This article focuses on the incidence of patient‐reported major toxicity for chemotherapy regimens commonly used in breast cancer, especially considering the need for patient‐centered assessments of treatment tolerability as an important complement to the NCI's CTCAE.
Keywords: Breast cancer, Chemotherapy, Patient‐reported symptoms
Abstract
Background.
This study explores the incidence of patient‐reported major toxicity—symptoms rated “moderate,” “severe,” or “very severe”—for chemotherapy regimens commonly used in early breast cancer.
Patients and Methods.
Female patients aged 21 years or older completed a validated Patient‐Reported Symptom Monitoring instrument and rated 17 symptoms throughout adjuvant or neoadjuvant chemotherapy. Fisher's exact tests compared differences in percentages in symptom ratings, and general linear regression was used to model the incidence of patient‐reported major toxicity.
Results.
In 152 patients, the mean age was 54 years (range, 24–77), and 112 (74%) were white; 51% received an anthracycline‐based regimen. The proportion of patients rating fatigue, constipation, myalgia, diarrhea, nausea, peripheral neuropathy, and swelling of arms or legs as a major toxicity at any time during chemotherapy varied significantly among four chemotherapy regimens (p < .05). The mean (SD) number of symptoms rated major toxicities was 6.3 (3.6) for anthracycline‐based and 4.4 (3.5) for non‐anthracycline‐based regimens (p = .001; possible range, 0–17 symptoms). Baseline higher body mass index (p = .03), patient‐reported Karnofsky performance status ≤80 (p = .0003), and anthracycline‐based regimens (p = .0003) were associated with greater total number of symptoms rated major toxicities (alternative model: chemotherapy duration, p < .0001). Twenty‐six percent of dose reductions (26 of 40), 75% of hospitalizations (15 of 20), and 94% of treatment discontinuations (15 of 16) were in anthracycline‐based regimens.
Conclusion.
Capturing multiple toxicity outcomes throughout chemotherapy enables oncologists and patients to understand the range of side effects as they discuss treatment efficacies. Continuous symptom monitoring may aid in the timely development of interventions that minimize toxicity and improve outcomes.
Implications for Practice.
This study investigated patient‐reported toxicities for 17 symptoms recorded prospectively during adjuvant and neoadjuvant chemotherapy regimens for early breast cancer. An analysis of four commonly used chemotherapy regimens identified significant differences among regimens in both individual symptoms and total number of symptoms rated moderate, severe, or very severe. Longer chemotherapy regimens, such as anthracycline‐based regimens followed by paclitaxel, had higher proportions of symptoms rated major toxicities. The inclusion of patient perspectives on multiple toxicity outcomes at the same time at multiple time points during chemotherapy has the potential for improving patient‐provider communication regarding symptom management, patient satisfaction, and long‐term clinical outcomes.
摘要
背景。本研究探索了在常用于治疗早期乳腺癌的化疗方案中患者报告的主要毒性(即被评为“中度”、“重度”或“极重度”的症状)的发生率。
患者和方法。年满 21 岁或以上的女性患者填写一份经验证的患者报告的症状监测文件并对辅助或新辅助化疗期间的 17 个症状进行评级。Fisher's精确检验对症状评级百分比之间的差异进行比较,一般线性回归用于模拟患者报告的主要毒性的发生率。
结果。在 152 名患者中,平均年龄为 54 岁(范围介于 24–77 岁之间),112 名患者 (74%) 为白种人;51% 的患者接受蒽环类方案治疗。在化疗期间的任何时间将疲劳、便秘、肌痛、腹泻、恶心、周围神经病变以及手臂或腿部肿胀评为主要毒性的患者的比例在 4 个化疗方案中显著不同 (p < 0.05)。在蒽环类方案和非蒽环类方案中,被评为主要毒性的症状的平均数 (SD) 分别为 6.3 (3.6) 和 4.4 (3.5)(p = 0.001;可能的范围,0–17 个症状)。基线较高的体质量指数 (p = 0.03)、患者报告的 Karnofsky 体力状态 ≤80 (p = 0.000 3) 以及蒽环类方案 (p = 0.000 3) 与被评为主要毒性的症状的总数较高相关(替代模式:化疗持续时间,p < 0.000 1)。在蒽环类方案中,剂量减少占 26%(40 名患者中的 26 名患者),住院治疗占 75%(20 名患者中的 15 名患者),治疗中断占 94%(16 名患者中的 15 名患者)。
结论。获取化疗过程中的多种毒性结果,可令肿瘤学家和患者在讨论治疗有效性时了解副作用的范围。持续的症状监测可能有助于及时地制定可以最大限度地减少毒性并改进预后的干预措施。
《肿瘤学家》
实践意义:本研究调查了患者报告的早期乳腺癌辅助和新辅助化疗方案中前瞻性记录的 17 个症状的毒性。针对 4 个常用化疗方案的分析在单项症状和被评为“中度”、“重度”或“极重度”的症状的总数方面确定了方案之间的显著差异。在较长的化疗方案中,如后续采用紫杉醇治疗的蒽环类方案,被评为主要毒性的症状占有较高的比例。同时,在化疗期间的多个时间点加入患者关于多种毒性结果的看法,这可能会改进患者和医疗服务提供者之间有关症状管理、患者满意度以及长期临床预后的沟通。
Introduction
Survival rates for women with breast cancer have steadily improved over the past several decades, in large part because of improvements in early detection and advances in treatment [1], [2]. Early breast cancers that are localized (stage I–II, 62% of new breast cancer cases) or regional (stage III, 31% of new cases) now have 5‐year survival rates of 98.9% and 85.2%, respectively [1]. Systemic therapy including endocrine therapy and chemotherapy are important components of treatment advances [3], [4]. Delivering protocol‐defined dosage and completing therapy are associated with improved outcomes [5] and depend on the patient's ability to tolerate treatment‐related toxicities. Metrics of treatment tolerability include hospitalization, treatment discontinuation, and clinician‐assessed toxicity monitored through treatment using the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) [6], [7].
