%, and 94.3% compared with 37.0%, 53.8%, 60.6%, and 71.8%, respectively (p < .001). The 1‐, 2‐, 3‐, and 5‐year OS rates in patients with ICC with and without fever were 19.4%, 3.9%, 3.9%, and 3.9% compared with 61.1%, 40.1%, 30.3%, and 19.4%, respectively (p < .001). Kaplan‐Meier analysis indicated that CRR and OS were significantly poorer for patients with ICC with fever than without fever (median TTR, 6.0 vs. 10.3 months; median OS, 8.0 vs. 15.4 months; all p < .001; Fig. 3A, 3B).
Figure 3.
Prognosis of patients with intrahepatic cholangiocarcinoma (ICC) with and without fever. Kaplan‐Meier analysis of overall survival (OS) rates and time to recurrence (TTR) between patients with ICC with and without fever before propensity score matching (PSM) analysis (A, B) and after PSM analysis (C, D). Kaplan‐Meier analysis of OS and TTR in patients with ICC with fever according to body temperature (E, F).
Univariate and multivariate Cox regression analyses of TTR and OS after curative resection of the entire cohort of patients with ICC with and without fever are presented in supplemental online Table 1. Multivariate Cox regression analysis of the entire cohort showed that fever was an independent risk factor associated with both TTR (hazard ratio [HR], 2.4; 95% confidence interval [CI], 1.5–3.8; p < .001) and OS (HR, 2.0; 95% CI, 1.3–3.1; p < .001).
These feverish patients had higher leucocytes (10.5 ± 4.3 × 109/L vs. 5.6 ± 1.6 × 109/L, p < .001), neutrophils (7.9 ± 3.8 × 109/L vs. 3.6 ± 1.3 × 109/L, p < .001), NLR (4.5 ± 2.1 vs. 2.8 ± 1.3; p < .001), and PLR (146.9 ± 74.4 vs. 123.4 ± 57.8; p = .036) than those without fever (Table 1).
Clinical Outcomes of Patients with ICC Presenting with Fever After Surgery After PSM Analysis
PSM analysis can overcome selection bias, balance differences in clinical features among groups, and increase the evidence level of a retrospective observational study. Therefore, we performed PSM analysis to investigate differences in the outcomes of patients with ICC with and without fever who had similar clinical features. PSM identified 81 patients according to a 2:1 ratio from each treatment group with similar characteristics, including 54 cases without fever and 27 cases with fever (all p > .05; Table 1). PSM revealed that patients with ICC with fever also had significantly shorter TTR and OS compared with those without fever (median 5.0 vs. 7.0 for TTR and 7.0 vs. 11.0 for OS; p < .05; Fig. 3C, 3D). The results of univariate and multivariate Cox regression analyses of TTR and OS after curative resection of ICC in the propensity‐matched cohort are presented in supplemental online Table 2. After univariable analysis, the significant variables with p < .05 were used in the multivariable analysis, which indicated that fever was an independent risk factor associated with TTR (HR, 3.2; 95% CI, 1.6–6.6; p = .001) and OS (HR, 1.9; 95% CI, 1.0‐3.4; p = .039) in the propensity‐matched cohort. The percentage of Ki‐67 index >20% was similar in ICC with and without fever in the propensity‐matched cohort.
Patients with fever also had higher leucocyte (10.6 ± 4.5 × 109/L vs. 5.6 ± 2.0 × 109/L; p < .001), neutrophil (8.0 ± 4.0 × 109/L vs. 3.4 ± 1.9 × 109/L; p < .001), NLR (4.6 ± 2.2 vs. 2.3 ± 1.5; p < .001), and PLR (153.2 ± 74.2 vs. 103.0 ± 45.4; p = .003) than those without fever in the propensity‐matched cohort (Table 1).
