This report describes results of a study that compared the clinicopathological features of young patients with gastric cancer in China and the U.S. and evaluated the dynamic survival of patients in the two regions, focusing on whether these factors predicted the survival of younger patients after resection over time.
Keywords: Gastric cancer, Young survivors, Conditional survival, China, U.S.
Abstract
Background.
Young survivors of gastric cancer (GC) have better prognoses than elderly patients, yet their disease‐specific survival (DSS) has received little attention.
Patients and Methods.
Data on young patients (aged ≤40 years) with GC undergoing resections at three Chinese institutions (n = 542) and from the SEER database (n = 533) were retrospectively analyzed. Three‐year conditional disease‐specific survival (CS3) was assessed. The effects of well‐known prognostic factors over time were analyzed by time‐dependent Cox regression.
Results.
Overall, young Chinese patients with GC had a better 5‐year DSS than U.S. patients (62.8% vs. 54.1%; p < .05). The disease‐specific mortality likelihood of the entire cohort was not constant over time, with most deaths occurring during the first 3 years after surgery but peaking at 1 and 2 years in China and the U.S., respectively. Based on 5‐year survivorship, the CS3 rates of both groups were similar (90.9% [U.S.] vs. 91.5% [China]; p > .05). Cox regression showed that for Chinese patients, site, size, T stage, and N stage were independent prognostic factors at baseline (p < .05). For U.S. patients, grade, T stage. and N stage significantly affected DSS at baseline (p < .05). In both groups, only T stage continuously affected DSS within 3 years after gastrectomy. However, for both groups, the initial well‐known prognostic factors lost prognostic significance after 5 years of survival (all p > .05). Although the 5‐year DSS rates of young Chinese patients with T3 and T4a disease were significantly better than those of young U.S. patients, in each T stage, the CS3 of both regions trended toward consistency over time.
Conclusion.
For young patients with GC, the factors that predict survival at baseline vary over time. Although the initial 5‐year DSS is heterogeneous, insight into conditional survival will help clinicians evaluate the long‐term prognoses of survivors while ignoring population differences.
Implications for Practice.
With the increasing number of young survivors of gastric cancer (GC), it is essential for clinicians to understand the dynamic prognosis of these patients. Based on large data sets from China and the U.S., this study found that the prognostic factors that predict survival for young patients with GC at baseline vary over time. Although the initial 5‐year disease‐specific survival is heterogeneous, insight into conditional survival will help clinicians evaluate the long‐term prognoses of survivors while ignoring population differences. This knowledge may be more effective in helping young patients with GC to manage future uncertainties, especially when they need to make important life plans.
摘要
背景。胃癌 (GC) 的年轻幸存者比老年患者具有更好的预后,但他们的疾病特异性生存率 (DSS) 却很少受到关注。
患者和方法。回顾性分析了三个中国机构 (n = 542) 和 SEER 数据库 (n = 533) 中进行 GC 切除的年轻患者(年龄 ≤ 40岁)的数据。评估了三年条件疾病特异性生存率 (CS3)。通过时间依赖性 Cox 回归分析了已知预后因素随时间的变化。
结果。总体而言,中国的年轻 GC 患者的 5 年 DSS 优于美国患者 (62.8% vs. 54.1%; p < 0.05)。整个队列的疾病特异性死亡率可能性并非一直恒定,大多数死亡发生在手术后的前 3 年,在中国和美国的峰值分别为 1 年和 2 年。根据 5 年生存率,两组的 CS3 率相似 [90.9%(美国) vs. 91.5% (中国);p> 0.05]。Cox 回归分析显示,对于中国患者,发病位置、大小、T 分期和 N 分期是基线时的独立预后因素 (p < 0.05)。对于美国患者,分级、T 分期和 N 分期在基线时显著影响 DSS (p < 0.05)。在两组中,只有 T 分期在胃切除术后 3 年内持续影响 DSS。然而,对于这两组,最初已知的预后因素在 5 年生存期后失去预后意义(所有 p > 0.05)。虽然中国年轻 T3 和 T4a 疾病患者的 5 年 DSS 率显著优于美国年轻患者,但在每个 T 分期,两个地区的 CS3 随时间推移趋于一致。
结论。对于患有 GC 的年轻患者,预测基线存活率的因素随时间而变化。虽然最初的 5 年 DSS 不均匀,但对条件生存率的洞察将有助于临床医生在忽略人群差异时评估幸存者的长期预后。
对实践的启示:随着胃癌 (GC) 年轻幸存者数量的增加,临床医生必须了解这些患者的动态预后。基于来自中国和美国的大量数据集,本研究发现,预测基线时年轻 GC 患者存活率的预后因素随时间而变化。虽然最初的 5 年疾病特异性生存率不均匀,但对条件生存率的洞察将有助于临床医生在忽略人群差异时评估幸存者的长期预后。这些认知可以更有效地帮助年轻的 GC 患者管理未来的不确定性,特别是当他们需要制定重要的人生计划时。
Introduction
