The prognosis of advanced osteosarcoma after failure of standard multimodal therapy is dismal. This article reports the results of a study that investigated the activity of apatinb in inoperable high‐grade osteosarcoma progressing upon chemotherapy.
Keywords: Osteosarcoma, Refractory to chemotherapy, Apatinib, Metastasis, Unresectable
Abstract
Background.
Antiangiogenesis tyrosine kinase inhibitors (TKIs) have been shown to prolong progression‐free survival (PFS) in advanced osteosarcoma. Methylsulfonic apatinib is a TKI that specifically inhibits vascular endothelial growth factor receptor‐2. We aim to assess apatinib in patients with advanced high‐grade osteosarcoma progressing upon chemotherapy.
Materials and Methods.
This phase II trial was conducted at Peking University People's Hospital. We enrolled participants (≥16 years of age) with progressive relapsed or unresectable osteosarcoma. Participants received 750 mg or 500 mg of apatinib according to body surface area once daily until disease progression or unacceptable toxicity. The primary endpoint was objective response rate and PFS at 4 months.
Results.
A total of 37 participants were finally included into the analysis. Until final follow‐up, the objective response rate (complete response + partial response) was 43.24% (16/37). The 4‐month PFS rate was 56.76% (95% confidence interval [CI], 39.43%–70.84%). Median PFS and overall survival were 4.50 (95% CI, 3.47–6.27) and 9.87 (95% CI 7.97–18.93) months, respectively. Toxic effects led to dose reductions or interruptions in a total of 25 of 37 (67.57%) patients. The most common grade 3–4 adverse events were pneumothorax in six (16.22%) patients, wound dehiscence in four (10.81%), proteinuria in three (8.11%), diarrhea in three (8.11%), and palmar‐plantar erythrodysesthesia syndrome in three (8.11%). No other serious adverse events were reported during the trial. There were no treatment‐related deaths.
Conclusion.
Apatinib is a sensitive drug for advanced osteosarcoma with a high response rate after failure of chemotherapy, with similar duration of response compared to other TKIs.
Implications for Practice.
For advanced osteosarcoma progressing upon chemotherapy, antiangiogenesis tyrosine kinase inhibitors (TKIs) have been proved to be effective in prolonging the progression‐free survival in previous multicenter trials and have been included into new National Comprehensive Cancer Network guidelines as second‐line therapy. Apatinib is a TKI that specifically inhibits vascular endothelial growth factor receptor‐2, which is domestically made in China. This phase II trial supports the use of apatinib in patients with advanced osteosarcoma progressing after chemotherapy.
摘要
背景。抗血管生成酪氨酸激酶抑制剂 (TKI) 已被证明可以在治疗晚期骨肉瘤时延长无进展生存期 (PFS)。甲磺酸阿帕替尼是一种可以特异性抑制血管内皮生长因子受体‐2 的 TKI。我们旨在于对经过化疗后出现疾病进展的晚期高级别骨肉瘤患者进行阿帕替尼评估。
材料和方法。本次 II 期试验于北京大学人民医院进行。我们招募了出现进展性复发或不可切除骨肉瘤的参与者(年龄 ≥16 岁)。根据体表面积的不同,参与者们每天服用一次 750 mg 或 500 mg 的阿帕替尼,直至出现疾病进展或不可接受的毒性。主要终点为客观缓解率和 4 个月 PFS。
结果。最终,我们一共将 37 名参与者纳入分析。直到最终随访时,客观缓解率(完全缓解 + 部分缓解)为 43.24% (16/37)。4 个月 PFS 率为 56.76% [95% 置信区间 (CI),39.43%–70.84%]。中位 PFS 和总生存期分别为 4.50 个月(95% CI,3.47–6.27)和 9.87 个月(95% CI,7.97–18.93)。毒性作用导致 37 名患者中的 25 名患者 (67.57%) 出现剂量减少或试验中断。最常见的 3–4 级不良反应如下:6 名患者 (16.22%) 出现气胸,4 名患者 (10.81%) 出现伤口裂开,3 名患者 (8.11%) 出现蛋白尿,3 名患者 (8.11%) 出现腹泻,以及 3 名患者 (8.11%) 出现手足综合征。在试验期间未报告其他重度不良反应。无治疗相关的死亡。
结论。阿帕替尼是一种治疗晚期骨肉瘤的敏感药物,在化疗失败后的缓解率较高,与其他 TKI 相比,具有相似的反应持续时间。
实践意义:对于经过化疗后出现疾病进展的晚期骨肉瘤,抗血管生成酪氨酸激酶抑制剂 (TKI) 在先前的多中心试验中已被证明可以有效地延长无进展生存期,并已作为二线治疗被收录在全新的国家综合癌症网络指南之中。阿帕替尼是一种在中国生产的可以特异性抑制血管内皮生长因子受体‐2 的 TKI。本次 II 期试验支持在经过化疗后出现疾病进展的晚期骨肉瘤患者中使用阿帕替尼。
Introduction
The prognosis of advanced osteosarcoma after failure of standard multimodal therapy has been dismal for decades [1], [2], with median progression‐free survival (PFS) and overall survival (OS) of only 4 (95% confidence interval [CI], 3.0–5.7) weeks and 5.9 (95% CI, 1.4–16.4) months, respectively [3]. Multiple agents that have been reported to be effective in preclinical tests have failed to prolong survival in patients with refractory osteosarcomas in the past 20 years [4], [5], [6], [7]. Recently, small molecule antiangiogenesis tyrosine kinase inhibitors (TKIs) have shown more promising prospects compared with other target therapies [3], [8], [9], [10], with median PFS and OS improved to 4–5 months and 7–11.3 months, respectively [3], [8], [9], [10]. However, progress has apparently come to a halt since Grignani et al. [9], [10] reported a phase II cohort trial of sorafenib and sorafenib plus everolimus for advanced osteosarcoma, whose data had been very promising in the second‐line therapy for osteosarcoma. At the 2018 American Society of Clinical Oncology meeting, regorafenib was demonstrated as prolonging median PFS to 13.7 (95% CI, 8.0–27.3) weeks, with acceptable toxicity with randomized trial [3], which gave more confidence in investigating these TKIs in advanced osteosarcoma. However, short duration of those TKIs and secondary drug resistance have become a growing concern, prompting researchers to attempt to combine TKIs with chemotherapy or even immunotherapy [11], [12], [13].
