This article addresses the question of whether adjuvant chemotherapy is necessary for patients who have had neoadjuvant radiotherapy and curative surgery.
Keywords: Rectal cancer, Adjuvant chemotherapy, Neo‐adjuvant chemoradiotherapy, Cancer‐specific survival
Abstract
Background.
Adjuvant chemotherapy is currently offered routinely, as standard, after radical resection for patients with rectal cancer receiving neo‐adjuvant chemoradiation. However, the efficacy of adjuvant chemotherapy in patients with ypTis‐2N0M0 has not been documented to the same extent, and the survival benefit remained controversial. The purpose of this work was to determine the role of chemotherapy in patients with ypTis‐2N0M0 classification.
Materials and Methods.
Data were obtained from the Surveillance, Epidemiology, and End Results database (n = 4,217). A propensity score model was utilized to balance baseline covariates.
Results.
Of the 4,217 included patients, 335 with ypTis‐2N0M0 did not receive adjuvant chemotherapy. There were comparable cancer‐specific survivals (CSS) between those undergoing adjuvant chemotherapy or not (log‐rank test = 0.136, p = .712) in the overall sample. After propensity score matching, the cancer‐specific survival did not differ between the chemotherapy and observation groups (log‐rank test = 0.089, p = .765). Additionally, the Cox model did not demonstrate adjuvant chemotherapy as the prognostic factor, with hazard ratio = 0.95 (95% confidence interval 0.69–1.32) for CSS. Furthermore, the 10‐year cumulative CSS was 78.7% and 79.4% between the chemotherapy and observation groups, indicating no significance, and no impact of adjuvant chemotherapy on survival was observed in different subgroups stratified by T stage, histological grade, histology, lymph nodes, and tumor size.
Conclusion.
Patients with ypTis‐2N0 rectal cancer did not benefit from adjuvant chemotherapy after preoperative radiology and radical surgery in this cohort study. These results provided new insight into the routine use of adjuvant chemotherapy for patients with rectal cancer with completed neo‐adjuvant radiotherapy and curative surgery.
Implications for Practice.
Inconsistent recommendations for patients with rectal cancer receiving neo‐adjuvant chemoradiation are offered by clinical guidelines. Adjuvant chemotherapy had no cancer‐specific survival benefit, not only in the whole cohort, but also in the propensity score‐matched cohort. A Cox model also confirmed adjuvant chemotherapy was not a significant prognostic factor in ypTis‐2N0 rectal cancer. No survival benefit conferred by adjuvant chemotherapy was observed, regardless of whether T stage, histological type, grade, lymph nodes and tumor size varied.
摘要
背景。目前,对于接受新辅助放化疗的直肠癌患者,在根治术后,作为标准会常规性地向其提供辅助化疗。但是,ypTis‐2N0M0 期患者的辅助化疗疗效尚未得到同等程度的记录证明,并且生存益处仍然存在争议。此项工作的目的是确定化疗在 ypTis‐2N0M0 类别患者中的作用。
材料和方法。数据获取自监测、流行病学和最终结果数据库 (n=4 217)。倾向评分模型用于平衡基线协变量。
结果。在收录的 4 217 名患者中,有 335 名 ypTis‐2N0M0期患者未接受辅助化疗。在整个样本中,对于接受或未接受辅助化疗(log‐rank检验 = 0.136, p =0.712)的患者,其肿瘤特异性生存率 (CSS) 具有可比性。在倾向评分匹配之后,化疗组和观察组之间的肿瘤特异生存率没有差异(log‐rank检验 = 0.089,p = 0.765)。此外,Cox 模型并未证明辅助化疗作为预后因素,CSS 的风险比为 0.95(95% 置信区间 0.69–1.32)。而且,化疗组和观察组之间的 10 年累积 CSS 分别为 78.7% 和 79.4%,提示无显著差异,且未观察到以 T 分期、组织学分级、组织学、淋巴结和肿瘤大小划分的不同亚组中辅助化疗对生存率造成的影响。
结论。患有 ypTis‐2N0期直肠癌的患者并未从本队列研究中的术前放射和根治术后的辅助化疗中获益。这些结果为完成新辅助放疗和治疗性手术的直肠癌患者常规使用辅助化疗提供了新的见解。
对实践的启示:临床指南为接受新辅助放化疗的直肠癌患者提供了不一致的建议。辅助化疗在整个队列与倾向评分匹配队列中没有肿瘤特异性生存率获益。Cox 模型还证实了辅助化疗并不是 ypTis‐2N0期直肠癌的显著预后因素。未观察到辅助化疗所带来的生存获益,无论是否有 T 分期、组织学类型、分级、淋巴结和肿瘤大小变化。
Introduction
Neo‐adjuvant chemoradiotherapy (CRT) followed by radical surgery is the recommended optimal treatment for locally advanced rectal cancer [1]. This is based on the fact that preoperative radiotherapy before curative surgery reduces recurrence and improves survival [2]. Similarly, adjuvant chemotherapy has an established role for patients with locally advanced rectal cancer, reducing the risk of recurrence and mortality by up to 20%–30% [3]. Therefore, is adjuvant chemotherapy necessary on the patients with completed neo‐adjuvant radiotherapy and curative surgery? The National Comprehensive Cancer Network recommended postoperative adjuvant chemotherapy for all patients undergoing preoperative chemoradiotherapy regardless of the final yield pathology [4], and the European Society for Medical Oncology guidelines stated that “similar to the situation in colon cancer Stages III (and “high‐risk” Stage II), adjuvant chemotherapy can be provided, even if the scientific support for sufficient effect is less” [5]. So, the efficacy of adjuvant chemotherapy in patients after preoperative CRT and surgery has not been documented to the same extent, and the survival benefit remained controversial [6]. For example, an analysis and a Cochrane review, performed to evaluate published studies, both showed that adjuvant chemotherapy seemed to improve 5‐year disease‐free survival (DFS) and overall survival (OS) rates [6], [7]. However, Breugom et al. did not identify an improvement in OS among patients with either node‐negative or node‐positive disease in the case of chemotherapy [8]. Apart from this retrospective trial, randomized phase III trials Chronicle and PROCTOR‐SCRIPT both could not show an apparent benefit of adjuvant chemotherapy after preoperative radiotherapy and total mesorectal excision (TME) on OS, DFS, and recurrence rate [9], [10]. However, the T stages and status of lymph nodes (LN) were not taken into consideration separately; ypTis‐2N0 patients, who were considered to have a good prognosis, were excluded. Is there any additional survival benefit posed by chemotherapy? So far, there has been no research concerning this concept. The additional use of adjuvant therapy, however, has potential implications in terms of financial costs, morbidity, and mortality. With these heterogeneous results and the potential harmful effects of chemotherapy, we determined to assess the value of adjuvant chemotherapy after preoperative radiology and surgery in ypTis‐2N0 patients.