Over the past decade, there has been growing support for patient‐centered assessments of treatment tolerability as an important complement to CTCAE [8], [9]. Standardized reporting is now available through the National Cancer Institute's Patient‐Reported Outcomes‐CTCAE (PRO‐CTCAE) scales [10], [11], [12], [13]. Efforts are underway to integrate PRO‐CTCAE measurement into clinical trials [14], [15], [16], [17], [18], [19], and interest is growing in the implementation and utilization of patient‐reported symptom monitoring in patient‐centered clinical practice [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. Like CTCAE, the focus of PRO‐CTCAE is continuous symptom monitoring throughout chemotherapy, not just at the beginning and end of chemotherapy.
To date, early breast cancer is relatively understudied in the PRO‐CTCAE literature. Some PRO‐CTCAE studies have included women with breast cancer within larger samples of diverse patient populations [9], [13], [15], [23]. We have identified only one study pertaining exclusively to breast cancer [30]. Our interest is in patent perspectives on the tolerability of specific chemotherapy regimens commonly used in current clinical practice for early breast cancer. As an ancillary investigation to two intervention studies conducted in this patient population, we investigate patient‐reported symptom severity for individual regimens using data collected prospectively throughout conventional adjuvant or neoadjuvant chemotherapy. Patient‐reported tolerability profiles are then combined with hospitalization, dose reduction, and treatment discontinuation rates to provide a more complete picture of regimen tolerability.
Patients and Methods
Study Participants
The sample was recruited between March 2014 and October 2017 from multiple institutions. The study sites included university‐affiliated hospitals (University of North Carolina [UNC] Cancer Hospital, Duke University Medical Center, MD Anderson Cancer Center, Ohio State University Comprehensive Cancer Center) and community‐based clinics (UNC Rex Healthcare). Women aged 21 years or older with histologically confirmed stage I–III breast cancer who were scheduled for adjuvant or neoadjuvant chemotherapy were offered participation in one of two identical studies that differed only in age criteria for inclusion (NCT02167932 for women under age 65 and NCT02328313 for women age 65 or older; see supplemental online Appendices 5 and 6 for protocols for these two studies). Both studies entailed interventions to encourage moderate walking during chemotherapy treatment. The analysis presented in this manuscript is a secondary analysis of prospective data collected through these intervention studies. The studies were approved by the UNC‐Chapel Hill Lineberger Comprehensive Cancer Center Protocol Review Committee and the institutional review boards of each study site. Written informed consent meeting university and federal guidelines was obtained from each participant.
Chemotherapy Regimens
The study protocols did not specify chemotherapy regimens; regimens were determined by treating oncologists in consultation with their patients depending on tumor stage [4] and phenotypes. The most common regimens for women in our studies were (a) doxorubicin/cyclophosphamide plus paclitaxel (AC‐T), (b) docetaxel/ cyclophosphamide (TC), (c) docetaxel/carboplatin with anti‐HER2 therapy (TCH), and (d) doxorubicin/ cyclophosphamide plus paclitaxel/carboplatin (AC‐TC). These four regimens accounted for 83% of women enrolled in the studies, and our analysis is limited to these four regimens. Growth factors (pegfilgrastim) were used for docetaxel/cyclophosphamide and docetaxel/carboplatin regimens and for doxorubicin/cyclophosphamide when administrated every 2 weeks (dose dense). Supplemental online Appendix 1 provides further details regarding chemotherapy regimens by phenotype, and supplemental online Appendix 2 provides details regarding the timing and order of chemotherapy drugs.
Measures
Patient‐Reported Chemotherapy Toxicities.
A patient‐tested outcomes instrument called Patient‐Reported Symptom Monitoring (PRSM) [31] was used to inquire about symptoms “in the past 7 days” for 17 chemotherapy‐related toxicities: trouble having a bowel movement (constipation); loose or watery stools (diarrhea); pain in the abdomen; pain in general; aching joints such as elbows, knees, shoulders (arthralgia); aching muscles (myalgia); numbness or tingling in the hands or feet (peripheral neuropathy); mouth or throat sores; nausea, vomiting; arm or leg swelling; tiredness, lack of energy, fatigue; sad or unhappy feelings (depression); anxiety (worrying); insomnia (difficulty falling asleep, staying asleep, waking up early); shortness of breath (dyspnea); and hot flashes. These 17 symptoms were selected a priori because they reflect what is recognized in clinical practice as the most common side effects of chemotherapy in early breast cancer. The PRSM [31] (supplemental online Appendix 3) is very similar to the PRO‐CTCAE [10], [13], which was not available to the general community when we launched the walking intervention trials. For each symptom, three questions inquired about (a) frequency of the symptom, (b) severity of the symptom, and (c) whether the symptom kept the patient from doing things she usually did (interference). For the current study, we have analyzed only the severity of symptoms, because it is the measure that is the most comparable to CTCAE. At scheduled infusion visits throughout their chemotherapy, patients rated their symptom severity as follows: 0 = none, 1 = mild, 2 = moderate, 3 = severe, or 4 = very severe.
Our specific interest was the incidence of symptoms rated by patients as a major toxicity—“moderate,” “severe,” or “very severe”—at any time during chemotherapy [32]. A maximum rating of moderate, severe, or very severe was recorded only once per symptom per patient, although the patient may have scored that symptom as a major toxicity at several time points during chemotherapy. This approach to toxicity assessment is analogous to CTCAE reporting in clinical trials, in which the percent of grade 2, 3, or 4 toxicities is the incidence of the highest toxicity score at any time during chemotherapy. For each of the 17 symptoms, we calculated the percent of patients whose maximum score at any time during chemotherapy was moderate, severe, or very severe. An individual patient could rate more than one symptom as a major toxicity, as reflected in the mean total number of major toxicity symptoms per regimen.
Study participants were asked by research staff to complete a PRSM form (printed or tablet) during infusion visits throughout their chemotherapy treatment, either shortly before or during each infusion visit, and generally after they had seen their treating oncologist. For regimens that entailed weekly infusions, symptom forms were completed every other week; otherwise, they were collected every other week (dose‐dense regimens) or every 3 weeks (triweekly regimens). By limiting all data analysis to every other week or every 3 weeks, we aimed to avoid over‐representing maximum toxicity reports for regimens that were administered weekly. In the event a patient had both neoadjuvant and adjuvant chemotherapy, symptom forms were collected only during neoadjuvant chemotherapy, to ensure all patients were chemotherapy naïve at baseline when they started symptom reporting. For patients whose anti‐HER2 treatment continued beyond their chemotherapy, PRSM reports were collected only for the chemotherapy portion. Symptom reports were not provided to the oncology provider or nurse. Standard clinical practice was used to manage side effects; the study protocols did not specify symptom management recommendations. Data were not collected on interventions used to manage toxicity other than dose delay or treatment discontinuation.
