The growing number of antiemetic regimens calls for a definition of the optimal regimen for chemotherapy‐induced nausea and vomiting (CINV). This meta‐analysis was conducted for direct and indirect comparisons of multiple randomized clinical trials to assess the efficacy and safety of antiemetic regimens for CINV of highly emetogenic chemotherapy.
Keywords: Antiemetic, Chemotherapy‐induced nausea and vomiting, Network meta‐analysis, Highly emetogenic chemotherapy
Abstract
Background.
It is important to control chemotherapy‐induced nausea and vomiting (CINV) to maintain dose intensity and patients' quality of life. The National Comprehensive Cancer Network guidelines suggest combination therapy of antiemetic agents. The growing number of antiemetic regimens, and in particular the growing use of regimens containing antagonists to the Nk‐1 receptor (NK1RAs) and the antipsychotic drug olanzapine (OLZ), call for the re‐evaluation of the optimal regimen for CINV. This study assessed the efficacy and safety of antiemetic regimens for highly emetogenic chemotherapy, using Bayesian network meta‐analysis.
Methods.
Randomized trials that compared different antiemetic regimens were included. We strictly followed Preferred Reporting Items for Systematic Reviews and Meta‐Analysis guidelines. The main outcomes were the odds ratio (OR) for overall complete response (absence of vomiting). We conducted network meta‐analysis within a Bayesian model to combine the direct and indirect evidence. Safety was assessed from the trial description. All statistical tests were two‐sided.
Results.
We systematically reviewed 27 randomized control trials (13,356 participants), which compared 12 different antiemetic regimens: serotonin‐3 receptor antagonist (5HT3), 5HT3 + dexamethasone (Dex), palonosetron (PAL), PAL + Dex, PAL at 0.75 mg (PAL0.75), PAL0.75 + Dex, NK1RA + 5HT3 + Dex, NK1RA + PAL + Dex, an oral combination of netupitant and palonosetron (NEPA) + Dex, OLZ + 5HT3 + Dex, OLZ + PAL + Dex, and OLZ + NK1RA + 5HT3 + Dex. An NK1RA + 5HT3 + Dex regimen and an NK1RA + palonosetron + Dex regimen gave a higher complete response (CR) rate than the reference regimen, 5HT3 + Dex (OR, 1.75; 95% credibility interval [95% CrI], 1.56–1.97, and OR, 2.25; 95% CrI, 1.66–3.03, respectively). A regimen containing NEPA was more effective in producing CR than conventional regimens without NEPA or olanzapine. Further analysis, based on the surface under the cumulative ranking probability curve, indicated that olanzapine‐containing regimens were the most effective in producing CR.
Conclusion.
Our meta‐analysis supports the conclusion that olanzapine‐containing regimens are the most effective for CINV of highly emetogenic chemotherapy. We confirmed that NK1RA + PAL + Dex is the most effective of conventional regimens. Substituting olanzapine for an Nk‐1 receptor antagonist may offer a less costly and more effective alternative for patients.
Implications for Practice.
Nausea and vomiting during chemotherapy often pose difficulties for patients and doctors, making it hard to continue the proper therapy and to maintain the quality of life. This article gives insights into the optimal choice of medicine to treat nausea during chemotherapy. The findings reported here provide readers with a robust efficacy ranking of antinausea medicine, which can be used as a reference for the best possible treatment. Furthermore, the 70% less costly drug, olanzapine, is suggested to be equally effective to aprepitant in reducing nausea and vomiting. The possibility of offering a cost‐effective treatment to a wider range of the population is discussed.
Introduction
It is important to control chemotherapy‐induced nausea and vomiting (CINV), which often pose difficulties in the treatment of highly emetogenic chemotherapy (HEC), making it hard to maintain dose intensity and patients' quality of life [1]. The growing use of new antiemetic agents, particularly Nk‐1 receptor antagonists and the antipsychotic agent olanzapine, has prompted us to conduct a meta‐analysis for definition of the optimal regimen for CINV.
CINV is caused by neurotransmitters and chemical substances stimulating the receptors in either the vomiting center or chemoreceptor trigger zone. Such substances include dopamine, serotonin, histamine, acetylcholine, and substance P (Nk‐1) [2], [3], [4], [5]. Antiemetic agents are designed to target one of these relevant receptors (supplemental online Table 1). Phenothiazine and metoclopramide, agents widely in use from the 1980s, inhibit the action of dopamine [6], [7]. The development of first‐generation serotonergic receptor antagonists (5HT3 inhibitors) in the 1990s effectively suppressed acute CINV [8] particularly when used in tandem with dexamethasone (Dex) [9], [10], and the combination has been regarded as the standard prophylaxis; however, delayed CINV still remained difficult to control [11] (acute complete response rates 57%–88%, delayed complete response rates 40%–63%). Newer antiemetic agents, that is, Nk‐1 receptor antagonist (NK1RA) [12], [13] and the second‐generation serotonin receptor antagonist (palonosetron; PAL) [14], [15], were both introduced in the 2000s and have been proven to be equally effective for both acute and delayed CINV. In addition to these “conventional” regimens, there are two newer options: NEPA (a combination of netupitant and palonosetron) and olanzapine. NEPA, first approved by the European Medicines Agency and the U.S. Food and Drug Administration in 2014, combines the highly selective Nk‐1 receptor antagonist netupitant (300 mg) and palonosetron (0.50 mg) [16], [17]. Olanzapine (OLZ), a second‐generation psychotropic for schizophrenia and bipolar disorder, was reported to have antiemetic effects in 2005 [18]. Olanzapine is efficacious not only in the prevention of CINV but also as a rescue agent for delayed CINV, owing to its affinity for multiple receptors such as serotonergic receptors (i.e., 5‐HT2a, 5‐HT2b, 5‐HT3, and 5‐HT6), dopaminergic receptors (D1, D2, D3, and D4), alpha‐1 adrenergic receptors, histaminic H1 receptors, and muscarinic receptors [19], [20]. The National Comprehensive Cancer Network (NCCN) guidelines of antiemesis include these two newer antiemetic agents as standard options, not to mention the older regimens already described above (version 2.2017) [21].
