Results from comprehensive genomic profiling in a cohort of patients with advanced parathyroid carcinoma are reported.
Keywords: Parathyroid cancer, Sequencing, Targeted therapy, Mutation, Profiling
Abstract
Background.
Parathyroid carcinoma (PC) is a rare endocrine malignancy that can cause life‐threatening hypercalcemia. We queried whether comprehensive genomic profiling (CGP) of PC might identify genomic alterations (GAs), which would suggest benefit from rationally matched therapeutics.
Methods.
We performed hybrid‐capture‐based CGP to identify GAs and tumor mutational burden (TMB) in tumors from patients with this malignancy.
Results.
There were 85 total GAs in 16 cases (5.3 GAs per case), and the median TMB was 1.7 mutations per megabase (m/Mb), with three cases having >20 m/Mb (18.7%). The genes most frequently harboring GA were CDC73 (38%), TP53 (38%), and MEN1 (31%). All MEN1‐mutated cases also had loss of heterozygosity at that locus, but in contrast all CDC73‐mutated cases retained heterozygosity. GAs suggesting potential benefit from matched targeted therapy were identified in 11 patients (69%) and most frequently found in PTEN (25%), NF1 (12.5%), KDR (12.5%), PIK3CA (12.5%), and TSC2 (12.5%). A patient whose tumor harbored KDR T668 K and who was treated with cabozantinib experienced a > 50% drop in parathyroid hormone level and radiographic partial response of 5.4 months with duration limited by toxicity.
Conclusion.
CGP identified GAs in PC that suggest benefit from targeted therapy, as supported by an index case of response to a matched tyrosine kinase inhibitor. Moreover, the unexpectedly high frequency of high TMB (>20 m/Mb) suggests a subset of PC may benefit from immune checkpoint inhibitors.
Implications for Practice.
Parathyroid carcinoma (PC) is a rare endocrine malignancy that can cause life‐threatening hypercalcemia. However, its molecular characteristics remain unclear, with few systemic therapeutic options available for this tumor. Hybrid‐capture‐based comprehensive genomic profiling of 16 primary cancers demonstrated presence of potentially actionable genomic alterations, including PTEN, NF1, KDR, PIK3CA, and TSC2, and a subset of hypermutated cancers with more than 20 mutations per megabase, the latter of which could benefit from immune checkpoint inhibitor therapy. A case benefiting from rationally matched targeted therapy for activating KDR mutation is also presented. These findings should be further investigated for their therapeutic potential.
Introduction
Parathyroid carcinoma (PC) is a rare malignant neoplasm accounting for less than 1% of all cases of hyperparathyroidism and approximately 0.005% of all cancers [1]. Most patients (>90%) with PC present with symptoms of hyperparathyroidism, which can include potentially life‐threatening hypercalcemia and end‐organ damage such as nephrolithiasis, renal failure, decreased bone mineral density and/or fracture, cardiac arrhythmia, and neurocognitive dysfunction [2]. Although PC is rare, its incidence between 1988 and 2003 increased by 60%, and 5‐ and 10‐year mortality rates were 16% and 33%, respectively [1]. Most patients achieve long‐term survival with surgical resection, but multiple operations and/or systemic therapy may be required for recurrent and/or metastatic disease [3].
Management of the latter is extremely challenging and has been focused on controlling hypercalcemia with agents such as bisphosphonates, denosumab, and calcimimetics. No cytotoxic regimen has been proven to be effective in a clinical trial involving patients with PC [4]. Thus, new active treatments are needed, and identification of genomic alterations (GAs) in the course of clinical care could lead to individualized treatment with rationally matched targeted agents or immunotherapies.
Herein, we report results from comprehensive genomic profiling (CGP) in a cohort of 16 patients with advanced PCs with a focus on identifying pathways for therapeutic benefit, and we present an index case benefiting from rationally matched therapy.
