The analysis presented in this article provides a guide to health care providers for the management of gastrointestinal high‐grade neuroendocrine carcinoma, based on data available to date.
Keywords: High‐grade, Gastrointestinal, Neuroendocrine carcinoma, Management
Abstract
Background.
High‐grade neuroendocrine carcinomas are rare in the gastrointestinal tract. However, treatment patterns and outcomes have not been well described.
Subjects, Materials, and Methods.
The National Cancer Database was analyzed. The primary objective was to describe the clinical outcomes and identify prognostic factors. Univariate and multivariate analyses were done to identify factors associated with patient outcome.
Results.
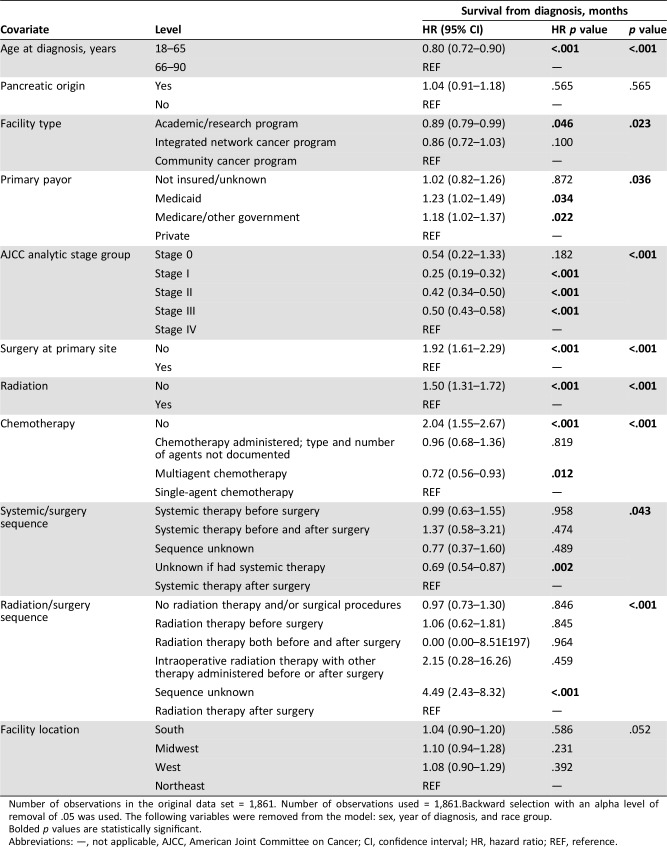
A total of 1,861 patients were identified between 2004 and 2013. The mean age was 63 years (standard deviation ±13). The majority of the patients (78.1%) were non‐Hispanic whites. The most common primary sites were pancreas (pancreatic neuroendocrine tumor [PNET] = 19.4%), large intestine (18.1%), esophagus (17.8%), and rectum (15.5%). Stage at presentation was I (6.6%), II (10.5%), III (18%) and IV (64.6%). Only 1.6% of the patients had brain metastases. Surgical resection was the primary therapy in 27.9%, and their median overall survival (OS) was 13.3 months. Patients treated with palliative chemotherapy had a median OS of 11.2 months, compared with 1.7 months for untreated patients. The median OS for high‐grade PNET was 6 months, compared with 9.9 months for other high‐grade gastrointestinal neuroendocrine carcinomas (HG GI NEC). On univariable analysis, age < 65 years (hazard ratio [HR] 0.72; 0.66–0.8; p < .001) and treatment at an academic center (HR 0.88; 0.79–0.99; p < .034) were associated with improved survival. Multivariable analysis confirmed prognostic advantage of treatment at an academic center.
Conclusion.
This is the largest series of HG GI NEC. Most patients present with metastatic disease, and overall survival remains poor. Treatment at an academic center, younger age, and use of chemotherapy were associated with improved survival. Multiagent chemotherapy was found to be associated with superior survival compared with single‐agent chemotherapy, which was superior to no chemotherapy. Temporal sequences of chemotherapy, surgery, and radiation administration were not found to be associated with survival differences on multivariable analysis.
Implications for Practice.
Management of patients with high‐grade gastrointestinal neuroendocrine carcinomas (HG GI NEC) is based on experience with small‐cell lung cancer. In this retrospective review, most patients had advanced disease and pancreatic primary had worse outcomes. Treatment at an academic center, younger age, and use of chemotherapy are associated with improved survival. Patients with early‐stage disease treated with resection alone had inferior outcomes compared with patients who received neoadjuvant or adjuvant therapy, suggesting that micrometastases contribute to poor surgical outcomes. The relatively high proportion of positive surgical margin favors downstaging with neoadjuvant therapy to improve resection and lower the risk of systemic recurrence.