For convenience, we defined regimens including the following agents as “conventional regimens”: dexamethasone, first‐generation serotonergic receptor antagonists (granisetron, ondansetron, azasetron, and ramosetron), second‐generation serotonergic receptor antagonists (palonosetron), and Nk‐1 receptor antagonists (aprepitant, fosaprepitant, and rolapitant). Also, we defined the NEPA and olanzapine regimen as a “new regimen.” Thus, there are many options for antiemesis. A comparison of the efficacy of these agents and combination regimens would be helpful for clinicians.
Chemotherapy is classified into four groups, based on the frequency of emesis in the absence of antiemetic agents [22]. Minimally emetogenic chemotherapy, which has an emesis occurrence of less than 10%, includes bevacizumab and vinorelbine. Low emetogenic chemotherapy, which has an emesis occurrence between 10% and 30%, includes agents such as fluorouracil, docetaxel, and trastuzumab. Moderately emetogenic chemotherapy, with the emesis occurrence between 30% to 90%, includes regimens with carboplatin, irinotecan, and oxaliplatin. HEC, which causes emesis in greater than 90% of subjects, includes cisplatin, doxorubicin plus cyclophosphamide, and epirubicin plus cyclophosphamide [23]. NCCN guidelines of antiemesis currently recommend six different regimens to treat HEC, although the choice among them largely depends on clinicians' experience, for the relative efficacy of the regimens has not been comprehensively evaluated.
A network meta‐analysis is suitable for clarifying such uncertainty, as it enables the direct and indirect comparisons of treatments. There are two types of comparisons. Direct outcome is assessed by a direct comparison of treatment A versus B, whereas indirect comparison between treatments A versus B is attained by assessing the relative efficacy of treatments A versus C and B versus C. Using this method, we can calculate the expected outcome of treatments that have not been directly compared head to head. It is also possible to derive a relative ranking of the efficacy of the regimens [24], [25], [26] and to base guidelines on network meta‐analysis studies [27], [28].
To address this issue, we conducted a network meta‐analysis for direct and indirect comparisons derived from the results of multiple randomized clinical trials, to assess the efficacy of antiemetic regimens for CINV of HEC.
Materials and Methods
Literature Search and Selection Criteria
We searched MEDLINE and the Cochrane Central Register of Controlled Trials; the date of the last search was October 8, 2017. The key search terms included “neoplasms AND nausea AND vomiting” OR “aprepitant” OR “rolapitant” OR “netupitant” OR “olanzapine.”
We limited our selection to randomized clinical trials of adult patients undergoing antiemetic treatment for HEC. We excluded pediatric trials and trials focusing on nausea caused by surgery or radiation therapy. We placed our focus on trials with solid carcinomas.
Data Collection and Quality Assessment
Two investigators (T.Y. and T.H.) independently conducted literature search, selected articles, and extracted data, using the criteria described above. We reached a consensus on all articles. We collected the following data: the first author, publication year, number of analyzed patients, treatment regimens, type of chemotherapy, cancer types, and the number of overall complete responses (CRs), defined as the absence of vomiting for a given subject during and after administration of a HEC. We collected data on the number of acute and delayed CRs and the types and occurrence of adverse events (AEs). We excluded one trial because its definitions of “acute” and “delayed” were inconsistent with the standard definition used in other studies (the standard definition of acute CINV is symptom in the period from 0 to 24 hours after chemotherapy, whereas delayed CINV is symptom in the period from 24 to 120 hours after chemotherapy). For multiple articles concerning a given trial, we included data from only the most complete and informative presentation.
We assessed the risk of bias of each trial using the Cochrane Collaboration's risk‐of‐bias tool [29]. Each study was assessed on the basis of selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other sources of bias.
Definition of Outcome and Treatment Arms
The primary outcome was the overall number of CRs. The secondary outcomes were acute and delayed CR and AEs. We defined CR as “no emesis, no rescue medication” to match the definition used in all studies. “Acute CR” was defined as CR for acute CINV symptoms in the period from 0 to 24 hours after chemotherapy. “Delayed CR” was defined as CR for delayed CINV symptoms that occurred in the interval from 24 to 120 hours after chemotherapy. Adverse events were graded using the National Cancer Institute Common Terminology Criteria, version 4.0 [30]. We used the odds ratio (OR) for the pooling effects size because all outcomes were binary variables and followed the binomial distributions.
We grouped the regimens within 12 different treatment arms: 5HT3, 5HT3 + Dex, PAL, PAL + Dex, PAL0.75, PAL0.75 + Dex, NK1RA + 5HT3 + Dex, NK1RA + PAL + Dex, NEPA + Dex, OLZ + 5HT3 + Dex, OLZ + PAL + Dex, and OLZ + NK1RA + 5HT3 + Dex. Treatments including dexamethasone were uniformly labelled “+ Dex” regardless of the dose and schedule of dexamethasone use. First‐generation serotonin receptor antagonists such as granisetron, ondansetron, azasetron, or ramosetron were classified as “5HT3.” Regimens with palonosetron, a second‐generation serotonin receptor antagonist, were classified into two different arms. When used either independently or with dexamethasone, regimens with palonosetron in dosage at 0.25 mg or 0.50 mg were labelled “PAL,” whereas the “PAL0.75” arm represented regimens using doses of 0.75 mg. Although studies suggest that there is no significant difference between the efficacies of palonosetron 0.25 mg and 0.75 mg [31], we chose to assess the 0.75 mg regimen as a separate regimen in consideration of its tendency to suppress delayed CINV more effectively than does 0.25 mg [32], [33]. When PAL was used with two or more drugs, no distinction was made regarding the amount of palonosetron dosage. We did not make distinctions as to whether palonosetron was dosed orally of intravenously [34]. The “NK1RA” arm included any of the following Nk‐1 antagonists: aprepitant, fosaprepitant, and rolapitant.
Statistical Analysis
First, we conducted direct comparisons (i.e., pairwise meta‐analysis) with the random‐effects model of DerSimonian and Laird [35], which takes account of the heterogeneity between studies. Results were reported with 95% confidence intervals, and statistical significance was defined as p less than .05. All statistical tests were two‐sided. To assess heterogeneity, we produced Cochrane Q statistics and I square (I2) statistics. The I2 statistics measure heterogeneity, which describes the proportion of heterogeneity degree (τ2) in the total variability between studies [36]. A value of I2 < 25% indicates low heterogeneity; 25%–50%, medium heterogeneity; and > 50%, high heterogeneity [37].