Physical Function.
At baseline, prior to chemotherapy treatment initiation, patients self‐assessed their Karnofsky performance status (Patient‐KPS) [33], with scores of 80 to 100 signifying ability to carry out normal activities of daily living.
Patient Characteristics, Breast Cancer Diagnosis and Treatment, and Adverse Events.
Research staff reviewed the electronic health record for data pertaining to study participant age, race, height, weight, body mass index (BMI), breast cancer stage (American Joint Committee on Cancer, version 7) [34] and phenotype, and treatment. Staff also extracted data regarding chemotherapy‐related adverse events, including hospitalizations, dose reductions, and treatment discontinuations.
Statistical Analysis
Fisher's exact tests for differences in symptom severity percentages and Wilcoxon rank sum and Kruskall‐Wallis tests were used to assess differences in number of moderate, severe, or very severe symptoms by regimen. General linear regression was used to test association of patient and clinical characteristics with the incidence of major toxicities. Multivariable models were fit based on significant unadjusted results. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC), and 2‐sided p < .05 was considered statistically significant.
Results
Participant Characteristics
In our sample of 152 patients (Table 1), average age at breast cancer diagnosis was 54 years (range, 24–77), 74% were white, and 9% had a high school education or less. High proportions were overweight (34%, BMI 25–29.9) or obese (38%, BMI ≥30). Twenty‐eight patients (18%) had HER2‐positive tumors, all of whom received anti‐HER2 therapy. Timing of chemotherapy was 43% neoadjuvant and 57% adjuvant. Planned duration of chemotherapy treatment was greater than 3 months for 67% of the sample. Half (51%) of the participants received an anthracycline‐based regimen. Among patients whose tumor was HR positive/HER2 negative, 55% received anthracycline‐based regimens and 45% nonanthracycline; among triple‐negative patients, 75% received anthracycline‐based regimens and 25% nonanthracycline (supplemental online Appendix 1). Taxane regimens included paclitaxel/nab‐paclitaxel (51%) and docetaxel (49%). Supplemental online Appendix 2 provides further details regarding order of administration of sequential anthracycline‐paclitaxel regimens, proportion of patients receiving neoadjuvant chemotherapy, and timing of infusions.
Table 1. Study participant (n = 152) characteristics at breast cancer diagnosis.
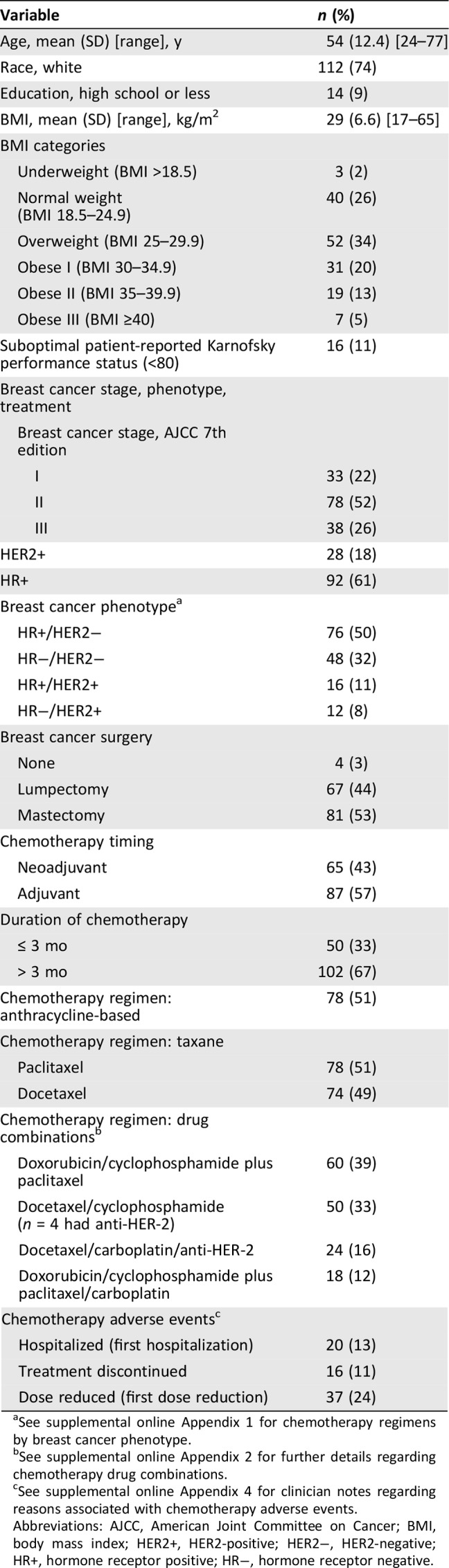
See supplemental online Appendix 1 for chemotherapy regimens by breast cancer phenotype.
See supplemental online Appendix 2 for further details regarding chemotherapy drug combinations.
See supplemental online Appendix 4 for clinician notes regarding reasons associated with chemotherapy adverse events.
Abbreviations: AJCC, American Joint Committee on Cancer; BMI, body mass index; HER2+, HER2‐positive; HER2−, HER2‐negative; HR+, hormone receptor positive; HR−, hormone receptor negative.