摘要
背景。高级别神经内分泌肿瘤在胃肠道领域很罕见。然而,人们尚未很好地描述治疗模式及预后。
受试者、材料和方法。我们对国家癌症数据库进行分析。主要目标为描述临床预后并识别预后因素。为了识别与患者预后相关的因素,我们执行了单变量和多变量分析。
结果。在 2004 年至 2013 年期间,一共找到 1 861 名患者。平均年龄为 63 岁(标准差 ±13 岁)。大多数患者 (78.1%) 为非西班牙裔白人。最常见的好发部位为胰腺 [胰腺神经内分泌肿瘤 (PNET)= 19.4%]、大肠 (18.1%)、食道 (17.8%) 和直肠 (15.5%)。患者出现症状的分期为 I 期 (6.6%)、II 期 (10.5%)、III 期 (18%) 和 IV 期 (64.6%)。仅有 1.6% 的患者出现脑转移。手术切除为首选治疗措施,占 27.9%,患者的中位总生存期 (OS) 为 13.3 个月。接受姑息化疗的患者的中位 OS 为 11.2 个月,而未经治疗的患者的中位 OS 为 1.7 个月。高级别 PNET 的中位 OS 为 6 个月,而其他高级别胃肠道神经内分泌肿瘤 (HG GI NEC) 的中位 OS 为 9.9 个月。在单变量分析中,年龄 < 65 岁 [风险比 (HR)0.72;0.66–0.8;p < 0.001]和在学术中心接受治疗(HR 0.88;0.79–0.99;p < 0.034)与延长生存期相关。多变量分析确认了在学术中心接受治疗的预后优势。
结论。这是最大系列的HG GI NEC。大多数患者出现了转移性疾病,总生存期仍然很短。在学术中心接受治疗、年龄较小以及采用化疗均与延长生存期相关。我们发现,与优于不采用化疗的单药剂化疗相比,多药剂化疗与更长的生存期相关。我们未发现化疗、手术和放疗的时间顺序与多变量分析中的生存期差异相关。
实践意义:高级别胃肠道神经内分泌肿瘤 (HG GI NEC) 患者的治疗以小细胞肺癌方面的经验为依据。在本次回顾性调查中,大多数患者患有晚期疾病,原发性胰腺疾病患者的预后较差。在学术中心接受治疗、年龄较小以及采用化疗均与延长生存期相关。与接受新辅助治疗或辅助治疗的患者相比,仅接受切除术治疗的早期疾病患者具有较差的预后,这表明微小转移会导致手术效果不好。手术切缘阳性比例相对较高,这促进了采用新辅助治疗降期,从而改进切除术并降低全身复发的风险。
Introduction
High‐grade poorly differentiated neuroendocrine carcinomas (HG NEC) are rare cancers. The incidence of high‐grade gastrointestinal neuroendocrine carcinomas (HG GI NEC) is increasing [1], [2], [3], [4], [5], [6]. These tumors are nonsecretory and demonstrate aggressive clinical behavior [1], [7]. The published literature regarding the molecular and biologic alterations in gastrointestinal HG NEC is very limited and is mostly based on single institutional series with small number of patients [8], [9], [10]. Although the biology and clinical behavior of these tumors are unique, current treatment paradigms are extrapolated from management guidelines for small‐cell lung cancer (SCLC) [2]. Metastatic disease is treated with systemic chemotherapy, with a doublet combination of etoposide and a platinum agent reported in a retrospective analysis [11]. HG GI NEC are also associated with variable clinical response [12]. The true response to etoposide‐based regimens is unknown, with reported response ranging from 30% to 67% in the first‐line setting [13]. Long‐term survival for patients with metastatic HG GI NEC remains poor. Surgery and radiation are typically reserved for select cases with limited extent of disease [9], [14]. Unfortunately, even patients with early‐stage disease invariably recur with minimal response on further lines of treatment [15].
The largest published series of advanced gastrointestinal neuroendocrine carcinomas have been the hospital‐based NORDIC NEC study [13]. A total of 252 patients who were diagnosed between 2000 and 2009 were retrospectively reviewed at 12 Nordic hospitals. Factors found to positively influence survival were performance status, colorectal primary, and elevated platelet counts and LDH levels. The median overall survival (OS) for patients with metastatic disease treated with a platinum‐etoposide doublet was 11 months. Given the rarity of these tumors and the complexity of their management, designing and conducting prospective randomized trials has been limited. Understanding the clinical presentation, management, prognostic factors, and outcomes of HG GI NEC is central to defining treatment guidelines and designing future trials. The National Cancer Database (NCDB) obtains data from all U.S. hospitals that contribute to this national cancer registry, capturing about 70% of incident cancer cases in the U.S. from more than 1,500 Commission‐on‐Cancer‐accredited cancer programs. In order to address these questions, the NCDB was used to evaluate current pattern and predictors of different treatment modalities for patients with HG GI NEC.
Subjects, Materials, and Methods
The NCDB contains clinical and demographic information and is a joint quality improvement initiative of the American College of Surgeons Commission on Cancer and the American Cancer Society.
Eligible patients were defined as high‐grade NET (International Classification of Diseases for Oncology, third edition) with morphological codes for high‐grade NET, small‐cell‐like, or poorly differentiated NET (8002/3, 8041/3) involving various parts of the gastrointestinal tract from the esophagus to the anus (C15, C16, C17, C18, C19, C20, C21, C22, C23, C24, C25, C26), between the years 2004 and 2013. The primary outcome was overall survival of patients with HG GI NEC. Patient‐specific covariates included age, gender, race, histology, insurance status, presence of metastatic disease and comorbid medical conditions, year of diagnosis, and location where treatment was received. The cancer staging manual (fifth and sixth editions) of the American Joint Committee on Cancer (AJCC) were used for coding by the contributing hospitals and were used for clinical and pathologic staging in the database. Ethical approval was not required for the study because patient information in the database is completely deidentified and the database is legally accessible to the public.
Statistical Analysis
The clinical and demographic characteristics of the patients were summarized using descriptive statistics as appropriate for variable type and distribution. All clinically meaningful variables were included and subsequently eliminated based on level of significance. Univariate and multivariate analyses were conducted to identify factors associated with patient outcome. To assess the association between patient characteristics and survival, Cox proportional hazards models were fitted with a backward elimination method (removal criteria p = .05). Likelihood ratio test (LRT) was used to compare the model with the covariate being assessed, both added with the model and with the assessed covariate dropped. An alpha level of .05 was used, and any covariate with LRT p value > .05 was removed from the final multivariate model. We used backward elimination to automate the LRTs, and determine the final model with the covariates presented. Kaplan‐Meier curves were generated for overall survival. All analyses were done using SAS 9.4 (SAS Institute, Inc., Cary, NC) with a significant level of 0.05.