Second, we conducted multiple‐treatment meta‐analysis, a network meta‐analysis, within a Bayesian framework using Markov chain Monte Carlo methods described by Caldwell [25], [38], [39]. This method uses information from direct and indirect comparisons to synthesize the OR of each pair of multiple treatments. All parameters were treated as random variables. We reported the outcome with 95% credibility intervals (CrIs), and statistical significance was defined as p less than .05. Multiple‐treatment meta‐analysis is based on a primary assumption that the analyzed network is consistent: direct and indirect evidence on the same comparisons must not disagree beyond chance. To assess inconsistency (i.e., disagreement between the direct and indirect comparisons), we applied the inconsistency model of Ian White [40], [41]. We also evaluated the rank probability, which can be presented as a “balloon plot.” This ranking probability can be summarized into simple heat map by calculating the surface under the cumulative ranking (SUCRA). When a treatment is certain to be the best, the SUCRA is 1, and when a treatment is certain to be the worst, the SUCRA is 0 [42].
Finally, we conducted a sensitivity analysis without the regimens that included olanzapine.
For all analyses, we used 5HT3 + Dex as the reference regimen because it was previously the most widely used until the introduction of palonosetron and Nk‐1 receptor antagonists.
Direct comparisons and risk of bias assessment were calculated by Review Manager, version 5.3 (Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark). The Bayesian network meta‐analysis was performed using WinBUGS, version 1.4 (Imperial College and Medical Research Council Biostatistics Unit, Cambridge, UK). OR, heterogeneity, and inconsistency were calculated, and diagrams and graphics were made using R, version 3.1.2 (R Foundation of Statistical Computing, Vienna, Austria). The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis guidelines [43]. Patient approval was waived because of the nature of the study.
Results
Overview of the Literature Search and Study Characteristics
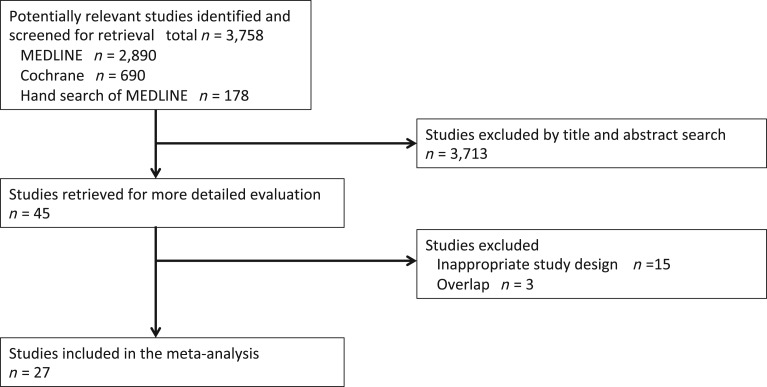
A flow diagram of our literature search is summarized in Figure 1. We identified 3,758 potentially eligible articles, of which 27 were ultimately included in analysis [12], [13], [15], [31], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65]. Table 1 and supplemental online Table 3 summarize the details of 27 studies included in our analysis. Supplemental online Table 2 shows the results of the quality assessments, based on the Cochrane Collaboration's risk‐of‐bias tool.
Figure 1.
The CONSORT (Consolidated Standards of Reporting Trials) flow diagram of the literature search and study selection. Search performed on October 8, 2017.
Table 1. Characteristics of the eligible studies.
Abbreviations: 5HT3, serotonin receptor antagonist; ABVD, doxorubicin hydrochloride, bleomycin sulfate, vinblastine sulfate, and dacarbazine; AC, anthracycline and cyclophosphamide; CPA, cyclophosphamide; CHOP, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone; Dex, dexamethasone; EC, epirubicin and cyclophosphamide; ECF, epirubicin, cisplatin, and 5‐fluorouracil; FAC, fluorouracil, doxorubicin, and cyclophosphamide; FEC, fluorouracil, epirubicin, and cyclophosphamide; GI, gastrointestinal; HEC, highly emetogenic chemotherapy; IAP, ifosfamide, doxorubicin, and cisplatin; MEC, moderately emetogenic chemotherapy; NEPA, oral combination of netupitant and palonosetron; NK1RA, Nk‐1 receptor antagonist; NSCLC, non‐small cell lung cancer; OLZ, olanzapine; PAL, palonosetron; PAL0.75, palonosetron at 0.75 mg; SCLC, small cell lung cancer.
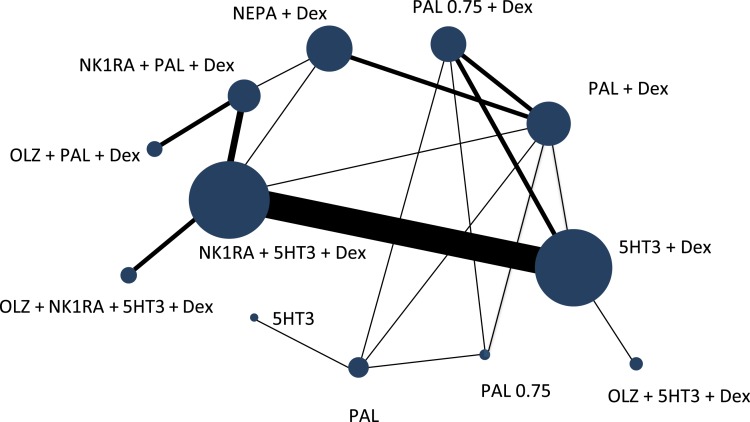
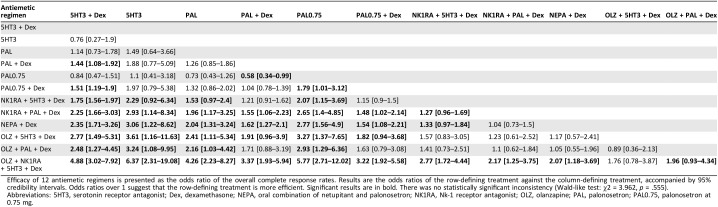
Figure 2 shows the network diagram of eligible comparisons for the multiple‐treatment meta‐analysis. Our study included 13,356 patients in 12 different treatment arms. The most extensively compared regimens, based on the number of subjects evaluated in cumulative studies, were NK1RA + 5HT3 + Dex versus 5HT3 + Dex (12 studies). No trial has been conducted comparing the following regimens: NK1RA + PAL/5HT3 + Dex versus PAL0.75 + Dex and NK1RA + PAL + Dex versus 5HT3 + Dex.