Moderate, Severe, or Very Severe Toxicities Overall and by Chemotherapy Regimen
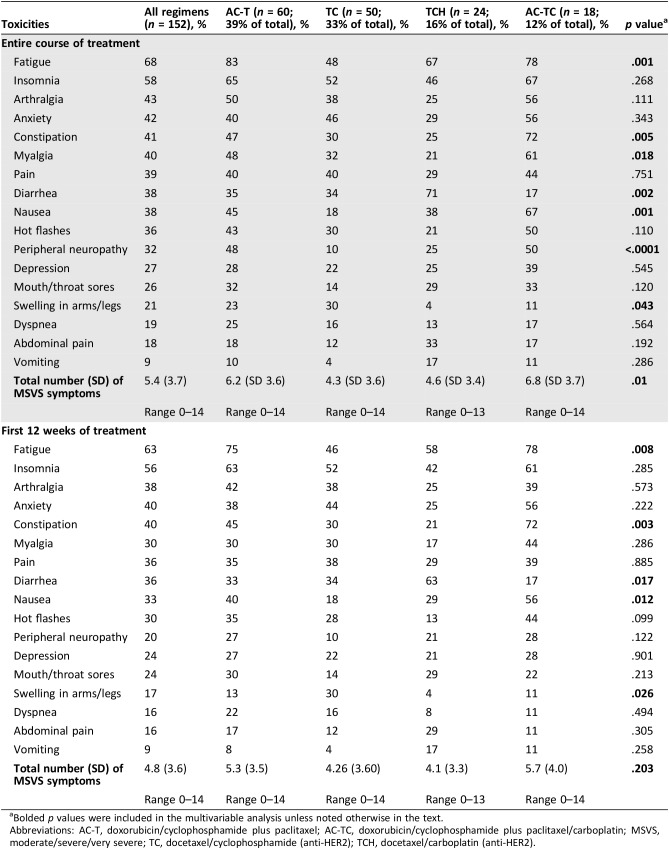
For the four most common chemotherapy regimens, the proportion of symptom reports collected as planned was 86% for AC‐TC, 81% for AC‐T, 100% for TCH, and 90% for TC. The most common reason for missing reports was staffing shortage. Table 2 presents patient‐reported toxicities rated “moderate,” “severe,” or “very severe” as the highest score at any time during the entire course of chemotherapy. For the four chemotherapy regimens combined, severity scores were highest for fatigue (68%), insomnia (58%), arthralgia (43%), anxiety (42%), constipation (41%), and myalgia (40%). Both individual symptoms (p ≤ .05) and total number of symptoms rated as major toxicities (p = .01) varied significantly among the four chemotherapy regimens. Mean (SD) total number of symptoms rated as major toxicities (possible range, 0–17 symptoms) was 6.3 (3.6) for anthracycline‐based regimens compared with 4.4 (3.5) for regimens that were not anthracycline based (p = .001).
Table 2. Incidence of patient‐reported symptoms rated MSVS by chemotherapy regimen (percent) during entire course of treatment and first 12 weeks of treatment.
Bolded p values were included in the multivariable analysis unless noted otherwise in the text.
Abbreviations: AC‐T, doxorubicin/cyclophosphamide plus paclitaxel; AC‐TC, doxorubicin/cyclophosphamide plus paclitaxel/carboplatin; MSVS, moderate/severe/very severe; TC, docetaxel/cyclophosphamide (anti‐HER2); TCH, docetaxel/carboplatin (anti‐HER2).
To account for varying durations of chemotherapy regimens, major toxicity percentages presented in the bottom half of Table 2 are limited to the first 12 weeks of chemotherapy for each regimen. In this sensitivity analysis, major toxicity incidences for myalgia and peripheral neuropathy no longer differed significantly among the four regimens, as these symptoms were most frequently due to the largely post‐12 weeks initiation of weekly paclitaxel. The total number of moderate, severe, or very severe symptoms did not differ significantly among the four regimens during the first 12 weeks of treatment (p = .203).
Association of Patient and Clinical Characteristics with Total Number of Items Rated Moderate, Severe, or Very Severe
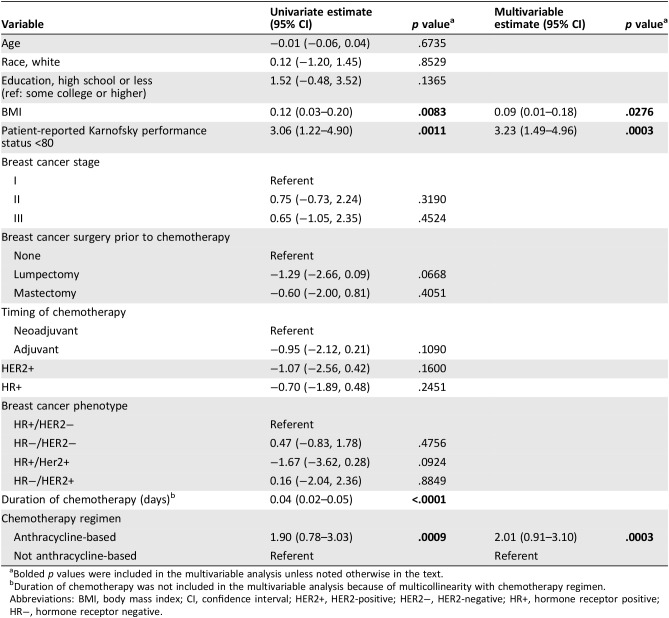
In Table 3, associations of patient characteristics, breast cancer stage and phenotype, and treatment with number of major toxicities are presented. In unadjusted analysis, higher BMI (p = .008), Patient‐KPS <80 (p = .001), longer duration of chemotherapy (p ≤ .001), and anthracycline‐based chemotherapy (p = .0009) were associated with higher number of major toxicities. In multivariable analysis, higher BMI (estimate 0.09; 95% confidence interval [CI], 0.01–0.18; p = .028), Patient‐KPS <80 (estimate 3.23; 95% CI, 1.49–4.96; p = .0003), and anthracycline‐based regimen (estimate 2.01; 95% CI, 0.91–3.10; p = .0003) remained independently associated with higher number of major toxicities. We did not include duration of chemotherapy in the multivariable model because of high correlation with anthracycline‐based chemotherapy. In multivariable analysis that substituted duration of chemotherapy for anthracycline‐based chemotherapy, Patient‐KPS (p = .0001) and BMI (p = .018) remained independently significant, as well as chemotherapy duration (p < .0001).
Table 3. Association of patient and clinical characteristics with total number of patient‐reported moderate/severe/very severe symptoms (n = 152).
Bolded p values were included in the multivariable analysis unless noted otherwise in the text.
