Abstract
Lessons Learned.
Nivolumab treatment at doses of 3 mg/kg once every 2 weeks (Q2W), 240 mg Q2W, and 360 mg once every 3 weeks was well tolerated in the Chinese population, with no new safety signals identified.
Comparison of intensive pharmacokinetic profiles of nivolumab at 3 mg/kg Q2W in Chinese versus global populations revealed no ethnic differences of nivolumab treatment.
Nivolumab shows promising preliminary antitumor activity in nasopharyngeal carcinoma.
Background.
This phase I/II study investigated the safety and pharmacokinetics (PK) of nivolumab (anti‐programmed cell death‐1 monoclonal antibody) in Chinese patients with nasopharyngeal carcinoma (NPC) and other solid tumors.
Methods.
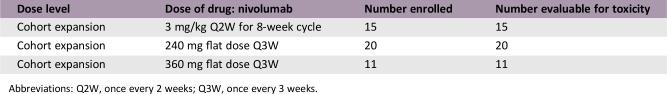
A dose evaluation phase (3 mg/kg once every 2 weeks [Q2W]) was followed by a cohort expansion phase (3 mg/kg Q2W or flat doses of 240 mg Q2W or 360 mg once every 3 weeks).
Results.
In the dose evaluation phase, 8/8 patients completed one cycle with no dose‐limiting toxicities. At data cutoff, 46/51 patients were evaluable for safety (all cohorts). Treatment‐related adverse events (TRAEs) occurred in 35 (76%) patients and were primarily grade 1–2; one patient (3 mg/kg Q2W) discontinued because of study drug toxicity. Intensive PK profiles at 3 mg/kg, 240 mg, and 360 mg were well characterized at single and multiple doses of nivolumab. An objective response was determined in six (6/46) patients, four (4/32) of whom had NPC tumors.
Conclusion.
Nivolumab monotherapy at 3 mg/kg and flat doses of 240 mg and 360 mg were well tolerated in this Chinese patient population, with PK profiles at 3 mg/kg being similar to those of global patients. Preliminary efficacy results showed promising antitumor activity of nivolumab in advanced NPC.
Abstract
经验总结
纳武单抗治疗剂量每 2 周 (Q2W) 3 mg/kg、240 mg Q2W、360 mg Q3W 的用药方式在中国人群中耐受性良好,没有发现新的安全信号。
对比 3 mg/kg Q2W 纳武单抗用药方式在中国与全球人群中的强化药代动力学特征,结果显示,纳武单抗治疗没有种族差异。
纳武单抗在鼻咽癌治疗中,具有良好的初步抗肿瘤活性。
摘要
背景。本项 I/II 期研究探讨了纳武单抗(抗程序性细胞死亡‐1 单克隆抗体)在中国鼻咽癌 (NPC)及其他实体肿瘤患者中的安全性与药代动力学 (PK) 特征。
方法。剂量评估阶段 [每 2 周(Q2W) 3 mg/kg]之后,开展队列扩展(3 mg/kg/Q2W 或 240 mg Q2W 或每 3 周 360 mg 的固定剂量)。
结果。在剂量评估阶段,8/8 例患者完成了第一个疗程,未出现剂量限制性毒性。数据截止时,46/51 名患者接受了安全性评估(所有队列)。35 例 (76%) 患者产生治疗相关不良事件 (TRAE),以 1‐2 级事件为主;1 例 (3 mg/kg Q2W) 因产生研究药物毒性而停药。3mg/kg,240 mg 和 360 mg强化PK特性在单剂量和多剂量纳武单抗得到良好表征。6 例 (6/46) 患者出现客观反应,4 例 (4/32) 为NPC。
结论。纳武单抗 3mg /kg、和 240 mg、360 mg 固定剂量单药治疗在中国患者中耐受性良好,3mg/kg 的 PK 分析结果与全球患者相似。初步疗效表明,纳武单抗对晚期NPC具有良好的抗肿瘤活性。
Discussion
Nivolumab is a human monoclonal antibody that targets the programmed cell death‐1 receptor. Nivolumab has been approved for the treatment of various types of cancer in >60 countries, and numerous clinical trials are ongoing. Although the overall efficacy, safety, and PK of nivolumab have been extensively studied, these data were established in predominantly Caucasian populations. The safety and intensive PK of nivolumab flat‐dose regimens (240 mg Q2W or 360 mg once every 3 weeks) have not been assessed in Chinese patients.
This single‐center, open‐label, phase I/II study assessed the safety and tolerability of nivolumab in Chinese adult patients with previously treated, advanced or recurrent NPC or other solid tumors. Secondary objectives were PK, immunogenicity, and preliminary antitumor activity.
