Abstract
Lessons Learned.
Single‐agent selinexor has limited activity in heavily pretreated patients with metastatic triple‐negative breast cancer.
Selinexor 60 mg by mouth twice weekly was generally well tolerated with a side‐effect profile consistent with previous clinical trials.
Future studies of selinexor in this population should focus on combination approaches and a biomarker‐driven strategy to identify patients most likely to benefit.
Background.
This phase II trial evaluated the safety, pharmacodynamics, and efficacy of selinexor (KPT‐330), an oral selective inhibitor of nuclear export (SINE) in patients with advanced triple‐negative breast cancer (TNBC).
Methods.
This phase II trial was designed to enroll 30 patients with metastatic TNBC. Selinexor was given at 60 mg orally twice weekly on days 1 and 3 of each week, three of each 4‐week cycle. The primary objective of this study was to determine the clinical benefit rate (CBR), defined as complete response + partial response + stable disease (SD) ≥12 weeks.
Results.
Ten patients with a median age of 60 years (range 44–71 years) were enrolled between July 2015 and January 2016. The median number of prior chemotherapy lines was 2 (range 1–5). A planned interim analysis for the first stage per protocol was performed. Three patients had SD and seven had progressive disease. On the basis of these results and predefined stoppage rules, the study was halted.
Conclusion.
Selinexor was fairly well tolerated in patients with advanced TNBC but did not result in objective responses. However, clinical benefit rate was 30%, and further investigation of selinexor in this patient population should focus on combination therapies.
Abstract
经验获取
对于既往接受多次治疗的转移性三阴性乳腺癌患者,单剂 Selinexor 的活性有限。
Selinexor 60mg口服,每周两次,总体耐受良好,副作用与先前临床试验结果一致。
未来针对 Selinexor 对于这类患者的研究应侧重于联合用药和以生物标志物为驱动的策略,确定最有可能从中受益的患者。
摘要
背景。本 II 期试验评估了口服选择性核输出抑制剂(SINE) Selinexor (KPT‐330) 在晚期三阴性乳腺癌 (TNBC) 患者治疗中的安全性、药效和有效性。
方法。本 II 期试验计划入组 30 例转移性 TNBC 患者。Selinexor 60mg口服,每周第 1 天和第 3 天共服用 2 次,服用 3 周,疗程为 4 周。本研究的主要目的是确定临床获益率 (CBR),具体定义为 ≥12 周的完全缓解率 + 部分缓解率 + 病情稳定率 (SD))。
结果。于 2015 年 7 月至 2016 年 1 月入组 10 例患者,中位年龄为 60 岁(范围为 44‐71 岁)。既往中位化疗线数为 2 (范围为 1‐5 )。根据方案对第一阶段进行计划期中分析。三名患者出现 SD,七名患者出现疾病进展。根据这些结果和预先确定的中止规则,停止了研究。
结论。晚期 TNBC 患者对 Selinexor 的耐受性较好,但没有引起客观缓解。临床获益率为 30%,对这类患者群体使用 Selinexor 的进一步研究应侧重于联合治疗。
Discussion
Selinexor (KPT‐330) is an oral SINE targeting Exportin 1 (XPO1). XPO1 functions as a nuclear exporter of major tumor suppressor proteins (TSPs), including p53, p21, BRCA1, BRCA2, and retinoblastoma protein [1]. TSPs require nuclear localization to regulate cell cycle progression and trigger apoptosis. XPO1 is overexpressed in many cancer cells, including TNBC, and can bypass normal TSP function. By binding to XPO1, selinexor prevents nuclear export of XPO1 cargo proteins [1]. Although not directly cytotoxic, treatment with selinexor retains tumor suppressor proteins in the nucleus where they can carry out their normal functions. Increased XPO1 mRNA production is a compensatory mechanism for selinexor‐induced loss of XPO1 function, and comparison of XPO1 mRNA levels predose and after administration of selinexor is a validated pharmacodynamic marker of appropriate drug engagement and inhibition of the target. Selinexor has single‐agent activity in diffuse large B‐cell lymphoma, multiple myeloma, and acute myeloid leukemia [2], [3], [4], [5]. It is currently under priority review for refractory multiple myeloma.