Results
Patient Demographics
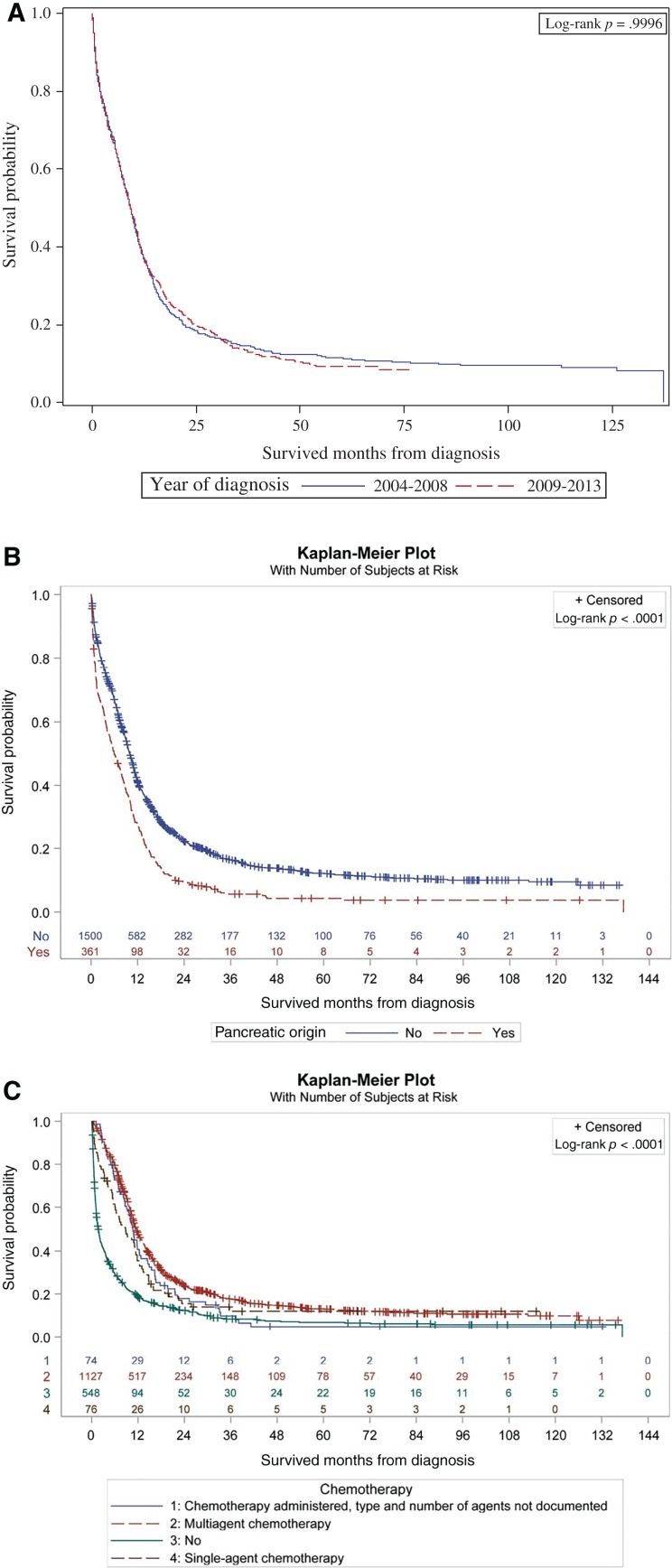
A total of 1,861 patients were identified for the 10 years of the study. The mean age was 63 years (standard deviation ±13), with a male preponderance (53.3%). About 78% were non‐Hispanic whites (Table 1). The most common primary sites were pancreas (pancreatic neuroendocrine tumor [PNET] = 19.4%), followed by large intestine (18.1%), esophagus (17.8%), and rectum (15.5%). Distribution across AJCC stages I–IV was 6.6%, 10.5%, 18%, and 64.6% consecutively. The most common sites of metastatic spread were liver (22.3%), bone (5%), lung (4.8%), and brain (1.6%). PNET was the most common primary site of brain metastasis (12 patients; 41%). About 53% of the patients were treated at community practices, whereas 32.5% were treated at academic or research cancer centers. Insurance coverage was mostly Medicare (45.4%) or private insurance policies (39.9%). The median interval from diagnosis to treatment was 21 days. At the time of data analysis, the mean follow‐up period was 15 months, with the longest follow‐up being 137 months. A higher number of patients were diagnosed between 2009 and 2013 (53.5%) compared with the 2004–2008 treatment period (46.5%). However, there was no difference in survival between these two treatment periods, with equal overall median survival of 9.3 months (hazard ratio [HR] 0.9996; 0.91–1.10; p = .9996; Fig. 1A).
Table 1. Descriptive statistics.
