Abstract
Lessons Learned.
Patients with metastatic colorectal cancer with good performance status or no liver metastasis could benefit from apatinib.
Circulating tumor DNA abundance may be a predictor in serial monitoring of tumor load.
Background.
Apatinib, an oral vascular endothelial growth factor (VEGF) receptor‐2 inhibitor, has been approved as third‐line treatment for metastatic gastric cancer in China. The aim of this study was to evaluate the efficacy and safety of apatinib, in the treatment of patients with refractory metastatic colorectal cancer after failure of two or more lines of chemotherapy.
Methods.
In this open‐label, single‐arm, phase II study, patients with histological documentation of adenocarcinoma of the colon or rectum were eligible if they had received at least two prior regimens of standard therapies including fluoropyrimidine, oxaliplatin, and irinotecan. These patients were treated with apatinib in a daily dose of 500 mg, p.o., in the third‐line or higher setting. Capture sequencing was dynamically performed to identify somatic variants in circulating tumor DNA (ctDNA) with a panel of 1,021 cancer‐related genes. The primary endpoint was progression‐free survival (PFS) and the tumor response was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Interim analysis was applied as predefined.
Results.
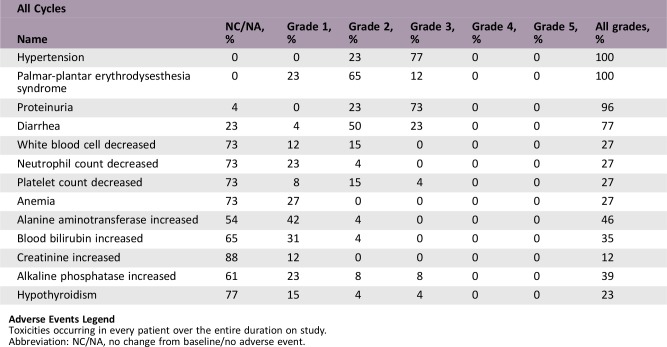
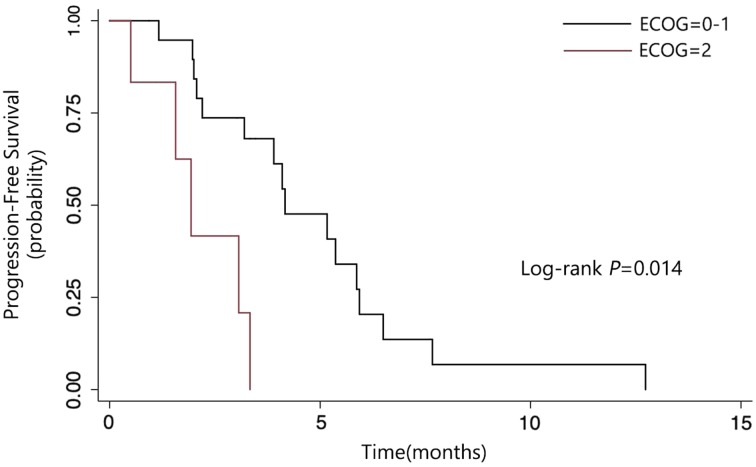
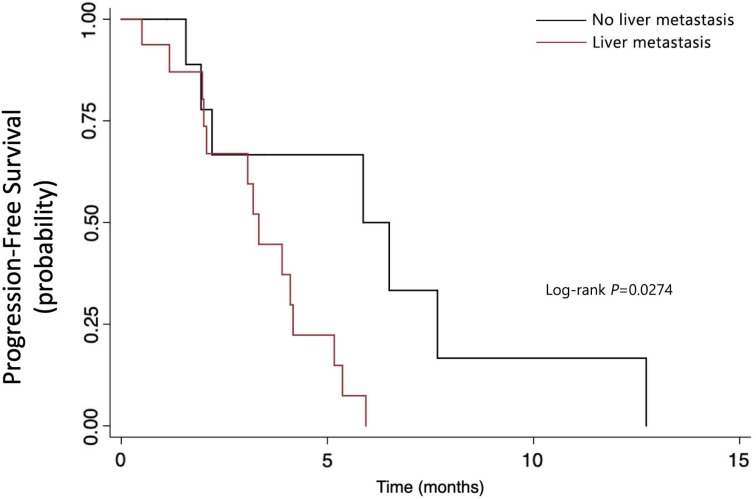
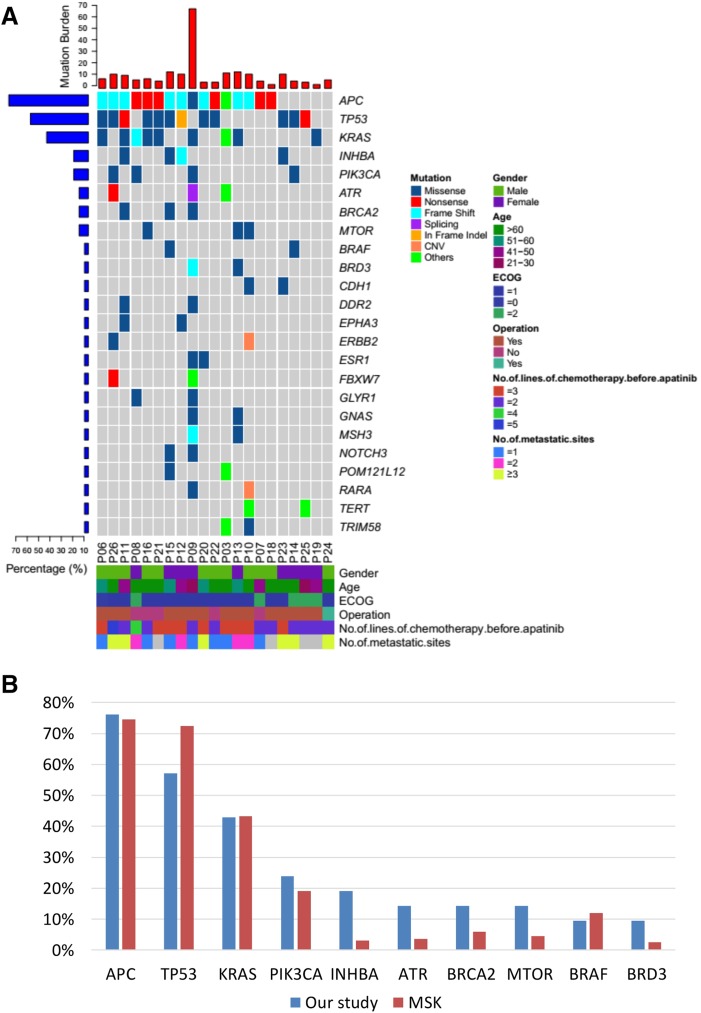
From June 1, 2016 to December 31, 2017, 26 patients were enrolled. The median PFS of the whole group was 3.9 months (95% confidence interval [CI]: 2.1–5.9). The median overall survival (OS) was 7.9 months (95% CI: 4.6–10.1+). Patients with performance status (PS) 0–1 had longer PFS than those with PS 2 (4.17 months vs. 1.93 months, p = .0014). Patients without liver metastasis also had longer PFS than those who had live metastasis (5.87 months vs. 3.33 months, p = .0274). The common side effects of apatinib were hypertension, hand‐foot syndrome, proteinuria, and diarrhea. The incidence of grade 3–4 hypertension, hand‐foot syndrome, proteinuria, and diarrhea was 76.92%, 11.54%, 73.08%, and 23.08%, respectively. All of the patients received dose reduction because of adverse effect. Results of capture sequencing showed APC, TP53, and KRAS were most frequently mutant genes. ctDNA abundance increased before the radiographic assessment in ten patients.
Conclusion.
Apatinib monotherapy showed promising efficiency for patients with refractory colorectal cancer, especially in patients with PS 0–1 or no liver metastasis. ctDNA abundance may be a predictor in serial monitoring of tumor load.
