This brief communication analyzes the long‐term efficacy and safety of a modified PET regimen in five cases of metastatic extramammary Paget's disease.
Abstract
Extramammary Paget's disease (EMPD) is a rare cutaneous adenocarcinoma that clinicopathologically resembles breast cancer. The prognosis of metastatic EMPD is poor. Although several chemotherapies have been tried, the effects are temporary; better drugs and combinations are required.
In the present study, we retrospectively analyze the efficacy and safety of combination of cisplatin, epirubicin, and paclitaxel in five metastatic EMPD cases. The efficacy was better than that for previously reported regimens: 80% partial responses, including two patients who were refractory to taxane‐ and/or platinum‐based regimens. In terms of safety, four patients who were able to continue treatment exhibited acceptable tolerability.
This is the first regimen to combine taxane and anthracycline. When treating breast cancer, anthracycline is regarded as the key cytotoxic agent, and anthracycline in combination with taxane constitutes a key chemotherapeutic regimen. Given our results, we speculate both drugs are critical chemotherapeutic agents for the treatment of metastatic EMPD.
Introduction
Extramammary Paget's disease (EMPD) is a rare intraepithelial adenocarcinoma that principally affects the genital and axillary regions. Although the pathogenesis remains to be clarified, it has been reported to resemble breast cancer (BC) in immunohistochemical and molecular profiles [1]. As EMPD typically grows slowly and is diagnosed in situ, the prognosis after surgical resection is generally favorable. However, once EMPD invades the dermis, the tumor readily metastasizes and the prognosis becomes extremely poor [2].
In terms of metastatic EMPD treatment, the effectiveness of several chemotherapeutic regimens has been described in case reports and small retrospective studies, including docetaxel (DTX) monotherapy, low‐dose 5‐fluorouracil‐plus‐cisplatin (FP), the 5‐fluorouracil, epirubicin, carboplatin, vincristine, and mitomycin (FECOM) combination, and the DTX + S‐1 regimen [3], [4], [5], [6]. Although these therapies shrink tumors in certain patient populations, the effects are usually temporary; further evaluation of other chemotherapeutic drugs, alone and in combination, is necessary.
A combination of CDDP, EPI and TXL therapy (PET) involves weekly administration of cisplatin (CDDP), epirubicin (EPI), and paclitaxel (TXL) and was developed in 2000 to treat metastatic BC [7]. In a phase II study of 68 patients with metastatic BC, the overall response rate (RR) was 87%, including 21 complete responses [7]. Based on the efficacy of PET and the pathobiological resemblance between EMPD and BC, we earlier adapted the PET regimen to treat two patients with metastatic EMPD; we modified the dosing intervals, and the regimen yielded partial responses (PRs) with acceptable tolerability [8]. Here, we retrospectively analyze the long‐term efficacy and safety of our modified PET regimen in five cases of metastatic EMPD.
Materials and Methods
This study was approved by the Ethics Committee of Keio University School of Medicine. Five metastatic EMPD cases treated via PET at Keio University Hospital from 2007 to 2018 were included. All patients received CDDP (30 mg/m2), EPI (50 mg/m2), and TXL (120 mg/m2) per cycle. The treatment intervals were modified and the doses decreased to 80% if necessary, depending on the severity of toxicity. Clinical data were retrieved from medical records. Clinical efficacy was assessed using the Response Evaluation Criteria in Solid Tumors, version 1.1; overall survival (OS) was the time from initiation of treatment to death. Adverse events were graded using the Common Terminology Criteria for Adverse Events, version 4.0.
Results
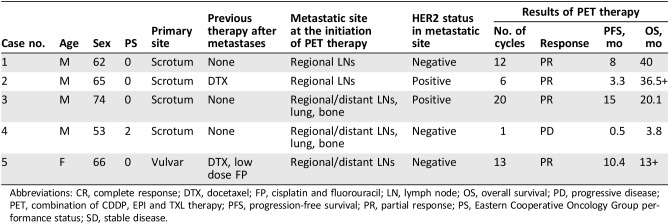
Patient characteristics are summarized in Table 1. The primary tumors were on the perineal or scrotal areas of all patients. Expression of HER2 was positive in 40% (2/5) of metastatic tumor samples. PET served as first‐line therapy for three patients, second‐line therapy for one patient who had failed DTX monotherapy, and third‐line therapy for one patient who was refractory to DTX monotherapy followed by failure of a low‐dose FP regimen. The dosing schedules were biweekly for patient no. 3, 2 weeks on/2 weeks off for patients no. 1 and 2, and triweekly for patient no. 5; patient no. 4 terminated treatment after the first cycle because of disease progression. Four patients exhibited PR after five cycles of treatment. The median OS and progression‐free survival (PFS) were 20.1 and 8 months, respectively; one case (no. 2) continues to exhibit a PR at the time of writing (Table 1). In terms of toxicity, four patients experienced adverse events (AEs) of grade 3 or 4; three required dose reductions. Hematotoxicity was observed in all five patients, including neutropenia (grade 3, n = 3; grade 4, n = 1), anemia (grade 1 or 2, n = 3), and thrombocytopenia (grade 4, n = 1; Table 2). Neutropenia was managed by administration of granulocyte colony‐stimulating factor (G‐CSF). All other AEs were of grade 1 or 2; no patient developed cardiac toxicity. All toxicities were minor, and no treatment‐related death was recorded.