Materials and Methods
Data Preprocessing
To evaluate patterns of utilization and the effect of adjuvant chemotherapy, this cohort study was performed on patients with completed neo‐adjuvant radiotherapy and curative surgery extracted from 1988 to 2014 in the Surveillance, Epidemiology, and End Results (SEER) program database [11]. The SEER data on cancer trends included multiple population‐specific cancer registries across the U.S. and created the largest volume of cancer‐relevant data and statistics. All patients with ypTis‐2N0M0 classification, defined as depth of invasion within the border of the muscular layer, and no nodal involvement after neo‐radiation and curative surgery were enrolled in the current analysis according to the American Joint Committee on Cancer staging system. In all rectal cancer patients, we restricted our analysis to those whose death was caused by cancer, date of diagnosis was after 1988, final tumor pathology was stage 0 or I, and treatment strategy was adjuvant chemotherapy and observation.
Outcome Variable and Covariates
The primary outcome variable for our study was cancer‐specific survival time. Patients were censored when they died of other causes or were still alive at the end of the follow‐up period. We selected clinical covariates associated with adjuvant chemotherapy. We also stratified patients by each covariate, performed cancer‐specific survival analysis, and constructed a Cox model in each subgroup, including histological grade, tumor size, regional nodes examined, T stages, and histology information.
Statistical Analysis
Propensity Score Matching.
We defined propensity score as the probability of patients with chemotherapy. To include prognosis‐related covariates, the propensity score model is preferable to those influencing the treatment‐selection process [12]. Briefly, those important prognostic factors in combination with records in SEER, including race, gender, age, tumor size, tumor number, T stage, histological grade, lymph node retrieved, etc., were utilized to create propensity score models. The propensity scores were calculated by a nonparsimonious multivariable logistic regression model, which used adjuvant chemotherapy as the outcome of interest and prognostic factors as covariates. A nearest neighbor and 1 to 2 matching algorithm was performed within default caliper (0.2) in SPSS version 22 (IBM, Chicago, IL) [13].
Descriptive and survival statistical analyses were adopted to assess the impact of adjuvant chemotherapy on patients with neo‐radiology and surgical treatment of rectal cancer. Categorized data were summarized using contingency tables and assessed by chi‐square test or Fisher's exact test for demographic and tumor characteristics of patients in groups (Adjuvant chemotherapy and No chemotherapy). We also performed multivariate Cox proportional hazards regressions for adjuvant chemotherapy, histological grade, and tumor size. Moreover, cancer‐specific survivals were calculated to evaluate the impact of chemotherapy using the Kaplan‐Meier analysis. The log‐rank test was used to determine whether the Kaplan‐Meier survival curves for the two groups were statistically equivalent. Above all, cut points for categorical variables including age, tumor size, and number of lymph nodes were chosen to identify the effect of chemotherapy in subanalysis, which allowed for estimation of the treatment effect on survival while minimizing bias. Last, hazard ratio (HR) and RR were calculated to assess the importance of chemotherapy, and 1‐, 3‐, 5‐, and 10‐year survival rates were calculated in different treatment groups. A p value of <.05 was considered statistically significant. Statistical analyses were performed using the statistical software package SPSS for Windows, version 22.
Results
Data Source and Propensity Score Matching
In the SEER database from 1988 to 2014, 4,217 patients with rectal cancer, who completed neo‐adjuvant radiotherapy and curative surgery, were confirmed as ypTis‐2N0M0 on final pathology. Among the enrollment of this study, 3,882 patients received adjuvant chemotherapy (chemo group) and the remaining 335 patients did not, only for observation (no‐chemo group). There existed systematic differences in demographics and preoperative data between chemo and no‐chemo groups in the overall samples. Compared with patients not receiving chemo treatment, those undergoing adjuvant chemotherapy had more common mucinous adenocarcinoma histology and fewer retrieved lymph nodes and were younger in age (p < .001 for all; supplemental online Table 1). The unbalanced distribution of those characters was indicated in the overall samples.