Abstract
经验获取
体力状态良好或无肝脏转移的转移性结直肠癌患者可受益于阿帕替尼。
循环肿瘤 DNA 丰度可能是肿瘤负荷连续监测的预测因子。
摘要
背景。阿帕替尼是一种口服血管内皮生长因子 (VEGF) 受体‐ 2 抑制剂,在中国已被批准作为转移性胃癌的三线治疗药物。本研究的目的是评估阿帕替尼治疗二线或二线以上化疗失败的难治性转移性结直肠癌患者的疗效和安全性。
方法。在这项开放标签单组 II 期研究中,如果组织学记录患有结肠或直肠腺癌的患者之前至少接受过两种标准疗法方案(包括氟尿嘧啶、奥沙利铂和伊立替康),则符合研究条件。这些患者接受阿帕替尼治疗,每日剂量为 500 毫克口服,该药物被作为三线或更高线数药物使用。动态执行捕获测序以鉴定循环肿瘤 DNA (ctDNA) 中的体细胞变异体,其具有 1 021 个癌症相关的基因面板。主要终点为无进展生存期 (PFS) 以及根据实体瘤疗效评定标准 (RECIST) 1.1 版确定肿瘤反应。中期分析按预定义应用。
结果。2016 年 6 月 1 日至 2017 年 12 月 31 日共入组 26 例患者。全组中位 PFS 为 3.9 个月 [95% 置信区间(CI):2.1–5.9]。中位总生存期 (OS) 为 7.9 个月 (95% CI:4.6–10.1+)。体力状态 (PS) 为 0‐1 的患者的 PFS 较 PS 为 2 的患者长 (4.17 个月 vs. 1.93 个月,p = 0.001 4)。无肝转移患者的 PFS 也比肝转移患者长 (5.87 个月 vs. 3.33 个月,p = 0.027 4)。阿帕替尼的常见副作用是高血压、手足综合征、蛋白尿和腹泻。3–4 级高血压、手足综合征、蛋白尿、腹泻发生率分别为 76.92%、11.54%、73.08% 和 23.08%。所有患者均因不良反应减少了给药剂量。捕获测序结果显示,APC、TP53 和 KRAS 是最常见的突变基因。10 例患者在影像学检查前的ctDNA 丰度增加。
结论。阿帕替尼单一疗法对难治性结直肠癌疗效良好,尤其是对 PS 0‐1 或无肝转移患者。ctDNA 丰度可作为肿瘤负荷序列监测的预测因子。
Discussion
Here we report results from an open‐label, single‐arm, phase II study aiming to evaluate the efficacy and safety of apatinib as a salvage treatment for refractory metastatic colorectal cancer. To our knowledge, this is the first prospective clinical trial to investigate the safety and efficacy of apatinib in refractory metastatic colorectal cancer.
After two or more lines of prior chemotherapy, efficacy of apatinib in our study seemed to be comparable with other tyrosine kinase inhibitors reported in previous studies. However, compared with other studies, it seems that a higher incidence of adverse events of apatinib occurred. Firstly, patients in our study already have received multiple lines of chemotherapy before apatinib, with cumulative toxicity of previous drugs. Secondly, patients in our trial were required to record their blood pressure and other symptoms every day and were closely followed up by online tools; thus more adverse events could be recorded. In addition, the relatively small sample size of our study might impair the generalization of our conclusions. Patients with PS 0–1 had longer PFS than those with PS 2 in our study. A possible reason might be that patients with good physical status showed better tolerance of apatinib and thus benefited from it.
In metastatic colorectal cancer, ctDNA was reported to be an early marker of chemotherapy. Increasing ctDNA has been found ahead of radiographic detection of progression in 10 of 13 patients who had serial ctDNA detections in our study, suggesting that detection of ctDNA was associated with radiographic tumor burden. A challenge to the application of angiogenesis inhibitors is finding suitable biomarkers to select patients who are most likely to benefit from it. Here we sequenced a panel of 1,021 cancer‐related genes in all patients. At least one mutation in ctDNA was detected in the baseline blood sample in 21 of 26 (81%) patients. The most frequently detected mutations were in APC (n = 16, 76%), P53 (n = 12, 57%), KRAS (n = 9, 43%), PIK3CA (n = 5, 24%), INHBA (n = 4, 19%), ATR (n = 4, 14%), BRCA2 (n = 4, 14%), MTOR (n = 4, 14%), BRAF (n = 2, 10%), and BRD3 (n = 2, 10%). We performed a survival analysis to determine whether APC, TP53, or KRAS mutation was associated with PFS and OS; however, no statistical difference was found, potentially confounded by the small size of the study.