Prognostic Factors for Patients with ICC with Fever After Surgery
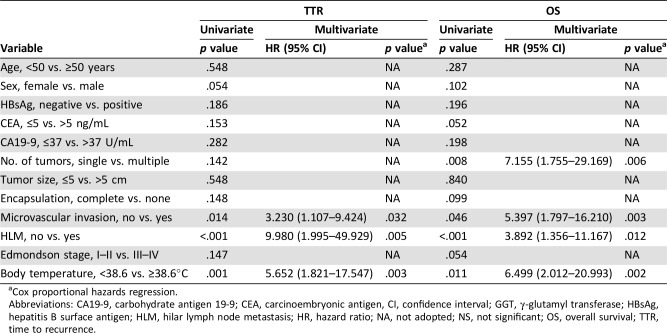
The prognostic factors of patients with ICC with fever were further identified. Univariate analysis showed that number of tumors, microvascular invasion, and hilar lymph node metastasis were prognostic factors for TTR and/or OS of patients with ICC with fever after resection. Multivariate analysis indicated that the independent factors for tumor recurrence were microvascular invasion and the presence of hilar lymph node metastasis; the factors for OS were number of tumors, microvascular invasion, and hilar lymph node metastasis (Table 2).
Table 2. Univariate and multivariate cox proportional regression analysis of factors associated with recurrence and OS in patients with intrahepatic cholangiocarcinoma with fever.
Cox proportional hazards regression.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen, CI, confidence interval; GGT, γ‐glutamyl transferase; HBsAg, hepatitis B surface antigen; HLM, hilar lymph node metastasis; HR, hazard ratio; NA, not adopted; NS, not significant; OS, overall survival; TTR, time to recurrence.
In addition to conventional prognostic factors, we found that body temperature ≥38.6°C was significantly related to TTR and OS in these feverish patients after surgery. Kaplan‐Meier analyses showed that patients with body temperature ≥38.6°C (n = 16) had higher CRR and shorter OS than those with body temperature <38.6°C (n = 15; all p < .001; Fig. 3E, 3F). The median TTR was 3.0 months for patients with temperature ≥38.6°C and 11.0 months for patients with temperature <38.6°C, whereas the median OS was 4.0 months for patients with temperature ≥38.6°C and 11.0 months for patients with temperature <38.6°C. Of 16 patients with body temperature ≥38.6°C, 12 patients (12/16, 75.0%) died within 6 months after surgery (11 patients died from liver recurrence and 1 patient died of unknown cause). The remaining 4 patients (4/16, 25.0%) died from liver recurrence within the subsequent 6 months. Of 15 patients with body temperature < 38.6°C, 2 patients (2/15, 13.3%) died from liver recurrence within 6 months after surgery. Seven patients (7/15, 46.7%) died within the subsequent 6 months, of whom four died from liver recurrence and three died of unknown cause. The remaining six patients (6/15, 40.0%) survived more than 1 year, of whom four died from liver recurrence, one was lost within follow‐up, and only one survived more than 2 years.
The 0.5‐, 1‐, 2‐, 3‐, and 5‐year CRR were 76.6%, 100.0%, 100.0%, 100.0%, and 100.0% in patients with temperature ≥38.6°C, and 20.0%, 42.2%, 88.4%, 88.4%, and 88.4% in patients with temperature <38.6°C. The 0.5‐, 1‐, 2‐, 3‐, and 5‐year OS rates were 25.0%, 0.0%, 0.0%, 0.0%, and 0.0% in patients with temperature ≥38.6°C, and 86.7%, 40.0%, 8.0%, 8.0%, and 8.0% in patients with temperature <38.6°C. Multivariate analysis indicated that body temperature ≥38.6°C was an independent prognostic factor for TTR and OS of patients with ICC with fever after surgery (for TTR, HR = 5.7 and p = .003; for OS, HR = 6.5 and p = .002; Table 2).
After PSM analyses, Kaplan‐Meier showed that patients with body temperature <38.6°C (n = 13) had higher CRR and shorter OS than matched patients without fever (n = 26, all p < .05; supplemental online Fig. 1A, 1B), and patients with body temperature ≥38.6°C (n = 14) also had higher CRR and shorter OS than matched patients without fever (n = 28, all p < .05; supplemental online Fig. 1C, 1D).