Duration of chemotherapy was not included in the multivariable analysis because of multicollinearity with chemotherapy regimen.
Abbreviations: BMI, body mass index; CI, confidence interval; HER2+, HER2‐positive; HER2−, HER2‐negative; HR+, hormone receptor positive; HR−, hormone receptor negative.
Hospitalization, Dose Reductions, and Treatment Discontinuations
First incidences of hospitalization and dose reduction were investigated, as well as early treatment discontinuation (supplemental online Appendix 4). Twenty patients (13%) were hospitalized, primarily because of neutropenic or other fever (45%). Supplemental online Appendix 2 provides information on timing of the first hospitalization, such as during doxorubicin/cyclophosphamide therapy in patients receiving AC‐T chemotherapy (ten patients). Sixteen patients (11%) had chemotherapy discontinued before completion of planned treatment, 25% due to peripheral neuropathy. In ten patients, discontinuation was during paclitaxel therapy in patients receiving AC‐T chemotherapy. Thirty‐five patients (23%) had at least one dose reduction, including 24% due to peripheral neuropathy, seven during paclitaxel therapy in patients receiving AC‐T chemotherapy, and nine during TC therapy in patients receiving AC‐TC chemotherapy.
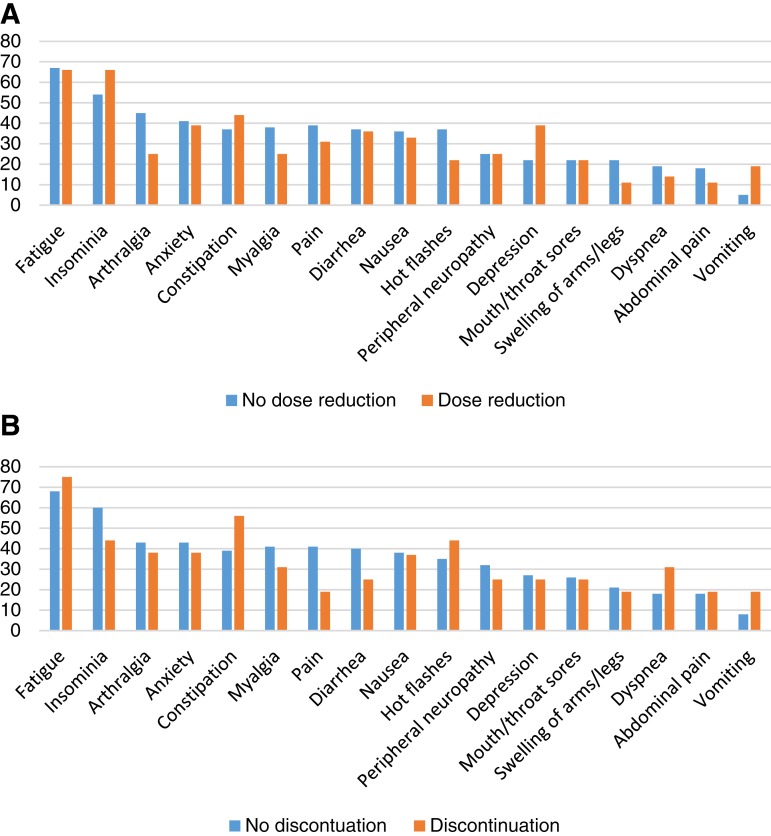
Figure 1A and B presents dose reduction and treatment discontinuation in patients reporting moderate, severe, or very severe toxicity. Significantly more patients reporting major depression had dose reduction (39%) compared with no dose reduction (22%; p = .041), and similarly for patients reporting vomiting (19% reduction compared with 5% no reduction; p = .008). Significantly fewer patients reporting major arthralgia had dose reduction (25%) compared with no reduction (45%; p = .032). All other dose reduction/no reduction and treatment discontinuation/no discontinuation pairings were not significantly different.
Figure 1.
Adverse events among patients reporting moderate/severe/very severe (MSVS) symptoms. (A): Percent of patients reporting MSVS symptoms by dose reduction and no dose reduction. (B): Percent of patients reporting MSVS symptoms by discontinuation and no discontinuation.
Conclusion
This study investigated patient‐reported toxicities recorded prospectively during neo(adjuvant) chemotherapy regimens for early breast cancer in current clinical practice. Our specific focus was on toxicities that patients rated moderate, severe, or very severe. An analysis of four commonly used chemotherapy regimens identified significant differences among the regimens in both individual symptoms and total number of symptoms rated as major toxicities. Not surprisingly, longer chemotherapy regimens, such as anthracycline‐based regimens followed by paclitaxel, had higher proportions of symptoms rated major toxicities. However, comparing the first 12 weeks of chemotherapy of the four regimens (duration of the shortest chemotherapy regimen), the total number of symptoms rated major toxicity nevertheless ranged from 4.1 to 5.7 for various regimens. Some symptoms, such as anxiety, may resolve as patients become accustomed to their chemotherapy treatment. Others—such depression, insomnia, constipation, diarrhea, and nausea—that are amenable to proven interventions, are important to monitor and address in a timely manner to improve patient satisfaction and quality of life during chemotherapy.
Regardless of regimen, ongoing patient‐reported symptom monitoring brings to light the extent to which patients perceive a variety of chemotherapy‐related symptoms as major toxicities. Patients with higher BMI, lower Patient‐KPS scores, and lengthy regimens reported a significantly greater number of severe toxicities. The finding pertaining to BMI may reflect the association between obesity and higher symptom severity for chemotherapy‐induced peripheral neuropathy that has been observed in prior studies [35], [36], [37], [38], [39].
We reviewed the breast cancer literature to identify studies that were comparable to our own, searching for prospective studies that included patient‐reported symptom toxicities collected during chemotherapy administration, instead of surveys after treatment asking patients to recall their experience. In the studies we identified, a variety of validated patient‐reported outcome measures (PROs) commonly used in symptom prevalence and severity research [40], [41] were used, such as the EORTC‐QLQ‐C30 (30 items) quality of life measure [42], [43], [44] or single‐symptom measures such as the Fatigue Scale from the Profile of Mood States [45]. The comparability of research‐oriented PRO scales to the single‐item PRO‐CTCAE [10], [11], [12], [13] or the Patient Reported Symptom Monitoring [31] form used in our study for tracking multiple toxicity outcomes at the same time remains to be determined and warrants further research. With regard to clinical practice implications, an important consideration is that research‐oriented PRO measures require knowledge of scale scoring and interpretation, whereas PRO‐CTCAEs are just one item per symptom and are as easy to use and understand as CTCAEs.