In conclusion, this study provides supporting evidence that apatinib exhibits efficacy for patients with refractory colorectal cancer, especially in patients with PS 0–1 or no liver metastasis. The common side effects of apatinib were hypertension, hand‐foot syndrome, proteinuria, and diarrhea. Given that sample size was small in this study, further investigation in a larger population is required in the future.
Trial Information
- Disease
Colorectal cancer
- Disease
Advanced cancer
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
More than two prior regimens
- Type of Study – 1
Phase II
- Type of Study – 2
Single arm
- Primary Endpoint
Progression‐free survival
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Drug 1
- Generic/Working Name
Apatinib
- Trade Name
Aitan
- Company Name
Jiangsu HengRui Medicine Co., Ltd.
- Drug Type
Small molecule
- Drug Class
Angiogenesis ‐ VEGF
- Dose
500 milligrams (mg) per flat dose
- Route
Oral (po)
- Schedule of Administration
28‐day cycle
Patient Characteristics
- Number of Patients, Male
16
- Number of Patients, Female
10
- Stage
IV
- Age
Median (range): 57 (28–75) years
- Number of Prior Systemic Therapies
Median (range): 4 (3–6)
- Performance Status: ECOG
-
0 — 2
1 — 18
2 — 6
3 — 0
Unknown — 0
- Cancer Types or Histologic Subtypes
Right or transverse colon, 6; left colon, 10; rectum, 10
Primary Assessment Method for Phase II Apatinib
- Title
Total patient population
- Number of Patients Screened
26
- Number of Patients Enrolled
26
- Number of Patients Evaluable for Toxicity
26
- Number of Patients Evaluated for Efficacy
26
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 0 (0%)
- Response Assessment SD
n = 14 (23%)
- Response Assessment PD
n = 9 (69%)
- Response Assessment OTHER
n = 3 (8%)
- (Median) Duration Assessments PFS
3.9 months
- (Median) Duration Assessments OS
7.9 months
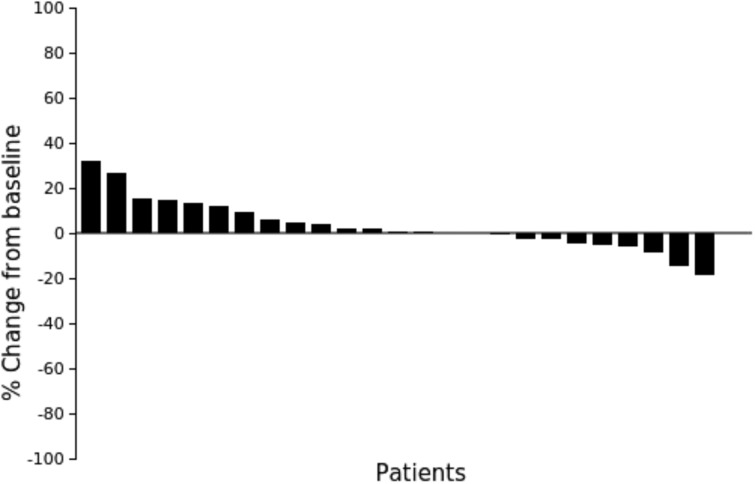
Waterfall plot showing the percentage of tumor diameter change at time of best response. Only 25 cases were recorded because one patient died before the first evaluation.