Therefore, to achieve a balanced distribution of these baseline covariates, a propensity score model was performed (ratio 1:2). After propensity score matching, the pairwise comparisons of all covariates were not significant (p > .05; supplemental online Table 2), which demonstrated that the characteristics were balanced between the two treatment groups. This matched cohort totaled 1,002 patients, and the number of chemo group patients was twofold that of the no‐chemo group (668:334). Furthermore, we conducted an inequality analysis to test whether the sample size was sufficient to reach a power of 0.8, and it showed that at least 548 and 302 patients were needed in two arms, respectively. Therefore, this propensity score‐balanced cohort (668:334) could achieve high power.
Cancer‐Specific Survival Analysis in Overall Samples
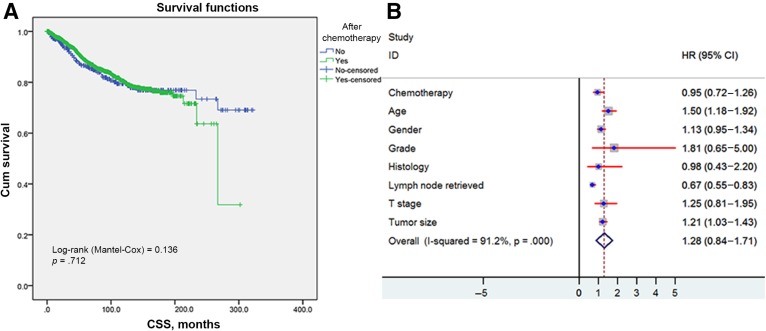
Kaplan‐Meier survival analysis revealed there were comparable cancer‐specific survivals of those undergoing adjuvant chemotherapies or not (log‐rank test = 0.136, p = .712; Fig. 1A). The per‐protocol analysis demonstrated an HR of 0.95 for cancer‐specific survivals (95% confidence interval [CI] 0.72–1.26) for adjuvant chemotherapy. It was also confirmed in multivariable analysis that adjuvant chemotherapy did not provide additional survival benefit (Fig. 1B). On the contrary, only younger age and more lymph nodes retrieved seemed to confer improved survival benefit. To consolidate the above result, the matched cohort and patients in the last 10 years (2004–2014) were analyzed thoroughly below.
Figure 1.
Kaplan‐Meier survival plots of cause‐specific survival. (A): Data based on the receipt of adjuvant chemotherapy in the entire sample. (B): HR of every clinical covariate from Cox model.
Abbreviations: CI, confidence interval; CSS, cancer‐specific survival; HR, hazard ratio; T, tumor.
As the modern standard for treating rectal cancer was established in 2004, we enrolled patients with ypTis‐2N0M0 only in the last 10 years (2004–2014) for validation. Consistently, no statistically significant difference was observed between the two groups (log‐rank test = 0.342, p = .559). However, the arm of the group without adjuvant chemotherapy only included 150 patients, not reaching a high statistical power.
Survival Effect of Adjuvant Chemotherapy in Propensity Score‐Balanced Cohort
The propensity score‐matched cohort demonstrated that mortality rate was 15.47% (n = 155) in all. Adjuvant chemotherapy was not associated with reduced cancer‐specific mortality at 1 year (vs. no chemo, 1.65%:2.99%), 3 years (5.33%:7.41%), 5 years (10.61%:12.67%), and 10 years (18.46%:19.85%; supplemental online Table 3). Furthermore, it was estimated that the 10‐year cumulative cancer‐specific survival was 78.7% and 79.4% in those receiving adjuvant chemotherapy or not, respectively. Additionally, the cumulative rectal cancer‐specific survival at 1, 3, and 5 years was presented in the same pattern regarding chemotherapy (supplemental online Table 4). No significance was demonstrated in cancer‐specific survival at any time point.
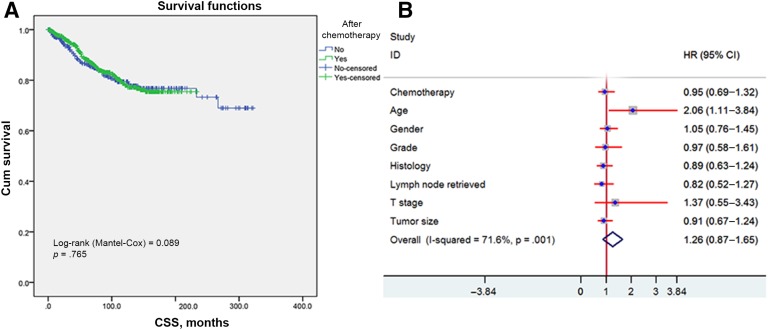
Overall, the cancer‐specific survival curves were similar between adjuvant chemo and no‐chemo groups. No statistically significant difference in CSS was observed (log‐rank test = 0.089, p = .765; Fig. 2A). In addition, the per‐protocol analysis demonstrated an HR of 0.95 for cancer‐specific survivals (95% CI 0.69–1.32) for adjuvant chemotherapy. We then adjusted other covariates by the Cox proportional model and got the same results: Adjuvant chemotherapy was not associated with a survival benefit. Consistently, only younger age was found to provide survival benefit with statistical significance (Fig. 2B).
Figure 2.
Comparison between adjuvant and no‐adjuvant chemotherapy groups in the propensity score‐matched cohort. (A): Kaplan‐Meier curves for cancer‐specific survival. (B): HR of every clinical covariate from the Cox model.