Figure 2.
Network diagram of antiemetic treatments for chemotherapy‐induced nausea and vomiting caused by highly emetogenic chemotherapy. The size of each navy‐blue node depicts the total sample size. The width of each link illustrates the number of studies.
Abbreviations: 5HT3, serotonin receptor antagonist; Dex, dexamethasone; NEPA, oral combination of netupitant and palonosetron; NK1RA, Nk‐1 receptor antagonist; OLZ, olanzapine; PAL, palonosetron; PAL0.75, palonosetron at 0.75 mg.
Results of the Direct Comparisons
First, we examined direct comparisons of regimens. Seven direct comparisons among the selected studies are as follows:
5HT3 + Dex versus NK1RA + 5HT3 + Dex
5HT3 + Dex versus PAL0.75 + Dex
PAL + Dex versus NEPA + Dex
PAL + Dex versus PAL0.75 + Dex
NK1RA + 5HT3 + Dex versus NK1RA + PAL + Dex
NK1RA + PAL + Dex versus OLZ + PAL + Dex
NK1RA + 5HT3 + Dex versus OLZ + NK1RA + 5HT3 + Dex
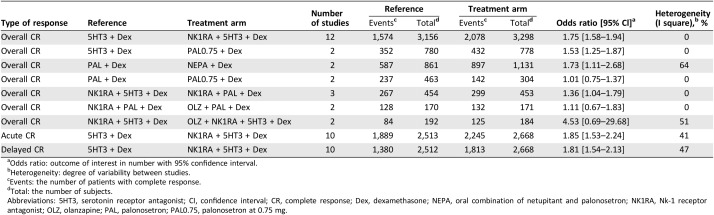
The number of patients who achieved overall CR was reported in 24 studies, for which the odds ratio in each study is shown in Table 2 and supplemental online Figure 1. The numbers of patients achieving acute and delayed CR were reported in 21 studies, and the ORs for some of these studies are shown in Table 2 and supplemental online Figure 2. The figures also show heterogeneity for all direct comparisons.
Table 2. Odds ratio for overall, acute, and delayed complete response rates in meta‐analysis of direct comparisons between each pair of antiemetic regimens.
Odds ratio: outcome of interest in number with 95% confidence interval.
Heterogeneity: degree of variability between studies.
Events: the number of patients with complete response.
Total: the number of subjects.
Abbreviations: 5HT3, serotonin receptor antagonist; CI, confidence interval; CR, complete response; Dex, dexamethasone; NEPA, oral combination of netupitant and palonosetron; NK1RA, Nk‐1 receptor antagonist; OLZ, olanzapine; PAL, palonosetron; PAL0.75, palonosetron at 0.75 mg.
NK1RA regimens and PAL regimens are more effective than first‐generation 5HT3‐based regimens. An NEPA regimen containing both NK1RA and PAL was better than PAL without NK1RA. PAL is preferred to 5HT3 in combination with NK1RA and Dex, despite the lack of significant difference in head‐to‐head trials. OLZ‐based regimens were equivalent to or better than NK1RA‐based regimens. NK1RA + 5HT3 + Dex was more efficacious in both acute and delayed CINV than 5HT3 + Dex.
Overall, there was no heterogeneity in most of the direct comparisons. We found moderate heterogeneity for the comparisons of PAL + Dex versus NEPA + Dex and NK1RA + 5HT3 + Dex versus OLZ + NK1RA + 5HT3 + Dex. In both cases, only two studies were included in the meta‐analysis.
Bayesian Network Meta‐Analysis
Multiple‐treatments meta‐analysis was conducted for 66 possible indirect comparisons from the 27 articles (Table 3). The results were presented as an OR with 95% CrIs. Forty‐two overall comparisons were made of the rates of CR for both acute (supplemental online Table 4) and delayed CR (supplemental online Table 5).
Table 3. Indirect comparison of overall complete response rates.
Efficacy of 12 antiemetic regimens is presented as the odds ratio of the overall complete response rates. Results are the odds ratios of the row‐defining treatment against the column‐defining treatment, accompanied by 95% credibility intervals. Odds ratios over 1 suggest that the row‐defining treatment is more efficient. Significant results are in bold. There was no statistically significant inconsistency (Wald‐like test: χ2 = 3.962, p = .555).
Abbreviations: 5HT3, serotonin receptor antagonist; Dex, dexamethasone; NEPA, oral combination of netupitant and palonosetron; NK1RA, Nk‐1 receptor antagonist; OLZ, olanzapine; PAL, palonosetron; PAL0.75, palonosetron at 0.75 mg.
Among the conventional regimens, for overall CR, NK1RA + PAL + Dex was the most effective. PAL + Dex and PAL0.75 + Dex were equally effective, irrespective of acute and delayed CR. NEPA was more effective than the conventional regimens, except for NK1RA + PAL + Dex, to which it still showed numerical superiority. OLZ‐containing regimens showed the highest OR, specifically, OLZ + NK1RA + 5HT3 + Dex. The other OLZ‐containg regimens were also found to be better than non‐OLZ regimens: OLZ + 5HT3 + Dex and OLZ + PAL + Dex. OLZ tended to be more effective than NK1RA, in combination with 5TH3 + Dex or PAL + Dex.
Analysis indicated that there was no statistically significant inconsistency in the overall, acute, and delayed CR analyses and sensitivity analysis, which indicated that the current results were trustworthy. The p values of assessing inconsistency are shown under each table.