Previously studied broad‐spectrum TKIs mainly affect osteosarcoma via vascular endothelial growth factor receptor (VEGFR)‐1 (Flt‐1) and VEGFR‐2 (kinase insert domain receptor) [14], [15], [16]. Apatinib is an orally active multikinase inhibitor that is domestically made in China; the median inhibition concentration (IC50) values of VEGFR‐2, VEGFR‐1, c‐kit, and platelet‐derived growth factor receptor‐β are 2 nM, 70 nM, 420 nM, and 537 nM, respectively [17]. Furthermore, our research group has shown that patients with osteosarcoma with high levels of VEGFR‐2 have poorer prognosis, and deactivation of VEGFR‐2/STAT3/BCL‐2 signal pathway would lead to apatinib‐induced growth inhibition of osteosarcoma [18]. Previously, this drug was approved by regulatory authorities for the treatment of adenocarcinoma of the stomach or gastroesophageal junction [19], [20]. Besides, this agent has been used off‐label in the treatment of patients with advanced sarcoma throughout the country in the past 3 years, most of which have been proven to be effective, although with diverse response rates [21], [22], [23]. To objectively assess its effectiveness and toxicity, we designed and conducted this prospective nonrandomized phase II trial to investigate the activity of apatinib in inoperable high‐grade osteosarcoma progressing upon chemotherapy.
Materials and Methods
Patients and Study Design
This open‐label, phase II trial was conducted prospectively and exclusively at Peking University People's Hospital. Eligibility criteria included (a) age ≥ 16 years; (b) histologically documented high‐grade osteosarcoma that was either unresectable or locally advanced or metastatic; (c) having measurable lesions according to Response Evaluation Criteria In Solid Tumors (RECIST 1.1) [24]; (d) progressing upon prior treatment (completed >4 weeks before trial entry) that consisted of standard high‐grade osteosarcoma chemotherapy agents, including high‐dose methotrexate, doxorubicin, cisplatin, and ifosfamide; (e) an Eastern Cooperative Oncology Group [25] performance status of 0–1, with a life expectancy >3 months; (f) adequate renal, hepatic, and hematopoietic function; (g) normal or controlled blood pressure; and (h) surgery completion at least 3 weeks before enrollment. Patients were excluded when they had been previously exposed to other TKIs; had central nervous system metastasis; had other kinds of malignant tumors at the same time; had cardiac insufficiency or arrhythmia; had uncontrolled complications such as diabetes mellitus, coagulation disorders, urine protein ≥ ++, and so on; had pleural or peritoneal effusion that needed to be handled by surgical treatment; and had other infections or wounds; or were pregnant or breastfeeding.
The trial was approved by the institutional review board and ethics committee of Peking University People's Hospital and complied with good clinical practice guidelines and the Declaration of Helsinki. Written informed consents about the purpose, the expected risks, and the investigational nature of the study were obtained from each patient. The protocol is available online (supplemental online Appendix 1).
Procedures
Patients were treated with a dose of apatinib 750 mg once daily for body surface area (BSA) ≥ 1.5 and 500 mg daily for BSA < 1.5. Apatinib was taken orally approximately half an hour after a meal, the timing of which, whenever it was, should be at the same time each day. Researchers and participants were not masked to drug assignments, and the study ran until disease progression, unacceptable toxic effects, or participants’ refusal. Other predefined reasons for patient removal from the trial were the following: investigator's decision, substantial noncompliance with study requirements, pregnancy, using simultaneously illicit drugs or other prohibited substances, development of concurrent illness that could jeopardize the patient's clinical status and trial endpoints, or interruption of study drugs for more than 30 days. The dose was reduced or temporarily suspended according to predefined rules and after considering any observed severe toxicity, which was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 [26].
Baseline assessment included chest computed tomography (CT; with each layer ≤5 mm), bone scan or [18F]2‐fluoro‐2‐deoxy‐D‐glucose‐positron emission tomography (FDG‐PET), and local CT and magnetic resonance imaging for musculoskeletal lesions if participants had lesions located at musculoskeletal sites. There is generally hardly any visceral metastasis in osteosarcoma, thus abdominal CT was not routinely conducted in all patients. However, an abdominal ultrasound was performed for each evaluation. If there was any suspicious lesion, then an abdominal contrast CT would be performed. Laboratory tests included full blood count, serum chemistry, electrocardiogram, thyroid and cortisol hormone levels, and urinalysis, in which elevated levels of protein in urine protein would be checked quantitatively for 24 hours. Physical examination was performed at each follow‐up. For this study design, the first time point for evaluation was set at 1 month (almost 4 weeks). If a patient showed a partial response (PR) according to RECIST 1.1, the next evaluation would be conducted 1 month later to confirm this PR, and then the evaluation would be repeated every 2 months thereafter. If a patient exhibited stable disease (SD), then the next evaluation would be performed 2 months later to confirm the SD and then repeated every 2 months thereafter. We strictly followed PR or progressive disease as described in RECIST 1.1 [23] and did not set a minor response as performed by Grignani et al. [9], [10].