Gastric cancer (GC) is the fifth most frequently diagnosed cancer and the third leading cause of death from cancer worldwide [1], with a majority of documented incidents occurring among individuals between 50 and 70 years of age [2], [3] and a rarely reported incidence among patients 40 years of age or younger [4], [5]. Although the overall incidence of GC has decreased over recent decades, many epidemiological surveys in the East and West have indicated that the incidence of GC among young patients is increasing [2], [6], [7]. For young patients, GC is still a serious health problem because even in areas with high incidences of GC, no regular screening programs include young people under 40 years of age. Meanwhile, because of a lack of obvious symptoms and rapid disease progression, diagnosis and treatment are often delayed in young patients with GC [8].
The clinicopathological features of young patients with GC are different from those of middle‐aged and elderly patients [4], [5]. GC in young patients has a more aggressive biological behavior than in middle‐aged and elderly patients. Because of the advanced stage of GC in young patients, undifferentiated and diffuse tumors are often predominant at diagnosis, and the prognosis is poor. However, recent studies have shown that after adjusting for the tumor stage, the survival of young patients with GC is not inferior to the survival of elderly patients and may actually be better [9], [10].
Until recently, little information has been available in the literature regarding the tumor characteristics and prognoses of GC among young patients in Eastern versus Western countries. More importantly, an assessment of survival changes in young survivors of GC has not been reported. Previous studies have shown that the disease‐specific survival (DSS) of patients with GC is not uniform over time; as the survival time is prolonged, the prognosis is improved [11], [12], [13]. The notion that the risk of tumor‐specific mortality decreases with the prolongation of the postoperative survival time provides a theoretical basis for the determination of whether survivors will reach a “cure” state, which is essential for young patients with GC and their families. Unlike static survival estimates, conditional survival (CS) can explain the dynamic changes in survival possibilities over time after surgery. Thus, CS is a better clinical indicator and can be used to predict the long‐term prognoses of GC survivors. Current oncology studies, including lung cancer, renal cell cancer, adrenal cortical tumors, colon cancer, and other tumors [11], [14], [15], [16], [17], [18], have reported that with prolonged survival, patients who were initially considered to have poor clinical pathological characteristics of prognosis can even achieve the state of “cured,” similar to those patients with good initial relative prognostic characteristics. Therefore, we hypothesized that the well‐known factors evaluated in the current staging system and nomograms would have a limited ability to predict CS in young patients with GC after curative‐intent gastrectomy. Prognostic factors that have a strong impact on DSS at baseline may weaken or even lose their differentiating abilities over time. Therefore, based on large data sets, this study not only compared the clinicopathological features of young patients with GC in China and the U.S. but also evaluated the dynamic survival of young survivors of GC in the two regions. Moreover, we aimed to determine whether these factors predicted the survival of young patients with GC after resection over time.
Subjects, Materials, and Methods
Population and Covariates
Data were selected from a multi‐institutional cohort comprising patients who underwent surgical resections between 2000 and 2012 from three high‐volume GC centers in China (Fujian Medical University Union Hospital, Sun Yat‐sen University Cancer Center, and Sun Yat‐sen University Sixth Hospital). Additionally, data from the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2012 in the U.S. were collected. The institutional review boards of all participating institutions approved the study.