Materials and Methods
CGP was performed in a Clinical Laboratory Improvement Amendments‐certified, College of American Pathologists‐accredited laboratory (Foundation Medicine, Inc., Cambridge, MA). Approval for this study, including a waiver of informed consent and a Health Insurance Portability and Accountability Act waiver of authorization, was obtained from the Western Institutional Review Board (Protocol no. 20152817). Written consent from the index patient for reporting clinical information and images was obtained and filed on the chart. The pathologic diagnosis of each case was confirmed on routine hematoxylin and eosin‐stained slides, and all samples forwarded for DNA extraction contained a minimum of 20% tumor nuclear area, compared with benign nuclear area. In brief, ≥50 ng DNA was extracted from 40 microns of 16 parathyroid carcinoma specimens in formalin‐fixed, paraffin‐embedded tissue blocks. The samples were assayed by CGP using adaptor ligation, and hybrid capture was performed for all coding exons from 287 (version 1) to 315 (version 2) cancer‐related genes plus select introns from 19 (version 1) to 28 (version 2) genes frequently rearranged in cancer. Sequencing of captured libraries was performed using the Illumina HiSeq technology (Illumina, San Diego, CA) to a mean exon coverage depth of at least ×500, and resultant sequences were analyzed for GAs, including short variant alterations (base substitutions, insertions and deletions), copy number alterations (focal amplifications and homozygous deletions), and select gene fusions or rearrangements, as previously described [5]. Germline variants documented in the dbSNP database (dbSNP142; http://www.ncbi.nlm.nih.gov/SNP/), with two or more counts in the ExAC database (http://exac.broadinstitute.org/) or recurrent variants of unknown significance that were predicted by an internally developed algorithm to be germline, were removed, except for known driver germline events (e.g., documented hereditary BRCA1/2 and deleterious TP53 mutations) [6]. Known confirmed somatic alterations deposited in the Catalog of Somatic Mutations in Cancer (COSMIC, version 62) were highlighted as biologically significant [7]. All inactivating events (i.e., truncations and deletions) in known tumor suppressor genes were also called significant. To maximize mutation detection accuracy (sensitivity and specificity) in impure clinical specimens, the test was previously optimized and validated to detect base substitutions at a ≥ 5% mutant allele frequency (MAF), indels with a ≥ 10% MAF with ≥99% accuracy, and fusions occurring within baited introns/exons with >99% sensitivity [5]. Tumor loss of heterozygosity was determined as described previously [8]. Copy number alteration were detected by fitting a statistical copy number to normalized coverage data at all sequenced exons and ~3,500 genome‐wide single‐nucleotide polymorphisms (SNPs). This profile was segmented and interpreted using allele frequencies of sequenced SNPs to estimate tumor purity and copy number at each segment. Fitting was performed using Gibbs sampling, assigning total copy number and minor allele count to all segments [5]. Loss of heterozygosity was called if the total copy number at a locus was 1 (LOH1), or if the copy number was 2 or more with a minor allele count of 0 (LOHx). The distortion of the germline alternate allele frequency from 50% because of LOH was calculated.
Tumor mutational burden (TMB) was determined on 0.8 megabase (Mb; version 1) or 1.1 Mb (version 2) of sequenced DNA for each sample based on the number of somatic base substitution or indel alterations per Mb after filtering to remove known functionally oncogenic somatic mutations, as previously described [9].
Results
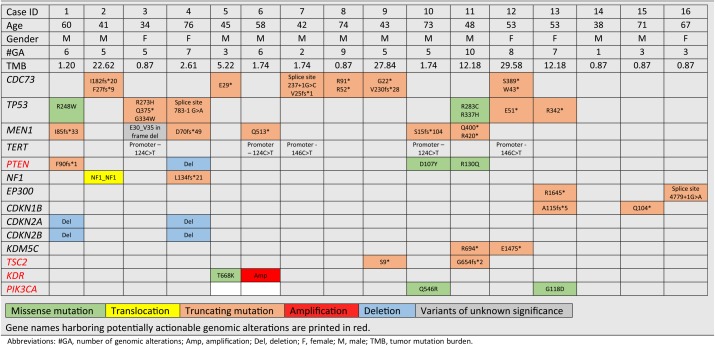
Of 16 patients with PC, 11 were male and 5 were female, and their median age was 56 years (range, 38–76). All cases (100%) were advanced or metastatic disease at the time of CGP. GAs suggesting potential benefit from matched targeted therapy were identified in 11 out of 16 patients (69%) and most frequently observed in PTEN, NF1, TSC2, KDR, and PIK3CA. Missense mutations in HRAS, PTCH2, and PIK3R1, amplifications of 4q12 (PDGFRA, KIT, and KDR) and 20q13 (AURKA, SRC, TOP1, ZNF217, ARFRP1, GNAS) were identified in one case each (6%; Table 1). Four cases (25%) harbored alterations in PTEN, and alterations in NF1, EP300, CDKN2A/B, KDR, PIK3CA, KDM5C, and TSC2 were seen in two cases (12%) each. There were 85 total GAs, with a mean of 5.3 GAs per case. The most frequent GAs were mutations in CDC73 and TP53, which were observed in six cases each (38%), and MEN1 and TERT alterations in five cases (31%) each. Mutations in CDC73 and MEN1 were mutually exclusive in this series (p = .09, Fischer's exact test). All three cases of high TMB also harbored GA in CDC73 but not MEN1.