Adverse events were assessed and graded according to CTCAE version 4.03 [26]. In case of grade 3 or 4 toxicity, apatinib was reduced by one dose level (from 750 mg to 500 mg daily or from 500 mg to 250 mg) or by two dose levels (750 mg once daily to 250 mg). Whenever feasible, patients would be returned to a higher dose. If the adverse event did not resolve by suspending treatment, the patient was removed from the trial. In the case of creatinine increase, febrile neutropenia, or any other clinically relevant unexpected toxic effects, apatinib would be stopped until resolution.
European Organization for Research and Treatment of Cancer (EORTC) 30‐item core Qol questionnaire (QLQ‐C30) was adopted to evaluate the quality of life in our study [27], [28], [29]. The QLQ‐C30 was administered at baseline, on day 1 of each assessment, and follow‐up. Baseline questionnaires were completed prior to the first prescription of apatinib. Subsequent questionnaires were completed before any study‐related procedures for that visit and before tumor assessment results were communicated to the patient.
Outcomes
The primary outcome measure was objective response rate (ORR) and 4‐month PFS. We defined ORR as CR + PR rate according to the best of response. PFS was calculated from the date of trial entry until the time of disease progression or death, whichever came first. Patients alive and free from progression were censored. The secondary endpoints included six‐month PFS; overall survival; clinical benefit rate (CBR), which was the proportion of patients who achieved disease control (CR + PR + SD for at least six months); duration of response; pain improvement; life quality score; and safety. OS was calculated from trial entry until death. We calculated duration of response from day of first response assessment until either progression/death (event) or last day of follow‐up (censored). In the absence of an event or loss to follow‐up, all survival endpoints were censored on the last date the patient was known to be event free.
We assessed any sign of tumor‐related pain improvement by the numeric rating scale score (NRS) [29], [31] and evaluated patients’ life quality by QLQ C‐30 [27], [28], [29]. To understand the real effect of the study drug on symptoms, we deemed assessable only those patients who had completed at least three forms (assessment at baseline, at least one during treatment, and at the off‐treatment visit).
Statistical Analysis
This was a single center, open‐label trial. For advanced osteosarcoma refractory to chemotherapy, we mainly based our data on the reports of Grignani et al. [9], [10] and Schuetze et al. [30]. We calculated the number of needed patients under a hypothesis of interest, in which apatinib PFS at 4 months was ≥30% (H1 = 30%) and a null hypothesis in which apatinib reached a PFS at 4 months ≤10% (H0 = 10%) as described by Grignani et al. [9], [10]. Thus, this trial consisted of two phases; stage I demanded at least 17 participants with at least six successful cases (PFS more than 4 months) and then this trial could move on to stage II, enrolling at least another 20 participants for further analysis.
The analysis included all patients who received therapy. The population assessable for treatment activity comprised all patients for whom at least one disease assessment (either clinical or radiological) was conducted. The primary endpoint was analyzed in the intention‐to‐treat population, which included all patients who received at least one dose of the drug. PFS and OS were estimated by the Kaplan‐Meier method. RECIST overall responses and disease control were calculated and reported with 95% CIs. Safety evaluation was based on the frequency and severity of toxicities was graded according to CTCAE version 4.03 [26]. Some potential predictor variables were evaluated among groups based on PFS using Cox regression analysis. All statistical analyses were two‐sided, significance level was set at .05, and 95% CIs were generated [10]. This trial is registered with ClinicalTrials.gov, with identifier NCT02711007.
Role of the Funding Source
The study was supported by the sponsor, Jiangsu HengRui Medicine (Lianyungang, China). However, pharmaceutical companies had no role in data collection and interpretation or writing of the report. The entire study was designed and conducted by physicians and oncologists from the Musculoskeletal Tumor Center of Peking University People's Hospital. Data were collected and entered into the study database by an independent data monitoring committee (Beijing Duheng for Drug Evaluation and Research Co., Ltd., Beijing, China), which was also responsible for data interpretation and analysis. The corresponding author had full access to all data in the study, and all authors listed had final responsibility for the decision to submit for publication.
Results
From March 17, 2016, to June 9, 2017, a total of 41 patients with continuous advanced osteosarcoma with locally advanced or inoperable metastatic lesions were registered and signed informed consent for this study. However, during the screening stage, one patient did not meet the inclusion criteria, two patients changed their mind and instead opted to use pazopanib, and one patient underwent radiation of lesions, and were thus all excluded from this trial. A total of 37 patients were finally enrolled into the study (supplemental online Fig. 1). Table 1 describes the patients’ characteristics at baseline. Twenty‐eight patients developed progression during MAP/I chemotherapy, seven relapsed with pulmonary metastasis after stopping chemotherapy within 3 months, and two developed unresectable lesions after chemo‐suspension longer than 6 months and refused to continue chemotherapy. All patients were treated according to the protocol and were included in the safety and activity analyses. The analysis was conducted 6 months after the last patient started therapy.
Table 1. Baseline characteristics.