Well‐known clinicopathological data were routinely collected. T stage and N stage were classified according to the criteria described in the American Joint Committee on Cancer (AJCC) staging manual (seventh and eighth editions) [19], [20]. The tumor site was divided into four subsites as follows: lower third (C16.3 and C16.4), upper third (C16.0 and C16.1), middle third (C16.2, C16.5, and C16.6), and overlapping (C16.8) [21]. The tumors were pathologically categorized as low grade (well and moderately differentiated) or high grade (poorly differentiated and undifferentiated). The histological types were categorized as general (8140–8389) and special (8440–8499). The use of adjuvant treatment was defined as receiving postoperative adjuvant chemotherapy and/or radiation.
Young patients were defined as those 40 years of age or younger. The inclusion criteria were defined as follows: the presence of primary GC, no combined malignancy, no preoperative chemotherapy and/or radiotherapy, no distant metastasis, complete basic patient information, and more than 1 month of exact survival. Exclusion criteria were defined as follows: histology showing a tumor type other than adenocarcinoma, remnant GC. The selection scheme of the SEER database is shown in supplemental online Figure 1.
Deaths from GC were coded as disease‐specific mortality. The causes of death among the SEER cohorts were defined using the SEER cause‐of‐death codes [22]. All patients from the other centers received standard postoperative follow‐up, including visits every 3–6 months for the first 2 years, every 6–12 months from the 3rd to 5th year, and annually thereafter. Most routine follow‐up appointments included a physical examination, laboratory testing, chest radiography, and abdominopelvic ultrasonography or computed tomography, and an annual endoscopic examination was also performed [23], [24], [25], [26]. All patients were observed until death or the final follow‐up date in December 2015, ensuring that at least 3 years of actual follow‐up occurred.
Conditional Survival Concept
CS, the origin of which is conditional probability in biostatistics, can be calculated using the life‐table method [14]. The 3‐year conditional disease‐specific survival (CS3) at x years indicates the likelihood of an additional 3‐year survivorship for a survivor who has already survived for x years after the initial treatment, calculated as follows: CS3 = DSS(x + 3) / DSS(x) [27]. Initial prognostic estimates for patients were usually based on individual characteristics after surgery. CS estimates were recalculated by incorporating the clinicopathological characteristics and the survival time (supplemental online Fig. 2).
Statistical Analysis
The associations between variables and DSS were assessed using a Cox proportional hazards model. CS analysis was then employed to assess possible changes in the prognostic impact of the aforementioned factors over time after resection. The difference in CS between the groups was compared via the standardized differences (d) method, which was first described by Cucchetti et al. [28] and has been employed by several groups [11], [29], where |d| < 0.1 indicates very small differences between groups, 0.1 ≤ |d| < 0.3 indicates small differences, 0.3 ≤ |d| < 0.5 indicates moderate differences, and |d| ≥ 0.5 indicates obvious differences.
The d value can represent the impact of each factor on prognosis but cannot assess the relative independence of several predictive factors. Therefore, we employed a second multivariable analysis (time‐dependent multivariable analysis) at a later time (1, 3, and 5 years after surgery) to assess the independent predictors of DSS among the patients who were alive after a certain number of postoperative years [18].
All data were processed using SPSS version 19.0 (IBM, Armonk, NY) and R software (version 3.4.3). All tests were two‐sided with a significance level set to p < .05.
