Abstract
Lessons Learned.
Adjuvant treatment with zoledronic acid did not decrease the recurrence rate of giant cell tumor of bone (GCTB) in this study. The efficacy could not be determined because of the small sample size.
GCTB recurrences, even in the denosumab era, are still an issue; therefore, a randomized study exploring the efficacy of zoledronic acid in the adjuvant setting in GCTB is still valid.
Background.
Bisphosphonates are assumed to inhibit giant cell tumor of bone (GCTB)‐associated osteoclast activity and have an apoptotic effect on the neoplastic mononuclear cell population. The primary objective of this study was to determine the 2‐year recurrence rate of high‐risk GCTB after adjuvant zoledronic acid versus standard care.
Methods.
In this multicenter randomized open‐label phase II trial, patients with high‐risk GCTB were included (December 2008 to October 2013). Recruitment was stopped because of low accrual after the introduction of denosumab. In the intervention group, patients received adjuvant zoledronic acid (4 mg) intravenously at 1, 2, 3, 6, 9, and 12 months after surgery.
Results.
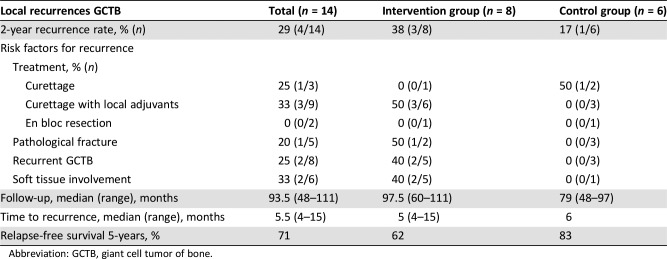
Fourteen patients were included (intervention n = 8, controls n = 6). Median follow‐up was long: 93.5 months (range, 48–111). Overall 2‐year recurrence rate was 38% (3/8) in the intervention versus 17% (1/6) in the control group (p = .58). All recurrences were seen within the first 15 months after surgery.
Conclusion.
Adjuvant treatment with zoledronic acid did not decrease the recurrence rate of GCTB in this study. The efficacy could not be determined because of the small sample size. Because recurrences, even in the denosumab era, are still an issue, a randomized study exploring the efficacy of zoledronic acid in the adjuvant setting in GCTB is still valid.
Abstract
经验总结
在本研究中,唑来膦酸辅助治疗未能降低骨巨细胞瘤 (GCTB) 的复发率。由于样本量小,无法确定疗效。
即使是Denosumab 治疗时代,仍存在 GCTB 复发问题,因此,探讨唑来膦酸辅助治疗 GCTB 的有效性随机研究仍然需要。
摘要
背景。双膦酸盐被认为可以抑制骨巨细胞瘤 (GCTB) 相关的破骨细胞活性,诱导肿瘤单核细胞群凋亡。本研究的主要目的是确定唑来膦酸辅助治疗与标准治疗对于高风险 GCTB 2 年复发率的影响。
方法。本项多中心随机开放标签 II 期试验的研究对象为高风险 GCTB 患者(2008 年 12 月至 2013 年 10 月)。Denosumab治疗出现后,由于入组率较低,停止了研究对象的招募。干预组患者于术后 1、2、3、6、9、12 个月后静脉注射唑来膦酸(4mg)辅助治疗。
结果。入组了 14 例患者(干预组 n = 8,对照组 n = 6)。随访时间较长,平均为:93.5 个月(范围:48–111个月)。干预组整体 2 年内复发率为 38% (3/8),对照组为 17% (1/6) (p = 0.58)。复发病情皆发生于术后 15 个月内。
结论。在本研究中,唑来膦酸辅助治疗未能降低 GCTB 的复发率。由于样本量小,无法确定疗效。即使是使用Denosumab治疗,仍存在 GCTB 复发问题,因此,探讨唑来膦酸辅助治疗 GCTB 的有效性随机研究仍然需要。
Discussion
GCTB are rare, locally aggressive bone tumors with the capacity to metastasize. The mainstay of treatment is surgical resection, either en bloc resection or curettage with or without local adjuvants like phenol, liquid nitrogen, or polymethylmethacrylate (PMMA). The majority of recurrences after primary intralesional surgery are seen in so‐called high‐risk GCTB. This group includes tumors with extension into surrounding soft tissue, (intra‐articular) pathologic fracture, recurrences, absence of local adjuvant therapy after primary curettage, and localization in the spine or sacrum. A systemic adjuvant treatment may be beneficial for this category of patients. Zoledronic acid has shown in different in vitro and animal studies to induce GCTB neoplastic stromal cell inhibition, apoptosis, and osteogenic differentiation. Further case reports and series support the beneficial use of zoledronic acid and other bisphosphonates as (neo)adjuvant or definitive treatment of GCTB.