Table 1. Patient characteristics and the outcomes of PET treatment.
Abbreviations: CR, complete response; DTX, docetaxel; FP, cisplatin and fluorouracil; LN, lymph node; OS, overall survival; PD, progressive disease; PET, combination of CDDP, EPI and TXL therapy; PFS, progression‐free survival; PR, partial response; PS, Eastern Cooperative Oncology Group performance status; SD, stable disease.
Table 2. Results of PET therapy.
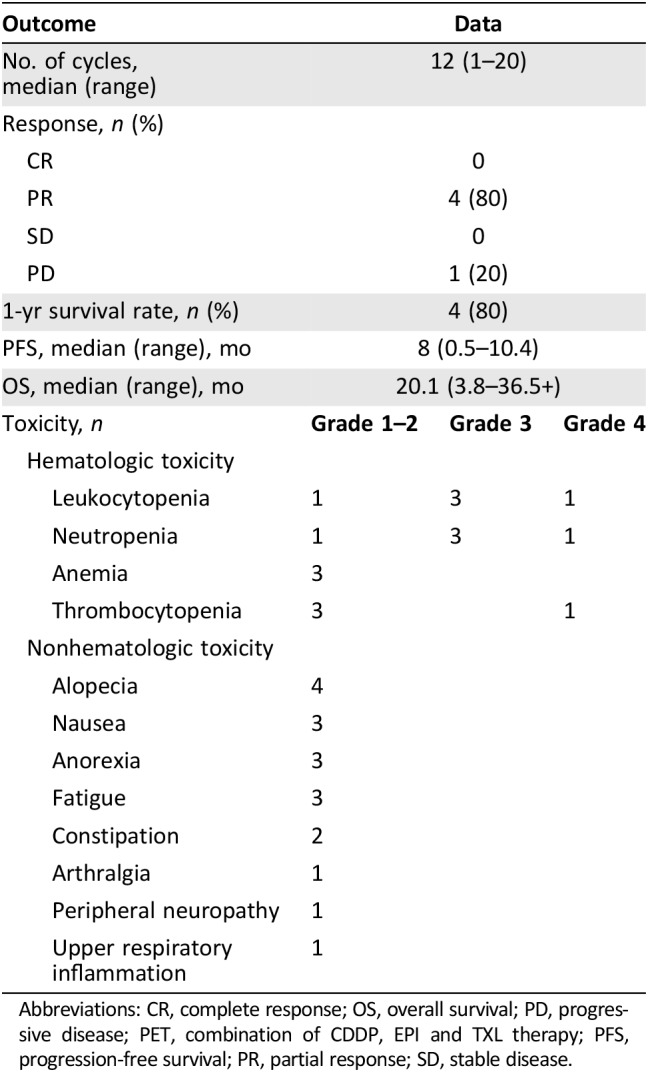
Abbreviations: CR, complete response; OS, overall survival; PD, progressive disease; PET, combination of CDDP, EPI and TXL therapy; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Discussion
Currently, no consensus on the optimal chemotherapeutic regimen for treatment of metastatic EMPD has been attained because of its rareness and lack of clinical trials. Of the regimens tested, low‐dose FP and the FECOM regimen (given as first‐line treatments) showed RRs of 59% and 57% and median PFSs of 5.2 and 6.5 months, respectively [3], [4]. However, the median OSs were less than 1 year. Furthermore, use of these drug combinations requires repeated hospitalization, compromising patient quality of life. Thus, DTX monotherapy is the preferred first‐line treatment based on its lower toxicity and the certain tumor shrinkage effect (RR of 58%). However, its efficacy is not durable, and the reported median PFS and OS were 7.1 and 16.6 months, respectively [5]. Of our five patients receiving PET, four patients showed PR. The median PFS and OS were 8 and 20.1 months, respectively. Although we included only a small number of cases because of the rarity of metastatic EMPD, PET therapy was more effective than the previously reported regimens.
