Because immune checkpoint inhibitor therapies (CPI) lack the toxicities of chemotherapy, oncologists may prescribe CPI to patients at the end of life. This article describes real‐world patterns of CPI initiation near the end of life among individuals with metastatic urothelial cell carcinoma.
Abstract
Several immune checkpoint inhibitor therapies (CPIs) have been approved to treat metastatic urothelial cell carcinoma (mUC). Because of the favorable toxicity profile of CPI compared with chemotherapy, oncologists may have a low threshold to prescribe CPI to patients near the end of life. We evaluated trends in initiation of end‐of‐life systemic therapy in 1,637 individuals in the Flatiron Health Database who were diagnosed with mUC between 2015 and 2017 and who died. Rates of systemic therapy initiation in the last 30 and 60 days of life were 17.0% and 29.8%, respectively. The quarterly proportion of patients who initiated CPI within 60 days of death increased from 1.0% to 23% during the study period (ptrend < .001). After CPI approval, end‐of‐life CPI initiation significantly increased among patients with poor performance status (ptrend = .020) and did not significantly change among individuals with good performance status. The quarterly proportion of patients who initiated any systemic therapy at the end of life doubled (17.4% to 34.8%) during the study period, largely explained by increased CPI use. These findings suggest a dramatic rise in CPI use at the end of life in patients with mUC, a finding that may have important guideline and policy implications.
Use of chemotherapy near the end of life subjects patients to toxicities and costs of cancer treatment without improving quality of life, and it is a well‐established metric of low‐value care [1], [2]. Because immune checkpoint inhibitor therapies (CPI) lack the classical toxicities of chemotherapy [3], oncologists may frequently prescribe CPI to patients at the end of life who would otherwise not be eligible for chemotherapy because of factors such as poor performance status (PS). End‐of‐life CPI use has been termed “desperation oncology” in the lay press [4]. CPI in metastatic urothelial cell carcinoma (mUC) was approved in the U.S. in 2016 for individuals whose cancers progressed on platinum‐based chemotherapy [5] and in 2017 as first‐line therapy in cisplatin‐ineligible individuals [6], [7]. We describe real‐world patterns of CPI initiation near the end of life among individuals with mUC who died. Given the perceived favorable toxicity profile of CPI, we hypothesized that the proportion of patients initiating CPI near the end of life has increased over time, particularly among individuals with poor PS.
We performed a secular trend analysis of 1,637 individuals in the Flatiron Health Network with mUC (stage IV at diagnosis or recurrent) who were diagnosed between January 1, 2015, and December 31, 2017, and who died from any cause prior to December 31, 2017 [8]. The Flatiron Health Database is derived using technology‐enabled abstraction from electronic health records. At the time of study conduct, the database consisted of over 2 million active patients with cancer seen at 280 primarily community oncology practices, in both urban to rural areas, and is broadly representative of the U.S. population [9]. We obtained approval and waiver of informed consent from the Copernicus Group and University of Pennsylvania institutional review boards prior to study conduct. We excluded patients receiving clinical trial treatments (including CPI as part of a clinical trial) or agents not listed in National Comprehensive Cancer Network guidelines for mUC [10]. We calculated the quarterly proportions of CPI and chemotherapy initiation in any line of treatment in the last 30 and 60 days of life. We used nonparametric tests of trend to test our hypothesis of increasing CPI use at the end of life after approval of CPI [11]. We used chi‐square tests to compare demographic characteristics of patients by treatment at the end of life, with level of significance being a two‐sided p < .05. Significant chi‐square results were further analyzed with post hoc chi‐square tests of proportions with Bonferroni adjustment. All analyses were performed using STATA (version 15.1; College Station, TX).