We performed a multicenter randomized open‐label phase II trial in patients with high‐risk GCTB. The primary objective was to determine the 2‐year recurrence rate of GCTB after adjuvant zoledronic acid versus standard care. The trial's low accrual and early closure were due to the clinical introduction of denosumab in the treatment of GCTB.
Zoledronate did not decrease the recurrence rate of GCTB in this study. Although this was a randomized study, the (nonsignificant) higher number of recurrences in the zoledronate arm may be explained by more patients with a recurrent GCTB and soft tissue involvement, as well as more patients who had received suboptimal primary treatment with curettage instead of en bloc resection at primary surgery. All four patients who had a recurrence were treated with curettage, one without local adjuvants. Recurrence rates for the high‐risk cases described here are comparable to average recurrence rates in our previously published report. All recurrences were seen within the first 2 years after surgery, which is comparable to the literature.
Two other prospective trials were performed on the effects of adjuvant treatment with bisphosphonates in GCTB. These small nonrandomized trials with different bisphosphonates resulted in recurrence rates of 0%–15% after a follow‐up of 25–58 months.
The position of zoledronic acid in the treatment of GCTB next to denosumab is undetermined. Its use in advanced GCTB has strongly increased over the past few years because of several larger clinical trials that demonstrated tumor growth inhibition and reduced surgical morbidity. Despite these positive outcomes, doubts are also raised regarding the risk of tumor recurrence after denosumab withdrawal. It is suggested that a complete pathological response cannot be achieved, because denosumab does not have an apoptotic effect on the neoplastic stromal cell population. Therefore, zoledronic acid might be a more suitable (neo)adjuvant treatment option. Larger randomized trials with zoledronic acid are needed to give us further insight to the optimal treatment strategy in advanced GCTB.
Trial Information
- Disease
Giant cell tumor of bone
- Stage of Disease/Treatment
Adjuvant
- Prior Therapy
None
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Randomized
- Primary Endpoint
2‐year recurrence rate
- Secondary Endpoint
Relapse‐free survival
- Additional Details of Endpoints or Study Design
- This study was designed as a randomized, open‐label, multicenter phase II trial. Patients were eligible if they had a high‐risk GCTB treated with surgery. The primary objective was to determine the 2‐year recurrence rate of high‐risk GCTB after adjuvant zoledronic acid compared with standard care in the control arm. High‐risk GCTB was defined as one or more of the following: localization in pelvis, spine, sacrum, or distal ulna; joint or soft tissue involvement; pathologic fracture; grade III; or absence of local adjuvant therapy or recurrent GCTB. Patients with metastases, malignant GCTB, or prior use of bisphosphonates were excluded.
- Statistical methods: In case 90 patients were randomized in the study (45 in each arm), the study would have 95% power (α = .1; P0 = 45%, one‐tailed test) to detect differences between the recurrence rate in the two arms of 30% and 85% power to detect a difference of 25%. Relapse‐free survival curves were calculated by means of the using Kaplan‐Meier technique.
- The target number of patients was not reached because of low accrual after the introduction of denosumab in this patient group. Given the low accrual numbers, Fisher's exact test was used to assess differences between the treatment groups.
- Investigator's Analysis
Active, but patient numbers too low for accurate comparison.
Drug Information: Zoledronic Acid Arm
- Drug 1
- Generic/Working Name
Zoledronic acid
- Drug Type
Small molecule
- Dose
4 milligrams (mg) per flat dose
- Route
IV
- Schedule of Administration
Monthly for 3 months followed by a 3‐monthly schedule for up to 1 year after surgery. All subjects received daily supplements of 500 mg calcium and 400 IU of vitamin D, unless documented hypercalcemia (albumin‐adjusted serum calcium >2.9 mmol/L [11.5 mg/dL] or ionized calcium >1.5 mmol/L) developed on study.