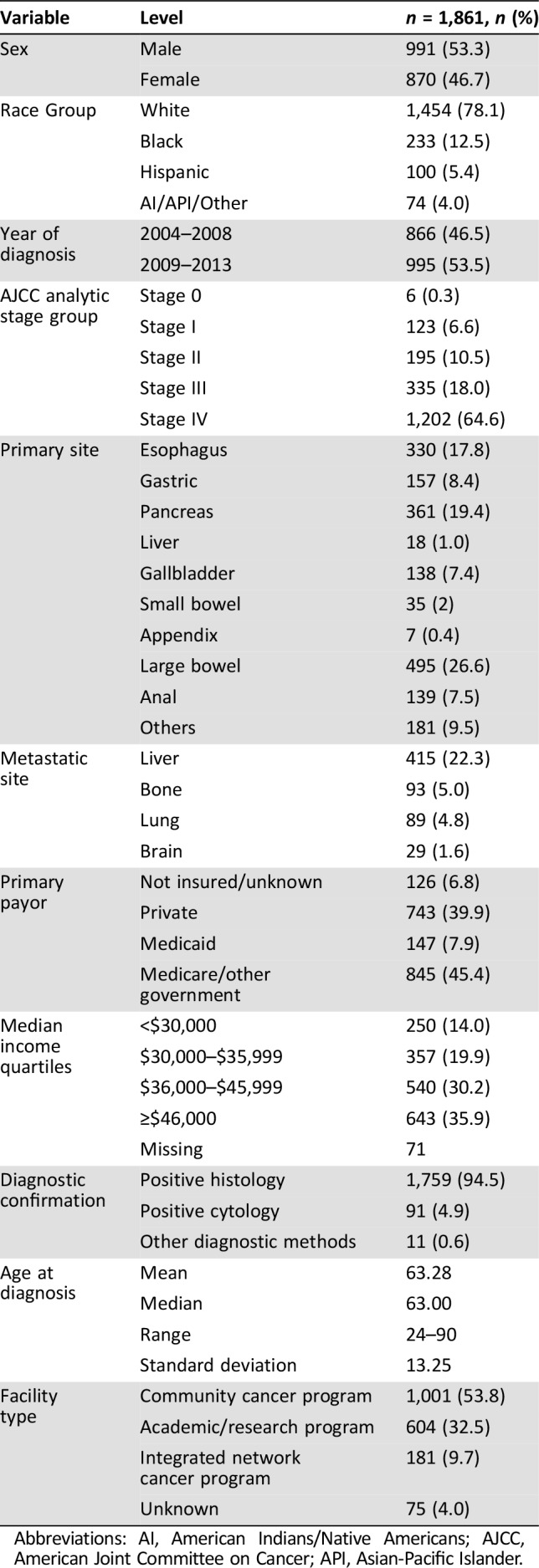
Abbreviations: AI, American Indians/Native Americans; AJCC, American Joint Committee on Cancer; API, Asian‐Pacific Islander.
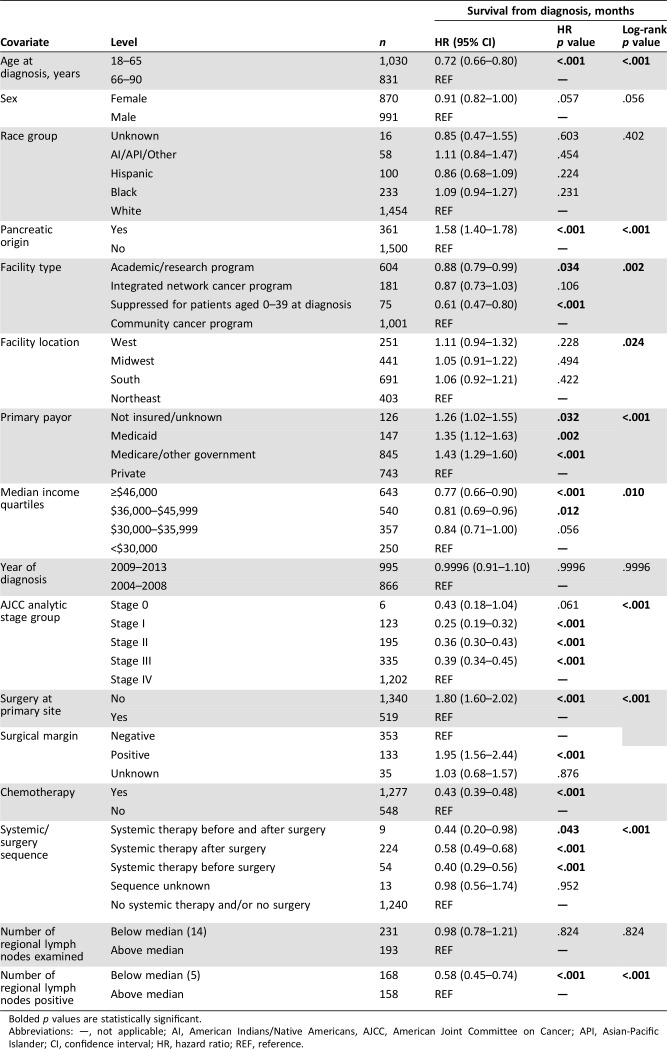
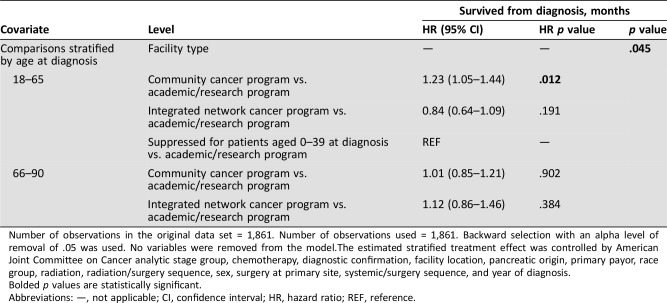
Figure 1.
Kaplan‐Meier plots. (A): Survival curves by year of diagnosis group (2004–2008 vs. 2009–2013; (B): primary (pancreatic origin vs. others; (C): chemotherapy treatment.
Surgical Treatment
About 28% of the study population were treated with surgical resection (Table 2). An equal number of patients (9 each) refused or died before planned surgical resection, and 67 patients (3.6%) had comorbid medical conditions prohibiting surgery. The median inpatient hospital stay was 7 days (range: 1–74), and 2.5% of the patients had unplanned readmission within 30 days following discharge. Median OS for patients who underwent surgical resection was 13.3 months, with 21.5% alive at 5 years. About a quarter of the patients who underwent resection had positive margins on pathologic evaluation, with unknown margin status in 6.8% of the resected cases. Positive margin at resection (HR 1.95; 1.56–2.44; p < .001) was associated with worse outcomes.
Table 2. Treatment pattern.