Results
Demographic and Clinicopathologic Characteristics
During the 12‐year study period, a total of 6,988 Chinese patients and 14,985 U.S. patients underwent curative‐intent resections. Of these, 542 (7.8%) young Chinese patients with GC and 533 (3.6%) young U.S. patients with GC met the inclusion criteria (Table 1). The mean age at diagnosis of the young Chinese patients with GC was 34.7 years, which was less than 35.4 years of the U.S. patients (p = .017). The female‐to‐male ratio of the patients was similar between the regions (1:1 [China] vs. 1:1.04 [U.S.]; p = .735). The average numbers of examined lymph nodes were 30.9 ± 11.5 and 18.0 ± 12.1 nodes in the Chinese cohort and the U.S. cohort, respectively (p < .001). Most of the patients in the two regions had poorly differentiated tumors at baseline (88.7% [China] vs. 84.8% [U.S.]). Additionally, the mean sizes of the tumors in both cohorts were similar (5 cm; p = .813). Significant differences in histological type, site, T stage, N stage, and adjuvant treatment were found between the China and U.S. cohorts (p < .05). Regardless of whether the seventh or eighth edition of the AJCC staging system was used, advanced pathological stage disease was considerably more common in the Chinese cohort than in the U.S. cohort (p < .05).
Table 1. Demographic and clinicopathological description of the study population.

Abbreviations: AJCC, American Joint Committee on Cancer; LN, lymph node.
Traditional Actual DSS
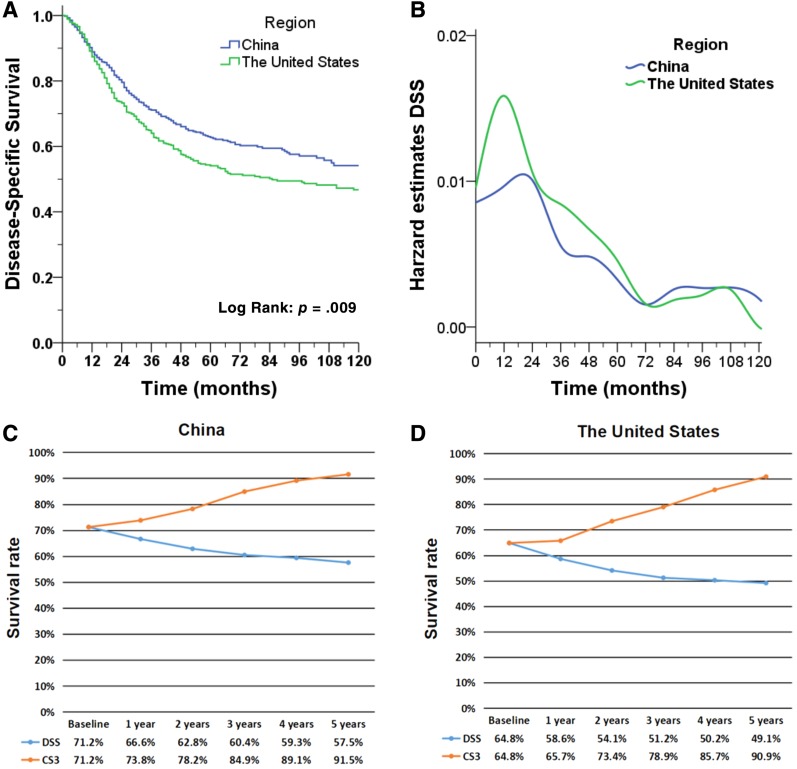
After a median follow‐up time of 78 months for the China cohort, 231 patients (42.6%) had died, and 205 of these deaths (88.7%) were attributed to GC. Among the 533 patients in the U.S. cohort, 267 died (50.1%), including 247 who died of cancer (92.5%). The survival curve (Fig. 1A) showed that the DSS of the Chinese patients was better than that of the U.S. patients (p = .009). The DSS rates of the Chinese patients were 90.3%, 71.2%, and 62.8% at 1, 3, and 5 years after surgery, respectively (supplemental online Table 1); for the U.S. cohort, the DSS probabilities were 89.1%, 64.8%, and 54.1%, respectively (supplemental online Table 2). With the prolongation of the survival time, the decrease in the DSS curve in both cohorts tended to be slow. When assessed over time, the hazard of death was not constant. For both groups, the majority of disease‐specific deaths occurred during the first 3 years after surgery but peaked at 1 year in China and 2 years in the U.S., diminishing thereafter (Fig. 1B).
Figure 1.