Patient Characteristics: Zoledronic Acid Arm
- Number of Patients, Male
4
- Number of Patients, Female
4
- Stage
High‐risk GCTB
- Age
Median (range): 34 (21–55) years
- Performance Status: ECOG
-
0 — 3
1 — 5
2 —
3 —
Unknown —
- Other
Complete baseline demographic and disease characteristics are presented in Table 1.
Table 1. Local recurrences after adjuvant systemic therapy with zoledronic acid compared with standard care, including known risk factors for recurrence.
Abbreviation: GCTB, giant cell tumor of bone.
Patient Characteristics: Control Arm
- Number of Patients, Male
4
- Number of Patients, Female
2
- Stage
High‐risk GCTB
- Age
Median (range): 45.5 (19–73) years
- Performance Status: ECOG
-
0 — 2
1 — 4
2 —
3 —
Unknown —
- Other
Complete baseline demographic and disease characteristics are presented in Table 1.
Primary Assessment Method: Control Arm
- Title
2‐year recurrence rate
- Number of Patients Enrolled
7
- Number of Patients Evaluable for Toxicity
6
- Number of Patients Evaluated for Efficacy
6
- Evaluation Method
Other (recurrence confirmed by imaging and histology)
- Outcome Notes
Control Arm: Response Assessment: 2‐year recurrence rate, 17% (n = 1); time to recurrence, median, 6 months.
Primary Assessment Method: Zoledronic Acid Arm
- Title
2‐year recurrence rate
- Number of Patients Enrolled
8
- Number of Patients Evaluable for Toxicity
8
- Number of Patients Evaluated for Efficacy
8
- Evaluation Method
Other (recurrence confirmed by imaging and histology)
- Outcome Notes
Zoledronic acid Arm: Response Assessment: 2‐year recurrence rate, 38% (n = 3); time to recurrence, median (range), 5 months (4–15 months).
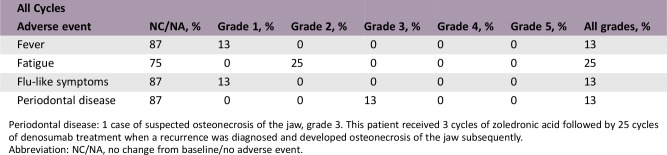
Adverse Events
Periodontal disease: 1 case of suspected osteonecrosis of the jaw, grade 3. This patient received 3 cycles of zoledronic acid followed by 25 cycles of denosumab treatment when a recurrence was diagnosed and developed osteonecrosis of the jaw subsequently.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Competing agents
- Investigator's Assessment
Active, but patient numbers too low for accurate comparison.
Giant cell tumors of bone (GCTBs) are rare, locally aggressive bone tumors with capacity to metastasize [1]. Their occurrence is most frequent in patients aged 30–40 years, in the metaphysis of long bones, but the tumors can also be found in other bones [2]. Histologically, GCTB consists of reactive osteoclast‐like giant cells expressing receptor activator of nuclear factor kappa‐B (RANK) with a CD33+CD14− phenotype [3], mononuclear osteoclast precursor cells, and neoplastic spindle‐shaped cells expressing RANK‐ligand [4]. RANK signaling promotes the generation of multinuclear osteoclast, which results in bone resorption among others by the production of the principal protease cathepsin K [5], [6], [7].
The mainstay of treatment is surgical resection, either en bloc resection or curettage with or without local adjuvants like phenol, liquid nitrogen, or polymethylmethacrylate (PMMA) [8], [9], [10], [11], [12]. The majority of recurrences after primary intralesional surgery are seen in so‐called high‐risk GCTB. This group includes tumors with extension into surrounding soft tissue, (intra‐articular) pathologic fracture, recurrences, absence of local adjuvant therapy after primary curettage, and localization in the spine or sacrum [13], [14]. A systemic adjuvant treatment may be beneficial for this category of patients.
Zoledronic acid is a bisphosphonate widely used for prevention of bone‐related complications in osseous metastatic cancer and tumor‐related hypercalcemia [15], [16]. Zoledronic acid has shown in different in vitro and animal studies to induce GCTB stromal cell inhibition, apoptosis, and osteogenic differentiation [17], [18], [19], [20], [21], [22]. Further case reports [23], [24], [25], [26], [27] and retrospective series support the beneficial use of zoledronic acid and other bisphosphonates as (neo)adjuvant [28], [29] or definitive treatment [30] of GCTB.