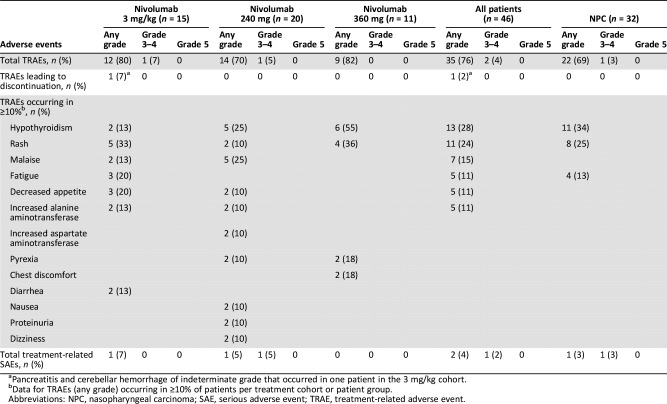
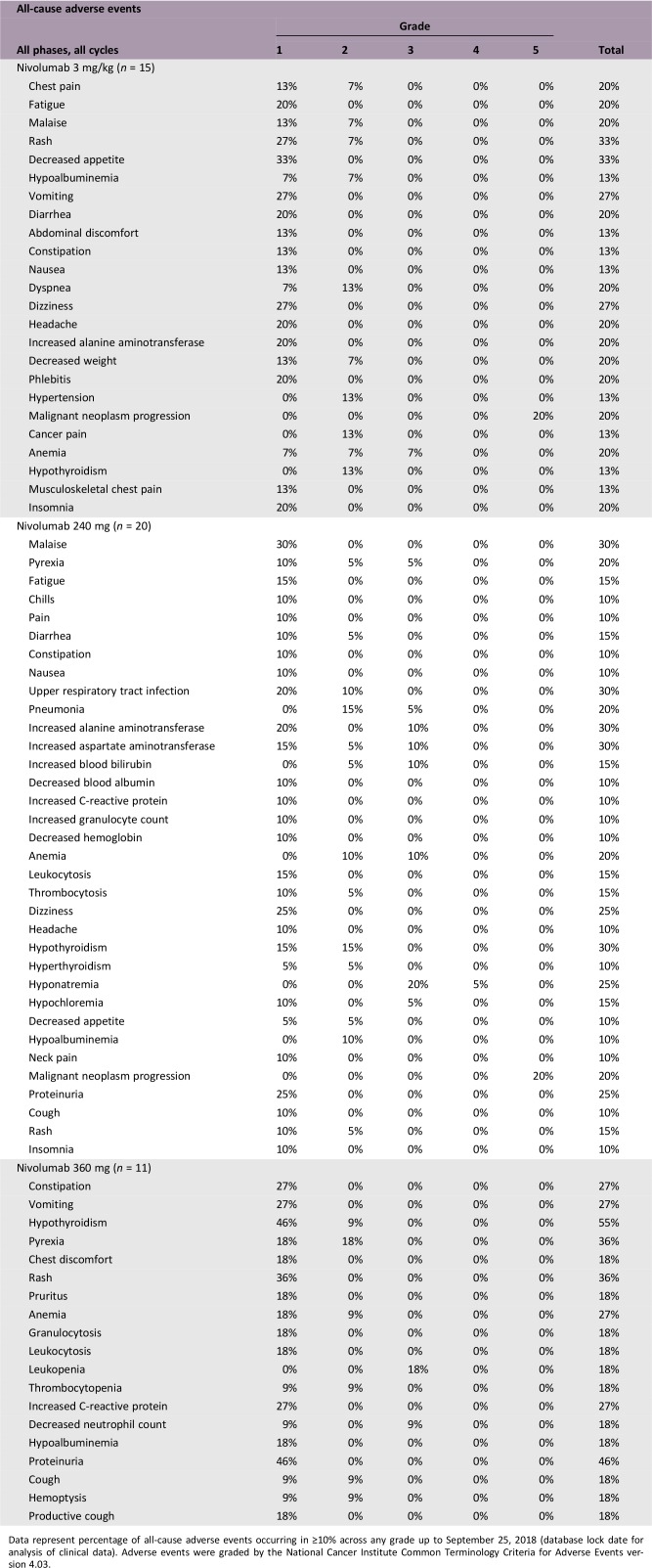
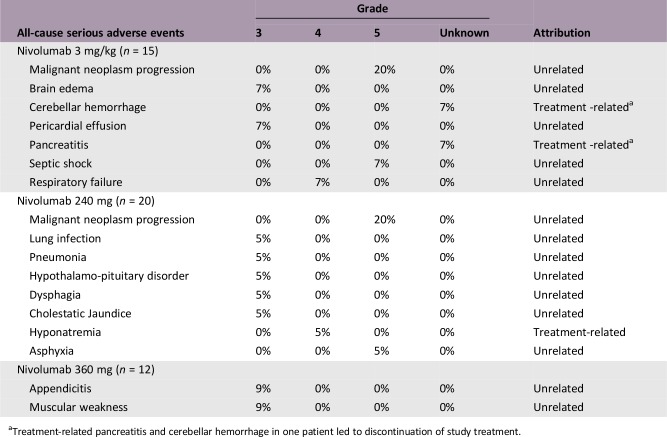
There were no new safety signals for nivolumab in Chinese patients, and safety profiles were consistent with prior clinical studies (Table 1). Of 46 evaluable patients, 35 experienced TRAEs; there were no grade 4–5 TRAEs, and only two were grade 3 (asthenia, 3 mg/kg cohort; hypochloremia, 240 mg cohort). Two serious TRAEs occurred, a grade 4 hyponatremia (240 mg cohort) and an indeterminate grade pancreatitis and cerebellar hemorrhage (3 mg/kg cohort) that led to discontinuation.