Demographics of patients with ICC with fever according to body temperature are presented in Table 3. For patients with ICC with fever, those with body temperature ≥38.6°C had worse tumor differentiation than those with temperature <38.6°C (9/15, 60.0% vs. 16/16, 100.0%; p = .007). Patients with body temperature ≥38.6°C had propensity with hilar lymph node metastasis (p = .054), another clinical index for dismal outcome. There were no significant differences in other tumor aspects.
Table 3. Baseline characteristics of body temperature.
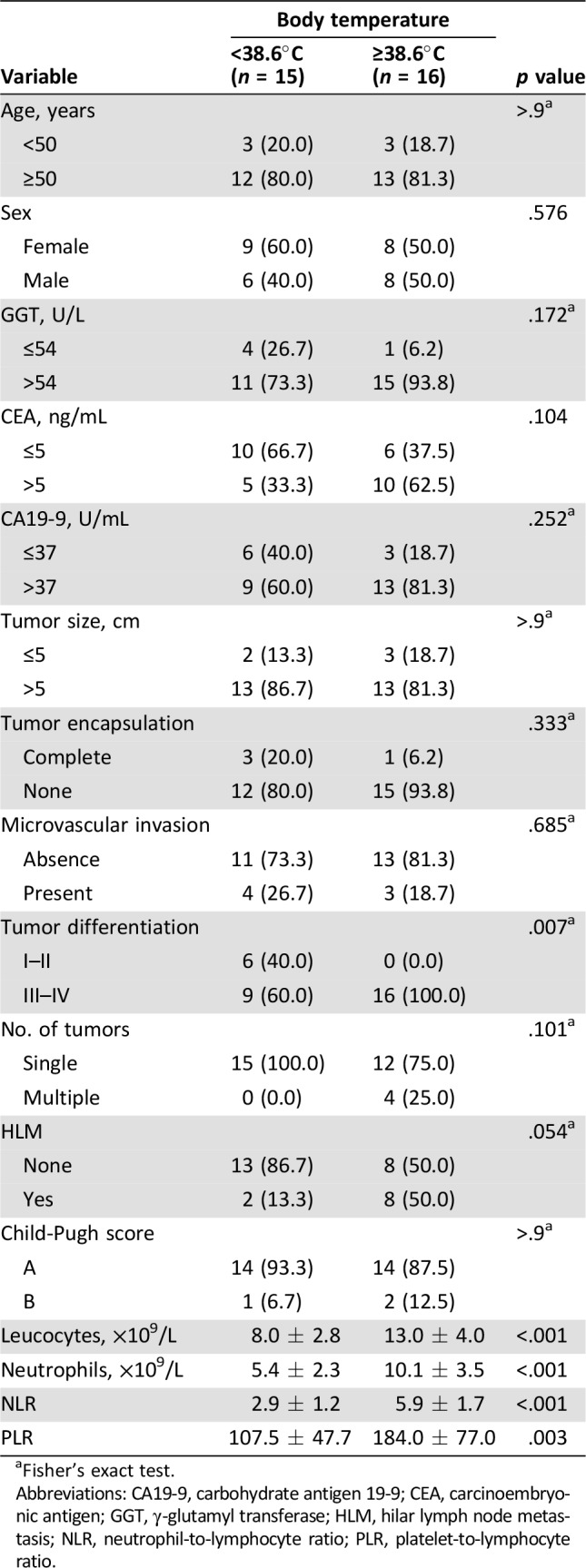
Fisher's exact test.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; GGT, γ‐glutamyl transferase; HLM, hilar lymph node metastasis; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio.