Table 1. Summary of clinical and sequencing data of 16 cases of patients with parathyroid carcinoma.
Abbreviations: #GA, number of genomic alterations; Amp, amplification; Del, deletion; F, female; M, male; TMB, tumor mutation burden.
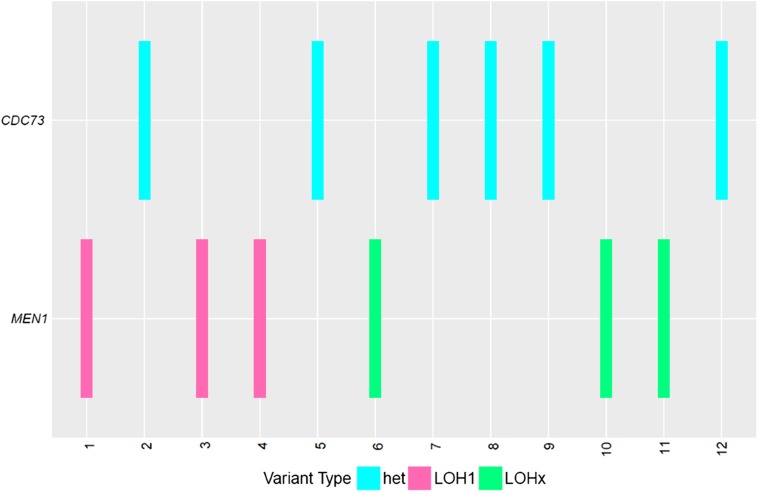
The median TMB for these cases of PC was 1.7 mutations per megabase (m/Mb), compared with a median of 3.6 m/Mb for all human cancers [9]. Three cases (19%) had high TMB, defined as greater than 20 m/Mb. LOH was assessed for CDC73 and MEN1. All CDC73 alterations were found to be heterozygous, whereas 50% of MEN1 alterations were under LOH (LOH1, one allele only), and the remainder exhibited copy‐number‐neutral LOH (LOHx, two or more identical alleles; Fig. 1). In one case, an inframe deletion (E30_V35 indel) in MEN1 was classified as a variant of unknown significance (VUS) but was also under LOH.
Figure 1.
Loss of heterozygosity in CDC73 and MEN1 for 12 patients (case IDs 1–12). All CDC73 alterations are heterozygous, whereas all MEN1 alterations exhibit evidences of LOH.
Abbreviations: Het, heterozygous; LOH1, loss of heterozygosity; LOHx, copy number neutral loss of heterozygosity.
Index Case
A 45‐year‐old man presented with nausea and bone pain and was found to be hypercalcemic, 14.5 mg/dL, in association with a markedly elevated intact parathyroid hormone (iPTH) level, 1,105 pg/mL. His family history was notable for a brother with successfully resected primary hyperparathyroidism. The patient was initially treated with partial parathyroidectomy and thyroidectomy. Surgical pathology revealed parathyroid carcinoma present at the margins, and his postoperative iPTH level remained elevated, 65 pg/mL. Eight months later, he developed recurrent fatigue and bone pain, a right neck mass, and rises in calcium and iPTH, which prompted performance of a right neck dissection, which confirmed recurrence of PC. Ten months later, he continued to have hypercalcemia, and a revision neck dissection was performed with no relief in symptoms or hypercalcemia. Three months thereafter, he underwent bilateral modified radical neck dissection, after which iPTH level dropped only to 202 pg/mL from 967 pg/mL. Over the next year, his iPTH continued to rise, peaking at 2,093 pg/mL, despite multiple computed tomography‐guided minimally invasive radiofrequency ablation procedures. During this period, a specimen from the initial dissection was submitted for CGP, which identified KDR (T668K), CDC73 (E29*), and KDM5A (S1403F) mutations. Estimated tumor mutational burden was 5.41 m/Mb. At the time, the patient opted for treatment with off‐label nivolumab, an anti‐PD1 antibody. Despite seven biweekly doses of nivolumab for 14 weeks, his iPTH level increased to 4,063 pg/mL with an increase as well in the size of supraclavicular and paratracheal lymph nodes. Later, a PD‐L1 stain with 22C3 antibody came back negative (0% by tumor proportion score). Treatment with nivolumab was discontinued, and the patient underwent a revision neck dissection including excision of the supraclavicular lesion; the iPTH level dropped to 841 pg/mL but quickly rebounded to 3,000 pg/mL within 2 months.