Table 1. Summary of treatment‐related adverse events and serious adverse events.
Pancreatitis and cerebellar hemorrhage of indeterminate grade that occurred in one patient in the 3 mg/kg cohort.
Data for TRAEs (any grade) occurring in ≥10% of patients per treatment cohort or patient group.
Abbreviations: NPC, nasopharyngeal carcinoma; SAE, serious adverse event; TRAE, treatment‐related adverse event.
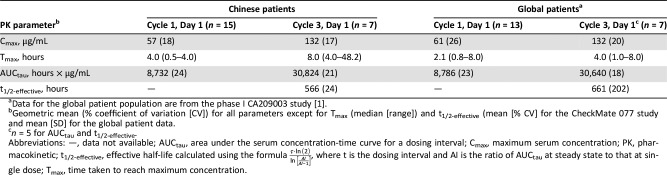
Intensive PK profiles were characterized after first nivolumab dose and at steady state in all cohorts; PK data were compared with results from global patients and revealed no ethnic sensitivity at 3 mg/kg Q2W (Table 2). At data cutoff, antidrug antibodies were detected in 1 of 41 patients tested (3 mg/kg cohort).
Table 2. Summary of pharmacokinetic parameters of nivolumab treatment (3 mg/kg, once every 2 weeks) in Chinese and global patient populations.
Geometric mean (% coefficient of variation [CV]) for all parameters except for Tmax (median [range]) and t1/2‐effective (mean [% CV] for the CheckMate 077 study and mean [SD] for the global patient data.
n = 5 for AUCtau and t1/2‐effective.
Abbreviations: —, data not available; AUCtau, area under the serum concentration‐time curve for a dosing interval; Cmax, maximum serum concentration; PK, pharmacokinetic; t1/2‐effective, effective half‐life calculated using the formula , where t is the dosing interval and AI is the ratio of AUCtau at steady state to that at single dose; Tmax, time taken to reach maximum concentration.
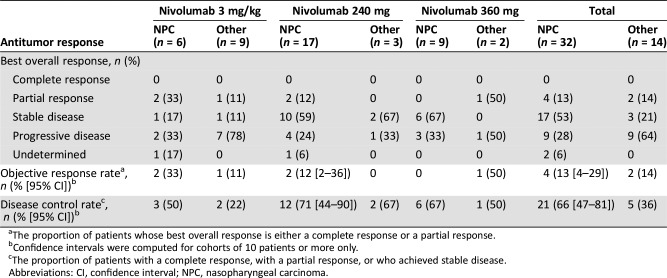
Among the 32 patients (70%) with NPC, the objective response rate was 13% (95% confidence interval [CI]: 4–29) and the disease control rate was 66% (95% CI: 47–81). The median follow‐up duration was 7.5 months (range: 0.8–24.7 months), and median progression‐free survival was 3.5 months (95% CI: 1.8–5.5).
In this pretreated group of patients with predominantly NPC, our study showed that the safety, tolerability, and PK (3 mg/kg) of nivolumab are similar in Chinese and global populations. Furthermore, promising antitumor activity of nivolumab in NPC suggests that further studies are warranted.
Trial Information
- Disease
Advanced cancer/solid tumor only
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
≥1 prior regimen
- Type of Study ‐ 1
Phase I/II
- Type of Study ‐ 2
Dose evaluation and cohort expansion
- Primary Endpoint
Safety and tolerability
- Secondary Endpoint
Pharmacokinetics
- Secondary Endpoint
Immunogenicity
- Secondary Endpoint
Efficacy
- Additional Details of Endpoints or Study Design
- Dose evaluation and cohort expansion phases: Based on global clinical experience, a nivolumab dose of 3 mg/kg was selected for the initial 8‐week dose evaluation phase. The study proceeded to the cohort expansion phase following the absence of dose‐limiting toxicities in any of the treated patients. In the cohort expansion phase, three patient cohorts received differing nivolumab regimens (3 mg/kg, 240 mg, and 360 mg) until disease progression or unacceptable toxicity, for a maximum of 2 years. At the time of data analysis, a fourth expansion cohort (480 mg flat dose, once every 4 weeks [Q4W]) was active and not yet recruiting.
- Investigator's Analysis
Drug tolerable, efficacy undetermined
Drug Information
- Drug 1
- Generic/Working Name
Nivolumab
- Trade Name
Opdivo
- Company Name
Bristol‐Myers Squibb Pharmaceuticals Ltd.