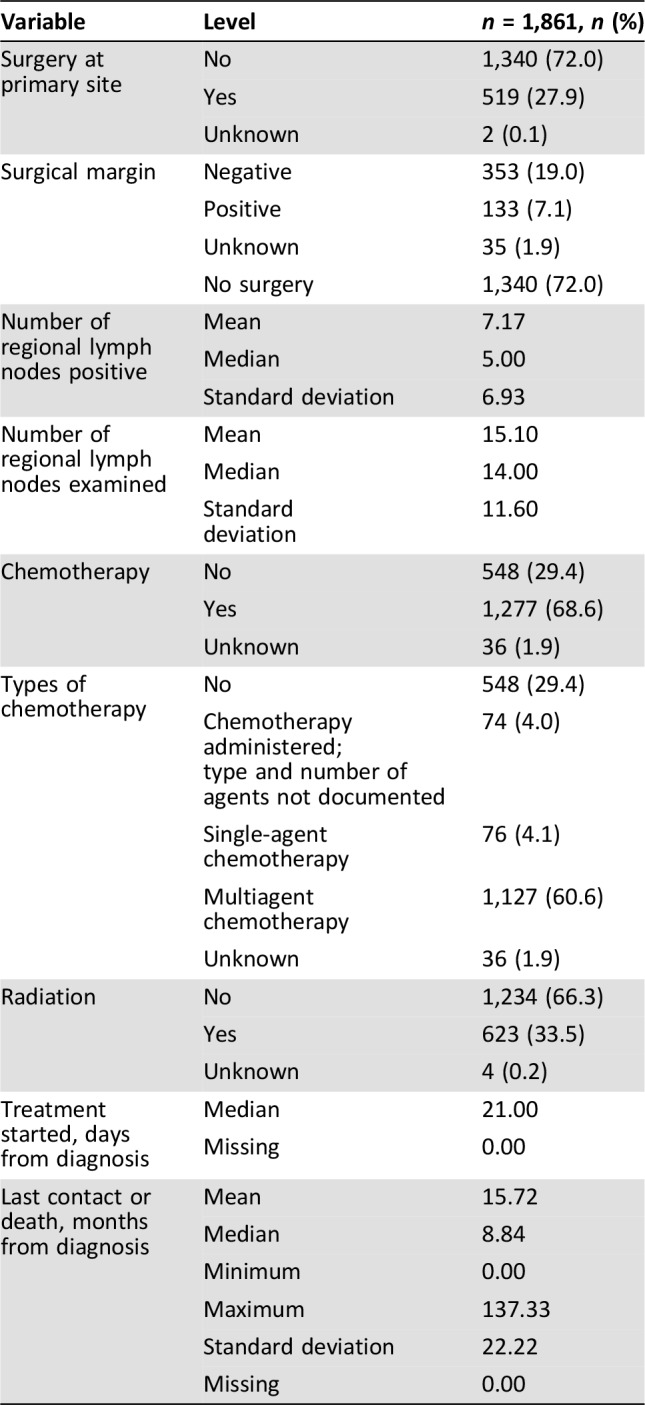
The median number of lymph nodes sampled was 14, with a positive rate of about 50%. There was no association between number of resected lymph nodes and survival (HR 0.98; 0.78–1.21; p = .824). An association between number of positive lymph nodes and survival was observed. Patients who had fewer than five positive lymph nodes demonstrated improved overall survival compared with patients with more than five positive lymph nodes (HR 0.58; 0.45–0.74; p < .001). Chemotherapy was administered preoperatively in about 3% of the patients, whereas 12% received postoperative chemotherapy. Nine patients (0.5%) received both preoperative and postoperative chemotherapy. Preoperative radiation was administered in 2.5% of the patients, and 5.7% received postoperative radiation therapy. Although the number of patients involved was rather small, preoperative chemotherapy administration (n = 54, HR 0.40; 0.29–0.56; p < .001), postoperative chemotherapy (n = 224, HR 0.58; 0.49–0.68; p < .001), and peri‐operative administration (n = 9, HR 0.44; 0.20–0.98; p = .043) were all associated with improved rates of survival.
Unresectable and Metastatic Disease
Seventy‐two percent of the patients had unresectable (7.4%) or metastatic disease (64.6%). These patients had worse overall outcomes when compared with those with resectable disease (HR 1.8; 1.6–2.02; p < .001). The most common site of metastases was liver (22.3%), followed by bone (5.0%) and lungs (4.8%). Less than 2% (1.6%) of the patients had brain metastases. Seventy‐six patients (4.1%) were treated with single‐agent chemotherapy, whereas 1,127 patients (60.6%) received multiagent or combined chemotherapy. Details of the specific chemotherapy agents are not reported in the database. Twenty‐eight patients (1.5%) died before planned chemotherapy, and 61 (3.3%) refused chemotherapy. Patients treated with chemotherapy had improved survival (HR 0.43; 0.39–0.48; p < .001), with a median OS of 11.2 months compared with 1.7 months for those who did not (Fig. 1B). When stratified by primary origin, patients with PNET (HR 0.27; 0.22–0.35; p < .001) and non‐PNET (HR 0.39; 0.33–0.45; p < .001) both benefitted from chemotherapy.
Prognostic Factors
Patients younger than 65 years of age had significantly improved survival when compared with those older than 65 years (HR 0.72; 0.66–0.8; p < .001; Table 3). Treatment at an academic center (HR 0.88; 0.79–0.99; p < .034) was also associated with improved survival. Multivariable analysis of interaction between younger age and treatment at an academic medical center was found to be statistically significant (Table 4). Patients 65 years and younger had improved survival when treated at an academic program compared with a community cancer center (HR 1.23; 1.05–1.44; p = .012).
Table 3. Univariate association with overall survival (from diagnosis).
Bolded p values are statistically significant.
Abbreviations: —, not applicable; AI, American Indians/Native Americans, AJCC, American Joint Committee on Cancer; API, Asian‐Pacific Islander; CI, confidence interval; HR, hazard ratio; REF, reference.
Table 4. Multivariable survival analysis of overall survival—interaction of age and facility type.