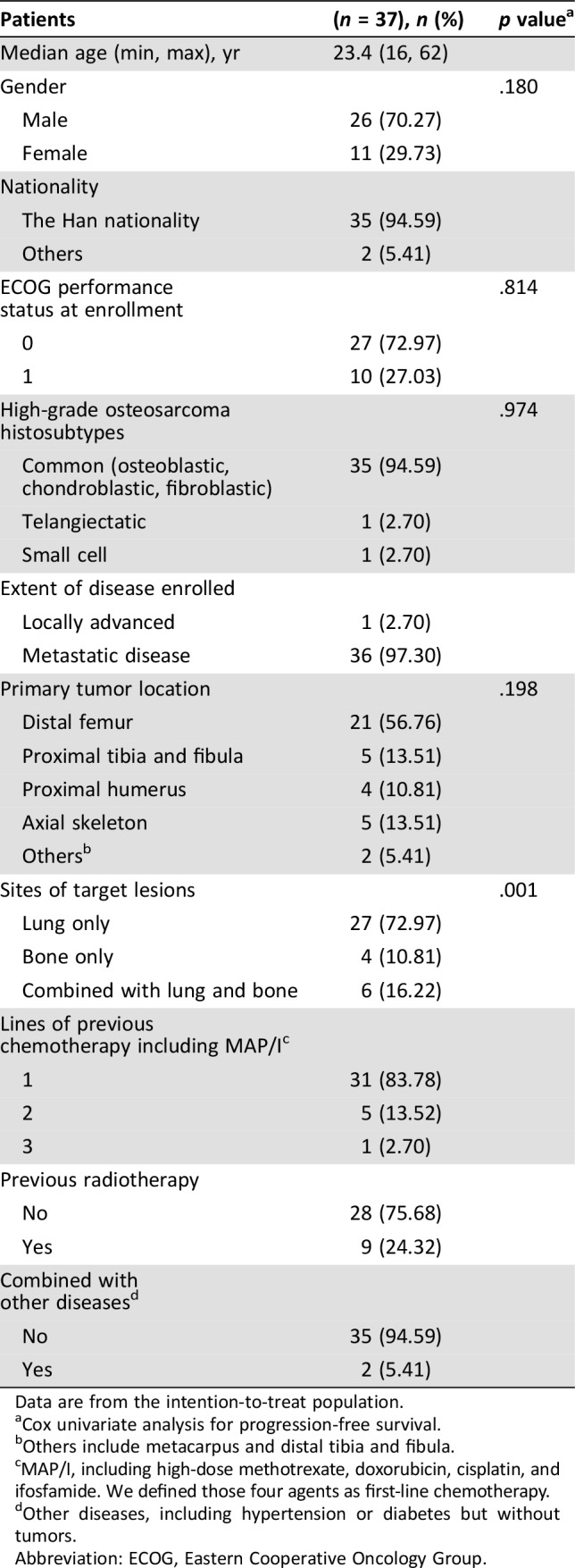
Data are from the intention‐to‐treat population.
Cox univariate analysis for progression‐free survival.
Others include metacarpus and distal tibia and fibula.
MAP/I, including high‐dose methotrexate, doxorubicin, cisplatin, and ifosfamide. We defined those four agents as first‐line chemotherapy.
Other diseases, including hypertension or diabetes but without tumors.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
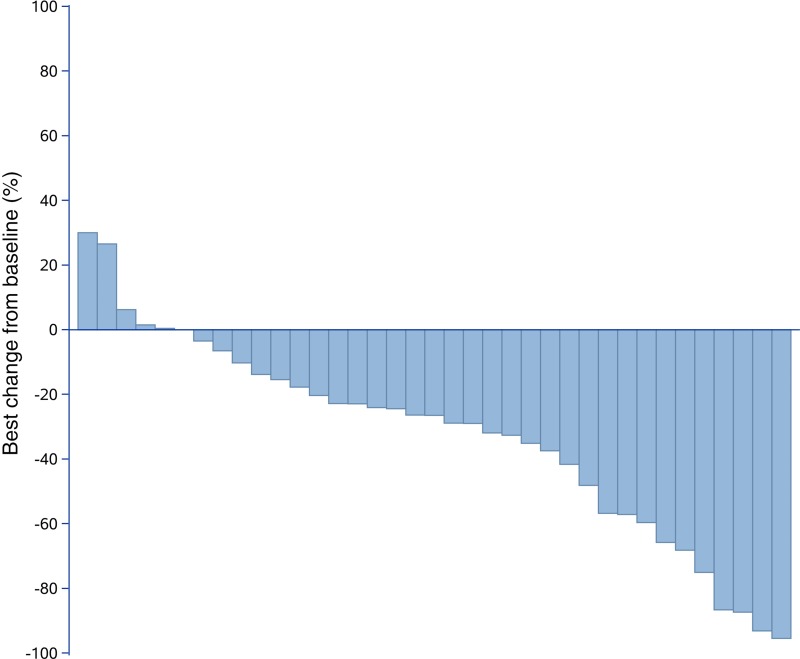
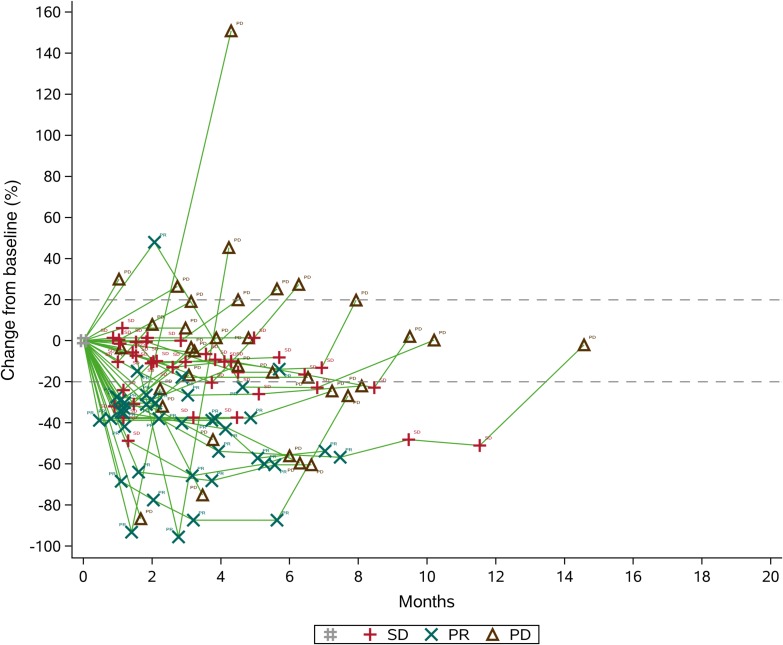
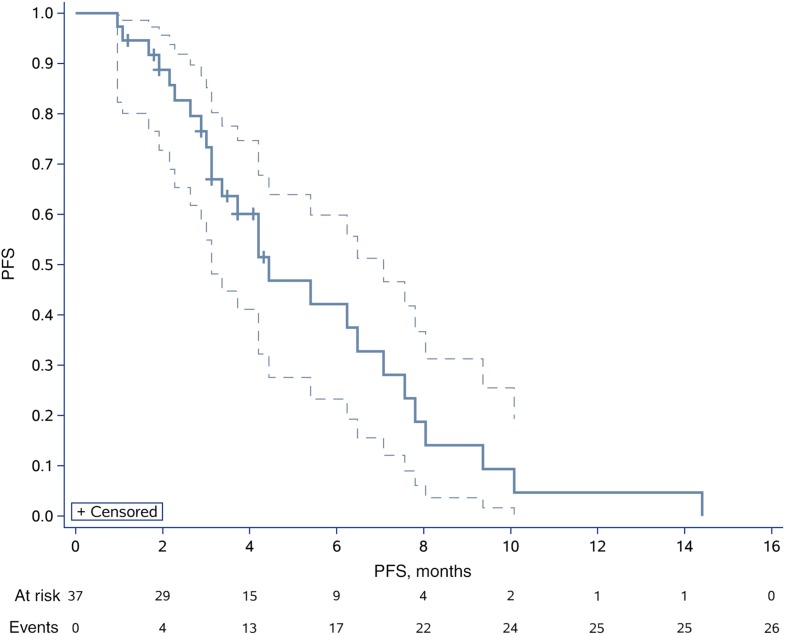
After completion of the initial phase, 10 of the 17 patients (58.82%) were progression‐free at 4 months. Therefore, we enrolled another 21 patients for phase II, and 21 of the 37 patients (56.76%) were free from progression at four months (Figs. 1, 2). The date of database cut‐off for the primary analysis was December 30, 2017. The median follow‐up for efficacy was 4.50 months (interquartile range [IQR], 3.13–6.63). Until last follow‐up, no CR had been observed. However, 16 of 37 (43.24%) patients demonstrated best overall response of PR according to our study design (Fig. 1). Approximately 13 of 37 (35.14%) had progressed, of whom four progressed through initial evaluation at 1 month, four progressed 4 weeks later after initial assessed as PR, and five failed to confirm stable disease 8 weeks later after initial assessed as SD (Fig. 2). The ORR was 43.24% (16/37; 95% CI, 27.10–60.0%), and CBR was 35.14% (13/37; 95% CI, 20.21–52.54%). The 4‐month PFS and 6‐month PFS were 56.76% (95% CI, 39.43%–70.84%) and 36.77% (95% CI, 21.48%–52.16%), respectively (Fig. 3). The median PFS and OS were 4.50 (95% CI, 3.47–6.27) and 9.87 (95% CI, 7.97–18.93) months, respectively (Fig. 4 and supplemental online Fig. 2).