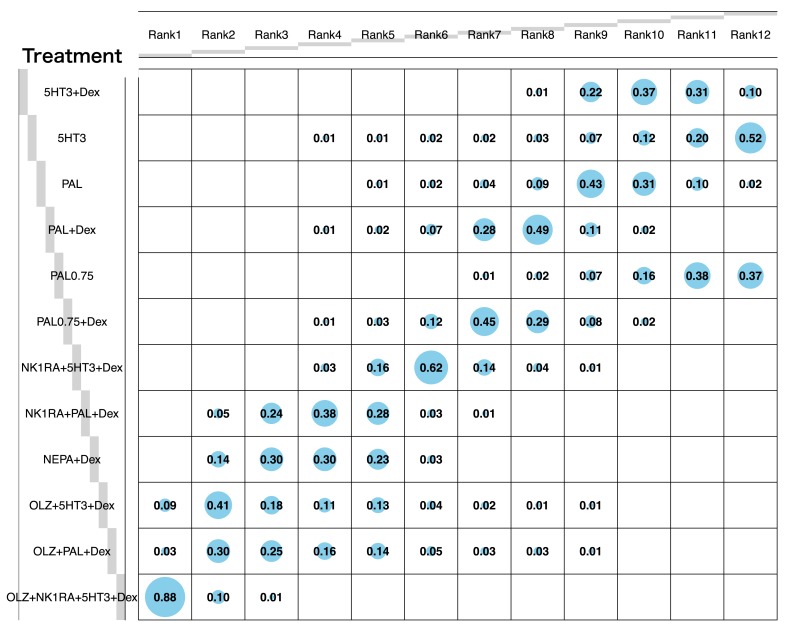
The rankings of the treatment arms according to efficacy in producing CR are shown in the probability balloon plot (Fig. 3). The regimen OLZ + NK1RA + 5HT3 + Dex had the highest probability of being the best treatment arm (rank 1, probability of 88%), followed by the other two OLZ‐containing regimens, which in turn were followed by NEPA + Dex. By contrast, single‐agent 5HT3 had the highest probability of being the least effective treatment (rank 12, probability of 52%).
Figure 3.
Balloon plot of ranking probability of the overall complete response (CR) rates. Each value represents the probability of each treatment to occupy a specific rank. Rank 1 is the most likely to prevent chemotherapy‐induced nausea and vomiting. The size of a blue balloon is proportional to the probability. For example, among all treatments, the probability of OLZ + NK1RA + 5HT3 + Dex to yield the best overall CR rates is 88%, and the probability of 5HT3 to yield the worst overall CR rates is 52%. All statistical tests are two‐sided.
Abbreviations: 5HT3, serotonin receptor antagonist; Dex, dexamethasone; NEPA, oral combination of netupitant and palonosetron; NK1RA, Nk‐1 receptor antagonist; OLZ, olanzapine; PAL, palonosetron; PAL0.75, palonosetron at 0.75 mg.
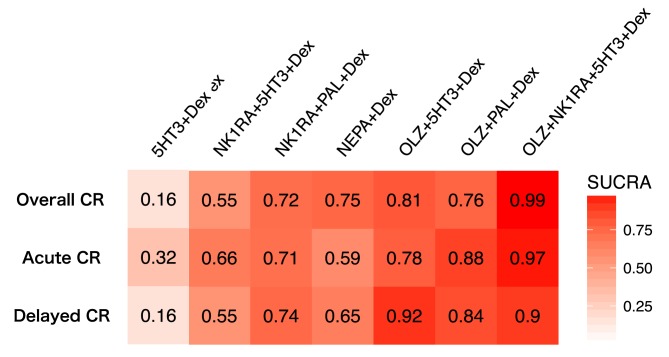
The SUCRA values indicated (Fig. 4 and supplemental online Fig. 3) that OLZ + NK1RA + 5HT3 + Dex (SUCRA = 0.99) had the highest probability of being the most effective treatment to suppress overall CINV, followed by OLZ + 5HT3 + Dex (SUCRA = 0.81), OLZ + PAL + Dex (SUCRA = 0.76), and NEPA + Dex (SUCRA = 0.75). As for the treatment to suppress delayed CINV, three regimens achieved the highest rank: NK1RA + PAL + Dex, NEPA‐containing regimens, and OLZ‐containing regimens.
Figure 4.
Heat map graph of efficacy, as assessed by the SUCRA. A heat map graph is a graphical representation of data using color to show the level of comparative strength of each treatment arm. Red represents a higher SUCRA value, which indicates a greater probability of being the best treatment arm, and white represents a lower SUCRA value, which indicates a lower probability of being the best treatment arm. The SUCRA values are described in each box. All statistical tests are two‐sided.
Abbreviations: 5HT3, serotonin receptor antagonist; CR, complete response; Dex, dexamethasone; NEPA, oral combination of netupitant and palonosetron; NK1RA, Nk‐1 receptor antagonist; OLZ, olanzapine; PAL, palonosetron; SUCRA, surface under the cumulative ranking.
Sensitivity Analysis
We conducted a sensitivity analysis to ensure that the relatively limited number of olanzapine‐containing trials did not create instability for the whole analysis. Supplemental online Table 6 illustrates the estimated OR of the overall CR in indirect comparisons without OLZ‐containing regimens. We found no major differences in the results of indirect comparisons with 12 regimens versus with nine non‐OLZ arms.
Safety
We were unable to assessed comparative safety profiles because not all articles conducted a consistent analysis of AEs. With regard to the conventional 5HT3 antagonist‐based regimens and NEPA, common AEs were constipation, headache, and fatigue. The main AEs of OLZ‐containing regimens were sedation and drowsiness [56], [63], but only grades 1 and 2 were reported. No grade 3 or 4 toxicity was attributable to any of the antiemetic regimens.
Discussion
By conducting network meta‐analysis for direct and indirect comparisons of multiple randomized clinical trials, we were able to rank antiemetic regimens with regard to the efficacy in treating CINV. Direct comparisons indicate that Nk‐1 antagonist‐based regimens (NK1RA + PAL + Dex and NK1RA + 5HT3 + Dex) were the most effective among the conventional regimens, with the former significantly better in the overall analysis, including direct and indirect comparisons. Using indirect comparisons, OLZ‐containing regimens, especially OLZ + NK1RA + 5HT3 + Dex, were the most effective of all. Randomized trials comparing OLZ + 5HT3 + Dex and NK1RA + 5HT3 + Dex are now underway and should provide further evidence.
Among the conventional regimens, NK1RA + PAL + Dex was the best in achieving overall CR for CINV. The second most effective of the conventional regimens was NK1RA + 5HT3 + Dex. The current result supports the NCCN guideline in that when using HEC, NK1RA should be added to either 5HT3 + Dex or PAL + Dex. With network meta‐analysis, we found that the CR rate of NK1RA + PAL + Dex was significantly higher than that of NK1RA + 5HT3 + Dex in both direct and indirect comparisons. The indirect comparisons support the conclusion that the second‐generation serotonergic receptor antagonist, PAL, should be preferred to first generation drugs of the same class.