Number of observations in the original data set = 1,861. Number of observations used = 1,861. Backward selection with an alpha level of removal of .05 was used. No variables were removed from the model.The estimated stratified treatment effect was controlled by American Joint Committee on Cancer analytic stage group, chemotherapy, diagnostic confirmation, facility location, pancreatic origin, primary payor, race group, radiation, radiation/surgery sequence, sex, surgery at primary site, systemic/surgery sequence, and year of diagnosis.
Bolded p values are statistically significant.
Abbreviations: —, not applicable; CI, confidence interval; HR, hazard ratio; REF, reference.
The income levels of the patients also correlated with overall survival. The highest income quartile ($46,000+) was associated with improved survival compared with patients earning less than $30,000 in the lowest income quartile (HR 0.77; 0.66–0.90; p < .001). The same trend was found with patients within the next highest income quartile ($36,000–$45,999) when compared with the lowest quartile (HR 0.81; 0.69–0.96; p = .012). Unresectable disease (HR 1.8; 1.6–2.02; p < .001) was associated with worse outcomes, and patients who had fewer than five lymph nodes positive for malignancy had improved overall survival (HR 0.58; 0.45–0.74; p < .001). There were, however, no survival outcome differences between gender (HR 0.91; 0.82–1.00; p = .057), racial groups, or geographic location of treatment facility on sensitivity analysis. Finally, multivariable analysis confirmed prognostic advantage of surgical resection, treatment with chemotherapy or radiation, and care at an academic center (Table 5). In addition, multiagent chemotherapy was found to be associated with superior survival when compared with single‐agent chemotherapy, which was superior to no chemotherapy. Temporal sequences of chemotherapy, surgery, and radiation administration were not found to be associated with survival differences on multivariable analysis.
Table 5. Multivariable survival analysis of overall survival (main effect).
Number of observations in the original data set = 1,861. Number of observations used = 1,861.Backward selection with an alpha level of removal of .05 was used. The following variables were removed from the model: sex, year of diagnosis, and race group.
Bolded p values are statistically significant.
Abbreviations: —, not applicable, AJCC, American Joint Committee on Cancer; CI, confidence interval; HR, hazard ratio; REF, reference.
Compared with other histologic subtypes, PNET was associated with less bone metastasis, but more brain, liver, and lung metastases. Pancreatic NET has statistically significantly worse outcomes when compared with other HG GI NEC on univariate analysis (HR 1.58; 1.40–1.78; p < .001). The median OS for high‐grade PNET was 6 months, compared with 9.9 months for other HG GI NEC (Fig. 1C). One‐year and 5‐year survival rates were 27.5% vs. 41% and 4.5% vs. 12.3%, respectively. Although PNET histology was associated with lower surgical resection rate on univariate analyses (6.09% vs. 33.1%; p < .001), it was significantly less associated with positive margins (0.83% vs. 8.67%; p < .001). When compared with other HG GI NEC types, PNET was also associated with lower rates of use of chemotherapy (59.8% vs. 70.7%; p < .001) and radiation treatment (18.9% vs. 37%; p < .001). PNET was associated with higher rate of diagnosis by cytology rather than core biopsy (17.5% vs. 1.9%; p < .001). There was no survival difference on multivariable analysis between patients with high‐grade pancreatic neuroendocrine carcinoma and other HG GI NECs.
Discussion
The incidence of HG GI NEC between the two treatment periods (2009–2013 vs. 2004–2008) has increased significantly. This observation confirms previously reported series using different registries [13]. The increase could be a reflection of the increasing awareness of the pathologic diagnosis of HG NEC, or a true rising incidence [9], [15]. The survival of patients with HG GI NEC has not, however, significantly changed over the study period. This may be a reflection of the limited research in this area and the lack of randomized trials aimed at identifying novel therapies. Chemotherapy has been the mainstay of treatment in unresectable or advanced HG GI NEC [13], with combination of platinum compounds (such as cisplatin or carboplatin) and etoposide accepted as the first‐line standard of care in these patients [13]. The response to etoposide‐based regimens can range from 30% to 67% in the first‐line setting. However, patients invariably recur with minimal response on further lines of treatment. There are no established second‐ or subsequent‐line therapies, although several studies have evaluated, or are currently evaluating, different anticancer agents such as irinotecan and cisplatin (NCT00353015), paclitaxel/carboplatin/etoposide (NCT00193531), and FOLFIRINOX chemotherapy (NCT03042780).
Overall, treatment modalities are patterned after experience with the more common SCLC. The morphology and immunohistochemical pattern of both SCLC and extrapulmonary small cell carcinomas (EPSCCa) have traditionally led to adoption of the same treatment paradigms. They both respond initially to platinum chemotherapy, with frequent relapses and shortened survival. However, the appropriateness of this comparison is unknown given emerging differences in disease presentations, tumor biology, and treatment outcomes. Clinically, SCLC tends to metastasize to brain, which has led to routine prophylactic cranial irradiation [16], [17]. This contrasts with the incidence of brain metastases in patients with HG GI NEC in this study, which was extremely low. An example of difference in biology is the expression of programmed death (PD)‐1 and its ligands (L) in 94 cases of small‐cell carcinomas (61 pulmonary—SCLC; and 33 extrapulmonary—EPSCCa). The analysis showed differential correlations of PD‐L1+ tumor‐associated macrophages and PD‐1+ tumor‐infiltrating lymphocytes between the SCLC and EPSCCa groups [18].