Figure 1.
Best of overall response (according to Response Evaluation Criteria In Solid Tumors version 1.1). Best change from baseline in target lesion size.
Figure 2.
Tumor volume change over time (according to Response Evaluation Criteria In Solid Tumors version 1.1). Change from baseline in target lesion.Abbreviations: PD, progressive disease; PR, partial response; SD, stable disease.
Figure 3.
Kaplan‐Meyer survival curve for progression‐free survival.Abbreviation: PFS, progression‐free survival.
We did notice dramatic tumor shrinkage, although with a short duration of response (Figs. 1, 2). All PR patients (16/37, 43.24%) showed at least 30% target lesion shrinkage from baseline. The median duration of response was 5.07 (95% CI, 2.70–6.53) months. From a total of 24 patients who have been observed with PR or SD at initial assessment, 13 of 37 (35.14%; 95% CI, 20.21%–52.54%) stayed no progression at 6 months.
At the same time, in our study, 27 of 37 (72.97%) patients had pulmonary lesions only, 4 of 37 (10.81%) had bone lesions (with measurable lesions according to RECIST 1.1) only, and 6 of 37 (16.22%) had both pulmonary and bone lesions as target lesions. Cox univariate analysis of PFS for different target lesions indicated different responses to apatinib (p = .001). Because it was the only obvious factor that influenced PFS, we did not subsequently inspect it in a multivariable Cox model. Besides, it is quite common to notice oligoprogression during observation. Although some patients dropped out of this trial during the follow‐up period, lesion progression in these patients may have been disrupted by this drug in combination with radiotherapy.