Both NEPA, a combination of Nk‐1 antagonist and PAL, and OLZ‐based regimens appear to be more effective than other Nk‐1‐based or serotonergic‐based regimens. This finding confirms preclinical work that netupitant in NEPA has longer‐acting efficacy than other Nk‐1 antagonists [16], [66]. Also, basic findings showed that inhibition of substance P‐mediated responses by netupitant is enhanced synergistically by palonosetron.
The OLZ‐containing regimens, and OLZ + NK1RA + 5HT3 + Dex in particular, were the most effective of all the antiemetic regimens. When adding olanzapine to the conventional regimens, we can expect extra effects, which might stem from the fact that the mechanisms of olanzapine differ from those of Nk‐1 receptor antagonists or 5‐HT3 receptor antagonists, although this should be tested. Olanzapine may be a good choice for patients with higher risks of CINV.
As for safety, AEs were tolerable in all regimens evaluated in this study, although an extensive safety analysis was not possible, given the limited and inconsistently presented data in the publications reviewed.
With regard to cost, olanzapine substituted for Nk‐1 receptor antagonist may offer a cost‐effective alternative. Olanzapine costs ¥1,959 in Japan (approximately U.S. $17.80) for a 4‐day treatment, whereas an Nk‐1 receptor antagonist, aprepitant for example, costs as much as ¥11,759 (approximately U.S. $106.90) for a 3‐day treatment. To elaborate, the OLZ + 5HT3 + Dex regimen costs ¥4,303 (approximately U.S. $39.10) per patient, whereas NK1RA + 5HT3 + Dex costs ¥14,102 (approximately U.S. $128.20) per patient. NK1RA + 5HT3 + Dex costs three times more than OLZ + 5HT3 + Dex. Given the current result from the indirect comparison that, in combinations with PAL + Dex or 5HT3 + Dex, the OR of OLZ turned out to be numerically higher than that of NK1RA, we suggest substituting olanzapine for NK1RA. By doing so, we are able to reduce the cost for CINV treatment by, for instance, approximately 70%. A trial testing the noninferiority of OLZ + 5HT3 + Dex compared with NK1RA + 5HT3 + Dex is awaited.
Our study has some limitations. First, because of the retrospective nature of meta‐analysis studies, publication biases and selective biases cannot be excluded. Also, even with a selection confined to randomized controlled trials, patient backgrounds varied across studies to a certain extent. These biases and variety might have cast some limited influence on the comparisons, but they would not compromise the whole analysis. Second, it was impossible to assess inconsistency in some of the comparisons, because they were not included in the loops of the whole network. Finally, because we treated all the dexamethasone‐containing regimens as “Dex,” this review cannot discuss whether to reduce the dosage of dexamethasone in order to minimize side effects such as infection and diabetes mellitus [67], [68], [69]. Further research is awaited.
Conclusion
Our meta‐analysis found, through both a direct and indirect analysis, that olazapine‐containing regimens are the most effective for CINV of HEC. We confirmed the validity of current guidelines that, among standard regimens, excluding olanzapine, NK1RA + PAL + Dex and NK1RA + 5HT3 + Dex are most effective. Further evaluation of olanzapine in combination with Nk‐1 and 5HT3 antagonists is underway. Substitution of olanzapine for an Nk‐1 receptor antagonist may be the most cost‐effective strategy.
See http://www.TheOncologist.com for supplemental material available online.
Author Contributions
Conception/design: Takamichi Yokoe, Tetsu Hayashida, Aiko Nagayama, Ayako Nakshoji, Hinako Maeda, Tomoko Seki, Maiko Takahashi, Toshimi Takano, Takayuki Abe, Yuko Kitagawa
Provision of study material or patients: Takamichi Yokoe, Tetsu Hayashida, Aiko Nagayama
Collection and/or assembly of data: Takamichi Yokoe, Tetsu Hayashida
Data analysis and interpretation: Takamichi Yokoe, Tetsu Hayashida, Aiko Nagayama, Toshimi Takano, Takayuki Abe
Manuscript writing: Takamichi Yokoe, Tetsu Hayashida
Final approval of manuscript: Takamichi Yokoe, Tetsu Hayashida, Aiko Nagayama, Ayako Nakashoji, Hinako Maeda, Tomoko Seki, Maiko Takahashi, Toshimi Takano, Takayuki Abe, Yuko Kitagawa
Disclosures
Tetsu Hayashida: Chugai, Eisai (RF), Novartis Pharma, Taiho, Chugai (H); Aiko Nagayama: Chugai (OI), Chugai, Roche (E, OI—family member); Toshimi Takano: Novartis, Chugai, Taiho, Takeda, Ono, Merck Sharp & Dohme, Merck Serono (RF), Daiichi‐Sankyo, Kyowa Hakko Kirin, Eisai (H); Yuko Kitagawa: Chugai, Novartis Pharma, Astellas, Taiho, Ono, Eli Lilly & Co. (RF, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
References
- 1.Coates A, Abraham S, Kaye SB et al. On the receiving end‐‐patient perception of the side‐effects of cancer chemotherapy. Eur J Cancer Clin Oncol 1983;19:203–208. [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ. Chemotherapy‐induced nausea and vomiting. N Engl J Med 2008;358:2482–2494. [DOI] [PubMed] [Google Scholar]
- 3.Mitchelson F. Pharmacological agents affecting emesis. A review (Part I). Drugs 1992;43:295–315. [DOI] [PubMed] [Google Scholar]
- 4.Bountra C, Gale JD, Gardner CJ et al. Towards understanding the aetiology and pathophysiology of the emetic reflex: Novel approaches to antiemetic drugs. Oncology 1996;53(suppl 1):102–109. [DOI] [PubMed] [Google Scholar]
