Until now, no studies have evaluated the effectiveness of PD‐1 inhibitors in classical Hodgkin lymphoma in a real‐world context (outside the context of study protocols). This article reports on clinical response to PD‐1 inhibitors, progression‐free and overall survival, and the prevalence and management of immune‐related adverse events in a population of patients with relapsed or refractory Hodgkin lymphoma treated with PD‐1 inhibitors.
Keywords: Hodgkin lymphoma, Immunotherapy, PD‐1 inhibitor, Real‐world, Checkpoint inhibitor
Abstract
Background.
Although classical Hodgkin lymphoma (cHL) is highly curable, 20%–30% of patients will not be cured with conventional treatments. The programmed death‐1 (PD‐1) inhibitors (PD‐1i) nivolumab and pembrolizumab have been Food and Drug Administration‐approved for relapsed/refractory (R/R) cHL. There is limited data on the real‐world experience with PD‐1i in cHL and it is unknown whether fewer selected patients treated with PD‐1i derive benefits similar to those observed in published trials.
Materials and Methods.
We performed a multicenter, retrospective analysis of R/R cHL patients treated with PD‐1i in the nontrial setting. The primary objective was to describe progression‐free survival (PFS) and overall survival (OS) in this population. Secondary objectives were to characterize response rates, toxicities, discontinuation patterns, and post‐PD‐1i therapies.
Results.
The study included 53 patients from nine U.S. centers. Overall response rate (ORR), complete response (CR), and partial response (PR) to PD‐1i were 68%, 45%, and 23%, respectively. Twelve‐month OS and PFS were 89% and 75%, respectively; median PFS was 29 months. Ninety‐six percent of patients with CR continue to respond at a median follow‐up of 20 months. Toxicities were similar to those previously described. Seventy percent of patients treated with systemic therapy after PD‐1i demonstrated objective responses.
Conclusion.
To our knowledge, this analysis is the first describing real‐world experience with PD‐1i in cHL patients in the U.S. Here, we demonstrate similar response rates compared to prior studies. The toxicity profile of PD‐1i was similar to that seen in previous studies; we further describe toxicity patterns in those with prior autoimmune disease or allogeneic transplant. Post‐PD‐1i systemic therapies appear active. These results support the effectiveness and tolerability of PD‐1i therapy in R/R cHL in a real‐world setting.
Implications for Practice.
Two PD‐1 inhibitors have recently been approved for patients with relapsed/refractory classical Hodgkin lymphoma based on results from nonrandomized clinical trials. However, to date, there have been no studies evaluating the effectiveness and toxicity profile of these drugs in the real‐world setting in the U.S. The present study demonstrates that patients treated in a real‐world context experience similar rates of overall effectiveness compared with published clinical trials. Patients who discontinue PD‐1 inhibitors may experience clinical responses to subsequent treatment with systemic chemotherapy or targeted therapy. This study provides clinicians with further insight into the effectiveness and tolerability of PD‐1 inhibitors and suggests that when patients progress while on these drugs, conventional systemic chemotherapy may be an effective treatment option.
Introduction
Classical Hodgkin lymphoma (cHL) represents about 10% of all lymphomas in the U.S. with approximately 8,500 new cases diagnosed annually [1]. Approximately 70%–80% of patients will be cured of their disease with standard frontline chemotherapy (i.e., ABVD) with or without radiotherapy [2], [3]. For those that relapse or are refractory to upfront therapy, salvage chemotherapy followed by high‐dose chemotherapy and autologous stem cell transplantation (autoSCT) results in cure for an additional 10% of patients. Patients who are unable to be salvaged with autoSCT have historically had few treatment options, limited primarily to allogeneic stem cell transplantation (alloSCT), various noncurative chemotherapy regimens, and clinical trials.
There has been a dramatic shift in the treatment landscape of relapsed or refractory (R/R) cHL in recent years. The introduction of the immunoconjugate brentuximab vedotin and checkpoint inhibitors has broadened the treatment armamentarium for patients with relapsed or refractory disease [4], [5], [6]. Several clinical trials have demonstrated significant efficacy of programmed death‐1 (PD‐1) inhibitors in treating R/R cHL [7], [8], [9], [10], resulting in Food and Drug Administration approval of nivolumab in 2016 and pembrolizumab in 2017.
Clinical trials of PD‐1 inhibitors in R/R cHL have consistently demonstrated high response rates (about 70%), even among patients whose disease progressed on brentuximab vedotin [9]. Moreover, studies demonstrate that these responses can be durable in heavily pretreated patients [11], [12]. Although checkpoint inhibitors have demonstrated efficacy in this disease, they can be associated with significant toxicity, namely immune‐related adverse events (IrAEs). The management approach to these toxicities may vary across practice settings.