The median follow up time for toxicity was 7.37 (IQR, 6.33–11.07) months. We tracked and recorded self‐perceived improvement in pain management. We did not record a reduction of pain in terms of NRS score (mean baseline 2.3, SD 1.5). We noticed that pain even became more severe during the initial 1–3 months, with the main complaint of back pain or myalgia/arthralgia, with mean score on treatment as 4.6 (SD 1.7; p = .119), which gradually returned to baseline afterwards. We did not record any improvement in 37 patients who were fully assessable by EORTC QLQ C‐30 due to a decline in physical, role, emotional, cognitive, and social functioning (p > .100). Constipation and diarrhea significantly increased (p = .024, .031) after the use of apatinib. A trend of appetite loss was observed but was not statistically significant (p = .137). The mean global health status was 59 (IQR, 42–75), 51 (IQR, 33–67), and 42 (IQR, 33–50) at baseline, mean scores on‐treatment assessment, and the time of disease‐progression, respectively. A significant degrease of global health status was found at the time of disease progression (p = .042).
The overall incidence of apatinib‐related adverse events was 89.19%. In general, drug‐related adverse events were limited to grade 1 or 2 (Table 2). With median follow‐up time of 7.37 months (95% CI, 6.80–9.33) for the safety analysis, 22 of 37 (59.50%) had dose‐reduced treatment, and 11 of 37 (29.73%) patients had treatment temporarily interrupted (Table 3). The frequency of apatinib administration was 40.5% of planned. The mean temporary interruption duration was eight days (95% CI, 4–10).
Table 2. Adverse events that arose in at least one participant.
Table 3. Dose reductions.
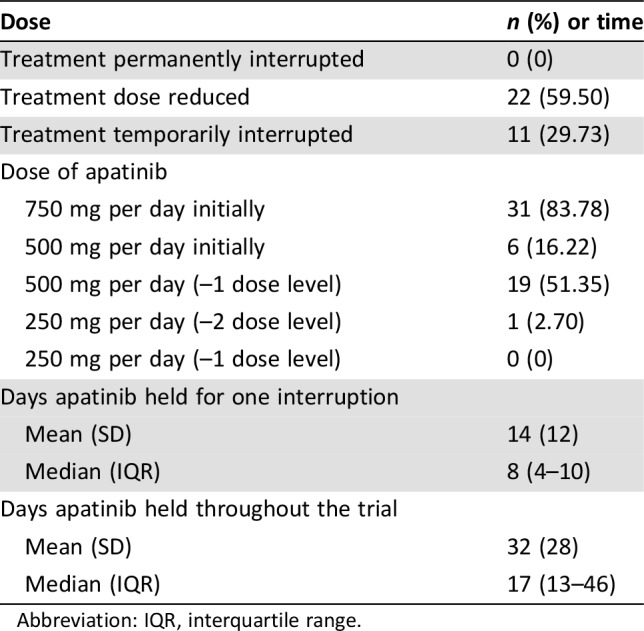
Abbreviation: IQR, interquartile range.
We noted the following grades 3 and 4 toxic effects that impacted dose reductions: pneumothorax (6, 16.22%), wound dehiscence (4, 10.81%), proteinuria (3, 8.11%), diarrhea (3, 8.11%), palmar‐plantar erythrodysesthesia syndrome (3, 8.11%), rash acneiform (2, 5.41%), abdominal cramps (2, 5.41%), anorexia (2, 5.41%), pleural infection (1, 2.7%), bladder perforation (1, 2.7%), hypertriglyceridaemia (1, 2.7%), weight loss (1, 2.7%), anemia (1, 2.7%), hypokalemia (1, 2.7%), palpitations (1, 2.7%), back pain (1, 2.7%), anorectal infection (1, 2.7%), cholecystitis (1, 2.7%) and fatigue (1, 2.7%; Table 2). All of these adverse events were causally related to the study drug. No deaths were related to the experimental treatment; all deaths were attributed to disease progression.
Discussion
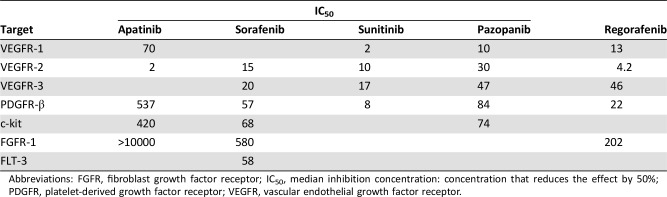
The results from this phase II, open‐label trial show that the administration of apatinib induced tumor shrinkage, showed a high rate of objective response (43.24%), with almost the same PFS rate at 4 months (56.76%) as sorafenib plus everolimus [10] (55.2% at 4 months) and the same PFS rate at 6 months (36.77%) as regorafenib [3] (35% at 24 weeks). Toxic effects were severe in our study but durable with high rates of dose reduction (59.50%). Approximately 35.14% (13/37) of the patients were free from progression at 6 months, which indicated that the duration of the response might be short, and the median duration of response was 5.07 months. The median inhibition concentration of those common antiangiogenesis TKIs [8] are listed in Table 4. Apatinib (YN968D1) is a novel and highly selective inhibitor of the VEGFR‐2 tyrosine kinase. Our preclinical study [18] on osteosarcoma demonstrates that high levels of VEGFR‐2 are related to short overall survival time (p = .021), and apatinib targets were expressed and effectively inhibited by this drug in vitro and in vivo, thereby providing a rationale to explore apatinib in patients with progressing osteosarcoma.
Table 4. Comparison of common antiangiogenic TKIs with different median inhibition concentrations (IC50).