- Drug Type
Antibody
- Drug Class
Immune therapy
- Dose
3 mg/kg, 240 mg, 360 mg
- Route
IV
- Schedule of Administration
- Dose evaluation phase: 3 mg/kg Q2W for 8 weeks. Cohort expansion phase: 3 mg/kg Q2W; or 240 mg flat dosing Q2W; 360 mg flat dosing once every 3 weeks (Q3W); 480 mg flat dosing Q4W. Note: At the time of data analysis, a fourth expansion cohort (480 mg flat dose Q4W) was active and not yet recruiting. The treatment cycles for the dose evaluation phase and 3 mg/kg Q2W and 240 mg Q2W dose expansion cohorts were 8 weeks' duration (four doses per cycle), whereas the treatment cycles for the 360 mg Q3W cohort were 3 weeks' duration (one dose per cycle).
Dose Escalation Table
Abbreviations: Q2W, once every 2 weeks; Q3W, once every 3 weeks.
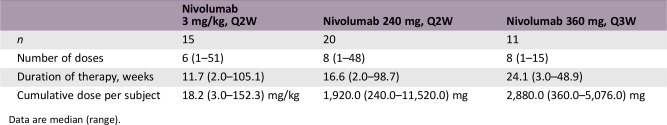
Dose Information: Evaluable Safety and PK Population
Data are median (range).
Patient Characteristics
- Number of Patients, Male
31
- Number of Patients, Female
15
- Stage
Advanced or recurrent
- Age
Median (range): 48 (27–72)
- Number of Prior Systemic Therapies
Median (range): 3 (1 to ≥4)
- Performance Status: ECOG
-
0 — 17
1 — 29
- Other
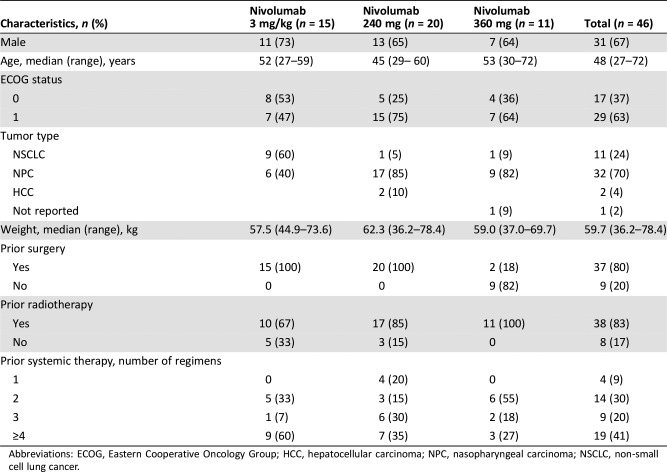
Race: Asian 100%; Complete baseline demographic and disease characteristics are presented in Table 3.
- Cancer Types or Histologic Subtypes
-
Hepatocellular carcinoma, 2
Lung, non‐small cell, 11
Nasopharyngeal carcinoma, 32
Not reported, 1
Table 3. Baseline patient characteristics and prior cancer therapy.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; NPC, nasopharyngeal carcinoma; NSCLC, non‐small cell lung cancer.
Primary Assessment Method
- Title
New assessment
- Title
Total patient population
- Number of Patients Screened
51
- Number of Patients Enrolled
51
- Number of Patients Evaluable for Toxicity
46
- Number of Patients Evaluated for Efficacy
46
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 6 (13%)
- Response Assessment SD
n = 20 (43%)
- Response Assessment PD
n = 18 (39%)
- Response Assessment OTHER
n = 2 (4%)
- Outcome Notes
Regarding “Response Assessment OTHER,” responses could not be determined for these patients.
Adverse Events
Data represent percentage of all‐cause adverse events occurring in ≥10% across any grade up to September 25, 2018 (database lock date for analysis of clinical data). Adverse events were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Serious Adverse Events
Treatment‐related pancreatitis and cerebellar hemorrhage in one patient led to discontinuation of study treatment.
Assessment, Analysis, and Discussion
- Completion
Interim analysis, study ongoing (480 mg cohort recruiting)
- Investigator's Assessment
Drug tolerable, efficacy undetermined
Nivolumab is a human monoclonal antibody that targets the programmed death receptor‐1 (PD‐1) and has been approved for the treatment of various types of cancer in ≥60 countries [2]. Early‐stage clinical trials of anti‐PD‐1 therapies have shown promising activity in recurrent or metastatic nasopharyngeal carcinoma (NPC), a common cancer in southeast Asia and North Africa [3], [4], with objective response rates (ORRs) of 21%–34% [5], [6], [7].
The overall efficacy, safety, and pharmacokinetics (PK) of nivolumab are based on clinical data from approximately 17,600 global (predominantly Caucasian) patients [8], [9], [10], [11], [12], [13], [14], [15]. The safety of nivolumab 3 mg/kg administered once every 2 weeks (Q2W) has been assessed in Chinese patients in the CheckMate 078 study (NCT2613507), leading to its approval for the treatment of non‐small cell lung cancer in China [16]; nivolumab 240 mg Q2W is approved in the U.S. [2] and Japan [17].