Although several prospective clinical trials have demonstrated significant and durable response rates to PD‐1 inhibitors in R/R cHL, there is a paucity of data evaluating the effectiveness of these agents in a real‐world (i.e., nontrial) context. We hypothesized that the effectiveness of PD‐1 inhibitors in a real‐world population of R/R cHL patients is similar to the efficacy observed in controlled trials. In this study, we aim to characterize clinical response to PD‐1 inhibitors and progression‐free and overall survival, as well as the prevalence and management of IrAEs in a population of patients with R/R cHL treated with PD‐1 inhibitors outside the context of study protocols. We also explore treatment alternatives and responses to therapy after progression on PD‐1 inhibitors.
Materials and Methods
Patient Selection and Study Design
We conducted a multicenter, retrospective analysis of patients with R/R cHL who received treatment with a PD‐1 inhibitor outside the context of a study protocol. Patients were included in the study if they were 18 years of age or older, had R/R cHL, had received at least two prior lines of therapy, and were treated with a PD‐1 inhibitor off of a clinical trial between January 1, 2014, and December 1, 2017. Patients who had received PD‐1 inhibitors while on trial but were then treated with PD‐1 inhibitors in an off‐trial context were included in the study.
Data Collection
The study protocol was approved by the Institutional Review Board (IRB) at the University of Pennsylvania. Participating institutions obtained approval from their respective internal IRBs as dictated by institutional policy. Data were abstracted from the medical record by a coinvestigator at each participating institution who compiled and deidentified each participant's data.
Data was collected on patient demographics, diagnosis of cHL, therapies and responses prior to PD‐1 inhibitor therapy, response to PD‐1 inhibitor therapy, toxicities resulting from therapy with PD‐1 inhibitors and the associated management of each toxicity, and post‐PD‐1 inhibitor treatment approaches and responses. All data were deidentified prior to release to the primary study investigator.
Study Endpoints
The primary study objective was to describe the overall survival (OS) and progression‐free survival (PFS) of patients with R/R cHL treated with checkpoint inhibitors outside the context of a study protocol. OS was defined as the time from initiation of PD‐1 inhibitor therapy to death or last follow‐up while alive, and PFS was defined as time from initiation of PD‐1 inhibitor therapy to progression or last follow‐up while in remission. Secondary objectives included characterization of objective response rates, toxicities of PD‐1 inhibitors, including toxicities associated with graft‐versus‐host disease (GVHD) in postallogeneic stem cell transplantation (SCT) patients, and the approach to management of toxicities. Response assessment was categorized according to the Lugano Classification response criteria [13]. Toxicities of PD‐1 inhibitors were evaluated using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading version 4.0. An additional secondary aim was to describe various treatment approaches and responses following discontinuation of PD‐1 inhibitors.
Statistical Analysis
We performed descriptive analyses of patient demographics, diagnostic data, and treatment data. OS and PFS was estimated using the Kaplan‐Meier method, and the log‐rank test was used to assess for differences between groups. Patients who were lost to follow‐up were censored on the date of their last known follow‐up visit. All statistical analysis was performed using Stata 15.0 (StataCorp, College Station, TX).
Results
Patient Characteristics
Fifty‐three patients from nine institutions across the U.S. were included in the analysis. All patients had R/R cHL and were treated with a PD‐1 inhibitor outside the context of a study protocol. Patient characteristics are summarized in Table 1. Median age at diagnosis was 36 years (range, 18–81) and median age at initiation of PD‐1 inhibitor therapy was 41 years (range, 20–89).
Table 1. Patient characteristics.
Frontline therapy was ABVD or AVD (doxorubicin, vinblastine, dacarbazine with or without bleomycin) in 46 patients (87%). Twelve patients (23%) were treated with consolidative radiation as part of frontline therapy. Response rates to frontline therapy are shown in Table 2. Upon relapse, 31 patients (59%) were treated with ifosfamide, carboplatin, and etoposide (ICE). Five patients (9%) were treated with brentuximab vedotin (BV) as initial salvage therapy. The remaining patients were treated with multiple regimens or other less common salvage regimens. Twenty‐eight (53%) and 10 (19%) patients underwent autologous SCT and allogeneic SCT, respectively, prior to checkpoint inhibitor therapy.
Table 2. Response to previous lines of therapy.

Abbreviations: CR, complete response; PD, progressive disease; PD‐1, programmed death‐1; PR, partial response; SD, stable disease.