We identified only one study using a multi‐symptom survey that was comparable to our study. This was a ten‐item CTCAE‐derived Italian questionnaire that was administrated after the first and third cycle of adjuvant chemotherapy [30]. Symptom incidence after the third cycle was highest for fatigue (78%), nausea (73%), dysgeusia (51%), anorexia (53%), and constipation (49%). Toxicity results were presented for all chemotherapy regimens combined, not separately for the chemotherapy regimens in the sample—5‐fluorouracil/epirubicin/ cyclophosphamide (64%), doxorubicin/cyclophosphamide or epirubicin/cyclophosphamide (22%), or docetaxel/cyclophosphamide (14%). In general, few studies report toxicities for individual chemotherapy regimens [43], and these were mostly for single‐symptom studies such as fatigue [46], [47]. A final observation regarding our search for comparable studies is that most studies limit the number of cycles at which patient‐reported symptoms are collected [30], [42], [43], [46], [47], [48] instead of aiming to collect patient reports throughout chemotherapy.
In the growing literature pertaining to patient‐reported symptom monitoring during active treatment, our study has important strengths. First, we have prospectively documented the spectrum of patient‐reported symptoms in early breast cancer throughout four commonly used chemotherapy regimens. Reflecting current clinical practice, our sample includes both anthracycline‐based and non‐anthracycline‐based regimens, and neoadjuvant as well as adjuvant chemotherapy. Our comparison of chemotherapy toxicities associated with different treatment regimens covers the entire course of treatment; however, we also conducted a sensitivity analysis limited to the first 12 weeks of chemotherapy to account for potential differences in symptom reporting that may be due to the duration of chemotherapy. Within both time frames, moderate, severe and very severe toxicities were reported by upwards of 40% of patients for multiple symptoms.
Second, our focus on 17 symptoms encouraged patients to report “no/mild” as well as more severe symptoms. The symptom reporting form used in our study has the advantage of validation in patients with cancer [31], using formatting that is familiar to clinicians (analogous to the CTCAE) and no scoring procedures that require specific knowledge of the measure (such as reverse scoring individual items or averaging a number of items to achieve an overall score). Our multisymptom report form is similar to the now readily available PRO‐CTCAE [10], [12], which was not available to us when we started our study, and which we suggest be used for patient‐reported symptom evaluation in future studies. Study participants were able to easily complete the symptom report form in a few minutes before or as they started their infusion. All of these features facilitate ease of use for patient‐reported symptom reporting in clinical practice.
A limitation of our study is that toxicity reports were collected from patients only during regularly scheduled chemotherapy visits, either biweekly or every 3 weeks. When patients were asked to report symptom severity “in the past 7 days,” the absence of weekly data collection could have resulted in under‐reporting of symptoms whose severity had declined from their peak toxicity outside the 7‐day time frame. Weekly symptom reports throughout chemotherapy would have increased the accuracy of major toxicity estimates. However, if symptom reporting were to be incorporated into clinical practice, our schedule of patient reports every other week or every third week during chemotherapy infusion would be feasible unless symptom reports were captured electronically during nontreatment weeks [21], [49], [50].
Another potential limitation is that patients receiving shorter regimens (e.g., TC) had less time to report symptom severity compared with patients with longer regimens. To address this concern, we presented data on both full treatment duration and the first 12 weeks of treatment (Table 2). During full treatment, severity differed significantly among regimens for seven symptoms (fatigue, constipation, myalgia, diarrhea, nausea, peripheral neuropathy, and swelling of arms/legs); within the 12‐week time frame, all toxicity levels remained significantly different by regimen, except myalgia and peripheral neuropathy.
Prior studies have reported how PRO‐CTCAE coupled with clinician alerts throughout treatment can trigger early interventions that, in turn, reduce adverse events such as hospitalizations, visits to the emergency room, and early discontinuation [28] and even improve overall survival in patients with metastatic disease [51]. Our study did not include alerts to oncology providers or nurses. However, we note that primary reasons for hospitalizations in our sample were neutropenic/other fever and infections—conditions that are not likely to be apparent from the 17 symptoms monitored in our study. The symptom of numbness/tingling in hands/feet is directly related to 25% of the treatment discontinuations and 24% of the dose reductions due to peripheral neuropathy. However, other prevalent reasons for these two adverse events pertain to neutropenic fever and blood counts—again, not apparent in the other 16 symptoms.
In general, we found that treatment discontinuation/continuation rates and dose reduction/no reduction rates were not significantly different even when patients rated their symptoms moderate to very severe. Patients appear to cope with multiple treatment toxicities, perhaps to ensure treatment efficacy through full completion of the treatment plan.
Patient‐reported symptom severity reflects overall quality of life during treatment and a patient's treatment experience but may also reveal the need for physician‐guided palliative intervention. The inclusion of patient perspectives on how treatment affects their quality of life [8] has the potential for improving symptom management, patient satisfaction, and long‐term clinical outcomes, including overall survival [28], [51], [52]. Capturing multiple toxicity outcomes in one report at multiple time points during chemotherapy enables oncologists and their patients to understand the range of side effects as they discuss treatment efficacies [40].
The chemotherapy regimens used in early breast cancer have major differences in toxicities [53], [54], [55]. For clinicians and patients these differences are of major importance as they may influence treatment decisions [56]. Future research should focus on how best to integrate an understanding of patient‐reported toxicity experiences with different chemotherapy regimens into patient‐centered decision‐making and care during chemotherapy, in ways that are feasible and meaningful to both patients and providers.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We greatly appreciate the active support of oncology clinicians and their research staff at multiple sites and, most importantly, the patients with breast cancer participating in our study. The sites are Duke University Medical Center/Cancer Institute, Ohio State University Comprehensive Cancer Center, MD Anderson Cancer Center, UNC Rex Healthcare, and UNC Cancer Center. We also thank Tucker Brenizer, Nicole Markowski, Nora Christopher, Emily Bell, Chad Wagoner, Will Pulley, Nancy Burns, Mary Watson, and Amy Garrett for their unwavering commitment to study implementation best practices. This study was supported by the Breast Cancer Research Foundation (New York, NY), the Kay Yow Cancer Fund (Raleigh, NC), and the UNC Lineberger Comprehensive Cancer Center/University Cancer Research Fund (Chapel Hill, NC).