- 5.Leslie RA. Neuroactive substances in the dorsal vagal complex of the medulla oblongata: Nucleus of the tractus solitarius, area postrema, and dorsal motor nucleus of the vagus. Neurochem Int 1985;7:191–211. [DOI] [PubMed] [Google Scholar]
- 6.Moertel CG, Reitemeier RJ, Gage RP. A controlled clinical evaluation of antiemetic drugs. JAMA 1963;186:116–118. [DOI] [PubMed] [Google Scholar]
- 7.Saller R, Hellenbrecht D. High doses of metoclopramide or droperidol in the prevention of cisplatin‐induced emesis. Eur J Cancer Clin Oncol 1986;22:1199–1203. [DOI] [PubMed] [Google Scholar]
- 8.Hesketh PJ, Gandara DR. Serotonin antagonists: A new class of antiemetic agents. J Natl Cancer Inst 1991;83:613–620. [DOI] [PubMed] [Google Scholar]
- 9.D'Olimpio JT, Camacho F, Chandra P et al. Antiemetic efficacy of high‐dose dexamethasone versus placebo in patients receiving cisplatin‐based chemotherapy: A randomized double‐blind controlled clinical trial. J Clin Oncol 1985;3:1133–1135. [DOI] [PubMed] [Google Scholar]
- 10.Strum SB, McDermed JE, Liponi DF. High‐dose intravenous metoclopramide versus combination high‐dose metoclopramide and intravenous dexamethasone in preventing cisplatin‐induced nausea and emesis: A single‐blind crossover comparison of antiemetic efficacy. J Clin Oncol 1985;3:245–251. [DOI] [PubMed] [Google Scholar]
- 11.Geling O, Eichler HG. Should 5‐hydroxytryptamine‐3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re‐evaluation of clinical evidence and drug cost implications. J Clin Oncol 2005;23:1289–1294. [DOI] [PubMed] [Google Scholar]
- 12.Hesketh PJ, Grunberg SM, Gralla RJ et al. The oral neurokinin‐1 antagonist aprepitant for the prevention of chemotherapy‐induced nausea and vomiting: A multinational, randomized, double‐blind, placebo‐controlled trial in patients receiving high‐dose cisplatin‐‐the Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112–4119. [DOI] [PubMed] [Google Scholar]
- 13.Poli‐Bigelli S, Rodrigues‐Pereira J, Carides AD et al. Aprepitant Protocol 054 Study Group. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy‐induced nausea and vomiting. Results from a randomized, double‐blind, placebo‐controlled trial in Latin America. Cancer 2003;97:3090–3098. [DOI] [PubMed] [Google Scholar]
- 14.Tonini G, Vincenzi B, Santini D. New drugs for chemotherapy‐induced nausea and vomiting: Focus on palonosetron. Expert Opin Drug Metab Toxicol 2005;1:143–149. [DOI] [PubMed] [Google Scholar]
- 15.Saito M, Aogi K, Sekine I et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: A double‐blind, double‐dummy, randomised, comparative phase III trial. Lancet Oncol 2009;10:115–124. [DOI] [PubMed] [Google Scholar]
- 16.Aapro M, Hesketh PJ, Jordan K et al. Safety of an oral fixed combination of netupitant and palonosetron (NEPA): Pooled data from the phase II/III clinical program. The Oncologist 2016;21:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stathis M, Pietra C, Rojas C et al. Inhibition of substance P‐mediated responses in NG108‐15 cells by netupitant and palonosetron exhibit synergistic effects. Eur J Pharmacol 2012;689:25–30. [DOI] [PubMed] [Google Scholar]
- 18.Navari RM, Einhorn LH, Passik SD et al. A phase II trial of olanzapine for the prevention of chemotherapy‐induced nausea and vomiting: A Hoosier Oncology Group study. Support Care Cancer 2005;13:529–534. [DOI] [PubMed] [Google Scholar]
- 19.Hocking CM, Kichenadasse G. Olanzapine for chemotherapy‐induced nausea and vomiting: A systematic review. Support Care Cancer 2014;22:1143–1151. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava M, Brito‐Dellan N, Davis MP et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003;25:578–582. [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology for Antiemesis. Version 2.2017. 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed October 13, 2017.
- 22.Roila F, Hesketh PJ, Herrstedt J; Antiemetic Subcommitte of the Multinational Association of Supportive Care in Cancer. Prevention of chemotherapy‐ and radiotherapy‐induced emesis: Results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 2006;17:20–28. [DOI] [PubMed] [Google Scholar]
- 23.Basch E, Prestrud AA, Hesketh PJ et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2011;29:4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salanti G, Higgins JP, Ades AE et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279–301. [DOI] [PubMed] [Google Scholar]
- 25.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: Combining direct and indirect evidence. BMJ 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–3124. [DOI] [PubMed] [Google Scholar]
- 27.Barbui C, Cipriani A. What are evidence‐based treatment recommendations? Epidemiol Psychiatr Sci 2011;20:29–31. [DOI] [PubMed] [Google Scholar]
- 28.Nagayama A, Hayashida T, Jinno H et al. Comparative effectiveness of neoadjuvant therapy for HER2‐positive breast cancer: A network meta‐analysis. J Natl Cancer Inst 2014;106:dju203. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Altman DG, Gotzsche PC et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Cancer Institute Common Terminology Criteria for Adverse Events . Version 4.0. Available at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed December 2016.