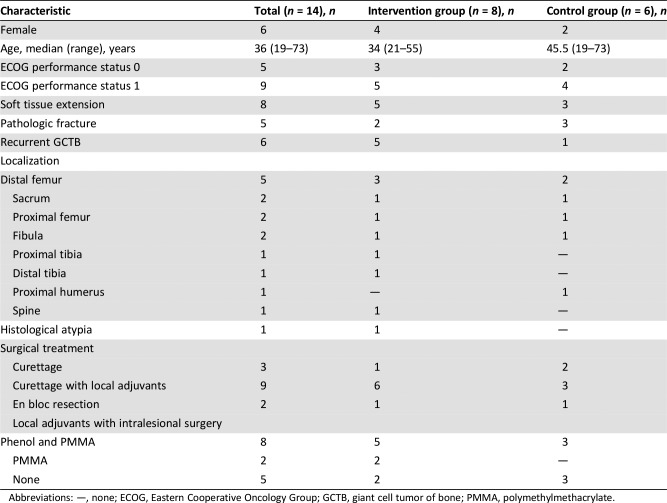
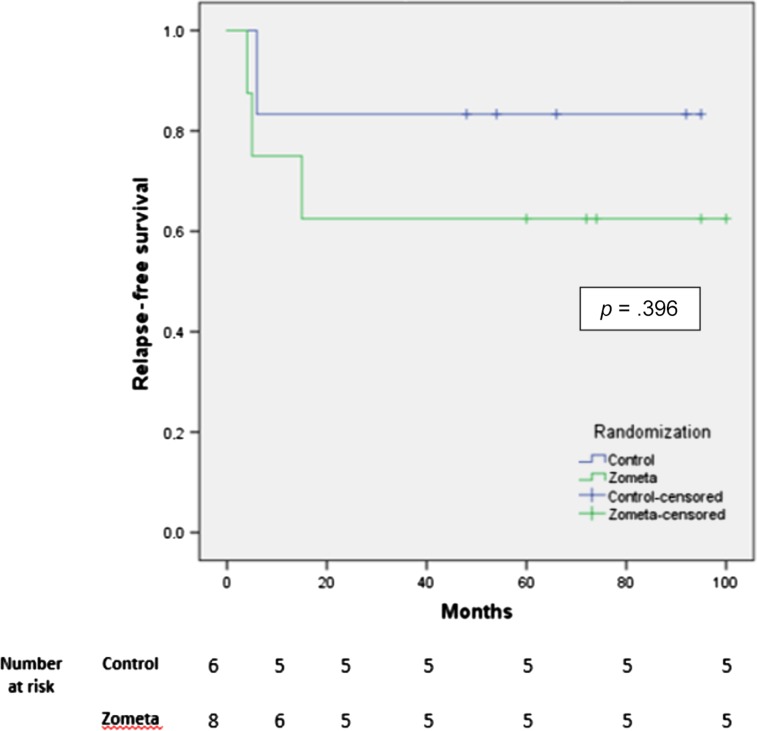
We performed a multicenter randomized open‐label phase II trial in patients with high‐risk GCTB. The primary objective was to determine the 2‐year recurrence rate of GCTB after adjuvant zoledronic acid versus standard care. The low accrual and early closure of the trial was due to the clinical introduction of denosumab in the treatment of GCTB. Adjuvant zoledronic acid was feasible, but it did not result in a decrease in 2‐years recurrence rate in this study. For patient, tumor and treatment characteristics, see Table 2. For relapse free survival, see Figure 1.
Table 2. Patient, tumor, and treatment characteristics.
Abbreviations: —, none; ECOG, Eastern Cooperative Oncology Group; GCTB, giant cell tumor of bone; PMMA, polymethylmethacrylate.
Figure 1.
Relapse free survival, Kaplan Meier.
One case of osteonecrosis of the jaw (ONJ) was seen among the eight patients treated in the intervention arm. This patient received three cycles of zoledronic acid, followed by 25 cycles of denosumab treatment when a recurrence was diagnosed, and developed ONJ subsequently. Therefore, the contributing factor of zoledronic acid is not clear. Other zoledronic acid‐related adverse events were grades 1–2. See also the Adverse Events table.