Abbreviations: CI, confidence interval; CSS, cancer‐specific survival; HR, hazard ratio, T, tumor.
Impact of Adjuvant Chemotherapy on Survival in Different Clinical Subgroups
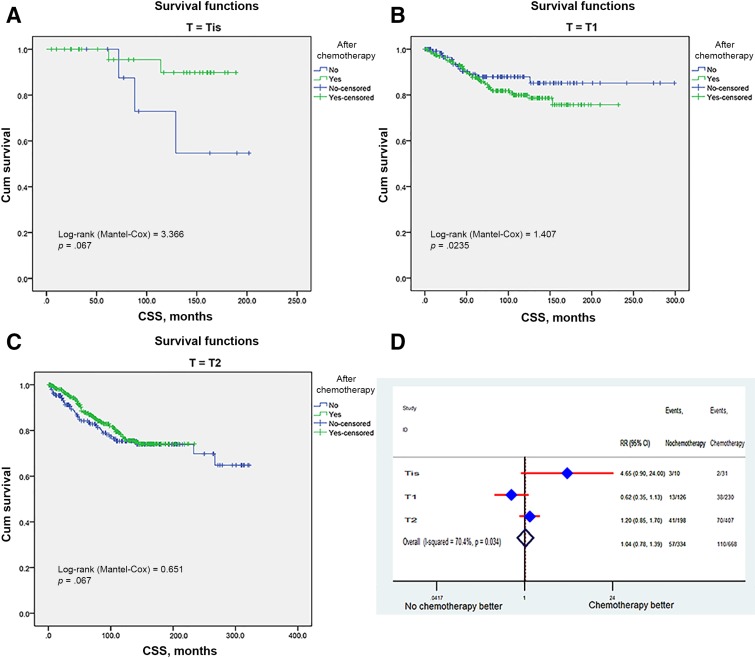
We then stratified patients according to the clinical covariates and identified the impact of adjuvant chemotherapy on survival in different subgroups. Based on the T stage, the cancer‐specific survival of adjuvant chemotherapy was comparable with that in the no‐chemo group, regardless of whether the T stage varied (Fig. 3A–3C). In T1‐stage patients (n = 356), the 3‐ and 5‐year survival rates were 92.4% ± 2.6% and 87.9% ± 3.3% in the no‐adjuvant‐chemo group and 94.5% ± 1.5% and 90.9% ± 2.0% in the adjuvant chemo group. In T2‐stage patients (n = 605), the 3‐ and 5‐year survival rates were 94.2% ± 1.7% and 88.1% ± 2.5% in the no‐adjuvant‐chemo group and 98.2% ± 0.7% and 96.3% ± 1% in the adjuvant chemo group. The multivariable Cox model also did not show apparent survival benefits for chemotherapy in various T‐stage groups (RR: 1.04, 95% CI 0.78–1.39; Fig. 3D).
Figure 3.
Survival curves and RR in different T‐stage subgroups. Kaplan‐Meier curves for CSS between adjuvant and no‐adjuvant chemotherapy groups in Tis (A), T1 (B), and T2 (C). Risk ratio of different T stage (D).
Abbreviations: CI, confidence interval; CSS, cancer‐specific survival; HR, hazard ratio; RR, risk ratio; T, tumor; Tis, tumor in situ.
Impact of Chemotherapy on Survival in Different Histological Type
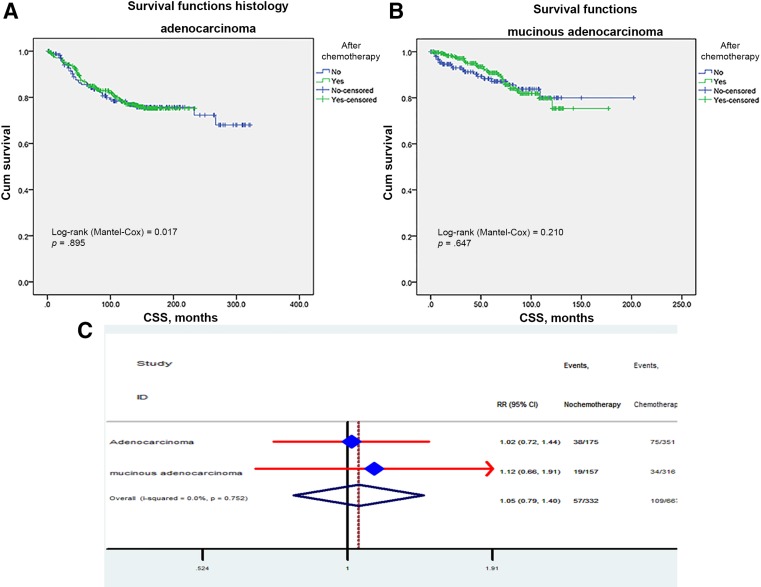
In line with stage subgroups, adjuvant chemotherapy did not have a significant role in subgroups of histological type. Chemotherapy did not seem to improve survival in adenocarcinoma patients, with 3‐ and 5‐year survival rates of 93.9% ± 1.3% and 89.6% ± 1.7% in the chemo group versus 90.2% ± 2.3% and 81.4 ± 3.1% in the no‐chemo group (Fig. 4A). Consistently, in the mucinous adenocarcinoma group (n = 473), 3‐ and 5‐year survival rates were 94.6% ± 1.9% and 91.2% ± 2.4% in the no‐chemo group versus 99% ± 0.6% and 97.9% ± 0.9% in the chemo group (Fig. 4B). Furthermore, we calculated the disease‐specific mortality rate by the Cox proportional model and got the same results in the adenocarcinoma group (RR: 1.02; 95% CI: 0.72–1.44) and in the mucinous adenocarcinoma group (RR: 0.66; 95% CI: 0.66–1.91; Fig. 4C).