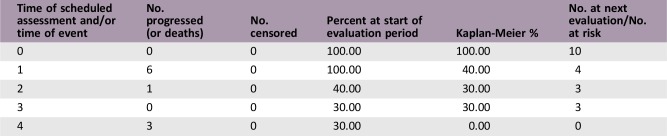
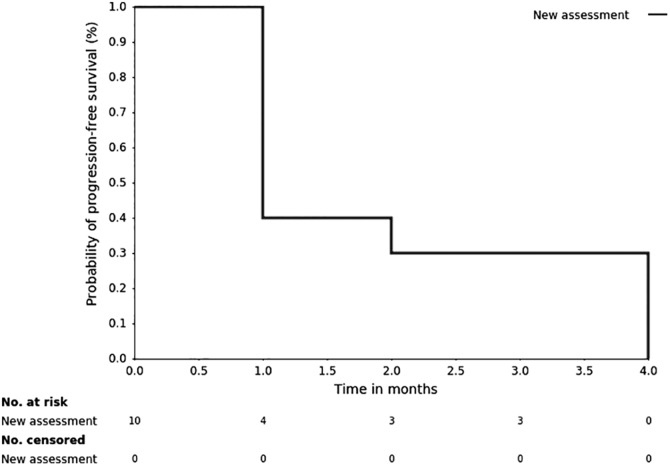
This study investigated the clinical benefit rate of selinexor in heavily pretreated patients with metastatic TNBC. Among the first 10 patients who were enrolled, we did not observe any objective responses; therefore, the study was terminated early for lack of efficacy per preplanned interim analysis. Three patients had a best response of stable disease, with two of the three patients having stable disease for ≥3 treatment cycles; however, this was not sufficient to warrant continuation of the study. The median PFS was 0.92 months (95% confidence interval [CI]: 0.62–3.58 months). The median overall survival (OS) was 5.98 months (95% CI: 1.68–10.39 months). Furthermore, we did not observe a correlation between XPO1 mRNA induction after treatment or p53 mutational status in patients who experienced clinical benefit.
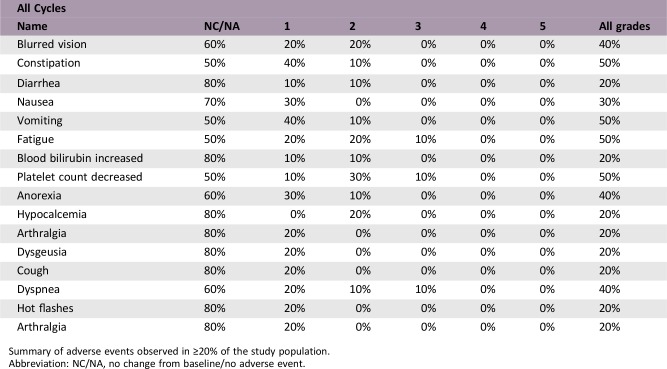
The side‐effect profile is consistent with that observed in the first‐in‐class, first‐in‐human study of selinexor in solid tumors, including nausea, fatigue, anorexia, and vomiting as the most common treatment‐related adverse events. Complete details of adverse events are available online. Thrombocytopenia was the most common hematologic toxicity; however, only one patient experienced grade ≥3 thrombocytopenia while on study. Although constitutional adverse events led to dose reductions in three patients in this study, there were no discontinuations due to selinexor treatment. In addition, there were no grade 4 or 5 adverse events observed in this study population.
Despite early termination of this trial for lack of efficacy as a single agent, interest remains in developing a niche for selinexor as a combination therapy in TNBC. A phase Ib clinical trial investigating the safety of combination selinexor and olaparib in patients with advanced solid tumors is currently ongoing (NCT02419495). Given the recent approval of olaparib for patients with metastatic breast cancer harboring BRCA1 or BRCA2 mutations, such a combination is intriguing [6].