Relationship Between Fever and Systemic Inflammation
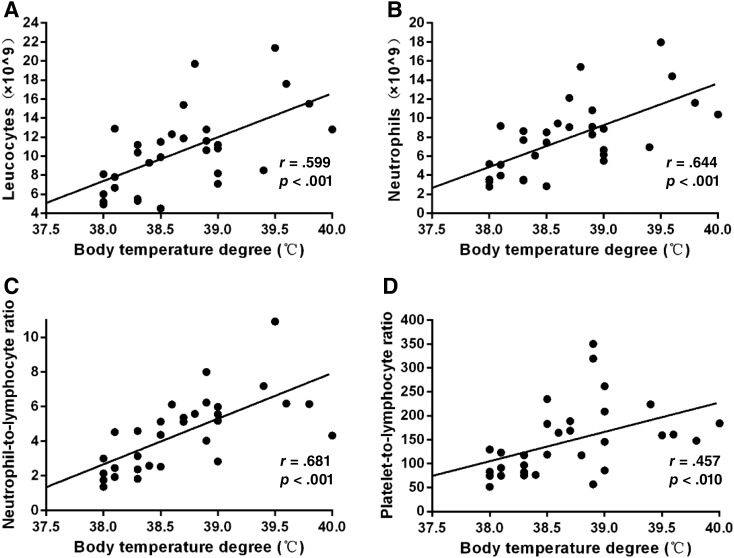
Clinically, fever is caused by inflammatory cytokines that are released by the tumor. We postulate that systemic inflammation might be involved in the fever of these patients with ICC. A systemic inflammatory response can be detected by elevation of leucocytes, neutrophils, NLR, and PLR in peripheral blood. The relationship between fever grade and inflammatory indices such as leucocytes, neutrophils, NLR, and PLR were analyzed. We found that body temperature was positively correlated with leucocytes (r = 0.599, p < .001), neutrophils (r = 0.644, p < .001), NLR level (r = 0.681, p < .001), and PLR level (r = 0.457, p = .010; Fig. 4). These feverish patients with temperature ≥38.6°C also had higher leucocytes (13.0 ± 4.0 × 109/L vs. 8.0 ± 2.8 × 109/L; p < .001), neutrophils (10.1 ± 3.5 × 109/L vs. 5.4 ± 2.3 × 109/L; p < .001), NLR (5.9 ± 1.7 vs. 2.9 ± 1.2; p < .001), and PLR (184.0 ± 77.0 vs. 107.5 ± 47.7; p = .003) than those with temperature <38.6°C (Table 3).
Figure 4.
Relationship between fever and systemic inflammation. Positive correlation between fever degree and leucocytes (r = 0.599, p < .001; A), neutrophils (r = 0.644, p < .001; B), neutrophil‐to‐lymphocyte ratio level (r = 0.681, p < .001; C), and platelet‐to‐lymphocyte ratio level (r = 0.457, p = .010; D) in peripheral blood in patients with intrahepatic cholangiocarcinoma with fever.
Discussion
ICC with fever as the initial symptom is rare [6] and poses a diagnostic challenge because of similar clinical symptoms and imaging features as liver abscess. The biological characteristics for those feverish patients are extremely aggressive, and the clinical outcomes are worse than those for patients without fever. Thus, diagnosis often occurs after the optimal treatment time, which leads to mean survival time of 3.5 months after diagnosis [5], [6]. Patients with ICC and fever and those with liver abscess both have fever, leukocytosis, and liver lobe hypodense lesion [21], [22]; thus, it is very challenging to distinguish ICC with fever from liver abscess. According to our clinical experience, most of these patients with fever can be diagnosed by contrast‐enhanced CT or MRI, which can detect hypodense lesion with irregular rim‐like hyperenhancement consistent with ICC without fever [23]. The diagnosis of liver malignancy also relies heavily on serum tumor markers such as serum CA19‐9 or CEA [24]. Elevated serum CA19‐9 or CEA levels might suggest the possibility of ICC. As the outcome of this ICC subtype is extremely dismal, early diagnosis and timely intervention is crucial to improve outcomes. Needle biopsy can be considered when it is difficult to differentiate those patients with nonresolving liver abscess, especially for elderly patients with persistent fever, no response to multiple courses of antibiotics, absence of biliary obstruction, repeatedly negative blood cultures, negative amoebic serology, and nonliquefaction of liver abscess on imaging.