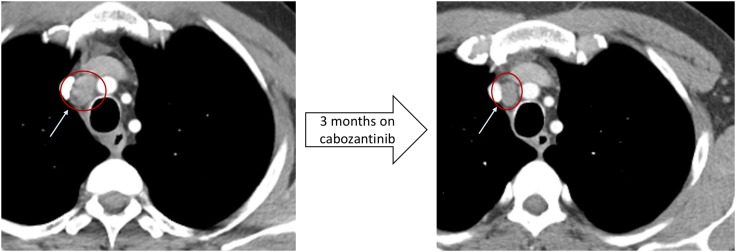
Based on the presence of KDR T668K, cabozantinib, a potent inhibitor of multiple receptor tyrosine kinases including VEGFR‐2 (protein product of KDR) [10], was started at 60 mg daily with disease burden evident only in the mediastinum. The patients iPTH level dropped from 3,000 pg/mL to 1,373 pg/mL, and the size of a paratracheal lymph node decreased from 1.4 cm to 1.1 cm over 3 months (Figs. 2 and 3). When the patient discontinued the medication because of treatment‐related toxicities, including hypertension, fatigue, diarrhea, and epistaxis, his iPTH level rose to 2,920 pg/mL over the next 3 months. He restarted cabozantinib, but the iPTH level kept trending upward to 3,778 pg/mL. He was then switched to ramucirumab, which is a monoclonal antibody against VEGFR‐2, but his iPTH continued to rise to 6,390 pg/mL. He was then switched to regorafenib, another tyrosine kinase inhibitor that inhibits VEGFR‐2, after which his iPTH level dropped to 5,040 pg/mL over 2 months, and which has been well tolerated to date.
Figure 2.
Trends of intact parathyroid hormone (pg/mL) in a patient with PC with activating KDR mutation. Cabozantinib dropped iPTH level by 60% in 5 months.
Abbreviation: iPTH, intact parathyroid hormone.
Figure 3.
Radiographic response with cabozantinib in a patient with PC with activating KDR mutation. There was a 28% reduction in tumor size in 3 months after initiating cabozantinib.
Discussion
Parathyroid carcinoma is one of the rarest of human cancers, and no proven systemic treatment option exists for patients with multiply recurrent or metastatic disease. Previous research has shown that somatic mutations in CDC73, a known tumor suppressor gene, are associated with sporadic PCs in 47% of cases [11]. Another tumor suppressor gene, MEN1, has been implicated in sporadic and familial PCs. However, neither of these genomic lesions is currently amenable to a targeted therapy [12]. This study reviews genomic profiles generated by CGP during clinical care to identify genomic alterations that may suggest benefit from targeted therapy.
A key recent study on PC performed whole exome sequencing on 25 sporadic and familial patients and demonstrated mutations in CDC73 (44%), FAT3 (24%,), and PIK3CA (16%). This study also showed that 5 out of 17 patients (29%) had biallelic inactivation in CDC73, which suggests germline mutations in familial PC cases [11].
Our analysis using CGP demonstrates that a significant proportion (69%) of patients harbor GAs, suggesting benefit from rationally matched targeted therapy. We also unexpectedly observed frequent hypermutation in PC, with nearly 19% of cases harboring >20 m/Mb, and all such cases were CDC73 mutated. An elevated tumor mutational burden correlates with response to immune checkpoint inhibitors (ICPIs), first observed in trials in lung cancer and melanoma and then across cancer types [13], [14], [15]. The etiology of hypermutation for these PC cases was unknown, as all were microsatellite stable and thus had intact DNA mismatch repair. Interestingly, the hypermutation could correlate to the two mutations of CDC73 and is not seen in MEN1‐mutated cases. It is unknown how this correlates to oncogenesis and warrants further investigation to confirm the frequency of hypermutation, although the rarity of PC may be prohibitive in accruing a series larger than this one in the next few years.