Figure 4.
Impact of histological type on the CSS survival between adjuvant and no‐adjuvant chemotherapy groups (A, B). Risk ratio of adenocarcinoma and mucinous adenocarcinoma (C).
Abbreviations: CI, confidence interval; CSS, cancer‐specific survival; HR, hazard ratio; RR, risk ratio.
Chemotherapy Effects Stratified by Histological Grade
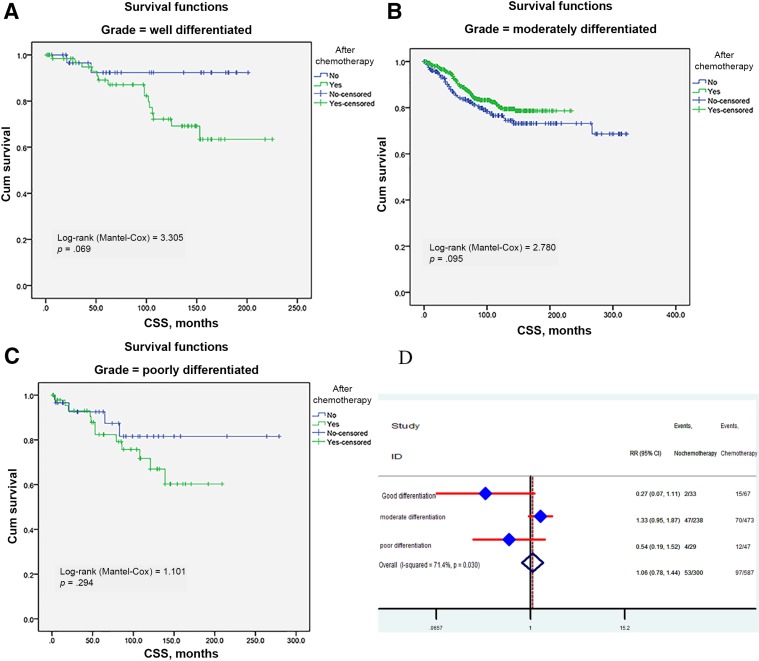
Because histological grade was significantly associated with benefit of adjuvant chemotherapy, we further split patients by grade to investigate their effects. Survival curves were constructed for three subgroups. Regardless of the histological grade, adjuvant chemotherapy did not have survival benefit in well‐, moderate‐, and poorly differentiated cancers (log‐rank test = 3.305, p = .069; log‐rank test = 2.78, p = .095; log‐rank test = 1.401, p = 0.294, respectively), and this trend was most apparent in poorly differentiated cancers (Fig. 5A–5C). Finally, we calculated the adjuvant chemotherapy's RRs in three groups (Fig. 5D). The result also indicated that chemotherapy did not play a critical role in different subgroups.
Figure 5.
Impact of histological grade on the CSS survival between adjuvant and no‐adjuvant chemotherapy groups: well differentiated (A), moderately differentiated (B), and poorly differentiated (C). Risk ratio of histological grade (D).
Abbreviations: CI, confidence interval; CSS, cancer‐specific survival; HR, hazard ratio; RR, risk ratio.
Subanalysis of Retrieved Regional Lymph Nodes and Tumor Size
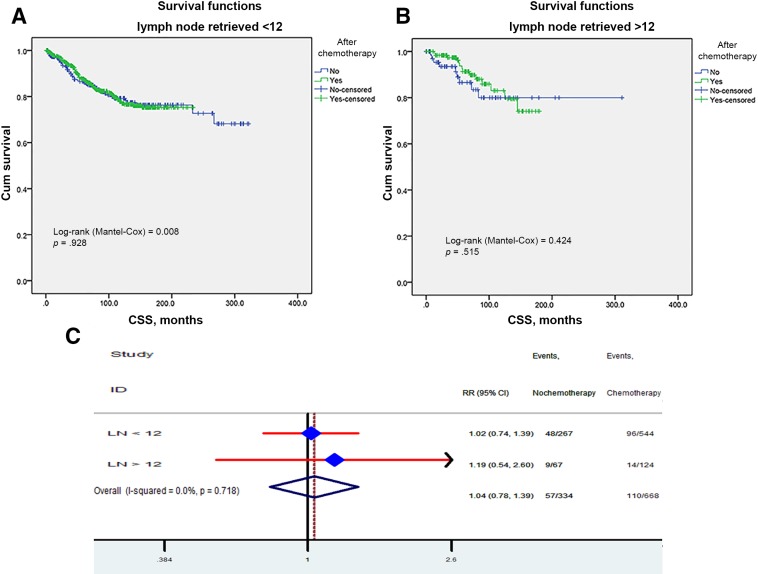
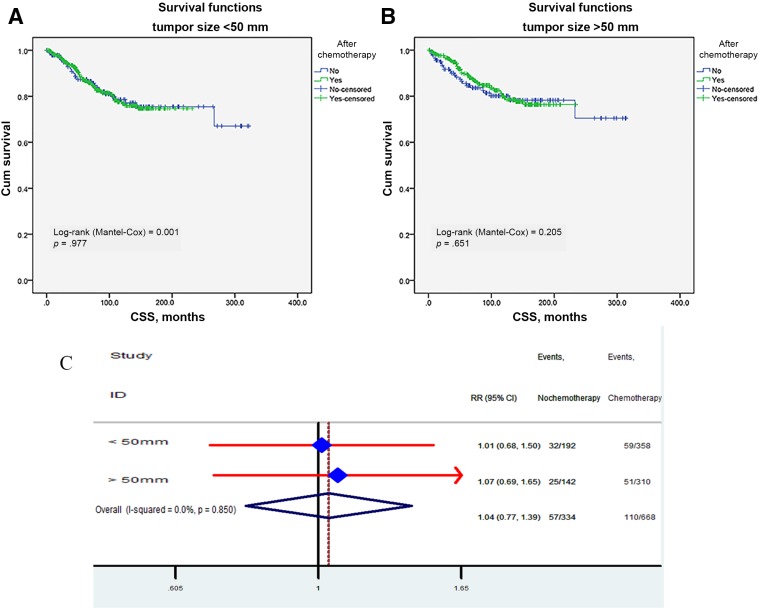
Kaplan‐Meier unadjusted survival analyses stratified by retrieved regional lymph nodes (LN <12 and LN ≥12) and tumor size (<50 mm and ≥50 mm) were used to compare survival between the no‐chemo group and the chemo group. No better outcome was produced by chemotherapy (all p > .05; Fig. 6A, 6B) relative to the no‐chemo group, regardless of the number of regional lymph nodes retrieved. In addition, we further analyzed the individual result using different cutoff values of retrieved regional lymph node numbers ranging from 5 to 20. The effects of CSS in patients with n (cutoff point) or more nodes and fewer than n nodes were calculated, respectively. The survival rates of patients with different cutoff values were all comparable between the chemo and no‐chemo groups. In the per‐protocol analysis, the HR was 1.04 (95% CI 0.78–1.39). No survival benefit conferred by adjuvant chemotherapy was observed when the tumor size was stratified by <50 mm and ≥50 mm (Fig. 7A, 7B). We then adjusted other covariates by Cox proportional model and got the same results: no reduced RR observed in chemo group (Fig. 7A), regardless of whether the tumor size varied.
Figure 6.
Impact of retrieved regional lymph nodes on the CSS survival between adjuvant and no‐adjuvant chemotherapy groups: fewer than 12 (A), more than 12 (B). Risk ratio of different retrieved regional lymph nodes (C).
Abbreviations: CI, confidence interval; CSS, cancer‐specific survival; HR, hazard ratio; RR, risk ratio.
Figure 7.
Impact of tumor size on the CSS survival between adjuvant and no‐adjuvant chemotherapy groups: smaller than 50 mm (A); larger than 50 mm (B). Risk ratio of different tumor size (C).
Abbreviations: CI, confidence interval; CSS, cancer‐specific survival; HR, hazard ratio; RR, risk ratio.
Discussion