The PD‐1 receptor‐ligand interaction is a major pathway exploited by tumors to suppress the body's natural immune control [19]. It has been shown to be expressed on activated lymphocytes including peripheral CD4+ and CD8+ T cells, B cells, T regs, and natural killer cells. Although healthy organs express little (if any) PD‐L1, aberrant expression of PD‐L1 has been demonstrated in various types of cancers. Previously published data show that the PD‐1/PD‐L1 pathway plays a critical role in tumor immune evasion and is a very attractive target for therapeutic intervention. An analysis of 32 metastatic gastroenteropancreatic (GEP)‐NEC cases showed a subgroup of 7 of 17 grade 3 GEP‐NECs/EPSCC (41.2%) patients with tumor expression of PD‐L1 [20]. In that analysis, PD‐L1 was significantly associated with high‐grade World Health Organization (WHO) classification (grade 3; p = .008). Clinical trials have established the efficacy and benefit of immune‐targeting agents in patients with SCLC and other malignancies who have progressed on standard chemotherapy, leading to U.S. Food and Drug Administration (FDA) approval in some of those disease subtypes. An objective response of 10% was achieved in the 98 recurrent small‐cell lung cancer patients who received single‐agent nivolumab 3 mg/kg on the CheckMate 032 trial [21]. These patients had previously been treated and become refractory to platinum‐based chemotherapy. More importantly, landmark analysis at 12 and 18 months showed that 20%–30% of patients were still alive, leading to the recent FDA approval. There are currently several trials evaluating pembrolizumab in econd‐and‐beyond lines of therapy in EPSCCa (NCT03290079, NCT03136055, and NCT03190213). Therefore, trials aimed at defining optimal management strategies for HG GI NEC are needed.
Factors contributing to poor outcomes in HG GI NEC patients include advanced unresectable or metastatic disease. However, the stage at presentation is not the only factor driving poor outcomes, because patients with early‐stage disease who underwent surgical resection in our series also had a relatively dismal median OS. Patients who received neoadjuvant or adjuvant therapy had better overall survival, suggesting that high incidence of micrometastasis contributes to the poor surgical outcomes. In addition, the relatively high proportion of margin‐positive resection raises the question of whether there is a role of downstaging with neoadjuvant therapy, aimed at enhancing resection and lowering risk of systemic recurrence. Clinical trials focused on multimodality management of early‐stage HG GI NEC are needed to establish best standards of care. Although small‐bowel NETs are the most common type of gastrointestinal neuroendocrine tumors [5], the low incidence of small‐bowel NEC (2%) in our series was consistent with previous reports [22].
The pancreas is the most common primary site of HG GI NECs and is associated with inferior median overall survival, 1‐year, and 5‐year survival rates compared with other GI NEC primaries. The reason for the poor outcome of pancreatic HG NEC could relate to the lower use of different treatment modalities (surgical resection, chemotherapy, and radiation) as shown in this study. Previously reported detrimental tumor biology of PNET invariably plays a crucial role in the disease course as well [23]. The widespread adoption of genomic profiling in GI malignancies may significantly improve our understanding of the differences in the biology of pancreatic HG NEC compared with HG GI NEC. This characterization may also provide new approaches for rational targeted treatment modalities [20], [24], [25], [26].
Higher median income was associated with improved survival as previously shown in a variety of clinical conditions [27]. Gender, racial groups, or geographic location of treatment facility were not shown to be associated with worse outcomes in patients with HG GI NEC. The other factors confirmed on multivariable analysis to be of prognostic advantage were surgical resection, treatment with chemotherapy or radiation, and care at an academic center. More than half the patients included in the analyses were treated at community cancer centers, but the prognostic advantage of care at an academic center follows previously reported trends for different rare diseases [28], [29]. Academic centers have a higher level of specialization and expertise in rare tumors. In addition, physicians in academic centers tend to rely more on multidisciplinary models of care, which have been shown to improve survival [30], [31]. On the other hand, potential for selection bias also exists in this analysis. Patients who seek care at academic institutions may have better performance status, higher levels of health literacy, or more resources (higher economic/income class), all of which could be impacting the better outcome in this setting.
Our analysis is the largest detailed review of the management approach employed for patients with HG GI NEC. Nonetheless, our findings have important limitations that should be considered. The retrospective nature of this work limits our ability to fully control for potential biases and confounders. The most recent (2017) WHO guidelines that include well‐differentiated G3 category are not included in NCDB data under analysis. Specific chemotherapy agents received by the patients cannot be identified from the database. However, chemotherapy options are limited for patients with HG GI NEC, and response patterns in clinical practice are similar across multiple regimens. Cancer‐specific survival can also not be calculated because the database does not capture the cause of the patient's death, and patterns of recurrence/progression‐free survival are not obtainable from the records. However, given the aggressive nature of this disease and short overall survival, it is plausible that the majority of the mortality is due to HG GI NEC. In clinical practice, treatment decisions are often based on the locoregional extent of the disease. Patients are classified as having limited or extensive/advanced stage, rather than the AJCC stages described in the database. However, the AJCC staging system allows for less interobserver variability because of the detailed tumor size, lymph node involvement, and metastatic status (TMN) ascribed to each case. Lastly, we observed that a significant number of cases had missing data regarding the specific surgical procedure employed.
Conclusion
Our study established the pattern of disease presentation and treatment outcomes for patients with HG GI NECs. These findings will guide future prospective research in this area, especially multi‐institutional/international studies that will prospectively define appropriate management strategies in early‐ and advanced‐stage HG GI NEC.
See http://www.TheOncologist.com for supplemental material available online.