Survival estimates of young patients. (A): Actual disease‐specific survival (Kaplan‐Meier survival curve), (B): hazard estimates of death from GC, and (C): actual 3‐year disease‐specific survival and 3‐year conditional disease‐specific survival are compared for young Chinese patients; (D): actual 3‐year disease‐specific survival and 3‐year conditional disease‐specific survival are compared for young American patients.
Abbreviations: CS3, 3‐year conditional disease‐specific survival; DSS, disease‐specific survival.
Changes in CS over Time
As shown in Figure 1C and D, the actual DSS was compared with CS3 for young patients with GC in China and the U.S. over time. For both regions, the CS3 increased with the prolongation of postoperative survival compared with the declining trend of DSS. For China, the CS3 of the patients was 78.2% after 2 years of survival, compared with an actual DSS at 5 years of 62.8% (p < .001). After 5 years of survival, the CS3 of the patients reached 91.5%, which was significantly higher than the actual 8‐year DSS (DSS at 8 years, 57.5%; p < .001). Similarly, in the U.S., the CS3 rate at 2 years was 73.4%, in contrast to the actual 5‐year DSS of 54.1% (p < .001). After 5 years of survival, the CS3 of the patients reached 90.9%, which was significantly higher than the actual 8‐year DSS of 49.1% (p < .001).
Univariate analysis (supplemental online Table 3) showed that for young patients with GC in China and the U.S., site, size, grade, T stage, N stage, surgical procedures, and adjuvant treatment were associated with DSS at baseline (p < .05). Figure 2 and supplemental online Figure 3 show the temporal trend of DSS and CS3 for each aforementioned prognostic factor of Chinese patients. The temporal trends of DSS and CS3 for each prognostic factor of U.S. patients are demonstrated in Figure 3 and supplemental online Figure 4. Stratified analysis showed that CS3 was higher than the actual DSS for any subgroup in China or the U.S., and this gap increased with time. Notably, in both China and the U.S., the difference between the actual DSS and CS3 was more significant over time for young patients who initially had poor prognostic characteristics. Conversely, for patients with relatively good initial prognoses, the corresponding CS3 did not increase significantly. For example, for young Chinese patients with T4b‐stage GC (unadjusted hazard ratio [HR], 12.65), the 8‐year DSS was only 34.7%, but after 5 years of survival, the CS3 reached 94.6% (Δ59.9%). However, the 8‐year actual DSS for patients with T1 disease was 91.2%, and the CS3 at 5 years was 98.2% (Δ7.0%). Similarly, for T4b‐stage U.S. patients (unadjusted HR, 17.83), the 8‐year DSS was only 23.6%, but after 5 years of survival, the CS3 reached 100% (Δ76.4%). However, patients with T1 disease had 90.9% DSS probability at 8 years and the CS3 at 5 years was 100% (Δ9.1%).
Figure 2.
A comparison of actual disease‐specific survival (A, C, E, G, I) with 3‐year conditional disease‐specific survival (B, D, F, H, J) of young Chinese patients is demonstrated. Patients were stratified according to tumor grade (A, B), tumor size (C, D), primary site (E, F), T stage (G, H), and N stage (I, J).
Figure 3.
A comparison of actual disease‐specific survival (A, C, E, G, I) with 3‐year conditional disease‐specific survival (B, D, F, H, J) of young U.S. patients is demonstrated. Patients were stratified according to tumor grade (A, B), tumor size (C, D), primary site (E, F), T stage (G, H), and N stage (I, J).
Meanwhile, the changes in CS3 were more pronounced in the patient subgroups that initially had the least favorable tumor characteristics (i.e., larger tumors, poor histological grade, advanced T stage, and advanced N stage). We also found that the difference in CS3 between subgroups gradually decreased over time. For example, for young Chinese patients with GC, the d value of CS3 between AJCC seventh edition stage I and stage III decreased from 0.84 (obvious differences) at baseline to 0.50 at 3 years and 0.26 at 5 years after surgery (small differences). Similar results were evident between AJCC eighth edition stage I and stage III, and CS3 also gradually changed from obvious differences at baseline (|d| = 0.91) to moderate differences after 5 years (|d| = 0.39) in U.S. patients. These findings suggest that patients with poor initial prognoses can gain similar survival benefits to those of patients with better initial prognoses after a period of survival.