Author Contributions
Conception/design: Kirsten A. Nyrop, Allison M. Deal, Hyman B. Muss
Provision of study material or patients: Carey K. Anders, Lisa A. Carey, Elizabeth C. Dees, Trevor A. Jolly, Katherine E. Reeder‐Hayes, Gretchen G. Kimmick, Meghan S. Karuturi, Raquel E. Reinbolt, JoEllen C. Speca, Hyman B. Muss
Collection and/or assembly of data: Kirsten A. Nyrop, Carey K. Anders, Lisa A. Carey, Elizabeth C. Dees, Trevor A. Jolly, Katherine E. Reeder‐Hayes, Gretchen G. Kimmick, Meghan S. Karuturi, Raquel E. Reinbolt, JoEllen C. Speca, Hyman B. Muss
Data analysis and interpretation: Allison M. Deal, Seul Ki Choi
Manuscript writing: Kirsten A. Nyrop, Shlomit S. Shachar, Hyman B. Muss
Final approval of manuscript: Kirsten A. Nyrop, Allison M. Deal, Shlomit S. Shachar, Ethan Basch, Bryce B. Reeve, Seul Ki Choi, Jordan T. Lee, William A. Wood, Carey K. Anders, Lisa A. Carey, Elizabeth C. Dees, Trevor A. Jolly, Katherine E. Reeder‐Hayes, Gretchen G. Kimmick, Meghan S. Karuturi, Raquel E. Reinbolt, JoEllen C. Speca, Hyman B. Muss
Disclosures
Ethan Basch: Noona Healthcare, Sivan Healthcare (C/A), PCORI (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Cancer Stat Facts: Female Breast Cancer . National Cancer Institute website. https://seer.cancer.gov/statfacts/html/breast/html. Accessed November 7, 2018.
- 2.DeSantis CE, Ma J, Goding Sauer A et al. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67:439–448. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi S, Fletcher GG, Eisen A et al. Adjuvant chemotherapy for early female breast cancer: A systematic review of the evidence for the 2014 Cancer Care Ontario systemic therapy guideline. Curr Oncol 2015;22(suppl 1):S82–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gradishar WJ, Anderson BO, Balassanian R et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:310–320. [DOI] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group E (EBCTCG) . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: An overview of the randomised trials. Lancet 2005;365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute . Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE). http://healthcaredelivery.cancer.gov/pro-ctcae. Accessed November 7, 2018.
- 7.Gwede CK, Johnson DJ, Daniels SS et al. Assessment of toxicity in cooperative oncology clinical trials: The long and short of it. J Oncol Manag 2002;11:15–21. [PubMed] [Google Scholar]
- 8.Basch E, Jia X, Heller G et al. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J Natl Cancer Inst 2009;101:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruner DW, Movsas B, Basch E. Capturing the patient perspective: Patient‐reported outcomes as clinical trial endpoints. Am Soc Clin Oncol Educ Book 2012:139–144. [DOI] [PubMed]
- 10.Basch E, Reeve BB, Mitchell SA et al. Development of the National Cancer Institute's patient‐reported outcomes version of the common terminology criteria for adverse events (PRO‐CTCAE). J Natl Cancer Inst 2014;106(9):dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza TR, Dueck AC, Bennett AV et al. Evaluation of different recall periods for the US National Cancer Institute's PRO‐CTCAE. Clin Trials 2017;14:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay JL, Atkinson TM, Reeve BB et al. Cognitive interviewing of the US National Cancer Institute's Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE). Qual Life Res 2014;23:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dueck AC, Mendoza TR, Mitchell SA et al. Validity and reliability of the US National Cancer Institute's Patient‐Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE). JAMA Oncol 2015;1:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basch EM, Reeve BB, Mitchell SA et al. Electronic toxicity monitoring and patient‐reported outcomes. Cancer J 2011;17:231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basch E, Dueck AC, Rogak LJ et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol 2017;3:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basch E, Pugh SL, Dueck AC et al. Feasibility of patient reporting of symptomatic adverse events via the Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE) in a chemoradiotherapy cooperative group multicenter clinical trial. Int J Radiat Oncol Biol Phys 2017;98:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basch E, Rogak LJ, Dueck AC. Methods for implementing and reporting patient‐reported outcome (PRO) measures of symptomatic adverse events in cancer clinical trials. Clin Ther 2016;38:821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett AV, Dueck AC, Mitchell SA et al. Mode equivalence and acceptability of tablet computer‐, interactive voice response system‐, and paper‐based administration of the U.S. National Cancer Institute's Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE). Health Qual Life Outcomes 2016;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Maio M, Basch E, Bryce J et al. Patient‐reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol 2016;13:319–325. [DOI] [PubMed] [Google Scholar]
- 20.Cowan RA, Suidan RS, Andikyan V et al. Electronic patient‐reported outcomes from home in patients recovering from major gynecologic cancer surgery: A prospective study measuring symptoms and health‐related quality of life. Gynecol Oncol 2016;143:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeksted C, Pappot H, Nissen A et al. Feasibility and acceptability of electronic symptom surveillance with clinician feedback using the Patient‐Reported Outcomes version of Common Terminology Criteria for Adverse Events (PRO‐CTCAE) in Danish prostate cancer patients. J Patient Rep Outcomes 2017;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basch E, Wood WA, Schrag D et al. Feasibility and clinical impact of sharing patient‐reported symptom toxicities and performance status with clinical investigators during a phase 2 cancer treatment trial. Clin Trials 2016;13:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Judson TJ, Bennett AV, Rogak LJ et al. Feasibility of long‐term patient self‐reporting of toxicities from home via the Internet during routine chemotherapy. J Clin Oncol 2013;31:2580–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stover AM, Basch EM. Implementation of symptom questionnaires into oncology workflow. J Oncol Pract 2016;12:859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung AE, Basch EM. Incorporating the patient's voice into electronic health records through patient‐reported outcomes as the “review of systems.” J Am Med Inform Assoc 2015;22:914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basch E. Missing patients’ symptoms in cancer care delivery‐‐The importance of patient‐reported outcomes. JAMA Oncol 2016;2:433–434. [DOI] [PubMed] [Google Scholar]