In this study, the largest baseline covariate‐balanced cohort of yielded early rectal cancer was created by propensity‐score matching, and impacts of adjuvant chemotherapy on survival were investigated. In all results, adjuvant chemotherapy had no cancer‐specific survival benefit, not only in the whole cohort, but also in the propensity score‐matched cohort. The Cox model also confirmed adjuvant chemotherapy was not a significant prognostic factor in ypTis‐2N0 rectal cancer. Regardless of whether T stage, histological type, differentiation grade, retrieved regional lymph nodes, and tumor size varied, there was still no survival benefit conferred by adjuvant chemotherapy.
This current study is unique in comparing any postoperative chemotherapy against observation alone in ypTis‐2N0 rectal cancer. Some small sample‐size trials or subgroup analyses concerning the impact of postoperative adjuvant chemotherapy for patients in the ypT0‐2N0 classification have previously been conducted. Consistently, lower ypStage subgroups, such as stage I/II, could not obtain survival benefit from adjuvant chemotherapy. In contrast, higher ypStage, such as ypStage III (ypN1‐2), were proposed to have obtained greater benefit from chemotherapy [14]. Subsequently, no improvement in survival was observed in patients with ypT0‐2 N0 classification after neo‐chemoradiation and curative surgery when postoperative adjuvant chemotherapy was applied [15]. Later, those results were confirmed by Govindarajan, who presented comparable 5‐year DFS between patients who did and did not receive adjuvant treatment regardless of T stage classifications [16]. In addition, two other trials [17], [18] also claimed that adding adjuvant chemotherapy did not significantly prolong the survival of patients with ypN0 classification. However, the retrospective design nature, small size sample, and selection bias of those studies were frequently considered to be potential drawbacks. Hence, no consensus was reached on the basis of yield pathologic stage of the patient in determining the necessity for adjuvant chemotherapy.
Here, the largest baseline covariates balanced by propensity score matching demonstrated that adjuvant chemotherapy had no cancer‐specific survival benefit in ypT0‐2N0 classification, which added more clear evidence of the lack of benefit of chemotherapy applied in ypStage I rectal cancers. The main reason was related to accurate estimation for postoperative pathology, as the final pathologic stage is most prognostic of oncologic outcomes in locally advanced rectal cancer patients after neo‐chemoradiation [19]. Prolonging overall survival may require better control of relapse; Chang Hyun Kim showed that the ypStage group and corresponding pStage group had identical local relapse rate and that both staging systems displayed similar discriminatory power in predicting relapse [20]. Especially at stage I, ypStage I, and pStage I, similar 10‐year local recurrence rates were demonstrated, and as a result, ypStage I in rectal cancer is assumed a good prognostic factor, much like pStage I. In fact, chemotherapy is only effective for patients with poor prognosis, such as those with stage III or T3‐4 disease, just as the ADORE trial examining the role of oxaliplatin and leucovorin as chemotherapy for rectal cancer reported. It turned out that patients with final pathological stage III disease received improved survival benefit but patients with stage I and II disease did not [21]. That is why we did not observe any survival benefit in ypStage I when adding chemotherapy.