Trial Information
- Disease
Breast cancer
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
No designated number of regimens
- Type of Study – 1
Phase II
- Type of Study – 2
Single arm
- Primary Endpoint
Clinical benefit rate
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Safety
- Secondary Endpoint
Tolerability
- Additional Details of Endpoints or Study Design
- This study used a Simon two‐stage design to test the null hypothesis of the CBR P = 5% versus the alternative P = 20%. Up to 30 patients could potentially be accrued during this trial with 10 patients for stage I and 20 patients for stage II. The alpha level of the design was set at 0.05 and the statistical power at 0.8. If one or more patients achieved objective response (complete or partial response) in the first 10 eligible patients (stage I), another 20 patients would be enrolled in stage II. If three or fewer clinical benefits are observed by the end of stage II, then no further investigation of the regimen is warranted. Therefore, under this design, there would be an 80% chance of detecting a tumor response rate of at least 20%. An objective response rate of 5% or less would lead to the conclusion that the regimen lacks antitumor activity at a .05 significance level in this design. Given that the “true” response probability was equal to or less than 5%, there was a 59.9% probability of ending the trial during stage I.
- Investigator's Analysis
Level of activity did not meet planned endpoint
Drug Information
- Drug 1
- Generic/Working Name
Selinexor
- Drug Type
Small molecule
- Drug Class
SINE
- Dose
60 mg per flat dose
- Route
p.o.
- Schedule of Administration
Two times weekly
Patient Characteristics
- Number of Patients, Female
10
- Stage
4
- Age
Median (range): 61 (44–71)
- Number of Prior Systemic Therapies
Median (range): 2 (1–5)
- Performance Status: ECOG
-
0 — 7
1 — 3
2 — 0
3 — 0
Unknown — 0
- Cancer Types or Histologic Subtypes
Triple‐negative breast cancer, 10
Primary Assessment Method
- Number of Patients Screened
13
- Number of Patients Enrolled
10
- Number of Patients Evaluable for Toxicity
10
- Number of Patients Evaluated for Efficacy
10
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 0 (0%)
- Response Assessment SD
n = 3 (30%)
- Response Assessment PD
n = 7 (70%)
- (Median) Duration Assessments PFS
0.92 months, CI: 0.62–3.58
- (Median) Duration Assessments OS
5.98 months, CI: 1.68–10.39
Kaplan‐Meier, Time Units, Months
Kaplan‐Meier plot: Progression‐free survival for all treated patients.
Adverse Events
Summary of adverse events observed in ≥20% of the study population.
Abbreviation: NC/NA, no change from baseline/no adverse event.
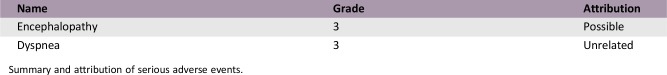
Serious Adverse Events
Summary and attribution of serious adverse events.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Level of activity did not meet planned endpoint
This study investigated the clinical benefit rate of selinexor in heavily pretreated patients with metastatic triple‐negative breast cancer (TNBC). Among the first 10 patients enrolled, we did not observe any objective responses; therefore, the study was terminated early for lack of efficacy per preplanned interim analysis. Three patients had a best response of stable disease with two of the three patients having stable disease for ≥3 treatment cycles; however, this was not sufficient to warrant continuation of study. Furthermore, we did not observe a correlation between XPO1 mRNA induction after treatment or p53 mutational status in patients who experienced clinical benefit.