Adverse Events
Adverse Events Legend
Toxicities occurring in every patient over the entire duration on study.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
Here we reported results from an open‐label, single‐arm, phase II study aiming to evaluate the efficacy and safety of apatinib as a salvage treatment for refractory metastatic colorectal cancer (mCRC). A total of 54 participants were planned to be enrolled. From June 1, 2016 to December 31, 2017, 26 patients were enrolled. The median PFS was 3.9 months and median OS was 7.9 months. As shown in Figure 1, patients with performance status (PS) 0–1 had longer PFS than those with PS 2 (4.17 months vs. 1.93 months, p = .0014). As shown in Figure 2, patients without liver metastasis also had longer PFS than those who had live metastasis (5.87 months vs. 3.33 months, p = .0274).
Figure 1.
Subgroup analysis of progression‐free survival by ECOG performance status.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Figure 2.
Subgroup analysis of progression‐free survival by whether patients had liver metastasis.
Apatinib has demonstrated encouraging anticancer activity across a broad range of malignancies, including epithelial ovarian cancer [1], glioma [2], breast cancer [3], [4], gastric cancer [5], [6], and hepatocellular carcinoma [7]. There are still another 181 undergoing studies registered at ClinicalTrials.gov (https://www.clinicaltrials.gov). Two retrospective results about apatinib in advanced mCRC had been reported. Recently a real‐world retrospective study explored the apatinib used in mCRC [8]. Median progression‐free survival (PFS) of this study was 3.82 months, and median overall survival (OS) was not reached. Another pilot study also suggested that apatinib was active as a third‐line treatment of refractory mCRC with PFS of 4.8 months and OS of 10.1 months [9]. Although there were data from pilot and real‐world retrospective studies of apatinib, this is the first prospective clinical trial to investigate the safety and efficacy of apatinib in refractory metastatic colorectal cancer to our knowledge.
After two or more lines of prior chemotherapy, the efficacy of apatinib in our study seemed to be comparable with other tyrosine kinase inhibitors (TKIs) reported in previous studies. The CORRECT [10] and CONCUR [11] trials compared regorafenib with placebo among patients with mCRC who had received at least two prior chemotherapy regimens. In the CORRECT study, median PFS and OS were 1.9 months and 6.4 months for the regorafenib group and 3.2 months and 8.8 months, respectively, in the CONCUR study. The FRESCO study [12] evaluated efficacy of oral fruquintinib as third‐line or later therapy among patients with mCRC, in which the median PFS and median OS were 3.71 and 9.30 months. In our study, apatinib conferred a 3.9‐month median PFS and 7.9‐month median OS, which is comparable to that of regorafenib and fruquintinib. The objective response rate was approximately 1%–4.7% in trials of fruquintinib and regorafenib in mCRC [2], [3], [4]. No objective responses were observed in our trial. The relatively small sample size of our study might be the possible reason. Objective response might be observed if more patients were included in our trial.
Regarding to the safety of apatinib, hypertension, hand‐foot syndrome, proteinuria, and diarrhea are the most common adverse events in our study. These events are also well‐known, common adverse events in most studies of small‐molecule vascular endothelial growth factor receptor (VEGFR) TKI [6], [13]. VEGFR TKI could reduce blood flow and vessel patency, which were noted not in only tumor cells, but also in normal vasculature, particularly a loss of endothelial cells fenestrae in renal glomeruli and several endocrine organs [14]. Besides, anti‐VEGF‐A/VEGFR therapy also contributed to constriction of the feeder arteries supplying tumors, leading to reduced downstream blood flow and systemic hypertension [14]. These findings provided an explanation for some side effects of VEGFR TKI. Compared with other studies, it seems that a higher incidence of adverse events of apatinib occurred in our study. Firstly, patients in our study already have received multiple lines of chemotherapy before apatinib, with cumulative toxicity of previous drugs. Secondly, patients in our trial were required to record their blood pressure and other symptoms every day and were closely followed‐up by online tools; thus more adverse events could be recorded. In addition, the relatively small sample size of our study might impair the generalization of our conclusions. All the patients received dose reduction because of adverse events, indicating that a dose of 500 mg each day is unacceptable in heavily pretreated patients with colorectal cancer, and the toxicity administration is very important. Patients with performance status (PS) 0–1 had longer PFS than those with PS 2 in our study. The possible reason might be that patients with good physical status showed better tolerance of apatinib and thus benefit from it.