HepPar‐1, Ki‐67, CK7, and CK19 were analyzed to explore the molecular features in these ICC tissues. These markers are not expressed in normal liver tissues and can be used to differentiate feverish patients with ICC from those with benign disease [25], [26]. The histochemical expression of HepPar‐1, CK7, and CK19 was similar in patients with ICC with and without fever. HepPar was a highly expressed and sensitive marker for HCC [27], [28], whereas HepPar‐1 had low expression in patients with ICC [29]. CK7 and CK19 are often used as biomarkers of ICC [30], [31]. HepPar‐1 could be combined with CK7 and CK19 to distinguish ICC with fever from HCC. Ki‐67 expression was higher in patients with ICC with fever than in those without fever before PSM. Ki‐67 is a proliferative marker that is only present in proliferating cells [32], [33]; thus, high Ki‐67 expression in these feverish patients suggests that tumor cell proliferation caused the larger tumor size.
There are several reasons for poor prognosis in patients with ICC with fever. First, patients with ICC with fever were usually not correctly diagnosed in the clinic because of similar clinical symptoms and imaging features as liver abscess, which delays correct treatment. Second, these feverish patients had more aggressive tumor features than those without fever such as higher serum GGT and CEA levels, larger tumor, and poorer tumor differentiation, which are all related to worse prognosis. Third, fever may be a crucial factor in survival; PSM analysis also verified that patients with ICC with fever had worse prognosis than those without fever. Fever is caused by inflammatory cytokines that are released by tumors, especially endogenous pyrogen‐related cytokines such as interleukin‐1, interleukin‐6, interleukin‐8, and tumor necrosis factor [34], [35], [36]. In renal cell carcinomas and lymphomas, these cytokines have been associated with increased incidence of neoplastic fever and advanced stage [37], [38]. We also found that patients with ICC with fever had higher levels of leucocytes, neutrophils, NLR, and PLR than those without fever. The fever grade was positively related to leucocytes, neutrophils, NLR, and PLR, which indicated more severe inflammatory and immunosuppressive status that hampers the adaptive antitumor response and causes tumor progression [39]. Anti‐inflammatory treatment may be beneficial for patients with ICC with fever.
In our study, the patients with ICC with fever underwent hepatectomy and their body temperatures returned to normal after surgery. Li et al. reported the outcomes of four ICC cases with fever; one underwent right hemihepatectomy and survived longer than the other three who had unresectable tumor or underwent exploratory laparotomy and surgical drainage. These four patients all died within 1 year (8 days, 28 days, 6 weeks, and 7.5 months) [5]. In our study of feverish patients with ICC with temperature <38.6°C (n = 15), six patients (6/15, 40%) survived more than 1 year after surgery. This outcome is better than the outcomes in Li's report, indicating that surgical intervention may improve the prognosis of patients with ICC with temperature <38.6°C. Patients with temperature ≥38.6°C (n = 16) all received surgical treatment; 12 patients (12/16, 75%) died within 6 months after surgery, and all died within 1 year after surgery (16/16, 100%). Almost all of these patients died from recurrence (15/16, 93.8%). Thus, surgical intervention might not be a rational choice for patients with temperature ≥38.6°C; anti‐inflammatory and immunotherapy might improve the outcome for patients with this extremely malignant disease. The molecular mechanism mediating disease progression for this ICC subgroup must be identified to develop new effective treatment strategies.
Conclusion
ICC with fever (≥38.0°C) is a rare subgroup of ICC with aggressive biological characteristics, severe inflammatory and immunosuppressive status, and poor prognosis. Patients with ICC with fever ≥38.6°C have an extremely dismal outcome and more aggressive clinical course. New therapeutic strategies are needed to improve survival for patients with temperature ≥38.6°C.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This study was supported by grants from the National Key Research and Development Program of China (2016YFF01400 and 2016YFC0902400), the National High Technology Research and Development Program (863 Program) of China (2015AA020401), the State Key Program of National Natural Science of China (81530077), the National Natural Science Foundation of China (81472676, 81572823, 81602543, 81602581, and 81672839), the Projects from the Shanghai Science and Technology Commission (14DZ1940300, 14411970200 and 14140902301), and the Resident Standardization Training Foundation of Zhongshan Hospital (2017006).
Contributed equally.