The median lines of treatment attempted prior to PD‐1 inhibitor therapy was 4 (range, 1 to >10). The majority (62%) had received between 3–5 prior lines of therapy. Fifty‐one of 53 patients (91%) were treated with brentuximab vedotin prior to receiving checkpoint inhibitor therapy. Forty‐eight percent of patients demonstrated an objective response to BV (Table 2). There was significant heterogeneity in the therapy given immediately prior to initiating PD‐1 inhibitor therapy, with the most common being BV (19 patients, 36%), bendamustine (6 patients, 11%), and radiation therapy alone (4 patient, 8%). Thirty patients (54%) achieved an objective response (either complete response [CR] or partial response [PR]) to the line of therapy immediately prior to checkpoint inhibitor therapy (Table 2). The median duration between the initiation of the line of therapy immediately preceding the PD‐1 inhibitor and initiation of the PD‐1 inhibitor was 6 months (range, 1–108 months).
Exposure to PD‐1 Inhibitors
Fifty‐two patients (98%) received nivolumab as the initial off‐trial PD‐1 inhibitor. The most commonly administered nivolumab dose was 3 mg/kg every 2 weeks (83%); two patients received nivolumab 3 mg/kg every 3 weeks. One patient was treated with pembrolizumab 200 mg every 3 weeks. At the time of data collection, 16 patients (31%) remained on the original PD‐1 inhibitor. For those patients who remained on therapy at the data cutoff, median duration of treatment with a PD‐1 inhibitor was 21 months (range, 2–34); among patients who discontinued therapy, median treatment duration was 6 months (range, 1–20). Nine of the 24 patients (38%) who achieved CR remained on the original PD‐1 inhibitor at the data cutoff; those with CR who discontinued the PD‐1 inhibitor did so because of toxicity (4, 17%), autoSCT (2, 8%), disease progression (1, 4%), or patient or physician preference (7, 29%). Of 34 patients in the entire cohort that discontinued therapy, 14 patients (39%) had done so due to progression, 8 patients (22%) discontinued due to toxicity, 2 patients (6%) went on to autologous SCT, and the remaining discontinued due to patient preference, physician preference, or nonadherence. Dose interruptions occurred in 24 patients overall (45%). Twelve patients (50%) had dose interruption due to toxicity, five patients (21%) due to patient preference, and the remainder due either to patient nonadherence or physician preference. Dose interruption did not impact on PFS (p = .42). No patients were treated with dose reduction.
Response to PD‐1 Inhibitor Therapy
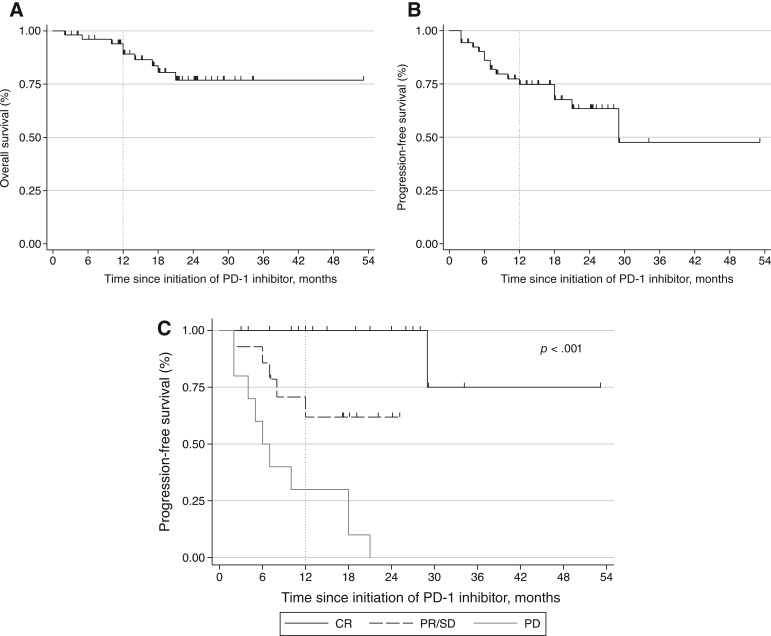
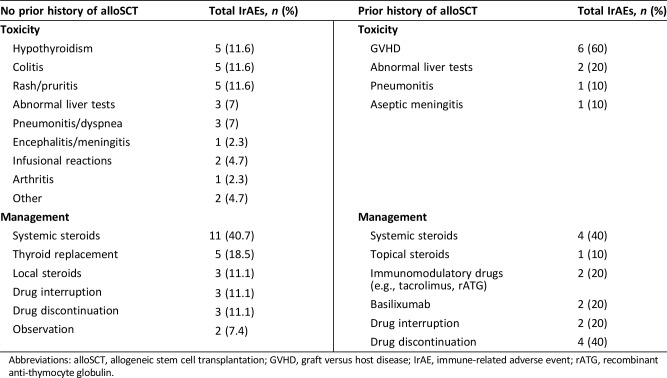
The best ORR to PD‐1 inhibitor therapy was 68% (45% CR, 23% PR). Only two patients (4%) were classified as having SD; 12 patients (19%) experienced PD as their best response. The median interval from initiation of PD‐1 inhibitor to the best overall response was 3 months (range, 1–16). One‐year OS for the entire cohort was 89% (95% confidence interval [CI:], 76–95; Fig. 1A). Median OS for the entire cohort was not reached. Overall, 12‐month PFS was 75% (95% CI, 60–85; Fig. 1A) and median PFS was 29 months (95% CI, 21–not reached; Fig. 1B). At 12 months, no patients who had achieved CR experienced progression, and during median follow‐up of 20 months, only 1 of 24 patients who had experienced CR subsequently had disease progression. The 12‐month PFS for patients who achieved PR or PD was 55% (95% CI, 22–78) and 30% (95% CI, 7–58). Neither of the two patients classified as having SD progressed during the first 12 months. For those who failed to achieve CR, the median time to progression was 18 months (95% CI, 7–not reached). Median follow‐up time for the entire cohort was 13 months.