This study therefore aimed to assess the safety, tolerability, and PK of nivolumab in Chinese patients with previously treated, advanced or recurrent NPC or other solid tumors. Importantly, this is the first study to assess the safety and intensive PK of 240 mg and 360 mg flat‐dose regimens in Chinese patients.
This was an open‐label, single‐center, phase I/II study (CheckMate 077; NCT02593786) of nivolumab monotherapy. The study comprised a dose evaluation phase (3 mg/kg Q2W, 60‐minute intravenous [IV] infusion) and cohort expansion phase (3 mg/kg Q2W, flat dose 240 mg Q2W and 360 mg once every 3 weeks [Q3W]; 30‐minute IV infusion) and consisted of a screening period (≤28 days), a treatment period (until disease progression or intolerable toxicities), and a follow‐up period (≤100 days).
Included patients were Chinese adults (aged ≥18 years), Eastern Cooperative Oncology Group Performance Status 0 or 1, with histologically or cytologically confirmed solid tumors that were clinically advanced or recurrent, who had progressed after ≥1 prior line of systemic therapy. Exclusion criteria included central nervous system metastases, prior malignancy (except for nonmelanoma or certain in situ cancers, or complete remission ≥2 years), autoimmune disease, prior immunotherapy, active tuberculosis infection, pregnancy, or immunosuppressive agent treatment.
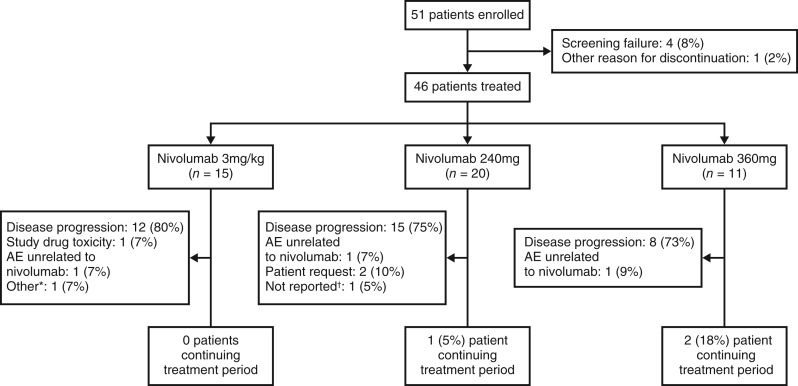
At data cutoff, 51 patients were enrolled, of whom 46 (90%) received ≥1 dose of study treatment and were evaluable for safety (Fig. 1). Baseline patient characteristics are presented in Table 3; the median patient age was 48 years (range: 27–72 years), 67% (31) were male, and 28 (61%) had received >2 prior lines of systemic anticancer therapy. Forty‐three patients (93%) had discontinued during the treatment period, mostly because of disease progression (35 patients, 76%), and only 1 patient because of study drug toxicity. Three patients (7%) discontinued because of adverse events (AEs) unrelated to nivolumab.
Figure 1.
Patient disposition flow chart. *Death due to septic shock considered unrelated to the study drug. †Treatment status unconfirmed at the time of database lock.
Abbreviation: AE, adverse event.
At data cutoff, median (range) duration of treatment was 11.7 (2.0–105.1) weeks for the 3 mg/kg cohort, 16.6 (2.0–98.7) weeks for the 240 mg cohort, and 24.1 (3.0–48.9) weeks for the 360 mg cohort. The median (range) cumulative dose per subject were 1,920.0 (240.0–11,520.0) mg and 2,880.0 (360.0–5,076.0) mg for the 240 mg and 360 mg cohorts, respectively, and 18.2 (3.0–152.3) mg/kg in the 3 mg/kg cohort.
No dose‐limiting toxicities were observed during the dose evaluation phase (n = 8) or dose expansion phase. Incidence of treatment‐related adverse events (TRAEs) was similar across cohorts (Table 1). Overall, no new safety signals were identified, with safety profiles consistent with prior global studies and in line with a phase III study in Chinese patients (NCT02613507) [8], [9], [10], [11], [12], [13], [14], [15], [16], [18], [19], [20]. There were two grade ≥3 TRAEs: one grade 3 asthenia in the 3 mg/kg cohort and one grade 3 hypochloremia in the 240 mg cohort. Treatment‐related serious adverse events were a grade 4 hyponatremia (240 mg cohort) and pancreatitis and cerebellar hemorrhage of indeterminate grade (one patient, 3 mg/kg cohort) that led to discontinuation of treatment. There were no incidences of death associated with nivolumab.
In terms of all‐cause AEs, incidence of hypothyroidism was 30% in the 240 mg cohort (three patients each experienced a grade 1 or 2 event) and 55% in the 360 mg cohort (five patients with a grade 1 event and one patient with a grade 2 event) compared with no such reported events in the 3 mg/kg cohort. This most likely reflects the increased proportions of patients with NPC in the flat‐dosing cohorts who had received prior radiotherapy.