Although responses to single‐agent selinexor were not seen in this study, combination approaches may provide therapeutic benefit to patients with TNBC. Chemotherapy resistance in TNBC is at least partly mediated by survivin, a pro‐survival molecule that plays a critical role in resistance to taxanes [7], [8], [9]. In pancreatic cell lines, the combination of selinexor and gemcitabine was synergistic and led to depletion of survivin and apoptosis, which was greater than either agent alone. Additionally, the combination demonstrated greater reduction in nuclear localization of DNA repair enzymes, leading to the accumulation of DNA damage. Because increased DNA repair enzymes CHK1 and RAD51 were seen in pretreatment tissue samples in biopsies from two patients, this suggests that a combination approach with cytotoxic chemotherapy could be investigated as a way to augment responses to chemotherapy in patients with TNBC. Preclinical data also suggest that single‐agent selinexor can lead to some level of poly ADP ribose polymerase (PARP) cleavage, which is associated with responses [10], [11]. The combination of a PARP inhibitor and selinexor appears to act synergistically in TNBC cell lines [12]. A phase Ib clinical trial investigating the safety of combination selinexor and olaparib in patients with advanced solid tumors is currently ongoing (NCT02419495). Given the recent approval of olaparib for patients with metastatic breast cancer harboring BRCA1 or BRCA2 mutations, such a combination is intriguing [6].
The side‐effect profile is consistent with that observed in the first‐in‐class, first‐in‐human study of selinexor in solid tumors including nausea, fatigue, anorexia, and vomiting as the most common treatment‐related adverse events [13]. Thrombocytopenia was the most common hematologic toxicity; however, only one patient experienced grade ≥3 thrombocytopenia while on study. This result is not unexpected, as a recent study showed that selinexor inhibits the maturation of hematopoietic stem cells to megakaryocytes, without affecting other aspects of platelet production. Although constitutional adverse events led to dose reductions in three patients in this study, there were no discontinuations due to selinexor treatment. In addition, there were no grade 4 or 5 adverse events observed in this study population. Patients were treated with antiemetics and oral dexamethasone in the first cycles to mitigate nausea, vomiting, and anorexia. If tolerated, these supportive medications could be tapered off during subsequent cycles. Side effects are a function of the dose and schedule.
This trial demonstrated that administration of selinexor 60 mg twice weekly with supportive care is well tolerated. In addition to the dose and schedule chosen, the supportive care measures implemented may have led to the relatively low incidence of observed nausea and anorexia compared with the first‐in‐human study. Serious adverse events occurred in three patients and included grade 3 dyspnea in two patients and grade 3 reversible encephalopathy, described as memory impairment. The first case of grade 3 dyspnea was unrelated to the study drug. Grade 2 sinus tachycardia and grade 2 blurry vision were associated with this serious adverse event, and whereas sinus tachycardia was unrelated to the study drug, blurry vision was possibly related. The second case of grade 3 dyspnea was also unrelated to study drug and definitely disease related, whereas the case of grade 3 reversible encephalopathy was possibly related to selinexor. No treatment‐emergent adverse event of grade 4 or 5 was observed. Dose reductions were required in two patients for fatigue and mood irritability, both related to the study drug. Treatment was temporarily interrupted in one patient for grade 2 thrombocytopenia related to selinexor. No treatment‐related events led to discontinuation of selinexor.
Despite early termination of this trial for lack of efficacy as a single agent, interest remains in developing a niche for selinexor as a combination therapy in TNBC. A recent publication demonstrated the ability of selinexor to inhibit proliferative and migratory processes in TNBC cells by restoring arrestin‐related domain‐containing protein 3 [14]. The preclinical evidence for an effective role of selinexor in TNBC remains intriguing, and our study highlights several areas for further exploration with selinexor in this disease. Outcomes seen in this trial are not generalizable, and patients with TNBC who are treatment naïve may show increased responsiveness to treatment as a single agent or in combination. Efforts are under way to develop a biomarker strategy to identify responsive subsets of patients upfront [3], [15].
Acknowledgments
We thank Sonya J. Smyk, Moffitt Cancer Center, for editorial support in the preparation of this manuscript.