Circulating tumor DNA (ctDNA) is tumor‐derived fragmented DNA in the bloodstream, acting as a surrogate for tumor biopsy that can be detected in patients with cancer by identifying genomic aberrations. Recent studies showed that quantitative ctDNA dynamics could predict therapeutic efficacy before tumor response was assessed by imaging or clinical symptoms [15], [16], [17]. In metastatic colorectal cancer, ctDNA was reported to be an early marker of chemotherapy [18], [19]. In our study, increasing ctDNA has been found ahead of radiographic detection in 10 of 13 patients who had serial ctDNA detected, suggesting that detection of ctDNA was associated with radiographic tumor burden, which is consistent with previous studies.
A challenge to the application of angiogenesis inhibitors is finding suitable biomarkers to select patients who are most likely to benefit from it. Unfortunately, there has been no success in identifying biomarkers for any antiangiogenic drugs. As we know, VEGF signal transduction plays an important role in angiogenesis. It is worth mentioning that therapeutic intervention by blocking VEGF signal transduction occurs not only in tumor‐associated blood vessels but also in nonmalignant endothelial cells and tumor microenvironment [20], [21]. Tumor microenvironment was considered as potential source of clinical biomarkers [22]. Circulating endothelial cells have been correlated with prognosis in several tumors and with response to bevacizumab in colorectal cancer [23], [24], [25]. Here we analyzed a panel of 1,021 cancer‐related gene in all of the patients. The mutations detected in baseline ctDNA were showed in Figure 3A, which is consistant with MSK‐IMPACT profiles (Fig. 3B). We assessed whether APC, TP53, or KRAS mutation was associated with PFS and OS. Unfortunately, no positive results were found. On one hand, this study was subject to limitations of small sample size; on the other hand, as we mentioned above, antiangiogenic therapies act at least in part on vascular microenvironment rather than on tumor cells, making it more difficult to identify a biomarker in circulating tumor DNA.
It should be noted that there were still some limitations in our study, including small size, possible information bias, and lack of a control group. Multicenter randomized controlled double‐blind clinical trials and further follow‐up are expected in the future.
In conclusion, this study provides supporting evidence that apatinib exhibits efficacy for patients with refractory colorectal cancer, especially in patients with PS 0–1 or no liver metastasis. The common side effects of apatinib were hypertension, hand‐foot syndrome, proteinuria, and diarrhea. Given that sample size was small in this study, the efficacy and safety of apatinib in mCRC requires further investigation in a larger population.
Figure
Figure 3.
Mutations detected. Mutations detected in baseline (A) and mutations of baseline circulating tumor DNA and MSK integrated mutation profiling of actionable cancer targets (IMPACT) profiles (B).
Abbreviations: CNV, copy number variation; ECOG, Eastern Cooperative Oncology Group; MSK, Memorial Sloan Kettering.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 81572389), Jiangsu 333 Project (grant BRA2016517), Jiangsu Province Key Medical Talents (grant ZDRCA2016026), and the Advanced Health Talent of Six‐One Project of Jiangsu Province (grant LGY2017069).
Contributed equally.
Footnotes
ClinicalTrials.gov Identifier: NCT03190616
Sponsor: The First Affiliated Hospital with Nanjing Medical University
Principal Investigators: Yanhong Gu, Xiaofeng Chen, Tianzhu Qiu, Hua Jiang
IRB Approved: Yes
Contributor Information
Hua Jiang, Email: czeyjh@njmu.edu.cn.
Yanhong Gu, Email: guyhphd@163.com.
Disclosures
The authors indicated no financial relationships.