Figure 1.
Outcomes among patients with relapsed/refractory Hodgkin lymphoma treated with PD‐1 inhibitors. (A): Overall survival of entire cohort from the initiation of PD‐1 inhibitor. (B): Progression‐free survival of entire cohort from the initiation of PD‐1 inhibitor. (C): Progression‐free survival stratified by best overall response to checkpoint inhibitor.
Abbreviations: CR, complete response; PD, progressive disease; PD‐1, programmed cell death protein‐1; PR, partial response; SD, stable disease.
Nine patients (17%) were deceased at the end of the study period. The cause of death was attributed to disease progression in three patients, sepsis in three patients, treatment‐related AML in one patient, and was unknown in two patients.
Response to Post‐PD‐1 Inhibitor Therapy
Twenty‐one patients went on to receive additional therapies after PD‐1 inhibition (40%). Treatment regimens following PD‐1 inhibitors were variable and consisted of pembrolizumab (n = 3), brentuximab (n = 3), lenalidomide (n = 3), and conventional chemotherapy (n = 4). Five patients went on to either autoSCT (n = 4) or alloSCT (n = 1). Of the 10 patients in whom the response to post‐PD‐1 inhibitor therapy was known, 7 patients (70%) experienced either CR (n = 3) or PR (n = 4). Specific post‐PD‐1 inhibitor therapies and clinical responses are shown in Table 3.
Table 3. Response to post‐PD‐1 therapy.
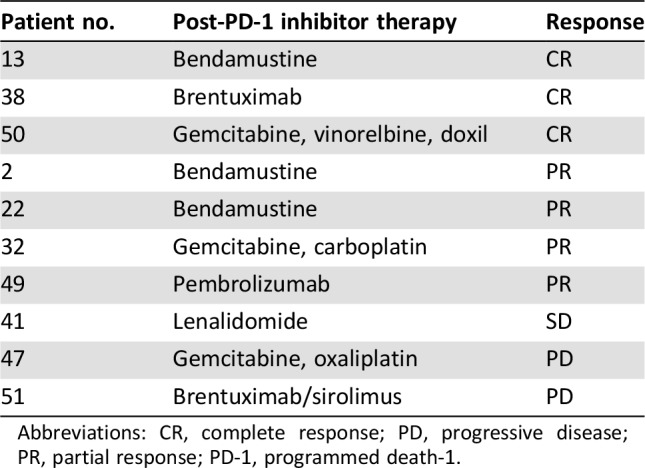
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; PD‐1, programmed death‐1.