Abbreviations: FGFR, fibroblast growth factor receptor; IC50, median inhibition concentration: concentration that reduces the effect by 50%; PDGFR, platelet‐derived growth factor receptor; VEGFR, vascular endothelial growth factor receptor.
Unlike Grignani et al. [9], [10], we chose objective response together with PFS at 4 months as our primary endpoint because during our off‐label use of apatinib in patients with osteosarcoma, we noticed that tumor shrinkage, especially in pulmonary metastatic lesions of high‐grade osteosarcoma, was not so difficult to achieve during the early stage of observation. We had sufficient confidence to evaluate lesions using the RECIST criteria [24] instead of minor response described as Grignani et al. [9], [10]. However, it seemed to us that the duration of the response might be short as off‐label use was conducted for only 2–3 months [32], which might also coincide with the characteristics of this agent (Table 4). To capture the best changes in tumor volume, the first evaluation was conducted almost four weeks after initiation of treatment. To confirm this change without violating the principle of RECIST 1.1, if PR was observed, then we re‐evaluated the lesion four weeks later. However, if SD was the initial evaluation, then we assessed the lesion eight weeks later. Thereafter, evaluations were performed every two months, similar to that of Grignani et al. [9], [10].
We were aware of the importance of the timing for secondary drug resistance; thus, similar to all the other investigations, CBR was defined as no progression at 6 months, and we had 13 of 37 (35.14%) patients surviving by then. Using the Kaplan‐Meier method, our PFS at 4 months and 6 months were 56.76% (95% CI, 39.43–70.84%) and 36.77% (95% CI, 21.48–52.16%), respectively, which were almost the same as that for sorafenib [5] as well as regorafenib [3] and might be a little shorter than the combination of sorafenib and everolimus [6]. However, owing to the small study population in all these trials, we considered that these were not significantly different. For unresectable osteosarcoma progression upon chemotherapy, we do not have accurate overall survival data without those TKIs, the prospective trials’ data for sorafenib [5] and sorafenib plus everolimus [6] were both single arm, and the trial for regorafenib had crossover from placebo to regorafenib [3]. However, according to the data of this trial, we think our agent might also improve the OS for these advanced osteosarcoma with median OS time of 9.87 (95% CI, 7.97–18.93) months compared with 5.9 (95% CI, 1.4–16.4) [3].
Our collected data of toxicity (Table 2) were differently distributed from what had already been described in clinical trials of apatinib [20], [23]. Our population was much younger than the participants in previous trials [20], [23], which might influence the distribution of the side effects. Hypertension was not as severe in adolescents. However, the observed toxicity was severe, with a dose reduction of 59.50% and a rate of total apatinib‐related adverse events of 89.19%. Although the tumor burden was significantly reduced by this agent, the quality of life did not improve or was even worse with a decline in physical, role, emotional, cognitive and social functioning (p > .100). The pain was either not significantly relieved or became more severe during the initial 1–3 months, and gradually fell back to baseline afterwards.
The major limitations of our trial are the relatively small study population and the absence of a control group. However, several other similar studies provide sufficient data that could be used for comparison of the effectiveness and toxicity of this drug. A randomized trial design would have needed much more time to complete for such a rare tumor. We have thus used similar comparative factors, such as CBR and PFS, at ≥4 months to identify the therapeutic and toxic effects of this agent.
Conclusion
This trial shows that apatinib is an effective treatment regimen for patients with advanced osteosarcoma with high objective response rates. The duration of response to this drug was almost the same as previous described [3], [8], [9]. The toxicity of apatinib was severe but tolerable in adolescent patients.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The Jiangsu HengRui Medicine Co., Ltd. supported this study. We thank all the patients and their families for participating in this clinical trial. We also thank Dr. Danhua Shen and Dr. Kunkun Sun for reviewing the pathological slides of these osteosarcoma patients and Dr. Yuan Li for conducting clinical PET/CT evaluation of our patients and LetPub (Shanghai, China) for language editing.
Author Contributions
Conception and design: Lu Xie, Jie Xu, Wei Guo
Provision of study materials or patients: Wei Guo, Xiaodong Tang, Taiqiang Yan, Rongli Yang
Collection and assembly of data: Lu Xie, Jie Xu, Xin Sun
Data analysis and interpretation: Lu Xie, Jie Xu, Wei Guo
Manuscript writing: Lu Xie
Final approval of manuscript: Lu Xie, Jie Xu, Xin Sun, Xiaodong Tang, Taiqiang Yan, Rongli Yang, Wei Guo
Accountable for all aspects of the work: Lu Xie, Jie Xu, Xin Sun, Xiaodong Tang, Taiqiang Yan, Rongli Yang, Wei Guo
Disclosures
The authors indicated no financial relationships.