Author Contributions
Conception/design: Zi‐Jun Gong, Xin‐Rong Yang
Provision of study material or patients: Shuang‐Jian Qiu, Jian Zhou, Jia Fan
Collection and/or assembly of data: Zi‐Jun Gong, Ao Huang, Yun‐Fan Sun, Kai‐Qian Zhou, Bo Hu
Data analysis and interpretation: Zi‐Jun Gong, Jian‐Wen Cheng, Pin‐Ting Gao
Manuscript writing: Zi‐Jun Gong, Jian‐Wen Cheng
Final approval of manuscript: Jia Fan, Xin‐Rong Yang
Disclosures
The authors indicated no financial relationships.
References
- 1.Zhang H, Yang T, Wu M et al. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett 2016;379:198–205. [DOI] [PubMed] [Google Scholar]
- 2.Bridgewater J, Galle PR, Khan SA et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268–1289. [DOI] [PubMed] [Google Scholar]
- 3.Esnaola NF, Meyer JE, Karachristos A et al. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer 2016;122:1349–1369. [DOI] [PubMed] [Google Scholar]
- 4.Rizvi S, Khan SA, Hallemeier CL et al. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Li G, Miao R et al. Primary liver cancer presenting as pyogenic liver abscess: Characteristics, diagnosis, and management. J Surg Oncol 2012;105:687–691. [DOI] [PubMed] [Google Scholar]
- 6.Shah V, Arora A, Tyagi P et al. Intrahepatic cholangiocarcinoma masquerading as liver abscess. J Clin Exp Hepatol 2015;5:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pramoolsinsap C, Kurathong S. Cholangiocarcinoma masquerading as liver abscess. Southeast Asian J Trop Med Public Health 1989;20:313–317. [PubMed] [Google Scholar]
- 8.Yao XI, Wang X, Speicher PJ et al. Reporting and guidelines in propensity score analysis: A systematic review of cancer and cancer surgical studies. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DH, Pieper CF, Ahmed A et al. Use and interpretation of propensity scores in aging research: A guide for clinical researchers. J Am Geriatr Soc 2016;64:2065–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittekind C. Pitfalls in the classification of liver tumors [in German]. Pathologe 2006;27:289–293. [DOI] [PubMed] [Google Scholar]
- 11.Zhou XD, Tang ZY, Fan J et al. Intrahepatic cholangiocarcinoma: Report of 272 patients compared with 5,829 patients with hepatocellular carcinoma. J Cancer Res Clin Oncol 2009;135:1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llovet JM, Di Bisceglie AM, Bruix J et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698–711. [DOI] [PubMed] [Google Scholar]
- 13.Gong ZJ, Guo W, Sun YF et al. Prognostic value of fever grade combined with neutrophil percentage in hepatocellular carcinoma patients presenting fever as the initial manifestation. Onco Targets Ther 2016;9:6281–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing CY, Fu YP, Huang JL et al. Prognostic nomogram based on histological characteristics of fibrotic tumor stroma in patients who underwent curative resection for intrahepatic cholangiocarcinoma. The Oncologist 2018;23:1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Q, Zhao YJ, Wang XY et al. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology 2014;146:1397–1407. [DOI] [PubMed] [Google Scholar]
- 16.Witjes CD, ten Kate FJ, Verhoef C et al. Immunohistochemical characteristics of hepatocellular carcinoma in non‐cirrhotic livers. J Clin Pathol 2013;66:687–691. [DOI] [PubMed] [Google Scholar]
- 17.Yang XR, Xu Y, Shi GM et al. Cytokeratin 10 and cytokeratin 19: Predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection. Clin Cancer Res 2008;14:3850–3859. [DOI] [PubMed] [Google Scholar]
- 18.Tsokos CG, Krings G, Yilmaz F et al. Proliferative index facilitates distinction between benign biliary lesions and intrahepatic cholangiocarcinoma. Hum Pathol 2016;57:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halazun KJ, Hardy MA, Rana AA et al. Negative impact of neutrophil‐lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009;250:141–151. [DOI] [PubMed] [Google Scholar]