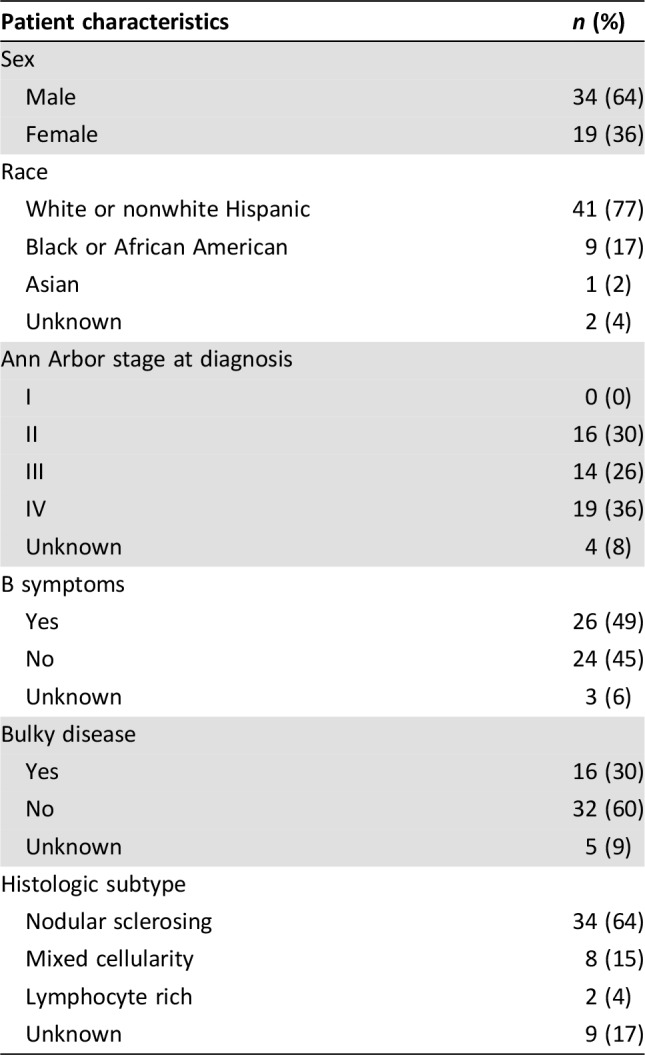
PD‐1 Inhibitor‐Associated Toxicity and Management
Among the 43 patients with no prior history of alloSCT, 22 patients (51%) experienced a total of 27 IrAEs. The most commonly reported toxicities and management strategies are shown in Table 4. In those without a history of alloSCT, grade 3 or 4 adverse events occurred in 16% and composed 26% of all adverse events in this group. Grade 4 toxicities included encephalitis and pneumonitis; the drug was discontinued in both of these patients. Dose interruptions occurred in three patients (11%). All patients experienced improvement or resolution of toxicity and there were no deaths attributed to checkpoint inhibitor therapy.
Table 4. Toxicities and management by prior alloSCT status.
Abbreviations: alloSCT, allogeneic stem cell transplantation; GVHD, graft versus host disease; IrAE, immune‐related adverse event; rATG, recombinant anti‐thymocyte globulin.
Of 10 patients who had undergone allogeneic transplantation prior to PD‐1 inhibitor therapy, 6 (60%) developed GVHD. Five patients had GVHD involving the skin and one patient had both skin and liver involvement. Management approaches included topical steroids (1), systemic steroids (4), PD‐1 inhibitor treatment interruption alone (2), topical tacrolimus (1) and anti‐thymocyte globulin (1). The patient with liver involvement was treated with PD‐1 inhibitor interruption, systemic steroids, and basiliximab. Three of these six patients were deceased at the completion of this study, all attributed to sepsis, although none of the cases were felt to be directly related to GVHD. One patient with GVHD also experienced grade 3 pneumonitis treated with systemic steroids. An additional patient who did not experience GVHD developed grade 2 aseptic meningitis that was treated with systemic steroids with resolution and was subsequently continued on treatment with a PD‐1 inhibitor. Three of the 10 post‐alloSCT patients (30%) developed neither IrAEs nor GVHD.
Of the four patients with a prior history of autoimmune disease (AD), three experienced IrAEs. One patient with a history of lupus experienced an infusional reaction and developed hypothyroidism. A second patient with a history of inflammatory bowel disease developed colitis. The third patient had a history of psoriasis and during PD‐1 inhibitor therapy developed colitis and a psoriasis flare. These three patients have all discontinued PD‐1 inhibitor therapy. In patients with no history of autoimmunity, 51% experienced toxicities related to PD‐1 inhibitor therapy, compared with 75% in those with a history of autoimmunity.
Discussion
Patients with R/R cHL who fail salvage chemotherapy and autoSCT are generally not considered curable and have a poor prognosis [14]. Over the last 5 years, however, the approval of several new agents has changed the treatment landscape for patients with R/R disease. Several clinical trials have evaluated the efficacy and safety of PD‐1 inhibitors in patients with R/R cHL [7], [8], [9], [11], [15], [16], [17]. To our knowledge, this study represents the first retrospective, real‐world analysis of checkpoint inhibitors in R/R cHL published in the U.S. We included 53 patients from nine institutions across the U.S. and evaluated survival endpoints and response rates to checkpoint inhibitor therapy, management of toxicity related to the use of checkpoint inhibitors, and the approach to treatment after progression on checkpoint blockade.
At 12 months, median OS was 89% and PFS was 75% in our cohort (Fig. 1A and 1B, respectively), which is comparable to survival trends in the published literature from phase I and II clinical trials. Durable responses to PD‐1 inhibitors have been demonstrated in several studies [10], [11], [17]. Our study extends these durability data to the real‐world setting; we observed that of the 24 patients in our cohort who achieved a CR, 96% continued to be in CR at the data‐cutoff, with a median follow‐up of 20 months. Median PFS among patients who experienced CR was not reached (Fig. 1C). Additionally, it has recently been recognized that patients who achieve PR to PD‐1 inhibitors can experience durable responses [11], [18]. In the present study, patients who achieved PR to checkpoint inhibitors had a 55% 12‐month PFS.