Zoledronate did not decrease the recurrence rate of GCTB in this study. Although this was a randomized study, the (nonsignificant) higher number of recurrences in the zoledronate arm may be explained by more patients with a recurrent GCTB and soft tissue involvement and by more patients who had received suboptimal primary treatment with curettage instead of en bloc resection at primary surgery. All four patients who had a recurrence were treated with curettage, in one case without the use of local adjuvants. Recurrence rates for the high‐risk cases described here are comparable to average recurrence rates in our previously published report [13]. All recurrences were seen within the first 2 years after surgery, which is comparable to literature [8], [11], [13], [14], [31].
Two other prospective trials were performed on the effects of adjuvant treatment with bisphosphonates in GCTB [32], [33]. Yu et al. included 16 patients with both primary and recurrent, nonaxial GCTB in a single‐arm prospective trial in which patients received 2 years of adjuvant bisphosphonate treatment (alendronate 10 mg per day for a period of 2 years) after intralesional curettage with PMMA. No recurrences were seen after a median follow‐up of 25 months [32]. Gouin et al. performed a single‐arm phase II trial among 24 patients with a primary GCTB treated with five adjuvant doses of zoledronic acid (4 mg every 3 weeks) after curettage with either PMMA or bone allograft. Recurrence rate was 15% with recurrences diagnosed 4, 24, and 58 months after surgery [33].
The benefit of adjuvant zoledronic acid, as well as optimal timing and duration of zoledronic acid treatment, is yet to be determined. The improved mineralization and marginalization [28], [34], [35] leading to easier curettage advocates the use of zoledronic acid in the neoadjuvant setting. In a recent comparative prospective study extended curettage was performed in 37 patients with GCTB with or without three preoperative zoledronic acid infusions (4 mg) at 3‐week intervals. Recurrences were seen in 1 out of 18 patients in the zoledronic acid group and 4 out of 19 patients in the control group (p = .47). Curettage tissue showed a decrease in stromal cells and increased calcification in the zoledronic acid group [34].
Local adjuvant bisphosphonate therapies, such as local zoledronic acid injections or irrigation, and zoledronic acid‐loaded bone cement have been tested in small series [36], [37]. Given the rationale of better bioavailability and fewer adverse effects because of lower systemic concentrations, this treatment might prove beneficial in preventing recurrences.
The position of zoledronic acid in the treatment of GCTB next to denosumab is undetermined. Denosumab is a monoclonal antibody that binds the receptor activator of RANK‐ligand, needed to develop and activate osteoclasts. Its use in advanced GCTB has strongly increased over the past few years because of several larger clinical trials that demonstrated tumor growth inhibition and reduced surgical morbidity [38], [39], [40], [41], [42]. Despite these positive outcomes, doubts are also raised, first of all regarding the neoadjuvant use of denosumab. Performing a complete curettage becomes more challenging because of the new bone formation and cortical thickening in these lesions after treatment with denosumab [43]. Further concerns exist in relation to the risk of tumor recurrence after denosumab withdrawal [17], [40], [44]. It is suggested that a complete pathological response cannot be achieved, because denosumab does not have an apoptotic effect on the neoplastic stromal cell population [17], [44], [45], [46]. The same studies suggested stromal cell inhibition and apoptosis after treatment with zoledronic acid [17], [45]; therefore, zoledronic acid might be a more suitable (neo)adjuvant treatment option. A combination of denosumab and bisphosphonate for optimal growth inhibition and apoptosis of the neoplastic cells might be feasible, although safety is an issue here given the overlapping toxicity profile. Larger randomized trials with zoledronic acid and longer follow‐up are needed to give us further insight to the optimal treatment strategy in advanced GCTB, which agents to choose, and the optimal treatment duration.
Figure and Table
Footnotes
ClinicalTrials.gov Identifier: NCT00889590
Sponsor: Leiden University Medical Center
Principal Investigator: Hans Gelderblom
IRB Approved: Yes
Disclosures
Paul C. Jutte: Stryker (C/A). The authors indicated no financial relationships.