References
- 1.Miao M, Deng G, Luo S et al. A phase II study of apatinib in patients with recurrent epithelial ovarian cancer. Gynecol Oncol 2018;148:286–290. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Liang L, Yang T et al. A pilot clinical study of apatinib plus irinotecan in patients with recurrent high‐grade glioma: Clinical trial/experimental study. Medicine 2017;96:e9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu X, Cao J, Hu W et al. Multicenter phase II study of apatinib in non‐triple‐negative metastatic breast cancer. BMC Cancer 2014;14:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu X, Zhang J, Xu B et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple‐negative breast cancer. Int J Cancer 2014;135:1961–1969. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Wei Y, Shen S et al. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression‐free survival in advanced gastric cancer patients. Oncotarget 2017;8:29346–29354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Qin S, Xu J et al. Apatinib for chemotherapy‐refractory advanced metastatic gastric cancer: Results from a randomized, placebo‐controlled, parallel‐arm, phase II trial. J Clin Oncol 2013;31:3219–3225. [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Jin XL, Yang C et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: A single‐center randomized controlled trial. Cancer Biol Ther 2017;18:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gou M, Si H, Zhang Y et al. Efficacy and safety of apatinib in patients with previously treated metastatic colorectal cancer: A real‐world retrospective study. Sci Rep 2018;8:4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang L, Wang L, Zhu P et al. A pilot study of apatinib as third‐line treatment in patients with heavily treated metastatic colorectal cancer. Clin Colorect Cancer 2018;17:e443–e44. [DOI] [PubMed] [Google Scholar]
- 10.Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Qin S, Xu R et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2015;16:619–629. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Qin S, Xu RH et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: The FRESCO randomized clinical trial. JAMA 2018;319:2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Zhao X, Chen L et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor‐2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sitohy B, Nagy JA, Dvorak HF. Anti‐VEGF/VEGFR therapy for cancer: Reassessing the target. Cancer Res 2012;72:1909–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson SJ, Tsui DW, Murtaza M et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368:1199–1209. [DOI] [PubMed] [Google Scholar]
- 16.Mok T, Wu YL, Lee JS et al. Detection and dynamic changes of EGFR mutations from circulating tumor DNA as a predictor of survival outcomes in NSCLC patients treated with first‐line intercalated erlotinib and chemotherapy. Clin Cancer Res 2015;21:3196–3203. [DOI] [PubMed] [Google Scholar]
- 17.Garcia‐Murillas I, Schiavon G, Weigelt B et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015;7:302ra133. [DOI] [PubMed] [Google Scholar]
- 18.Tie J, Kinde I, Wang Y et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 2015;26:1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garlan F, Laurent‐Puig P, Sefrioui D et al. Early evaluation of circulating tumor DNA as marker of therapeutic efficacy in metastatic colorectal cancer patients (PLACOL study). Clin Cancer Res 2017;23:5416–5425. [DOI] [PubMed] [Google Scholar]
- 20.Lacal PM, Graziani G. Therapeutic implication of vascular endothelial growth factor receptor‐1 (VEGFR‐1) targeting in cancer cells and tumor microenvironment by competitive and non‐competitive inhibitors. Pharmacol Res 2018;136:97–107. [DOI] [PubMed] [Google Scholar]
- 21.Romero‐Laorden N, Doger B, Hernandez M et al. Predictive biomarker candidates to delineate efficacy of antiangiogenic treatment in renal cell carcinoma. Clin Transl Oncol 2016;18:1–8. [DOI] [PubMed] [Google Scholar]
- 22.Genova C, Rijavec E, Grossi F. Tumor microenvironment as a potential source of clinical biomarkers in non‐small cell lung cancer: Can we use enemy territory at our advantage? J Thorac Dis 2017;9:4300–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamori Y, Masago K, Ohmori K et al. Increase in circulating endothelial progenitor cells predicts response in patients with advanced non‐small‐cell lung cancer. Cancer Sci 2012;103:1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S, Ward MM, O'Loughlin J et al. Incremental increase in VEGFR1(+) hematopoietic progenitor cells and VEGFR2(+) endothelial progenitor cells predicts relapse and lack of tumor response in breast cancer patients. Breast Cancer Res Treat 2012;132:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manzoni M, Mariucci S, Delfanti S et al. Circulating endothelial cells and their apoptotic fraction are mutually independent predictive biomarkers in bevacizumab‐based treatment for advanced colorectal cancer. J Cancer Res Clin Oncol 2012;138:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]