In published clinical trials, the ORR to checkpoint inhibitors has ranged from 65%–87% [7], [8], [9], [10], [15]. In these studies, observed CR rates are 20% or less, and PR rates are reported to be nearly 50% or more (see Table 5). The present study demonstrated a best overall response rate of 68%, similar to that observed in published trials; however, marked differences are noted in the reported best overall response in our cohort relative to published trial data. Forty‐five percent of patients in the present study achieved a CR and 23% achieved PR (see Table 5 for comparison), representing a significant deviation from previously published results. Several possible explanations exist for the higher CR rate observed in this study. First, assessments of clinical response in the present study are made by the treating clinician, rather than a study team or independent radiology review, which may introduce bias (i.e., recall bias, misclassification bias) into this assessment. For example, it is possible that treating clinicians inadvertently applied Deauville criteria less stringently, thereby overestimating rates of CR in their patients when compared with independent reviewers. A recently published extended analysis of nivolumab in patients with R/R cHL who progress after autoSCT (CheckMate 205) also reported differences between independent radiology review committee (IRC) assessment and investigator assessment of response [10]. In this study, IRC‐assessed ORR, CR, and PR were 69%, 16%, and 53%, respectively. Investigator‐assessed ORR was similar to the IRC assessment (72%), but the investigator‐assessed CR was 33%. There may be differences in the disease biology among patients in our cohort compared with those in previously published studies. For example, only about half of the patients in our cohort underwent autoSCT prior to treatment with checkpoint inhibitors, which is significantly lower than that in published trials. Although the results of this retrospective analysis must be interpreted with caution, these data suggest that real‐world outcomes in patients with R/R cHL treated with checkpoint inhibitors are similar to those reported in trials of highly selected and potentially more treatment‐refractory patients.
Table 5. Previously reported outcomes with PD‐1 inhibitors in patients with R/R cHL.
Abbreviations: AutoSCT, autologous stem cell transplantation; BOR, best overall response; cHL, classical Hodgkin lymphoma; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; LOT, line of therapy; NS, nodular sclerosing; ORR, overall response rate; OS, overall survival; PD, progressive disease; PD‐1, programmed death‐1; PFS, progression‐free survival; PR, partial response; R/R, relapsed or refractory; SD, stable disease.
Approximately half (53%) of the patients in the present cohort experienced toxicity related to checkpoint inhibition (see Table 4). The most common toxicities included hypothyroidism, colitis, liver enzyme abnormalities, pneumonitis, and rash, each occurring in slightly less than 10% of patients. Encephalitis, meningitis, arthritis, and infusional reactions were less commonly observed (see Table 4). Our cohort included 10 patients who underwent alloSCT prior to PD‐1 inhibition. The rate of GVHD (primarily skin GVHD) was high, at 60%, but there was no apparent death related to GVHD. Because the present study is a retrospective analysis, toxicities were likely under‐reported compared with published clinical trials of these therapies in R/R cHL. Twenty‐three percent of reported toxicities were considered to be grade 3 or 4 in severity. Drug discontinuation occurred in four patients due to grade 4 toxicity (encephalitis, pneumonitis, transaminitis, and GVHD). Dose interruptions were common and did not appear to impact on response.
The most common approach to management of checkpoint inhibitor‐associated toxicity in our cohort was systemic corticosteroids, which was utilized in 40% of adverse events. All patients who developed thyroid dysfunction (n = 5) while on checkpoint inhibitors were managed with thyroid replacement. Other approaches to toxicity management included topical steroids, dose interruption or discontinuation, or observation. In general, the management of reported toxicities were in line with recently published guidelines for the management of immune‐related adverse events [19].
Patients with a history of allogeneic SCT were excluded from initial studies of checkpoint inhibitors in R/R cHL because of the concern over increased toxicity. To date, no controlled prospective studies have evaluated the safety or efficacy of checkpoint blockade in this setting, although several case reports and retrospective series have been published suggesting an increased risk of GVHD but also overall response rates as high as 85%. In our cohort, 10 patients were treated with checkpoint blockade following allogeneic SCT. Seventy percent (7 patients) had either a CR (n = 4) or PR (n = 3); response was unknown in three patients because of discontinuation prior to response assessment, in each case due to toxicity. Treatment‐emergent GVHD was seen in 60% of patients (n = 6) treated with PD‐1 inhibitors following allogeneic SCT. All six patients developed skin GVHD; one patient also experienced liver GVHD after a single dose of nivolumab and was treated with drug interruption, systemic corticosteroids, and basiliximab. The patient died shortly thereafter of a sepsis‐like syndrome. Recently published consensus‐based guidelines have been put forth regarding the use and toxicity of checkpoint inhibitors either prior to or following allogeneic SCT [20].