CDC73 mutations were the most common GA in PC in this series. CDC73 (also known as HRPT2) encodes for parafibromin, and mutations in the gene have been associated with hyperparathyroidism‐jaw tumor syndrome, a rare autosomal dominant disease, characterized by parathyroid tumors, ossifying fibromas of the mandible and maxilla, renal hamartomas, and cystic kidney disease [16]. We observed 11 distinct truncating mutations in six cases, none of which were under LOH. From this, it is not currently possible to ascertain whether biallelic inactivation of CDC73 is occurring via compound mutations in these cases. In an animal model, inactivation of a single allele of CDC73 induced parathyroid tumors [17]. In this series, the presence of two CDC73 mutations in five of six cases intuitively suggests that the Knudson two‐hit hypothesis for ablation of a tumor suppressor is at play, despite the heterozygosity. This would suggest a trans genotype of mutations on different alleles, but cis or trans status of these mutations could not be assessed because of the distance between the mutations, as has been done for T790M and C797S for EGFR‐mutant lung cancer that is osimertinib resistant [18].
We also observed truncating mutations in MEN1 in five patients with PC. MEN1 mutation causes a genetic disorder, multiple endocrine neoplasia type 1 (MEN1), which is characterized by tumors of the parathyroid, pancreatic islet cells, and anterior pituitary [19]. Most parathyroid tumors in patients affected by MEN1 are benign, but PCs have been reported among these patients [20]. In contrast to CDC73‐mutated PC cases, all MEN1‐mutated patients had either definitive LOH (single allele) or copy number neutral LOH (homozygous alleles all mutated), which suggests biallelic inactivation of MEN1 is critical for some PC cases. Moreover, a case harboring a MEN1 deletion classified as a VUS also exhibited LOH at the MEN1 locus. Given the co‐occurrence of LOH and MEN1 mutation in other cases, this is quite likely is a functionally inactivating MEN1 event and should be reassessed.
Our analysis also showed frequent GAs of PTEN (18%, 4/16) and PIK3CA (13%, 2/16), core components of PI3K/AKT/mTOR pathway, which has a critical role in controlling cell growth and metabolism [21], [22]. These GAs suggest potential benefit from mTOR pathway inhibitors.
Truncating mutation and rearrangement in NF1 were seen in two cases. Mutations of NF1 are linked to neurofibromatosis type 1 (NF1), a tumor syndrome associated with malignant peripheral nerve sheath tumors, central nervous system tumors, and breast cancers [23]. PC has previously been observed in a single patient with NF1 [24], and a few other case reports suggest some association of NF1 and parathyroid adenoma [25], [26], [27]. This series further links NF1 mutation to PC, which could be verified with further studies.
We identified TP53 mutations, all truncating or gain of function and thus likely pathogenic, in six patients with PC. To our knowledge, TP53 mutation has not been previously reported in PC, although these mutations are not therapeutic targets [11].
Similarly, two hotspot mutations in the TERT promoter, 124C > T and 146C > T, have been well documented to be activating mutations and identified in high frequency in various human cancers such as melanoma [28], [29]. Five patients with PC harbored these TERT hotspot mutations.
Notably, CDC73‐ or MEN1‐mutated tumors from PC patients also harbored other alterations, indicating that canonical oncogenic drivers for PC can coexist with targetable alterations. The clinical relevance is exemplified by the index case of a patient with CDC73‐mutated PC with KDR mutation who responded to both cabozantinib and regorafenib.
KDR encodes for VEGFR‐2, which plays a crucial role in tumor biology independent of angiogenesis [30]. KDR T668K occurs in the immunoglobulin‐like (Ig‐like) C2‐type domain 7 of VEGFR‐2 and has been observed in a lung adenocarcinoma in the COSMIC database, suggesting that it is functionally active [31]. Cabozantinib, given as anti‐VEGFR2 therapy, is a multikinase inhibitor that also inhibits MET, RET, AXL, KIT, TIE2, and FLT3. Notably, a monoclonal antibody that binds domain VEGFR‐2, ramucirumab, did not affect the iPTH level. Ramucirumab binds to the Ig‐like C2‐type type 3 domain of VEGFR‐2 [32], and T668K is in the possibly adjacent type 7 domain. A previous report linked KDR T771R to outgrowth of an angioma by the likely inhibition of ramucirumab binding, which may be a relevant analogy to this case [33]. The patient also responded to and, importantly, better tolerated another regorafenib, which inhibits RET, Raf‐1, KIT, and VEGFR‐1 in addition to VEGFR‐2. The cumulative benefit from targeted therapy for this patient illustrates the benefit of CGP in patients with PC with matched therapy even in the presence of mutations in CDC73 or MEN1, likely independent of the latter's somatic or germline origin.