References
- 1.Arshi A, Sharim J, Park DY et al. Prognostic determinants and treatment outcomes analysis of osteosarcoma and Ewing sarcoma of the spine. Spine J 2017;17:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luetke A, Meyers PA, Lewis I et al. Osteosarcoma treatment ‐ Where do we stand? A state of the art review. Cancer Treat Rev 2014;40:523–532. [DOI] [PubMed] [Google Scholar]
- 3.Duffaud Florence, Blay JY, Mir, O et al. Results of randomized, placebo (PL)‐controlled phase II study evaluating efficacy and safety of regorafenib (REG) in patients (pts) with metastatic osteosarcoma (metOS), on behalf of the French Sarcoma Group (FSG) and Unicancer. Abstract presented at ASCO 2018 Annual Meeting; June 1–5, 2018; Chicago, IL; 11504. [Google Scholar]
- 4.Glade Bender JL, Adamson PC, Reid JM et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: A Children's Oncology Group Study. J Clin Oncol 2008;26:399–405. [DOI] [PubMed] [Google Scholar]
- 5.Freeman BB 3rd, Daw NC, Geyer JR et al. Evaluation of gefitinib for treatment of refractory solid tumors and central nervous system malignancies in pediatric patients. Cancer Invest 2006;24:310–317. [DOI] [PubMed] [Google Scholar]
- 6.Fox E, Aplenc R, Bagatell R et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan‐vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol 2010;28:5174–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois SG, Shusterman S, Ingle AM et al. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: A children's oncology group study. Clin Cancer Res 2011;17:5113–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie L, Ji T, Guo W. Anti‐angiogenesis target therapy for advanced osteosarcoma (Review). Oncol Rep 2017;38:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grignani G, Palmerini E, Dileo P et al. A phase II trial of sorafenib in relapsed and unresectable high‐grade osteosarcoma after failure of standard multimodal therapy: An Italian Sarcoma Group study. Ann Oncol 2012;23:508–516. [DOI] [PubMed] [Google Scholar]
- 10.Grignani G, Palmerini E, Ferraresi V et al. Sorafenib and everolimus for patients with unresectable high‐grade osteosarcoma progressing after standard treatment: A non‐randomised phase 2 clinical trial. Lancet Oncol 2015;16:98–107. [DOI] [PubMed] [Google Scholar]
- 11.Ramjiawan RR, Griffioen AW, Duda DG. Anti‐angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017;20:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014;26:605–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoluzzi L, Cacavio A, Ghesani M et al. Response to anti‐PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res 2016;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steeghs N, Nortier JW, Gelderblom H. Small molecule tyrosine kinase inhibitors in the treatment of solid tumors: An update of recent developments. Ann Surg Oncol 2007;14:942–953. [DOI] [PubMed] [Google Scholar]
- 15.Kuhnen C, Lehnhardt M, Tolnay E et al. Patterns of expression and secretion of vascular endothelial growth factor in malignant soft‐tissue tumours. J Cancer Res Clin Oncol 2000;126:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potti A, Ganti AK, Tendulkar K et al. Determination of vascular endothelial growth factor (VEGF) overexpression in soft tissue sarcomas and the role of overexpression in leiomyosarcoma. J Cancer Res Clin Oncol 2004;130:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding J, Chen X, Gao Z et al. Metabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor‐2 inhibitor apatinib in humans. Drug Metab Dispos 2013;41:1195–1210. [DOI] [PubMed] [Google Scholar]
- 18.Liu K, Ren T, Huang Y et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL‐2 signaling in osteosarcoma. Cell Death Dis 2017;8:e3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Qin S, Xu J et al. Apatinib for chemotherapy‐refractory advanced metastatic gastric cancer: Results from a randomized, placebo‐controlled, parallel‐arm, phase II trial. J Clin Oncol 2013;31:3219–3225. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Qin S, Xu J et al. Randomized, double‐blind, placebo‐controlled phase III trial of apatinib in patients with chemotherapy‐refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–1454. [DOI] [PubMed] [Google Scholar]
- 21.Zhu B, Li J, Xie Q et al. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: An observational study. Cancer Biol Ther 2017;19:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F, Liao Z, Zhao J et al. Efficacy and safety of Apatinib in stage IV sarcomas: Experience of a major sarcoma center in China. Oncotarget 2017;8:64471–64480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y, Tang F, Wang Y et al. Advanced alveolar soft part sarcoma responds to apatinib. Oncotarget 2017;8:50314–50322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 25.Verger E, Salamero M, Conill C. Can Karnofsky performance status be transformed to the Eastern Cooperative Oncology Group scoring scale and vice versa? Eur J Cancer 1992;28A:1328–1330. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services . Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Bethesda, MD: National Cancer Istitute, 2010. [Google Scholar]
- 27.Ayana BA, Negash S, Yusuf L et al. Reliability and validity of amharic version of EORTC QLQ‐C 30 questionnaire among gynecological cancer patients in Ethiopia. PLoS One 2016;11:e0157359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chien TW, Lin SJ, Wang WC et al. Reliability of 95% confidence interval revealed by expected quality‐of‐life scores: An example of nasopharyngeal carcinoma patients after radiotherapy using EORTC QLQ‐C 30. Health Qual Life Outcomes 2010;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wierzbicka M, Kuśnierkiewicz M, Wójtowicz JG et al. The quality of life in head and neck cancer patients: Description of randomized examination formula based on standardized questionnaires EORTC QLQ C‐30, EORTC QTQ‐H‐N35 and Kiel Questionnaire [in Polish]. Otolaryngol Pol 2001;55:287–292. [PubMed] [Google Scholar]
- 30.Schuetze SM, Zhao L, Chugh R et al. Results of a phase II study of sirolimus and cyclophosphamide in patients with advanced sarcoma. Eur J Cancer 2012;48:1347–1353. [DOI] [PubMed] [Google Scholar]
- 31.Ruan X, Padnos IW, Kaye AD. Validation of a new “objective pain score” vs. “numeric rating scale” for the evaluation of acute pain: A comparative study. Anesth Pain Med 2016;6:e38886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie L, Guo W, Wang Y et al. Apatinib for advanced sarcoma: Results from multiple institutions’ off‐label use in China. BMC Cancer 2018;18:396. [DOI] [PMC free article] [PubMed] [Google Scholar]