A history of autoimmunity has previously been thought to confer a higher risk of toxicity to PD‐1 inhibitors; therefore, patients with such a history have been excluded from clinical trials with these drugs. Existing retrospective data suggests that PD‐1 inhibitors can safely be used in patients with a history of AD and although flares of the pre‐existing AD are common, they are often of mild severity and manageable [21], [22]. Four patients (8%) in the present cohort had a history of pre‐existent AD, and three of these four patients experienced either a flare of their underlying AD or another IrAE with PD‐1 inhibitor therapy. Only one grade 3 episode of colitis was observed, which was treated with systemic corticosteroids; all other IrAEs in this cohort were grades 1 or 2. All IrAEs in patients with prior AD resolved. These observations are consistent with the limited published data describing PD‐1 inhibitor use in patients with AD.
As previously discussed, the overall response rate in our cohort was comparable to what has previously been reported (68%), and responses were durable. For patients who experienced disease progression or intolerable toxicity while on checkpoint inhibitors, next‐line treatment options were variable, but we found that high objective response rates were seen in this group of heavily pretreated patients (median of 4 prior lines of therapy). Twenty patients (38%) received another line of therapy following treatment with a checkpoint inhibitor. Five patients were taken to either autologous or allogeneic SCT. Of the remaining 15 patients, the best overall response was known in 10 patients (5 patients had either died, were lost to follow‐up, or were not on the next line therapy long enough to assess response). That 70% of patients responded to salvage therapy with systemic chemotherapy or brentuximab vedotin suggests that immunotherapy with PD‐1 inhibitors may be associated with increased effectiveness of subsequent conventional salvage therapies. Although interpretation of these results is limited by the number of patients, these findings warrant larger and more systematic analyses.
The primary limitation of this study is the retrospective design, which has the potential of introducing unanticipated bias. Although we included data from nine institutions, it is important to point out that community oncology practices were not included in this analysis, which does limit the external validity of the study. Additionally, the power of the study is limited somewhat by the modest sample size which increases the likelihood of a type 2 error, although given the largely descriptive nature of the present study, this is a minor concern.
Conclusion
Checkpoint blockade therapy has emerged as a key treatment approach for patients with R/R cHL. Controlled clinical trials have documented high overall response rates and durable responses in patients with relapsed or refractory disease, although data supporting the effectiveness of these therapies in real‐world, less selected patients is lacking. We hypothesized that PD‐1 inhibitor therapy effectiveness in the nontrial setting would produce response rates and rates of survival similar to that observed in published controlled trials. Our study not only demonstrated the effectiveness of these drugs in the real‐world setting but also suggests that unselected patients can achieve durable responses to PD‐1 inhibition. Furthermore, the study provides some evidence that heavily pretreated patients with relapsed and/or refractory disease who progress on PD‐1 inhibitor therapy can respond to systemic therapy, suggesting that checkpoint inhibitors might modify the therapeutic milieu in some way to render the disease more susceptible to conventional therapies. These results provide additional insight into the effectiveness and safety of PD‐1 inhibitors in patients with relapsed or refractory Hodgkin lymphoma.
Author Contributions
Conception/design: Steven M. Bair, Lauren E. Strelec, Jakub Svoboda
Provision of study material or patients: Tatyana A. Feldman, Philippe Armand, Nirav N. Shah, Nishitha Reddy, Nadia Khan, Charalambos Andreadis, Scott F. Huntington, Chaitra Ujjani, Sunita D. Nasta, Daniel J. Landsburg, Stephen J. Schuster, Jakub Svoboda
Collection and/or assembly of data: Steven M. Bair, Lauren E. Strelec, Tatyana A. Feldman, Gulrayz Ahmed, Philippe Armand, Nirav N. Shah, Nishitha Reddy, Nadia Khan, Charalambos Andreadis, Khoan Vu, Scott F. Huntington, Smith Giri, Chaitra Ujjani, Christina Howlett, Malik Faheem, Matthew R. Youngman
Data analysis and interpretation: Steven M. Bair
Manuscript writing: Steven M. Bair, Jakub Svoboda
Final approval of manuscript: Steven M. Bair, Tatyana A. Feldman, Philippe Armand, Nirav N. Shah, Scott F. Huntington, Sunita D. Nasta, Daniel J. Landsburg, Stephen J. Schuster, Jakub Svoboda
Disclosures