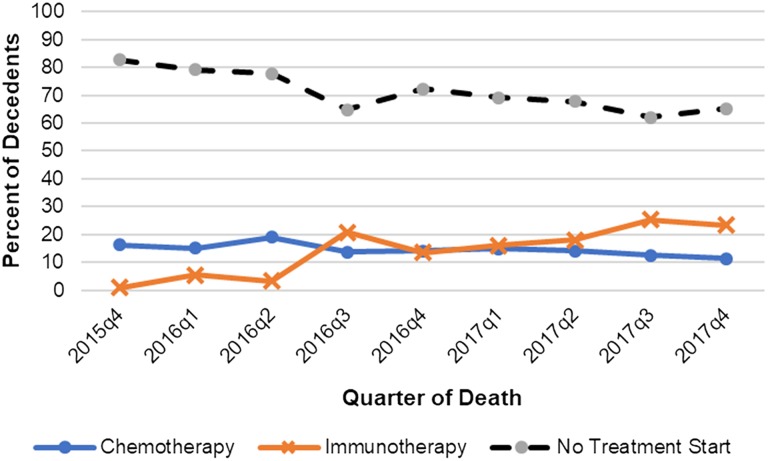
Among our decedent cohort, the median age at diagnosis was 74 years (interquartile range [IQR], 66–80), 73.1% were male, and 72.7% were white. In the last 30 and 60 days of life, 278 (17.0%) and 488 (29.8%) of decedents, respectively, initiated a new line of systemic therapy. Most patients who initiated end‐of‐life chemotherapy or CPI received salvage rather than first‐line therapy (Table 1). Proportions of CPI initiation within 60 days of death increased from 1.0% of all decedents in the final quarter of 2015 (2015q4) to 23% in the final quarter of 2017 (2017q4; Fig. 1; ptrend < .001). CPI accounted for 66% of treatment starts within 60 days of death in 2017q4, and most CPI initiators (20/32) had a recorded Eastern Cooperative Oncology Group (ECOG) PS ≥2. Following U.S. Food and Drug Administration (FDA) approval of CPI (2016 quarter 3 and onward), the quarterly proportions of decedents using CPI increased; although patients with ECOG PS ≥2 who had received a CPI at the end of life significantly increased (ptrend = .020), these proportions did not significantly change among individuals with ECOG PS 0–1. CPI initiators at the end of life had slightly worse PS than chemotherapy initiators (37.9% vs. 31.1% with ECOG PS ≥2; Table 1), although this difference was not statistically significant on post hoc testing. There were no appreciable differences in age and gender between chemotherapy and CPI initiators. Patients who did not initiate any systemic therapy near the end of life were significantly older and had worse PS than patients who initiated therapy (Table 1). The proportion of individuals with mUC initiating any systemic therapy within 60 days of death doubled from 17.4% in 2015q4 to 34.8% in 2017q4.
Table 1. Demographic characteristics of individuals within 60 days of death since 2016, stratified by treatment type.
Note: We only compare demographic characteristics for patients diagnosed in 2016 and 2017 to account for the fact that checkpoint inhibitor therapy (CPI) was approved in 2016 for metastatic urothelial cell carcinoma.
The p values are from Pearson's chi‐square tests for homogeneity comparing categorical variables between the three arms (chemotherapy, checkpoint inhibitor therapy, or no treatment start). Post hoc testing of significant results revealed significant differences in age (p = .003) and performance status (.01) between individuals receiving no treatment versus any systemic therapy. There were no significant differences in age or performance status between patients receiving chemotherapy versus CPI.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; F, female; M, male; NA, not applicable.
Figure 1.
Initiation of new therapy in the last 60 days of life in patients with metastatic urothelial carcinoma, by treatment type.
Abbreviations: q1, first quarter; q2, second quarter; q3, third quarter; q4, fourth quarter.
Using a real‐world nationally representative data set, we show that since the first FDA approval of CPI in mUC, significantly increasing proportions of patients with mUC initiate CPI near the end of life. In the final quarter of 2017, approximately one‐fourth of patients who died had initiated CPI near the end of life. After CPI approval, end‐of‐life CPI use increased significantly for patients with poor PS but did not increase significantly for patients with good PS. This may be explained by the approval of CPI in mid‐2017 for cisplatin‐ineligible patients, who may have poorer PS than cisplatin‐eligible patients [12]. Importantly, clinical trials of CPI in mUC exclude patients with ECOG PS 3–4 and include only a minority of patients with ECOG PS 2. Finally, the proportions of any systemic treatment initiation within 60 days of death doubled from 2015q4 to 2017q4, largely driven by increasing CPI use. This may reflect physicians’ and patients’ perceptions of CPI's favorable risk‐benefit profile, even among individuals with limited life expectancy or PS who may not live long enough to derive a survival benefit from CPI.