Tumor biology modification from chemoradiation may be another reason for the lack of benefit from chemotherapy. The molecular characteristics of cancer cells are dynamic and changeable and respond to chemo or radiology treatments, a process through which minor and dormant lineages can become dominant. After exposure to chemo or radiology treatments, cancer stem cells are able to survive to repopulate a tumor refractory to chemotherapy [22], [23]. Concerning chemotherapy resistance in colorectal cancer, there might be various mechanisms that are linked, including the evolution of mutational profiles [24] and epithelial‐mesenchymal transition (EMT) changes [25], in addition to stem cells dynamics [26].
However, contrary to the results of our study, a potential advantage from adjuvant chemotherapy was sometimes demonstrated in some retrospective studies [27], [28]. This can be attributed to the fact that the majority of these reports only presented overall survival rather than CSS in their analyses, which allowed non‐cancer‐related comorbidities to affect survival outcomes. Given that the tumor behavior is routinely evaluated by cancer‐specific survival, CSS is one of the most sensitive factors of the intended effects of biological characteristics. Unlike overall survival, CSS is less influenced by disparities in the treatment of relapsed disease, management of comorbidities, and differential rates of death from competing causes unrelated to cancer. Additionally, the retrospective design and unbalanced baseline of those studies may be considered to be other potential reasons. However, our propensity score matching was utilized to mitigate these limitations by matching the comorbidity levels, ages, T stages, and other relevant characteristics between the chemo‐ and observation group. Furthermore, the T stages and status of lymph nodes were not considered separately, because patients with positive lymph nodes had different responses to chemotherapy than patients with negative nodes [29]. Therefore, the heterogeneity from the combined stages may explain some of the contradictions concerning the prognosis of patients with chemo. Last, the different schedules of follow‐up periods may explain some of the contradictions relative to the effectiveness of adjuvant chemotherapy. For instance, in short‐term follow‐up, a subset of data from the European Organisation for Research and Treatment of Cancer (EORTC) Trial 22921 revealed that adjuvant chemotherapy prolonged survival in ypT0‐2, but not in ypT3‐4 patients [27]. But when the EORTC Trial was completed with prolonged follow‐up, it turned out that chemotherapy after preoperative radiotherapy did not affect DFS or OS, which was confirmed in both ypT0‐2 and ypT3‐4 classifications [30]. Consistent with our results, the 10‐year cumulative cancer‐specific survival was comparable, with 78.7% and 79.4% CSS in those receiving adjuvant chemotherapy or not, respectively; on the contrary, the 3‐year CSS in the chemo group was up to 2% greater than the observation group.
Although SEER is characterized by a large sample of patients for identifying gross trends and interesting patterns, some potential drawbacks of this study need to be considered. First, a different interval before starting adjuvant chemotherapy is inevitable in any real‐world database, so appropriate adjustment for this interval should be performed to determine the effect of an intervention. Second, the data set also does not include certain important prognostic indicators that could confound the survival analyses, such as type and course of chemotherapy or dose and fractionation of radiation. Last, chemotherapy compliance and clinical tumor node metastasis (TNM) staging, which has usually been the main focus in most rectal adjuvant trials, should be accounted for in the data.
Conclusion
Rectal cancer patients with ypTis‐2N0 did not benefit from adjuvant chemotherapy after preoperative radiology and radical surgery, which supported the results of previous studies investigating the role of adjuvant chemotherapy in ypTis‐2N0. Further refinement is necessary to identify the subset of patients that is most likely to benefit from adjuvant chemotherapy. It is also essential to determine the optimal duration and intensity of the adjuvant chemotherapy regimen.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant 81672374).
This article was published online on 19 April 2018. After online publication, revisions were made to figure legends 6 and 7. This notice is included in the online and print versions to indicate that it has been corrected on 23 May 2018.
Editor's Note: See the related commentary, “The Increasingly Complicated Approach to Rectal Cancer,” by David P. Ryan, on page 728 of this issue.
Author Contributions
Conception/design: Xiang Hu, San‐Jun Cai
Provision of study material or patients: Xiang Hu, Yan‐Lei Ma, Jun‐Jie Peng
Data analysis and interpretation: Xiang Hu, Ya‐Qi Li, Qing‐Guo Li
Manuscript writing: Xiang Hu
Final approval of manuscript: Xiang Hu, Ya‐Qi Li, Qing‐Guo Li, Yan‐Lei Ma, Jun‐Jie Peng, San‐Jun Cai
Disclosures
The authors indicated no financial relationships.