Tatyana A. Feldman: Bristol‐Myers Squibb, Seattle Genetics, Pharmacyclics/Abbvie, Takeda, KITE (C/A, H); Philippe Armand: Merck, Bristol‐Myers Squibb, Pfizer, Affimed (C/A), Merck, Bristol‐Myers Squibb, Affimed, Adaptive, Roche (institutional RF); Nishitha Reddy: Bristol‐Myers Squibb (C/A), Merck (RF); Sunita D. Nasta: Celgene (H), Roche Takeda/Millenium, Incyte, Debiopharm, Aileron, Rafael, Pharmacyclics, Atara (RF), Merck (C/A); Jakub Svoboda: Kite, Seattle Genetics, Bristol‐Myers Squibb, Kyowa (C/A), Merck, Bristol‐Myers Squibb, Seattle Genetics, Celgene, Pharmacyclics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Teras LR, CE DeSantis, Cerhan JR et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–459. [DOI] [PubMed] [Google Scholar]
- 2.Viviani S, Zinzani PL, Rambaldi A et al. ABVD versus BEACOPP for Hodgkin's Lymphoma When High‐Dose Salvage Is Planned. N Engl J Med 2011;365:203–212. [DOI] [PubMed] [Google Scholar]
- 3.Gordon LI, Hong F, Fisher RI et al. Randomized phase III trial ofabvdversus stanfordvwith or without radiation therapy in locally extensive and advanced‐stage hodgkin lymphoma: An intergroup study coordinated by the eastern cooperative oncology group (E2496). J Clin Oncol 2013;31:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bair SM, Mato A, Svoboda J. Immunotherapy for the treatment of Hodgkin lymphoma: An evolving paradigm. Clin Lymphoma Myeloma Leuk 2018;18:380–391. [DOI] [PubMed] [Google Scholar]
- 5.Rashidi A, Bartlett NL. Biologic agents in the management of Hodgkin lymphoma. J Natl Compr Canc Netw 2015;13:587–596. [DOI] [PubMed] [Google Scholar]
- 6.Matsuki E, Younes A. checkpoint inhibitors and other immune therapies for Hodgkin and non‐Hodgkin lymphoma. Curr Treat Options Oncol 2016;17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansell SM, Lesokhin AM, Borrello I et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younes A, Santoro A, Shipp M et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem‐cell transplantation and brentuximab vedotin: A multicentre, multicohort, single‐arm phase 2 trial. Lancet Oncol 2016;17:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R, Zinzani PL, Fanale MA et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017;35:2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armand P, Engert A, Younes A et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow‐up of the multicohort single‐arm Phase II CheckMate 205 Trial. J Clin Oncol 2018;36:1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanale M, Engert A, Younes A et al. Nivolumab for relapsed/refractory classical Hodgkin lymphoma after autologous transplant: Full results after extended follow‐up of the phase 2 Checkmate 205 Trial. Hematol Oncol 2017;35(suppl 2):135–136.26078106 [Google Scholar]
- 12.Timmerman JM, Engert A, Younes A et al. Checkmate 205 update with minimum 12‐month follow up: A phase 2 study of nivolumab in patients with relapsed/refractory classical Hodgkin lymphoma. Blood 2016;128:1110. [Google Scholar]
- 13.Cheson BD, Fisher RI, Barrington SF et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non‐hodgkin lymphoma: The Lugano classification. J Clin Oncol 2014;32:3059–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bair SM, Strelec LE, Nagle SJ et al. Outcomes in patients with relapsed/refractory Hodgkin lymphoma progressi after autologous stem cell transplant in the current era of novel therapeutics: A retrospective analysis. Am J Hematol 2017;92:879–884. [DOI] [PubMed] [Google Scholar]
- 15.Armand P, Shipp MA, Ribrag V et al. Programmed death‐1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 2016;34:3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armand P, Shipp MA, Ribrag V et al. Pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: Long‐term efficacy from the Phase 1b Keynote‐013 study. Blood 2016;128:1108a. [Google Scholar]
- 17.Zinzani PL, Fanale MA, Chen R et al. Pembrolizumab monotherapy in patients with primary refractory classical Hodgkin lymphoma: Subgroup analysis of the phase 2 Keynote‐087 study. Hematol Oncol 2017;35(suppl 2):136–137. [Google Scholar]
- 18.Cohen JB, Engert A, Ansell SM et al. Nivolumab treatment beyond investigator‐assessed progression: Outcomes in patients with relapsed/refractory classical Hodgkin lymphoma from the phase 2 Checkmate 205 study. Blood 2017;130(suppl 1):650a. [Google Scholar]
- 19.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbaux C, Merryman R, Devine S et al. Recommendations for managing PD‐1 blockade in the context of allogeneic HCT in Hodgkin lymphoma: Taming a necessary evil. Blood 2018;132:9–16. [DOI] [PubMed] [Google Scholar]
- 21.Menzies AM, Johnson DB, Ramanujam S et al. Anti‐PD‐1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–376. [DOI] [PubMed] [Google Scholar]
- 22.Leonardi GC, Gainor JF, Altan M et al. Safety of programmed death‐1 pathway inhibitors among patients with non‐small‐cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 2018;36:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]