- 20.Tan DW, Fu Y, Su Q et al. Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of cholangiocarcinoma: A meta‐analysis. Sci Rep 2016;6:33789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Beers BE. Diagnosis of cholangiocarcinoma. HPB (Oxford) 2008;10:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LD, Xu HX, Xie XY et al. Enhancement patterns of intrahepatic cholangiocarcinoma: Comparison between contrast‐enhanced ultrasound and contrast‐enhanced CT. Br J Radiol 2008;81:881–889. [DOI] [PubMed] [Google Scholar]
- 23.Fowler KJ, Brown JJ, Narra VR. Magnetic resonance imaging of focal liver lesions: Approach to imaging diagnosis. Hepatology 2011;54:2227–2237. [DOI] [PubMed] [Google Scholar]
- 24.Patel AH, Harnois DM, Klee GG et al. The utility of CA 19‐9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol 2000;95:204–207. [DOI] [PubMed] [Google Scholar]
- 25.Cong WM, Wu MC. New insights into molecular diagnostic pathology of primary liver cancer: Advances and challenges. Cancer Lett 2015;368:14–19. [DOI] [PubMed] [Google Scholar]
- 26.Rizvi S, Gores GJ. Emerging molecular therapeutic targets for cholangiocarcinoma. J Hepatol 2017;67:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiqui MT, Saboorian MH, Gokaslan ST et al. Diagnostic utility of the heppar1 antibody to differentiate hepatocellular carcinoma from metastatic carcinoma in fine‐needle aspiration samples. Cancer 2002;96:49–52. [PubMed] [Google Scholar]
- 28.Shiran MS, Isa MR, Sherina MS et al. The utility of hepatocyte paraffin 1 antibody in the immunohistological distinction of hepatocellular carcinoma from cholangiocarcinoma and metastatic carcinoma. Malays J Pathol 2006;28:87–92. [PubMed] [Google Scholar]
- 29.Fan Z, van de Rijn M, Montgomery K et al. Hep par 1 antibody stain for the differential diagnosis of hepatocellular carcinoma: 676 tumors tested using tissue microarrays and conventional tissue sections. Mod Pathol 2003;16:137–144. [DOI] [PubMed] [Google Scholar]
- 30.Strnad P, Stumptner C, Zatloukal K et al. Intermediate filament cytoskeleton of the liver in health and disease. Histochem Cell Biol 2008;129:735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiggers JK, Ruys AT, Groot Koerkamp B et al. Differences in immunohistochemical biomarkers between intra‐ and extrahepatic cholangiocarcinoma: A systematic review and meta‐analysis. J Gastroenterol Hepatol 2014;29:1582–1594. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura K, Hatano E, Higashi T et al. Proliferative activity in hepatocellular carcinoma is closely correlated with glucose metabolism but not angiogenesis. J Hepatol 2011;55:846–857. [DOI] [PubMed] [Google Scholar]
- 33.Xia J, Zhou Y, Ji H et al. Loss of histone deacetylases 1 and 2 in hepatocytes impairs murine liver regeneration through Ki67 depletion. Hepatology 2013;58:2089–2098. [DOI] [PubMed] [Google Scholar]
- 34.Su B, Luo T, Zhu J et al. Interleukin‐1beta/iinterleukin‐1 receptor‐associated kinase 1 inflammatory signaling contributes to persistent gankyrin activation during hepatocarcinogenesis. Hepatology 2015;61:585–597. [DOI] [PubMed] [Google Scholar]
- 35.Foggo V, Cavenagh J. Malignant causes of fever of unknown origin. Clin Med (Lond) 2015;15:292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti B, Tabarean I, Andrei C et al. Cytokines and fever. Front Biosci 2004;9:1433–1449. [DOI] [PubMed] [Google Scholar]
- 37.Seymour JF, Talpaz M, Cabanillas F et al. Serum interleukin‐6 levels correlate with prognosis in diffuse large‐cell lymphoma. J Clin Oncol 1995;13:575–582. [DOI] [PubMed] [Google Scholar]
- 38.Kim HL, Belldegrun AS, Freitas DG et al. Paraneoplastic signs and symptoms of renal cell carcinoma: Implications for prognosis. J Urol 2003;170:1742–1746. [DOI] [PubMed] [Google Scholar]
- 39.Gomez D, Morris‐Stiff G, Toogood GJ et al. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2008;97:513–518. [DOI] [PubMed] [Google Scholar]