- 27.Basch E. New frontiers in patient‐reported outcomes: Adverse event reporting, comparative effectiveness, and quality assessment. Annu Rev Med 2014;65:307–317. [DOI] [PubMed] [Google Scholar]
- 28.Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 2016;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falchook AD, Tracton G, Stravers L et al. Use of mobile device technology to continuously collect patient‐reported symptoms during radiation therapy for head and neck cancer: A prospective feasibility study. Adv Radiat Oncol 2016;1:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montemurro F, Mittica G, Cagnazzo C et al. Self‐evaluation of adjuvant chemotherapy‐related adverse effects by patients with breast cancer. JAMA Oncol 2016;2:445–452. [DOI] [PubMed] [Google Scholar]
- 31.Reeve BB, Mitchell SA, Dueck AC et al. Recommended patient‐reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst 2014;106:dju129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkinson TM, Hay JL, Dueck AC et al. What do “none,” “mild,” “moderate,” “severe,” and “very severe” mean to patients with cancer? Content validity of PRO‐CTCAE response scales. J Pain Symptom Manage 2018;55:e3–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loprinzi CL, Laurie JA, Wieand HS et al. Prospective evaluation of prognostic variables from patient‐completed questionnaires. North Central Cancer Treatment Group. J Clin Oncol 1994;12:601–607. [DOI] [PubMed] [Google Scholar]
- 34.American Joint Commission on Cancer . AJCC Cancer Staging Manual. 7th ed Chicago, IL: American Joint Commission on Cancer, 2010. [Google Scholar]
- 35.Bandos H, Melnikow J, Rivera DR et al. Long‐term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B‐30. J Natl Cancer Inst 2018;110:djx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckhoff L, Feddersen S, Knoop AS et al. Docetaxel‐induced neuropathy: A pharmacogenetic case‐control study of 150 women with early‐stage breast cancer. Acta Oncol 2015;54:530–537. [DOI] [PubMed] [Google Scholar]
- 37.Mustafa Ali M, Moeller M, Rybicki L et al. Long‐term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat 2017;166:519–526. [DOI] [PubMed] [Google Scholar]
- 38.Song SJ, Min J, Suh SY et al. Incidence of taxane‐induced peripheral neuropathy receiving treatment and prescription patterns in patients with breast cancer. Support Care Cancer 2017;25:2241–2248. [DOI] [PubMed] [Google Scholar]
- 39.Bao T, Basal C, Seluzicki C et al. Long‐term chemotherapy‐induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res Treat 2016;159:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reilly CM, Bruner DW, Mitchell SA et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer 2013;21:1525–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkinson TM, Stover AM, Storfer DF et al. Patient‐reported physical function measures in cancer clinical trials. Epidemiol Rev 2017;39:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Browall M, Ahlberg K, Karlsson P et al. Health‐related quality of life during adjuvant treatment for breast cancer among postmenopausal women. Eur J Oncol Nurs 2008;12:180–189. [DOI] [PubMed] [Google Scholar]
- 43.Hall E, Cameron D, Waters R et al. Comparison of patient reported quality of life and impact of treatment side effects experienced with a taxane‐containing regimen and standard anthracycline based chemotherapy for early breast cancer: 6 year results from the UK TACT trial (CRUK/01/001). Eur J Cancer 2014;50:2375–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckhoff L, Knoop A, Jensen MB et al. Persistence of docetaxel‐induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Cancer 2015;51:292–300. [DOI] [PubMed] [Google Scholar]
- 45.Jacobsen PB, Hann DM, Azzarello LM et al. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlates. J Pain Symptom Manage 1999;18:233–242. [DOI] [PubMed] [Google Scholar]
- 46.de Jong N, Candel MJ, Schouten HC et al. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol 2004;15:896–905. [DOI] [PubMed] [Google Scholar]
- 47.Junghaenel DU, Cohen J, Schneider S et al. Identification of distinct fatigue trajectories in patients with breast cancer undergoing adjuvant chemotherapy. Support Care Cancer 2015;23:2579–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanford SD, Beaumont JL, Butt Z et al. Prospective longitudinal evaluation of a symptom cluster in breast cancer. J Pain Symptom Manage 2014;47:721–730. [DOI] [PubMed] [Google Scholar]
- 49.Basch E, Artz D, Dulko D et al. Patient online self‐reporting of toxicity symptoms during chemotherapy. J Clin Oncol 2005;23:3552–3561. [DOI] [PubMed] [Google Scholar]
- 50.Basch E, Artz D, Iasonos A et al. Evaluation of an online platform for cancer patient self‐reporting of chemotherapy toxicities. J Am Med Inform Assoc 2007;14:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basch E, Deal AM, Dueck AC et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neugut AI, Hillyer GC, Kushi LH et al. A prospective cohort study of early discontinuation of adjuvant chemotherapy in women with breast cancer: The breast cancer quality of care study (BQUAL). Breast Cancer Res Treat 2016;158:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah AN, Gradishar WJ. Adjuvant anthracyclines in breast cancer: What is their role? The Oncologist 2018;23:1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barcenas CH, Niu J, Zhang N et al. Risk of hospitalization according to chemotherapy regimen in early‐stage breast cancer. J Clin Oncol 2014;32:2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muss HB, Berry DA, Cirrincione C et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node‐positive breast cancer: The Cancer and Leukemia Group B experience. J Clin Oncol 2007;25:3699–3704. [DOI] [PubMed] [Google Scholar]
- 56.Crozier JA, Swaika A, Moreno‐Aspitia A. Adjuvant chemotherapy in breast cancer: To use or not to use, the anthracyclines. World J Clin Oncol 2014;5:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]