Analysis of Prognostic Factors over Time
Time‐dependent multivariable analysis was performed to evaluate whether all prognostic factors could influence the DSS independently and continuously (Table 2, supplemental online Table 5). At the time of surgery, primary site, tumor size, T stage, and N stage were independent prognostic factors for young Chinese patients with GC (p < .05). After 1 year of survival, the tumor size no longer independently affected DSS (p > .05), whereas site, T stage, and N stage remained significant predictors of DSS. In patients who survived 3 years after surgery, only T stage remained an independent prognostic factor (p < .05). Notably, T stage lost prognostic significance after 5 years of survival. For young U.S. patients, high tumor grade, advanced T stage, and advanced N stage predicted worse DSS at baseline. However, after 1 year of survival, grade no longer predicted future DSS. Additionally, in patients who survived 3 years after surgery, advanced T stage was the only predictor of worse survival (p < .05). After 5 years of survival, as observed with the Chinese patients for whom T stage was no longer a significant predictor of DSS, advanced T stage was no longer a predictor.
Table 2. Time‐dependent multivariate analysis of the prognostic factors for young patients with gastric cancer.

With consideration of all significantly important prognostic factors in univariate analysis at baseline, including primary site, tumor size, grade, T stage, N stage, surgical procedures, and adjuvant treatment.
Abbreviation: HR, hazard ratio.
Subgroup analysis (Fig. 4) of the two groups showed that there were no significant differences in 5‐year DSS between the Chinese and U.S. groups for patients with stage T1, T2, and T4b disease. However, the 5‐year DSS rates of the Chinese patients with T3 and T4a disease were significantly better than those of U.S. patients. However, in each T stage, the CS3 of the two regions tended to be consistent over time.
Figure 4.
Actual disease‐specific survival of both regions stratified by T1 stage (A), T2 stage (C), T3 stage (E), T4a stage (G), and T4b stage (I); 3‐year conditional disease‐specific survival stratified by T1 stage (B), T2 stage (D), T3 stage (F), T4a stage (H), and T4b stage (J).
Discussion
Over the last half century, the GC incidence has stably declined in most populations worldwide. Nevertheless, the incidence of GC among young patients has increased [2], [4], [6], [7], [30]. The various potential reasons include the emergence of new carcinogens [31], changes in microbial flora [32], [33], the increased intake of foods high in salt, and the consumption of a high‐fat diet [34].
The clinical and prognostic characteristics of young patients with GC are still controversial. As in previous reports [4], [9], [30], we observed that the female‐to‐male ratio in the diagnosis of young patients with GC in China and the U.S. was approximately 1:1, which was significantly higher than the ratio for middle‐aged and elderly patients [35]. Additionally, most of the young patients with GC in China and the U.S. had poorly differentiated and undifferentiated tumors. Moreover, lower GCs were more common in Chinese patients, and upper GCs were more common in U.S. patients, which may be associated with the high incidence of gastroesophageal reflux and obesity in the U.S. population [36], [37].
Currently, upper gastrointestinal endoscopic screening has not been popularized in China, especially in rural areas, resulting in many patients being diagnosed only after experiencing severe cancer‐related symptoms [38]. This may be why more T4 tumors and lymph node metastasis (N3) were found in Chinese patients than in U.S. patients. Therefore, we believe that upper gastrointestinal endoscopy is helpful for the early detection of GC. Generally, compared with elderly patients with GC, young patients have a better physical status and can better tolerate surgery and postoperative chemotherapy, with a relatively low incidence of comorbidities and surgery‐related complications. Non‐tumor‐related deaths also rarely occur in young patients with GC. Our study inferred that the Chinese population had higher 5‐year OS and DSS than the U.S. group, even at a later stage, which may be related to population differences [39], [40].