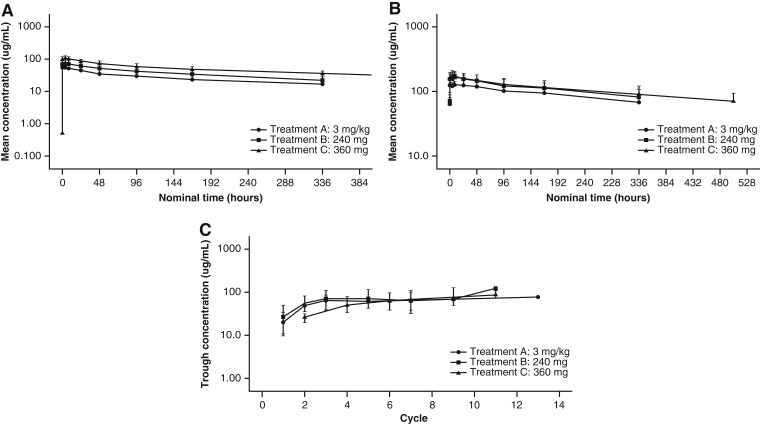
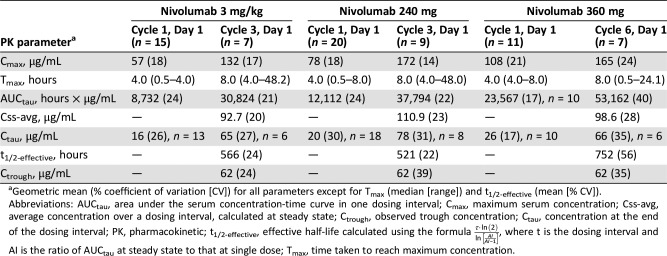
Intensive PK profiles of nivolumab were characterized following a single dose (Cycle 1, Day 1) and multiple doses (3 mg/kg and 240 mg: Cycle 3, Day 1; 360 mg: Cycle 6, Day 1). Single‐dose nivolumab resulted in rank‐order differences in the mean serum concentration versus time profiles, with the highest mean concentrations observed for the 360 mg Q3W flat dose, followed by the 240 mg Q2W flat dose and the 3 mg/kg Q2W dose (Fig. 2A). PK parameters were assessed via noncompartmental analysis and showed similar rank‐order relationships for the geometric mean area under the serum concentration‐time curve for a dosing interval (AUCtau), maximum serum concentration and concentration at the end of the dosing interval (Table 4).
Figure 2.
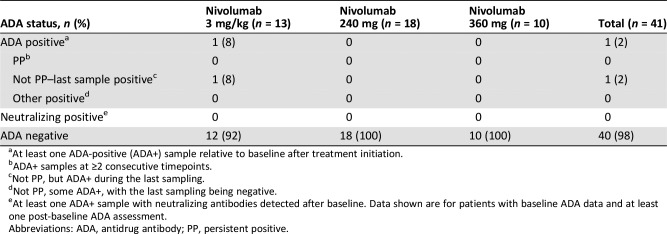
Pharmacokinetics of nivolumab. (A): Mean serum concentration‐time profiles of nivolumab after single‐dose administration. (B): Mean serum concentration‐time profiles of nivolumab at steady state. (C): Geometric mean trough concentration profiles of nivolumab. For all figures: A, 3 mg/kg; B, 240 mg; C, 360 mg. Logarithmic scale. Bars indicate SD.
Table 4. Summary pharmacokinetic parameters of nivolumab.
Geometric mean (% coefficient of variation [CV]) for all parameters except for Tmax (median [range]) and t1/2‐effective (mean [% CV]).
Abbreviations: AUCtau, area under the serum concentration‐time curve in one dosing interval; Cmax, maximum serum concentration; Css‐avg, average concentration over a dosing interval, calculated at steady state; Ctrough, observed trough concentration; Ctau, concentration at the end of the dosing interval; PK, pharmacokinetic; t1/2‐effective, effective half‐life calculated using the formula , where t is the dosing interval and AI is the ratio of AUCtau at steady state to that at single dose; Tmax, time taken to reach maximum concentration.
Steady state was reached by approximately Cycle 3 (Week 17: 3 mg/kg and 240 mg flat dose) or Cycle 6 (Week 16: 360 mg flat dose; Fig. 2C). At steady state, the mean serum nivolumab concentration profiles over time were similar between the flat‐dose cohorts and appeared lower for the 3 mg/kg cohort (Fig. 2B). The geometric mean AUCtau for the 360 mg flat‐dose cohort was approximately 41% and 72% higher than for the 240 mg flat‐dose and 3 mg/kg cohorts, respectively. However, when normalized by dosing interval, the AUCtau values were comparable, indicating that the different dosing regimens yielded comparable average concentrations at steady state. The mean effective half‐life varied between cohorts (521–752 hours, or 21.7–31.3 days) but was within ±5 days of the mean elimination half‐life of 25 days reported previously [2].