PET therapy differs from previous regimens principally by combining taxane with anthracycline. In patients with BC, the RRs for chemotherapy regimens featuring anthracycline were better than those for regimens lacking anthracycline, which was thus regarded as the key cytotoxic agent [9]. In particular, addition of taxane to anthracycline improved OS; this combination is now considered to be a key therapeutic regimen [10]. Considering the molecular biological similarity between BC and EMPD, not only taxane and fluoropyrimidine but also anthracycline may be critical to counter metastatic EMPD; the combination may further benefit patients. In support of this, patients no. 2 and 5, who were refractory to taxane monotherapy or fluoropyrimidine‐based therapy, evidenced PRs to the taxane/anthracycline combination.
In terms of safety, all four patients who were able to continue treatment showed acceptable tolerability and manageable toxicity. The major safety concern associated with the original weekly PET regimen was hematological toxicity requiring hospital‐based G‐CSF support for 3 consecutive days of every cycle [7]. After adjustment of the dosing interval, patients were able to continue treatment on an outpatient basis, without experiencing severe toxicity.
Conclusion
PET therapy is the first regimen to combine anthracycline and taxane and was highly and consistently effective in patients with metastatic EMPD compared with previously reported regimens. Modification of the dosing interval reduced toxicity and patients continued treatment on an outpatient basis. Further exploration of the efficacy and safety of PET therapy is required.
Acknowledgments
This research was supported by Japan Agency for Medical Research and Development under Grant Number JP17lk0201063.
Disclosures
The authors indicated no financial relationships.
References
- 1.Tessier‐Cloutier B, Asleh‐Aburaya K, Shah V et al. Molecular subtyping of mammary‐like adenocarcinoma of the vulva shows molecular similarity to breast carcinomas. Histopathology 2017;71:446–452. [DOI] [PubMed] [Google Scholar]
- 2.Ohara K, Fujisawa Y, Yoshino K et al. Proposal for a TNM staging system for extramammary Paget disease: Retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci 2016;83:234–239. [DOI] [PubMed] [Google Scholar]
- 3.Tokuda Y, Arakura F, Uhara H. Combination chemotherapy of low‐dose 5‐fluorouracil and cisplatin for advanced extramammary Paget's disease. Int J Clin Oncol 2015;20:194–197. [DOI] [PubMed] [Google Scholar]
- 4.Oashi K, Tsutsumida A, Namikawa K et al. Combination chemotherapy for metastatic extramammary Paget disease. Br J Dermatol 2014;170:1354–1357. [DOI] [PubMed] [Google Scholar]
- 5.Yoshino K, Fujisawa Y, Kiyohara Y et al. Usefulness of docetaxel as first‐line chemotherapy for metastatic extramammary Paget's disease. J Dermatol 2016;43:633–637. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita S, Yonekura K, Mera K et al. Successful treatment of metastatic extramammary Paget's disease with S‐1 and docetaxel combination chemotherapy. J Dermatol 2011;38:996–998. [DOI] [PubMed] [Google Scholar]
- 7.Frasci G, D'Aiuto G, Comella P et al. Cisplatin‐epirubicin‐paclitaxel weekly administration with G‐CSF support in advanced breast cancer. A Southern Italy Cooperative Oncology Group (SICOG) phase II study. Breast Cancer Res Treat 2000;62:87–97. [DOI] [PubMed] [Google Scholar]
- 8.Hirai I, Funakoshi T. Modified weekly regimen of cisplatin, epirubicin and paclitaxel induced a durable response in two cases of metastatic extramammary Paget's disease. J Dermatol 2017;44:1148–1151. [DOI] [PubMed] [Google Scholar]
- 9.Fossati R, Confalonieri C, Torri V et al. Cytotoxic and hormonal treatment for metastatic breast cancer: A systematic review of published randomized trials involving 31,510 women. J Clin Oncol 1998;16:3439–3460. [DOI] [PubMed] [Google Scholar]
- 10.De Laurentiis M, Cancello G, D'Agostino D et al. Taxane‐based combinations as adjuvant chemotherapy of early breast cancer: A meta‐analysis of randomized trials. J Clin Oncol 2008;26:44–53. [DOI] [PubMed] [Google Scholar]