Author Contributions
Conception/design: Olatunji B. Alese, Renjian Jiang, Walid Shaib, Christina Wu, Mehmet Akce, Madhusmita Behera, Bassel F. El‐Rayes
Provision of study material or patients: Olatunji B. Alese, Renjian Jiang, Walid Shaib, Christina Wu, Mehmet Akce, Madhusmita Behera, Bassel F. El‐Rayes
Collection and/or assembly of data: Renjian Jiang, Madhusmita Behera
Data analysis and interpretation: Olatunji B. Alese, Renjian Jiang, Walid Shaib, Christina Wu, Mehmet Akce, Madhusmita Behera, Bassel F. El‐Rayes
Manuscript writing: Olatunji B. Alese, Renjian Jiang, Walid Shaib, Christina Wu, Mehmet Akce, Madhusmita Behera, Bassel F. El‐Rayes
Final approval of manuscript: Olatunji B. Alese, Renjian Jiang, Walid Shaib, Christina Wu, Mehmet Akce, Madhusmita Behera, Bassel F. El‐Rayes
Disclosures
Christina Wu: Vaccinex, Bristol‐Myers Squibb, Boston Biomedical Inc, Lycera, Seattle Genetics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Staren ED, Gould VE, Jansson DS et al. Neuroendocrine differentiation in "poorly differentiated" colon carcinomas. Am Surg 1990;56:412–419. [PubMed] [Google Scholar]
- 2.Garcia‐Carbonero R, Sorbye H, Baudin E et al. ENETS consensus guidelines for high‐grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016;103:186–194. [DOI] [PubMed] [Google Scholar]
- 3.Heetfeld M, Chougnet CN, Olsen IH et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2015;22:657–664. [DOI] [PubMed] [Google Scholar]
- 4.Yao JC, Hassan M, Phan A et al. One hundred years after "carcinoid": Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Oberg K, Chung DC et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61–72. [DOI] [PubMed] [Google Scholar]
- 6.Dasari A, Shen C, Halperin D et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaffey MJ, Mills SE, Lack EE. Neuroendocrine carcinoma of the colon and rectum. A clinicopathologic, ultrastructural, and immunohistochemical study of 24 cases. Am J Surg Pathol 1990;14:1010–1023. [DOI] [PubMed] [Google Scholar]
- 8.Aytac E, Ozdemir Y, Ozuner G. Long term outcomes of neuroendocrine carcinomas (high‐grade neuroendocrine tumors) of the colon, rectum, and anal canal. J Visc Surg 2014;151:3–7. [DOI] [PubMed] [Google Scholar]
- 9.Ye L, Ye H, Zhou Q et al. A retrospective cohort study of pancreatic neuroendocrine tumors at single institution over 15 years: New proposal for low‐ and high‐grade groups, validation of a nomogram for prognosis, and novel follow‐up strategy for liver metastases. Int J Surg 2016;29:108–117. [DOI] [PubMed] [Google Scholar]
- 10.Basturk O, Tang L, Hruban RH et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: A clinicopathologic analysis of 44 cases. Am J Surg Pathol 2014;38:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patta A, Fakih M. First‐line cisplatin plus etoposide in high‐grade metastatic neuroendocrine tumors of colon and rectum (MCRC NET): Review of 8 cases. Anticancer Res 2011;31:975–978. [PubMed] [Google Scholar]
- 12.Raj N, Valentino E, Capanu M et al. Treatment response and outcomes of grade 3 pancreatic neuroendocrine neoplasms based on morphology: Well differentiated versus poorly differentiated. Pancreas 2017;46:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorbye H, Welin S, Langer SW et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol 2013;24:152–160. [DOI] [PubMed] [Google Scholar]
- 14.Mosquera C, Koutlas NJ, Fitzgerald TL. Localized high‐grade gastroenteropancreatic neuroendocrine tumors: Defining prognostic and therapeutic factors for a disease of increasing clinical significance. Eur J Surg Oncol 2016;42:1471–1477. [DOI] [PubMed] [Google Scholar]
- 15.Saclarides TJ, Szeluga D, Staren ED. Neuroendocrine cancers of the colon and rectum. Results of a ten‐year experience. Dis Colon Rectum 1994;37:635–642. [DOI] [PubMed] [Google Scholar]
- 16.Gregor A, Cull A, Stephens RJ et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: Results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer 1997;33:1752–1758. [DOI] [PubMed] [Google Scholar]
- 17.Auperin A, Arriagada R, Pignon JP et al. Prophylactic cranial irradiation for patients with small‐cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–484. [DOI] [PubMed] [Google Scholar]
- 18.Schultheis AM, Scheel AH, Ozretic L et al. PD‐L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 2015;51:421–426. [DOI] [PubMed] [Google Scholar]
- 19.McDermott DF, Atkins MB. PD‐1 as a potential target in cancer therapy. Cancer Med 2013;2:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim ST, Ha SY, Lee S et al. The impact of PD‐L1 expression in patients with metastatic GEP‐NETs. J Cancer 2016;7:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonia SJ, Lopez‐Martin JA, Bendell J et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 22.Dasari A, Mehta K, Byers LA et al. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018;124:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehehalt F, Saeger HD, Schmidt CM et al. Neuroendocrine tumors of the pancreas. The Oncologist 2009;14:456–467. [DOI] [PubMed] [Google Scholar]
- 24.Klempner SJ, Gershenhorn B, Tran P et al. BRAFV600E mutations in high‐grade colorectal neuroendocrine tumors may predict responsiveness to BRAF‐MEK combination therapy. Cancer Discov 2016;6:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim ST, Lee SJ, Park SH et al. Genomic profiling of metastatic gastroenteropancreatic neuroendocrine tumor (GEP‐NET) patients in the personalized‐medicine era. J Cancer 2016;7:1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meder L, Konig K, Ozretic L et al. NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int J Cancer 2016;138:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adler NE, Ostrove JM. Socioeconomic status and health: What we know and what we don't. Ann N Y Acad Sci 1999;896:3–15. [DOI] [PubMed] [Google Scholar]
- 28.Birkmeyer JD, Siewers AE, Finlayson EV et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 29.Begg CB, Cramer LD, Hoskins WJ et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 30.Bilimoria KY, Bentrem DJ, Ko CY et al. Multimodality therapy for pancreatic cancer in the U.S.: Utilization, outcomes, and the effect of hospital volume. Cancer 2007;110:1227–1234. [DOI] [PubMed] [Google Scholar]
- 31.La Torre M, Nigri G, Ferrari L et al. Hospital volume, margin status, and long‐term survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 2012;78:225–229. [PubMed] [Google Scholar]