At 3 mg/kg, nivolumab exposure in Chinese patients was similar to that of patients in a global phase I study (NCT00730639), as demonstrated by a cross‐study comparison (Table 2) [1]. These data are also in agreement with a recent phase I study of nivolumab in advanced solid tumors in Korean patients, in which PK data were shown to be comparable to U.S. and Japanese populations [21].
Immunogenicity was assessed via antidrug antibody (ADA) status (Table 5). Of 41 evaluable patients, only 1 patient (3 mg/kg cohort) was ADA positive after treatment. None of the patients were persistently ADA positive or neutralizing antibody positive.
Table 5. Nivolumab treatment immunogenicity.
At least one ADA‐positive (ADA+) sample relative to baseline after treatment initiation.
ADA+ samples at ≥2 consecutive timepoints.
Not PP, but ADA+ during the last sampling.
Not PP, some ADA+, with the last sampling being negative.
At least one ADA+ sample with neutralizing antibodies detected after baseline. Data shown are for patients with baseline ADA data and at least one post‐baseline ADA assessment.
Abbreviations: ADA, antidrug antibody; PP, persistent positive.
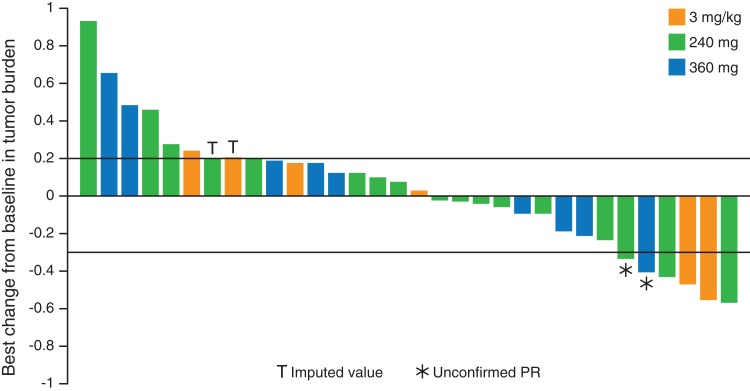
Preliminary antitumor activity was assessed for patients with NPC tumors and for patients with all other solid tumors. Of 32 patients with NPC, 15 (47%) had a reduction in tumor burden from baseline following nivolumab treatment (Fig. 3). Four patients (13%) achieved a partial response (two patients each, 3 mg/kg and 240 mg cohorts), and 17 (53%) had stable disease. The ORR and disease control rate (DCR) in this patient group were 13% (95% confidence interval [CI]: 4–29) and 66% (95% CI: 47–81), respectively (Table 6; Fig. 4). Patients with other solid tumor types achieved an ORR of 14% and a DCR of 36%.
Figure 3.
Waterfall plot of best reduction from baseline in target lesions for patients with nasopharyngeal carcinoma.
Abbreviation: PR, partial response.
Table 6. Antitumor responses.
The proportion of patients whose best overall response is either a complete response or a partial response.
Confidence intervals were computed for cohorts of 10 patients or more only.
The proportion of patients with a complete response, with a partial response, or who achieved stable disease.
Abbreviations: CI, confidence interval; NPC, nasopharyngeal carcinoma.
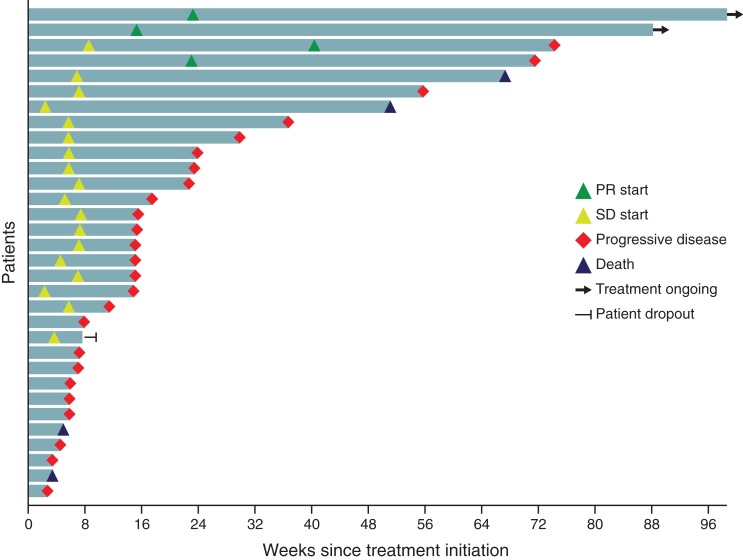
Figure 4.
Tumor swimmer plot for the subpopulation of patients with nasopharyngeal carcinoma.
Abbreviations: PR, partial response; SD, stable disease.
The median (range) follow‐up duration for patients with NPC was 7.5 (0.8–24.7) months, with six patients (19%) having received nivolumab treatment for >1 year. Median progression‐free survival (PFS) was 3.5 months (95% CI: 1.8–5.5 months), and median overall survival (OS) was not reached. The 3‐month PFS and OS rates were 64.2% (95% CI: 44.7–78.4) and 87.5% (95% CI: 70.0–95.1), respectively.