References
- 1.Athanasou N, Bansal M, Forsyth R et al. Giant cell tumour of bone In: Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, eds. WHO Classification of Tumours in Soft Tissue and Bone. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC), 2013. [Google Scholar]
- 2.Liede A, Bach BA, Stryker S et al. Regional variation and challenges in estimating the incidence of giant cell tumor of bone. J Bone Joint Surg Am 2014;96:1999–2007. [DOI] [PubMed] [Google Scholar]
- 3.Forsyth RG, De Boeck G, Baelde JJ et al. CD33+ CD14‐ phenotype is characteristic of multinuclear osteoclast‐like cells in giant cell tumor of bone. J Bone Miner Res 2009;24:70–77. [DOI] [PubMed] [Google Scholar]
- 4.Branstetter DG, Nelson SD, Manivel JC et al. Denosumab induces tumor reduction and bone formation in patients with giant‐cell tumor of bone. Clin Cancer Res 2012;18:4415–4424. [DOI] [PubMed] [Google Scholar]
- 5.Atkins GJ, Kostakis P, Vincent C et al. RANK Expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. J Bone Miner Res 2006;21:1339–1349. [DOI] [PubMed] [Google Scholar]
- 6.Lindeman JH, Hanemaaijer R, Mulder A et al. Cathepsin K is the principal protease in giant cell tumor of bone. Am J Pathol 2004;165:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maggiani F, Forsyth R, Hogendoorn PC et al. The immunophenotype of osteoclasts and macrophage polykaryons. J Clin Pathol 2011;64:701–705. [DOI] [PubMed] [Google Scholar]
- 8.Arbeitsgemeinschaft K, Becker WT, Dohle J et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am 2008;90:1060–1067. [DOI] [PubMed] [Google Scholar]
- 9.Algawahmed H, Turcotte R, Farrokhyar F et al. High‐speed burring with and without the use of surgical adjuvants in the intralesional management of giant cell tumor of bone: A systematic review and meta‐analysis. Sarcoma 2010;2010:586090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balke M, Schremper L, Gebert C et al. Giant cell tumor of bone: Treatment and outcome of 214 cases. J Cancer Res Clin Oncol 2008;134:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kivioja AH, Blomqvist C, Hietaniemi K et al. Cement is recommended in intralesional surgery of giant cell tumors: A Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop 2008;79:86–93. [DOI] [PubMed] [Google Scholar]
- 12.van der Heijden L, Dijkstra PD, van de Sande MA et al. The clinical approach toward giant cell tumor of bone. The Oncologist 2014;19:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Heijden L, van de Sande MA, Dijkstra PD. Soft tissue extension increases the risk of local recurrence after curettage with adjuvants for giant‐cell tumor of the long bones. Acta Orthop 2012;83:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klenke FM, Wenger DE, Inwards CY et al. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res 2011;469:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neville‐Webbe HL, Coleman RE. Bisphosphonates and RANK ligand inhibitors for the treatment and prevention of metastatic bone disease. Eur J Cancer 2010;46:1211–1222. [DOI] [PubMed] [Google Scholar]
- 16.Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584–593. [DOI] [PubMed] [Google Scholar]
- 17.Lau CP, Huang L, Wong KC et al. Comparison of the anti‐tumor effects of denosumab and zoledronic acid on the neoplastic stromal cells of giant cell tumor of bone. Connect Tissue Res 2013;54:439–449. [DOI] [PubMed] [Google Scholar]
- 18.Lau CP, Wong KC, Huang L et al. A mouse model of luciferase‐transfected stromal cells of giant cell tumor of bone. Connect Tissue Res 2015;56:493–503. [DOI] [PubMed] [Google Scholar]
- 19.Cheng YY, Huang L, Lee KM et al. Bisphosphonates induce apoptosis of stromal tumor cells in giant cell tumor of bone. Calcif Tissue Int 2004;75:71–77. [DOI] [PubMed] [Google Scholar]
- 20.Yang T, Zheng XF, Li M et al. Stimulation of osteogenic differentiation in stromal cells of giant cell tumour of bone by zoledronic acid. Asian Pac J Cancer Prev 2013;14:5379–5383. [DOI] [PubMed] [Google Scholar]
- 21.Chang SS, Suratwala SJ, Jung KM et al. Bisphosphonates may reduce recurrence in giant cell tumor by inducing apoptosis. Clin Orthop Relat Res 2004:103–109. [DOI] [PubMed] [Google Scholar]
- 22.Balke M, Neumann A, Szuhai K et al. A short‐term in vivo model for giant cell tumor of bone. BMC Cancer 2011;11:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arpornchayanon O, Leerapun T. Effectiveness of intravenous bisphosphonate in treatment of giant cell tumor: A case report and review of the literature. J Med Assoc Thai 2008;91:1609–1612. [PubMed] [Google Scholar]