References
- 1. Scott NA, Susnerwala S, Gollins S et al. Preoperative neo‐adjuvant therapy for curable rectal cancer–Reaching a consensus 2008. Colorectal Dis 2009;11:245–248. [DOI] [PubMed] [Google Scholar]
- 2. Folkesson J, Birgisson H, Pahlman L et al. Swedish rectal cancer trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644–5650. [DOI] [PubMed] [Google Scholar]
- 3.Quasar Collaborative Group , Gray R, Barnwell J et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007;370:2020–2029. [DOI] [PubMed] [Google Scholar]
- 4.Rectal cancer v.3. NCCN Clinical Practice Guidelines in Oncology, 2016. Available at http://www.nccn.org/professionals/physician_gls/. Accessed June 4, 2016.
- 5. Glimelius B, Tiret E, Cervantes A et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013;24(suppl 6):vi81–vi88. [DOI] [PubMed] [Google Scholar]
- 6. Petersen SH, Harling H, Kirkeby LT et al. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 2012;14:CD004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petrelli F, Coinu A, Lonati V et al. A systematic review and meta‐analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorectal Dis 2015;30:447–457. [DOI] [PubMed] [Google Scholar]
- 8. Breugom AJ, Swets M, Bosset JF et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: A systematic review and meta‐analysis of individual patient data. Lancet Oncol 2015;16:200–207. [DOI] [PubMed] [Google Scholar]
- 9. Glynne‐Jones R, Counsell N, Quirke P et al. Chronicle: Results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 2014;25:1356–1362. [DOI] [PubMed] [Google Scholar]
- 10. Breugom AJ, van Gijn W, Muller EW et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol 2015;26:696–701. [DOI] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results: SEER public‐use data (1973–2015). Available at http://www.seer.cancer.gov/publicdata/. Accessed November 2015 Submission (released April 2016).
- 12. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: A Monte Carlo study. Stat Med 2007;26:734–753. [DOI] [PubMed] [Google Scholar]
- 13. Thoemmes F. Propensity score matching in SPSS. Available at https://arxiv.org/ftp/arxiv/papers/1201/1201.6385.pdf. Accessed October 4, 2017.
- 14. Das P, Skibber JM, Rodriguez‐Bigas MA et al. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol 2006;29:219–224. [DOI] [PubMed] [Google Scholar]
- 15. Huh JW, Kim HR. Postoperative chemotherapy after neoadjuvant chemoradiation and surgery for rectal cancer: Is it essential for patients with ypt0–2n0? J Surg Oncol 2009;100:387–391. [DOI] [PubMed] [Google Scholar]
- 16. Govindarajan A, Reidy D, Weiser MR et al. Recurrence rates and prognostic factors in ypn0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Ann Oncol 2011;18:3666–3672. [DOI] [PubMed] [Google Scholar]
- 17. Fietkau R, Barten M, Klautke G et al. Postoperative chemotherapy may not be necessary for patients with ypn0‐category after neoadjuvant chemoradiotherapy of rectal cancer. Dis Colon Rectum 2006;49:1284–1292. [DOI] [PubMed] [Google Scholar]
- 18. Kiran RP, Kirat HT, Burgess AN et al. Is adjuvant chemotherapy really needed after curative surgery for rectal cancer patients who are node‐negative after neoadjuvant chemoradiotherapy? Ann Surg Oncol 2012;19:1206–1212. [DOI] [PubMed] [Google Scholar]
- 19. Quah HM, Chou JF, Gonen M et al. Pathologic stage is most prognostic of disease‐free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 2008;113:57–64. [DOI] [PubMed] [Google Scholar]
- 20. Kim CH, Lee SY, Kim HR et al. Pathologic stage following preoperative chemoradiotherapy underestimates the risk of developing distant metastasis in rectal cancer: A comparison to staging without preoperative chemoradiotherapy. J Surg Oncol 2016;113:692–699. [DOI] [PubMed] [Google Scholar]
- 21. Hong YS, Nam BH, Kim KP et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): An open‐label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 2014;15:1245–1253. [DOI] [PubMed] [Google Scholar]
- 22. Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: The 4 R's of radiobiology revisited. Stem Cells 2010;28:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Touil Y, Igoudjil W, Corvaisier M et al. Colon cancer cells escape 5FU chemotherapy‐induced cell death by entering stemness and quiescence associated with the c‐Yes/YAP axis. Clin Cancer Res 2014;20:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kreso A, O'Brien CA, van Galen P et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 2013;339:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhangu A, Wood G, Brown G et al. The role of epithelial mesenchymal transition and resistance to neoadjuvant therapy in locally advanced rectal cancer. Colorectal Dis 2014;16:O133–O143. [DOI] [PubMed] [Google Scholar]
- 26. Dallas NA, Xia L, Fan F et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin‐like growth factor‐i receptor inhibition. Cancer Res 2009;69:1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collette L, Bosset JF, den Dulk M et al. Patients with curative resection of cT3‐4 rectal cancer after preoperative radiotherapy or radiochemotherapy: Does anybody benefit from adjuvant fluorouracil‐based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007;25:4379–4386. [DOI] [PubMed] [Google Scholar]
- 28. Janjan NA, Crane C, Feig BW et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol 2001;24:107–112. [DOI] [PubMed] [Google Scholar]
- 29. Jung KU, Kim HC, Park JO et al. Adjuvant chemotherapy after neoadjuvant chemoradiation and curative resection for rectal cancer: Is it necessary for all patients? J Surg Oncol 2015;111:439–444. [DOI] [PubMed] [Google Scholar]
- 30. Bosset JF, Calais G, Mineur L et al. Fluorouracil‐based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long‐term results of the EORTC 22921 randomised study. Lancet Oncology 2014;15:184–190. [DOI] [PubMed] [Google Scholar]