This study confirms the safety profile of nivolumab at 3 mg/kg in Chinese patients and is the first to report tolerability at flat doses of 240 mg and 360 mg in this population. Additionally, these data indicate that the PK of nivolumab monotherapy are ethnically insensitive. In this population of pretreated patients with advanced or recurrent disease, preliminary antitumor responses in NPC are encouraging; confirmation is required in a larger patient population.
Figures and Tables
Acknowledgments
The authors thank the patients and families that made this trial possible; the clinical study teams who participated in the trial; Bristol‐Myers Squibb; ONO Pharmaceutical Company Ltd. (Osaka, Japan). BMS policy on data sharing may be found at https://www.bms.com/researchersand‐partners/independent‐research/data‐sharing‐requestprocess.html. This study was funded by Bristol‐Myers Squibb. Editorial assistance in manuscript preparation was provided by Susannah Thornhill and Tim Stentiford of MediTech Media, Asia Pacific, and funded by Bristol‐Myers Squibb China.
Contributed equally.
Footnotes
ClinicalTrials.gov Identifier: NCT02593786
Sponsor: Bristol‐Myers Squibb Pharmaceuticals Ltd.
Principal Investigator: Li Zhang
IRB Approved: Yes
Contributor Information
Hongyun Zhao, Email: zhaohy@sysucc.org.cn.
Li Zhang, Email: zhangli@sysucc.org.cn.
Disclosures
Amol Tendolkar: Bristol‐Myers Squibb (E, OI); Lu Chen: Bristol‐Myers Squibb (E); Dong Xu: Bristol‐Myers Squibb (E); Jennifer Sheng: Bristol‐Myers Squibb (E, OI); Li Zhang: Hengrui Medicine Co. Ltd., Eli Lilly, Novartis, Roche, Bristol‐Myers Squibb (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Center for Drug Evaluation and Research . Clinical Pharmacology and Biopharmaceutics Reviews. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125554Orig1s000ClinPharmR.pdf. Accessed April 2019.
- 2.Opdivo. Prescribing Information. United States Food and Drug Administration. April 2018, 12 December 2018. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl.pdf. Accessed April 2019.
- 3.Carioli G, Negri E, Kawakita D et al. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: Focus on low‐risk areas. Int J Cancer 2017;140:2256–2264. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Shen C, Lu X et al. The incidence and prognosis of nasopharyngeal carcinoma patients with family history. Oncotarget 2017;8:97323–97330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu C, Lee SH, Ejadi S et al. Safety and antitumor activity of pembrolizumab in patients with programmed death‐ligand 1‐positive nasopharyngeal carcinoma: Results of the KEYNOTE‐028 study. J Clin Oncol 2017;35:4050–4056. [DOI] [PubMed] [Google Scholar]
- 6.Fang W, Yang Y, Ma Y et al. Camrelizumab (SHR‐1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single‐arm, phase 1 trials. Lancet Oncol 2018;19:1338–1350. [DOI] [PubMed] [Google Scholar]
- 7.Ma BBY, Lim WT, Goh BC et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: An international, multicenter study of the Mayo Clinic Phase 2 Consortium (NCI‐9742). J Clin Oncol 2018;36:1412–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younes A, Santoro A, Shipp M et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem‐cell transplantation and brentuximab vedotin: A multicentre, multicohort, single‐arm phase 2 trial. Lancet Oncol 2016;17:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansell SM, Lesokhin AM, Borrello I et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris RL, Blumenschein G Jr, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P, Retz M, Siefker‐Radtke A et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single‐arm, phase 2 trial. Lancet Oncol 2017;18:312–322. [DOI] [PubMed] [Google Scholar]
- 15.Overman MJ, McDermott R, Leach JL et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): An open‐label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Lu S, Cheng Y et al. Abstract CT114: Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non‐small cell lung cancer (NSCLC): Results of the phase 3 CheckMate 078 study. Cancer Res 2018;78:CT114a. [Google Scholar]
- 17.Ono Pharmaceutical Co., Ltd., Bristol‐Myers Squibb. Opdivo approved for supplemental applications for expanded indications of malignant pleural mesothelioma and adjuvant treatment of melanoma change in dosage and administration of single dosing regimen and expanded indication of renal cell carcinoma in Opdivo and Yervoy combination therapy. Available at https://www.ono.co.jp/eng/news/pdf/sm_cn180821.pdf. Accessed December 22, 2018.
- 18.Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 19.Weber JS, D'Angelo SP, Minor D et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): A randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 20.Gettinger S, Horn L, Jackman D et al. Five‐year follow‐up of nivolumab in previously treated advanced non‐small‐cell lung cancer: Results From the CA209‐003 study. J Clin Oncol 2018;36:1675–1684. [DOI] [PubMed] [Google Scholar]
- 21.Lee KW, Lee DH, Kang JH et al. Phase I pharmacokinetic study of nivolumab in Korean patients with advanced solid tumors. The Oncologist 2018;23:155–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]