- 24.Chaudhary P, Khadim H, Gajra A et al. Bisphosphonate therapy is effective in the treatment of sacral giant cell tumor. Onkologie 2011;34:702–704. [DOI] [PubMed] [Google Scholar]
- 25.Gille O, Oliveira Bde A, Guerin P et al. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine (Phila Pa 1976) 2012;37:E396–E399. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Zhang Y, Li P et al. Administration of sodium ibandronate in the treatment of complicated giant cell tumor of the spine. Spine (Phila Pa 1976) 2011;36:E1166–E1172. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto N, Nakagawa K, Seichi A et al. A new bisphosphonate treatment option for giant cell tumors. Oncol Rep 2001;8:643–647. [DOI] [PubMed] [Google Scholar]
- 28.Tse LF, Wong KC, Kumta SM et al. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: A case‐control study. Bone 2008;42:68–73. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Wang Y, Wang J et al. Long‐term administration of bisphosphonate to reduce local recurrence of sacral giant cell tumor after nerve‐sparing surgery. J Neurosurg Spine 2017;26:716–721. [DOI] [PubMed] [Google Scholar]
- 30.Balke M, Campanacci L, Gebert C et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer 2010;10:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Donnell RJ, Springfield DS, Motwani HK et al. Recurrence of giant‐cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am 1994;76:1827–1833. [DOI] [PubMed] [Google Scholar]
- 32.Yu X, Xu M, Xu S et al. Clinical outcomes of giant cell tumor of bone treated with bone cement filling and internal fixation, and oral bisphosphonates. Oncol Lett 2013;5:447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gouin F, Rochwerger AR, Di Marco A et al. Adjuvant treatment with zoledronic acid after extensive curettage for giant cell tumours of bone. Eur J Cancer 2014;50:2425–2431. [DOI] [PubMed] [Google Scholar]
- 34.Kundu ZS, Sen R, Dhiman A et al. Effect of intravenous zoledronic acid on histopathology and recurrence after extended curettage in giant cell tumors of bone: A comparative prospective study. Indian J Orthop 2018;52:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cornelis F, Truchetet ME, Amoretti N et al. Bisphosphonate therapy for unresectable symptomatic benign bone tumors: A long‐term prospective study of tolerance and efficacy. Bone 2014;58:11–16. [DOI] [PubMed] [Google Scholar]
- 36.Chen KH, Wu PK, Chen CF. Zoledronic acid‐loaded bone cement as a local adjuvant therapy for giant cell tumor of the sacrum after intralesional curettage. Eur Spine J 2015;24:2182–2188. [DOI] [PubMed] [Google Scholar]
- 37.Nishisho T, Hanaoka N, Miyagi R et al. Local administration of zoledronic acid for giant cell tumor of bone. Orthopedics 2015;38:e25–e30. [DOI] [PubMed] [Google Scholar]
- 38.Thomas D, Henshaw R, Skubitz K et al. Denosumab in patients with giant‐cell tumour of bone: An open‐label, phase 2 study. Lancet Oncol 2010;11:275–280. [DOI] [PubMed] [Google Scholar]
- 39.Chawla S, Henshaw R, Seeger L et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open‐label, parallel‐group, phase 2 study. Lancet Oncol 2013;14:901–908. [DOI] [PubMed] [Google Scholar]
- 40.Gaston CL, Grimer RJ, Parry M et al. Current status and unanswered questions on the use of denosumab in giant cell tumor of bone. Clin Sarcoma Res 2016;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueda T, Morioka H, Nishida Y et al. Objective tumor response to denosumab in patients with giant cell tumor of bone: A multicenter phase II trial. Ann Oncol 2015;26:2149–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutkowski P, Ferrari S, Grimer RJ et al. Surgical downstaging in an open‐label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol 2015;22:2860–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Errani C, Ruggieri P, Asenzio MA et al. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat Rev 2010;36:1–7. [DOI] [PubMed] [Google Scholar]
- 44.Mak IW, Evaniew N, Popovic S et al. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am 2014;96:e127. [DOI] [PubMed] [Google Scholar]
- 45.Shibuya I, Takami M, Miyamoto A et al. In vitro study of the effects of denosumab on giant cell tumor of bone: Comparison with zoledronic acid. Pathol Oncol Res 2019;25:409–419. [DOI] [PubMed] [Google Scholar]
- 46.van der Heijden L, van de Sande MA, Hogendoorn PC et al. Neoadjuvant denosumab for extensive giant cell tumor in os ischium: A case report. Acta Orthop 2015;86:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]