This study has several limitations. Because we limited our cohort to decedents, our study should not be used to make inferences about treatment strategies in mUC and should be considered hypothesis generating. To quantify the risk‐benefit ratio of CPI relative to chemotherapy or no therapy among patients with poor performance status, future studies should assess overall survival in a full cohort of poor‐prognosis patients, along with patient‐centered outcomes such as safety, quality of life, and end‐of‐life care. Such studies require data sources with detailed clinical and treatment data and methodology to reduce confounding by indication. Greater inclusion of patients with poorer performance status in randomized trials would also increase generalizability of such trials to real‐world populations. Missing PS data (25%–34%) across subgroups was a limitation of this study, although it was equally distributed between chemotherapy and CPI initiators. Also, we could not assess cause of death from this database, and patients who died of noncancer causes could be captured in this analysis. Finally, clinician experience with CPI near the end of life and the recent FDA indication changes for first‐line CPI in mUC may have an impact on future prescribing patterns at the end of life [13].
To our knowledge, this study provides the first estimate of the proportion of patients with advanced urothelial cell carcinoma who initiate CPI near the end of life. Existing clinical guidelines regarding systemic therapy initiation near the end of life should account for the increasing use of newer treatment modalities such as CPI, especially among patients with poor PS. These findings, if replicated across other malignancies, may have important policy implications given the high cost of CPI compared with chemotherapy.
Acknowledgments
This work was supported by the National Cancer Institute of the National Institutes of Health 5‐T32‐CA009615 (to R.B.P.) and K23‐CA187185 (to R.M.).
Disclosures
Matthew D. Galsky: Janssen, Dendreon, Merck, GlaxoSmithKline, Ely Lilly & Co, Astellas, Genentech, Bristol‐Myers Squibb, Novartis, Pfizer, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Aileron Therapeutics, Dracen Pharmaceuticals, Inovio Pharmaceuticals (C/A), Janssen, Dendreon, Novartis, Bristol‐Myers Squibb, Merck, AstraZeneca, Genentech/Roche (RF), RAPPTA Therapeutics (OI); Blythe Adamson: Flatiron Health (E); Cary P. Gross: National Comprehensive Cancer Network/Pfizer, J&J (RF), Flatiron (other‐travel); Neal Meropol: Flatiron Health (E); Ronac Mamtani: Roche (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Prigerson HG, Bao Y, Shah MA et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 2015;1:778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnipper LE, Smith TJ, Raghavan D et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol 2012;30:1715–1724. [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 4.Kolata G. ‘Desperation oncology’: When patients are dying, some cancer doctors turn to immunotherapy. The New York Times. April 26, 2018. https://www.nytimes.com/2018/04/26/health/doctors‐cancer‐immunotherapy.html. Accessed August 27, 2018. [Google Scholar]
- 5.Bellmunt J, de Wit R, Vaughn DJ et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balar AV, Galsky MD, Rosenberg JE et al. Atezolizumab as first‐line therapy in cisplatin‐ineligible patients with locally advanced and metastatic urothelial carcinoma: A single‐arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balar AV, Castellano D, O'Donnell PH et al. First‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE‐052): A multicentre, single‐arm, phase 2 study. Lancet Oncol 2017;18:1483–1492. [DOI] [PubMed] [Google Scholar]
- 8.Curtis MD, Griffith SD, Tucker M et al. Development and validation of a high‐quality composite real‐world mortality endpoint. Health Serv Res 2018;53:4460–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flatiron Health Database . Flatiron Health. https://flatiron.com/real-world-evidence/. Published June 2018. Accessed August 26, 2018.
- 10.Flaig TW. NCCN Clinical practice guidelines in oncology: Bladder cancer. Bladder Cancer. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf. Published July 3, 2018. Accessed August 13, 2018.
- 11.Cuzick J. A Wilcoxon‐type test for trend. Stat Med 1985;4:87–90. [DOI] [PubMed] [Google Scholar]
- 12.Galsky MD, Hahn NM, Rosenberg J et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin‐based chemotherapy. J Clin Oncol 2011;29:2432–2438. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration . FDA alerts health care professionals and oncology clinical investigators about an efficacy issue identified in clinical trials for some patients taking keytruda (pembrolizumab) or tecentriq (atezolizumab) as monotherapy to treat urothelial cancer with low expression of PD‐L1. https://www.fda.gov/Drugs/DrugSafety/ucm608075.htm. Updated August 16, 2018. Accessed September 26, 2018.