Conclusion
It is important to note that these results are based on observations in patients with PC and have not been compared to normal parathyroid tissue or parathyroid adenomas. Also, family history information on presence of familial PC syndrome was not available to the investigators, which remains a major limitation of the study. Nonetheless, genomic profiling of patients with PC revealed subsets of patients with genomic features that suggest the rational matching of therapy. For the hypermutated PC cases, validation in a larger series would aid a more definitive estimation of the frequency of high TMB genotype, but the rarity of PC may prove prohibitive in accruing patients in a timely fashion. Regardless, the suggestion that patients with high TMB PC may benefit from ICPIs is a compelling avenue for clinical investigation. For targeted therapy, identification of druggable alterations beyond the characteristic lesions CDC73 or MEN1 may offer clinical benefit to patients with this rare cancer, as exemplified by the index case.
See http://www.TheOncologist.com for supplemental material available online.
Author Contributions
Conception/design: Hyunseok Kang, Paul W. Ladenson, Douglas W. Ball, Alexa B. Schrock, Russell Madison, Garrett M. Frampton, Jeffrey S. Ross
Provision of study material or patients: Vincent A. Miller, Siraj M. Ali
Collection and/or assembly of data: Hyunseok Kang, Dean Pettinga, Adrian D. Schubert, Siraj M. Ali
Data analysis and interpretation: Hyunseok Kang, Dean Pettinga, Adrian D. Schubert, Paul W. Ladenson, Douglas W. Ball, Jon H. Chung, Alexa B. Schrock, Russell Madison, Garrett M. Frampton, Phil J. Stephens, Jeffrey S. Ross, Vincent A. Miller, Siraj M. Ali
Manuscript writing: Hyunseok Kang, Dean Pettinga, Adrian D. Schubert, Paul W. Ladenson, Douglas W. Ball, Jon H. Chung, Alexa B. Schrock, Russell Madison, Garrett M. Frampton, Phil J. Stephens, Jeffrey S. Ross, Vincent A. Miller, Siraj M. Ali
Final approval of manuscript: Hyunseok Kang, Dean Pettinga, Adrian D. Schubert, Paul W. Ladenson, Douglas W. Ball, Jon H. Chung, Alexa B. Schrock, Russell Madison, Garrett M. Frampton, Phil J. Stephens, Jeffrey S. Ross, Vincent A. Miller, Siraj M. Ali
Disclosures
Dean Pettinga: Foundation Medicine, Inc. (E); Jon H. Chung: Foundation Medicine, Inc. (E, OI); Alexa B. Schrock: Foundation Medicine, Inc. (E, OI); Russell Madison: Foundation Medicine, Inc. (E, OI); Garrett M. Frampton: Foundation Medicine, Inc. (E, OI); Jeffrey S. Ross: Foundation Medicine, Inc. (E, OI); Vincent A. Miller: Foundation Medicine, Inc. (E); Siraj M. Ali: Foundation Medicine, Inc. (E, OI, IP), Incysus Inc. (SAB). Foundation Medicine, Inc. is a wholly owned subsidiary of Roche Pharmaceuticals. The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Lee PK, Jarosek SL, Virnig BA et al. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer 2007;109:1736–1741. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins BJ, Lewis JS Jr. Non‐functional parathyroid carcinoma: A review of the literature and report of a case requiring extensive surgery. Head Neck Pathol 2009;3:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandelin K, Auer G, Bondeson L et al. Prognostic factors in parathyroid cancer: A review of 95 cases. World J Surg 1992;16:724–731. [DOI] [PubMed] [Google Scholar]
- 4.Sharretts JM, Kebebew E, Simonds WF. Parathyroid cancer. Semin Oncol 2010;37:580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frampton GM, Fichtenholtz A, Otto GA et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun JX, He Y, Sanford E et al. A computational approach to distinguish somatic vs. germline origin of genomic alterations from deep sequencing of cancer specimens without a matched normal. PLoS Comput Biol 2018;14:e1005965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes SA, Beare D, Gunasekaran P et al. COSMIC: Exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res 2015;43:D805–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goetz MP, Sun JX, Suman VJ et al. Loss of heterozygosity at the CYP2D6 locus in breast cancer: Implications for germline pharmacogenetic studies. J Natl Cancer Inst 2014;107:dju401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalmers ZR, Connelly CF, Fabrizio D et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grüllich C. Cabozantinib: A MET, RET, and VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res 2014;201:207–214. [DOI] [PubMed] [Google Scholar]