Footnotes
ClinicalTrials.gov Identifier: NCT02402764
Sponsor: Karyopharm
Principal Investigator: Hyo S. Han
IRB Approved: Yes
Disclosures
Michael Shafique: GlaxoSmithKline (C/A); Roohi Ismail‐Khan: Karyopharm (RF‐institutional); Dan Sullivan: Karyopharm (RF‐institutional); Hatem Soliman: Pfizer, Eli Lilly, Novartis, PUMA, Astrazeneca (C/A); Hyo S. Han: Karyopharm (RF‐institutional). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol 2012;83:1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner JG, Dawson J, Cubitt CL et al. Inhibition of CRM1‐dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol 2014;27:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garzon R, Savona M, Baz R et al. A phase 1 clinical trial of single‐agent selinexor in acute myeloid leukemia. Blood 2017;129:3165–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez M, Shah BD, Gabrail NY et al. Preliminary evidence of anti tumor activity of selinexor (KPT‐330) in a phase I trial of a first‐in‐class oral selective inhibitor of nuclear export (SINE) in patients (pts) with relapsed / refractory non‐Hodgkin's lymphoma (NHL) and chronic lymphocytic leukemia (CLL). Blood 2013;122:90. [Google Scholar]
- 5.Chen CI, Gutierrez M, De Nully Brown P et al. Anti tumor activity of selinexor (KPT‐330), a first‐in‐class oral selective inhibitor of nuclear export (SINE) XPO1/CRM1 antagonist in patients (pts) with relapsed / refractory multiple myeloma (MM) Or Waldenstrom's macroglobulinemia (WM). Blood 2013;122:1942. [Google Scholar]
- 6.U.S. Food and Drug Administration . FDA approves first treatment for breast cancer with a certain inherited genetic mutation. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm592347.htm. Accessed December 5, 2018.
- 7.Virrey JJ, Guan S, Li W et al. Increased survivin expression confers chemoresistance to tumor‐associated endothelial cells. Am J Pathol 2008;173:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreger BT, Johansen ER, Cerione RA et al. The enrichment of survivin in exosomes from breast cancer cells treated with paclitaxel promotes cell survival and chemoresistance. Cancers (Basel) 2016;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parvani JG, Davuluri G, Wendt MK et al. Deptor enhances triple‐negative breast cancer metastasis and chemoresistance through coupling to survivin expression. Neoplasia 2015;17:317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Holloway MP, Nguyen K et al. XPO1 (CRM1) inhibition represses STAT3 activation to drive a survivin‐dependent oncogenic switch in triple‐negative breast cancer. Mol Cancer Ther 2014;13:675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravina GL, Mancini A, Sanita P et al. KPT‐330, a potent and selective exportin‐1 (XPO‐1) inhibitor, shows antitumor effects modulating the expression of cyclin D1 and survivin [corrected] in prostate cancer models. BMC Cancer 2015;15:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marijon H, Gery S, Friedlander S et al. Selinexor, a selective inhibitor of nuclear export (SINE) compound, shows enhanced antitumor activity in combination with the PARP inhibitor, olaparib, in models of triple‐negative breast cancer. Exp Mol Ther 2015;75(suppl 15):LB‐255a. [Google Scholar]
- 13.Abdul Razak AR, Mau‐Soerensen M, Gabrail NY et al. First‐in‐class, first‐in‐human phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol 2016;34:4142–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soung YH, Kashyap T, Nguyen T et al. Selective inhibitor of nuclear export (SINE) compounds block proliferation and migration of triple negative breast cancer cells by restoring expression of ARRDC3. Oncotarget 2017;8:52935–52947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heong VYM, Koe P, Yong WP et al. RAS/AKT pathway mutations as predictive biomarkers in patients with colorectal cancer treated with the exportin 1 (XPO1) inhibitor selinexor (SEL) – Inhibition of nuclear‐cytoplasmic translocation of p27 as a mechanism of anti‐tumour activity. Ann Oncol 2016;27(suppl 6):383. [Google Scholar]