- 31.Aapro MS, Grunberg SM, Manikhas GM et al. A phase III, double‐blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy‐induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 2006;17:1441–1449. [DOI] [PubMed] [Google Scholar]
- 32.Segawa Y, Aogi K, Inoue K et al. A phase II dose‐ranging study of palonosetron in Japanese patients receiving moderately emetogenic chemotherapy, including anthracycline and cyclophosphamide‐based chemotherapy. Ann Oncol 2009;20:1874–1880. [DOI] [PubMed] [Google Scholar]
- 33.Maemondo M, Masuda N, Sekine I et al. A phase II study of palonosetron combined with dexamethasone to prevent nausea and vomiting induced by highly emetogenic chemotherapy. Ann Oncol 2009;20:1860–1866. [DOI] [PubMed] [Google Scholar]
- 34.Karthaus M, Tibor C, Lorusso V et al. Efficacy and safety of oral palonosetron compared with IV palonosetron administered with dexamethasone for the prevention of chemotherapy‐induced nausea and vomiting (CINV) in patients with solid tumors receiving cisplatin‐based highly emetogenic chemotherapy (HEC). Support Care Cancer 2015;23:2917–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 38.Jackson D, Riley R, White IR. Multivariate meta‐analysis: Potential and promise. Stat Med 2011;30:2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mavridis D, Salanti G. A practical introduction to multivariate meta‐analysis. Stat Methods Med Res 2013;22:133–158. [DOI] [PubMed] [Google Scholar]
- 40.White IR, Barrett JK, Jackson D et al. Consistency and inconsistency in network meta‐analysis: Model estimation using multivariate meta‐regression. Res Synth Methods 2012;3:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson D, Barrett JK, Rice S et al. A design‐by‐treatment interaction model for network meta‐analysis with random inconsistency effects. Stat Med 2014;33:3639–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: An overview and tutorial. J Clin Epidemiol 2011;64:163–171. [DOI] [PubMed] [Google Scholar]
- 43.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chawla SP, Grunberg SM, Gralla RJ et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy‐induced nausea and vomiting. Cancer 2003;97:2290–2300. [DOI] [PubMed] [Google Scholar]
- 45.de Wit R, Herrstedt J, Rapoport B et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin‐based chemotherapy. J Clin Oncol 2003;21:4105–4111. [DOI] [PubMed] [Google Scholar]
- 46.de Wit R, Herrstedt J, Rapoport B et al. The oral NK(1) antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin‐based chemotherapy: A combined analysis of two randomised, placebo‐controlled phase III clinical trials. Eur J Cancer 2004;40:403–410. [PubMed] [Google Scholar]
- 47.Rapoport BL, Jordan K, Boice JA et al. Aprepitant for the prevention of chemotherapy‐induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: A randomized, double‐blind study. Support Care Cancer 2010;18:423–431. [DOI] [PubMed] [Google Scholar]
- 48.Saito H, Yoshizawa H, Yoshimori K et al. Efficacy and safety of single‐dose fosaprepitant in the prevention of chemotherapy‐induced nausea and vomiting in patients receiving high‐dose cisplatin: A multicentre, randomised, double‐blind, placebo‐controlled phase 3 trial. Ann Oncol 2013;24:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warr DG, Hesketh PJ, Gralla RJ et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy‐induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 2005;23:2822–2830. [DOI] [PubMed] [Google Scholar]
- 50.Schmoll HJ, Aapro MS, Poli‐Bigelli S et al. Comparison of an aprepitant regimen with a multiple‐day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high‐dose cisplatin treatment. Ann Oncol 2006;17:1000–1006. [DOI] [PubMed] [Google Scholar]
- 51.Aapro M, Rugo H, Rossi G et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed‐dose combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 2014;25:1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hesketh PJ, Rossi G, Rizzi G et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting following highly emetogenic chemotherapy: A randomized dose‐ranging pivotal study. Ann Oncol 2014;25:1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan L, Liu J, Liu X et al. Clinical research of olanzapine for prevention of chemotherapy‐induced nausea and vomiting. J Exp Clin Cancer Res 2009;28:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong X, Huang J, Cao R et al. Palonosetron for prevention of acute and delayed nausea and vomiting in non‐small‐cell lung carcinoma patients. Med Oncol 2011;28:1425–1429. [DOI] [PubMed] [Google Scholar]
- 55.Gralla RJ, Bosnjak SM, Hontsa A et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed‐dose combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol 2014;25:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy‐induced nausea and vomiting: A randomized phase III trial. J Support Oncol 2011;9:188–195. [DOI] [PubMed] [Google Scholar]
- 57.Mizukami N, Yamauchi M, Koike K et al. Olanzapine for the prevention of chemotherapy‐induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: A randomized, double‐blind, placebo‐controlled study. J Pain Symptom Manage 2014;47:542–550. [DOI] [PubMed] [Google Scholar]
- 58.Boccia R, Grunberg S, Franco‐Gonzales E et al. Efficacy of oral palonosetron compared to intravenous palonosetron for the prevention of chemotherapy‐induced nausea and vomiting associated with moderately emetogenic chemotherapy: A phase 3 trial. Support Care Cancer 2013;21:1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohzawa H, Miki A, Hozumi Y et al. Comparison between the antiemetic effects of palonosetron and granisetron in breast cancer patients treated with anthracycline‐based regimens. Oncol Lett 2015;9:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wenzell CM, Berger MJ, Blazer MA et al. Pilot study on the efficacy of an ondansetron‐ versus palonosetron‐containing antiemetic regimen prior to highly emetogenic chemotherapy. Support Care Cancer 2013;21:2845–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babu G, Saldanha SC, Kuntegowdanahalli Chinnagiriyappa L et al. The efficacy, safety, and cost benefit of olanzapine versus aprepitant in highly emetogenic chemotherapy: A pilot study from South India. Chemother Res Pract 2016;2016:3439707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki K, Yamanaka T, Hashimoto H et al. Randomized, double‐blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy‐induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol 2016;27:1601–1606. [DOI] [PubMed] [Google Scholar]
- 63.Navari RM, Qin R, Ruddy KJ et al. Olanzapine for the prevention of chemotherapy‐induced nausea and vomiting. N Engl J Med 2016;375:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rapoport B, Chua D, Poma A et al. Study of rolapitant, a novel, long‐acting, NK‐1 receptor antagonist, for the prevention of chemotherapy‐induced nausea and vomiting (CINV) due to highly emetogenic chemotherapy (HEC). Support Care Cancer 2015;23:3281–3288. [DOI] [PubMed] [Google Scholar]
- 65.Rapoport BL, Chasen MR, Gridelli C et al. Safety and efficacy of rolapitant for prevention of chemotherapy‐induced nausea and vomiting after administration of cisplatin‐based highly emetogenic chemotherapy in patients with cancer: Two randomised, active‐controlled, double‐blind, phase 3 trials. Lancet Oncol 2015;16:1079–1089. [DOI] [PubMed] [Google Scholar]
- 66.Rizzi A, Campi B, Camarda V et al. In vitro and in vivo pharmacological characterization of the novel NK(1) receptor selective antagonist netupitant. Peptides 2012;37:86–97. [DOI] [PubMed] [Google Scholar]
- 67.Italian Group For Antiemetic Research . Double‐blind, dose‐finding study of four intravenous doses of dexamethasone in the prevention of cisplatin‐induced acute emesis. Italian Group for Antiemetic Research. J Clin Oncol 1998;16:2937–2942. [DOI] [PubMed] [Google Scholar]
- 68.Italian Group For Antiemetic Research . Randomized, double‐blind, dose‐finding study of dexamethasone in preventing acute emesis induced by anthracyclines, carboplatin, or cyclophosphamide. J Clin Oncol 2004;22:72572–72579. [DOI] [PubMed] [Google Scholar]
- 69.Barbour SY. Corticosteroids in the treatment of chemotherapy‐induced nausea and vomiting. J Natl Compr Canc Netw 2012;10:493–499. [DOI] [PubMed] [Google Scholar]