- 11.Pandya C, Uzilov AV, Bellizzi J et al. Genomic profiling reveals mutational landscape in parathyroid carcinomas. JCI Insight 2017;2:e92061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haven CJ, van Puijenbroek M, Tan MH et al. Identification of MEN1 and HRPT2 somatic mutations in paraffin‐embedded (sporadic) parathyroid carcinomas. Clin Endocrinol (Oxf) 2007;67:370–376. [DOI] [PubMed] [Google Scholar]
- 13.Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Allen EM, Miao D, Schilling B et al. Genomic correlates of response to CTLA‐4 blockade in metastatic melanoma. Science 2015;350:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman AM, Kato S, Bazhenova L et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpten JD, Robbins CM, Villablanca A et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism‐jaw tumor syndrome. Nat Genet 2002;32:676–680. [DOI] [PubMed] [Google Scholar]
- 17.Walls GV, Stevenson M, Lines KE et al. Mice deleted for cell division cycle 73 gene develop parathyroid and uterine tumours: Model for the hyperparathyroidism‐jaw tumour syndrome. Oncogene 2017;36:4025–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niederst MJ, Hu H, Mulvey HE et al. The allelic context of the C797S mutation acquired upon treatment with third‐generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res 2015;21:3924–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thakker RV, Newey PJ, Walls GV et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 2012;97:2990–3011. [DOI] [PubMed] [Google Scholar]
- 20.Christakis I, Busaidy NL, Cote GJ et al. Parathyroid carcinoma and atypical parathyroid neoplasms in men1 patients; a clinico‐pathologic challenge. The MD Anderson case series and review of the literature. Int J Surg 2016;31:10–16. [DOI] [PubMed] [Google Scholar]
- 21.Fruman DA, Rommel C. PI3K and cancer: Lessons, challenges and opportunities. Nat Rev Drug Discov 2014;13:140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandoth C, McLellan MD, Vandin F et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uusitalo E, Rantanen M, Kallionpaa RA et al. Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol 2016;34:1978–1986. [DOI] [PubMed] [Google Scholar]
- 24.Demirjian AN, Grossman JM, Chan JL et al. Parathyroid carcinoma and neurofibromatosis. Surgery 2008;144:827–829. [DOI] [PubMed] [Google Scholar]
- 25.Favere AM, Tsukumo DM, Matos PS et al. Association between atypical parathyroid adenoma and neurofibromatosis. Arch Endocrinol Metab 2015;59:460–466. [DOI] [PubMed] [Google Scholar]
- 26.Cinamon U, Avinoach I, Harell M. Neurofibromatosis type 1, hyperparathyroidism, and osteosarcoma: Interplay? Eur Arch Otorhinolaryngol 2002;259:540–542. [DOI] [PubMed] [Google Scholar]
- 27.Altinova AE, Toruner F, Cimen AR et al. The association of neurofibromatosis, bilateral pheochromocytoma and primary hyperparathyroidism. Exp Clin Endocrinol Diabetes 2007;115:468–470. [DOI] [PubMed] [Google Scholar]
- 28.Borah S, Xi L, Zaug AJ et al. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 2015;347:1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell RJ, Rube HT, Xavier‐Magalhães A et al. Understanding TERT promoter mutations: A common path to immortality. Mol Cancer Res 2016;14:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013;13:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forbes SA, Beare D, Boutselakis H et al. COSMIC: Somatic cancer genetics at high‐resolution. Nucleic Acids Res 2017;45:D777–D783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spratlin JL, Cohen RB, Eadens M et al. Phase I pharmacologic and biologic study of ramucirumab (IMC‐1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor‐2. J Clin Oncol 2010;28:780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim YH, Odell ID, Ko CJ et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol 2015;151:1240–1243. [DOI] [PubMed] [Google Scholar]