The National Comprehensive Cancer Network and Japanese Gastric Cancer Association recommend surgery as the only curative treatment for GC [41], [42]. However, some differences in the surgical pattern exist between the East and West. Gastrectomy with D2 lymphadenectomy is the standard treatment for curable GC in eastern Asia. In Western countries, extended lymph node dissection of distant lymph nodes contributes to accurate staging of the disease, but the role of prolonging survival remains unclear. Therefore, D2 lymphadenectomy is only recommended in the West instead of therapeutic norms [43], [44]. The number of lymph nodes retrieved in the Chinese population of young patients with GC was significantly higher than in the U.S. population in the current study.
Although there are some differences in the initial clinicopathological features of young patients with GC in China and the U.S., the CS3 of both groups was over 90% after 5 years of survival, which was astonishingly consistent. A few studies have shown that DSS in patients with GC after surgery is not constant [12], [13], [45]; as disease‐free survival is prolonged, the prognosis of patients will gradually improve. However, these studies evaluate the overall population of gastric cancer, and there are no reports on assessing survival changes in young survivors of GC. With the increasing number of survivors of GC, especially young patients [46], [47], understanding the increased likelihood of survival over time will be important, as the overall survival of young patients is longer than that of older patients and is of greater social and family significance. Young patients with cancer always pessimistically believe that “The life of others has just started, but I'm going to end” [48]. Current psychosocial care guidelines for patients with cancer advise that clinicians pay attention to the establishment of hope based on reality when discussing prognosis [49], [50]. We believe that when young survivors of GC inquire about their prognoses during follow‐up, an accurate risk assessment should be given based on the survival time. This knowledge may be more effective in helping young patients with GC to manage future uncertainties, especially when they need to make important life plans.
CS is a new assessment of survival that can dynamically assess the changes in the risk of postoperative death in patients based on the survival time. Hence, CS can provide more accurate and valuable prognostic information for clinicians and patients. In this study, we evaluated the postoperative DSS and CS3 of young patients with GC in the East and West, based on a large multicenter data set. The results showed that, for both Chinese and U.S. patients, with the prolongation of survival time, the CS3 of patients initially diagnosed as having poor tumor characteristics gradually approached or even surpassed those with better initial tumor characteristics. For example, the actual 8‐year DSS rates of Chinese patients with T1, T3, and T4b stage disease were 91.2%, 56.4%, and 34.7%, respectively. After 5 years of survival, the CS3 rates of patients with T1, T3, and T4b stage disease compared with the CS3 rates of patients with T1, T3, and T4b stage disease at baseline increased by 1.3% (96.9%–98.2%), 11.3% (74.4%–85.7%), and 53.6% (41%–94.6%), respectively. Similar results could be obtained in the U.S. population. We found that the change in the postoperative survival of young survivors of GC was also consistent with Zamboni's “natural selection effect” hypothesis [15]. That is, most patients with cancer who have a high risk of cancer death die soon after surgery. In turn, the gradual deaths of high‐risk patients promote the natural selection of low‐risk patients, making the prognosis of the survivor more favorable. Understanding the possibility of continued survival over time will help alleviate the anxiety of young patients with GC, especially of those who are initially judged as having poor prognoses, and will improve their quality of life.
Current guidelines recommend routine follow‐up for 5 years after surgery for patients with GC [41], [42], [51]. Other surveillance programs should be sought beyond the fifth year. Our results showed that the overall patient CS3 exhibited almost no increased mortality after 5 years (90.9% [U.S.] vs. 91.5% [China]), which implied that most patients had reached a state of “tumor cure” compared with the general population. However, we also found that even after 5 years of survival, there were still some high‐risk patients with low CS3, suggesting that some patients still had high tumor‐related mortality, including Chinese patients with multiple primary sites (CS3 at 5 years, 79.8%) and young patients with N3a stage GC (CS3 at 5 years, 72.9%). With the increase in the number of young long‐term survivors of GC, arbitrarily defining the follow‐up time 5 years after surgery is unreasonable. CS evaluation can provide more valuable information to determine the follow‐up strategy. Therefore, the development of a follow‐up strategy after 5 years should incorporate CS status into the decision‐making process.
Multivariate Cox regression analysis showed that site, size, T stage, and N stage were independent prognostic factors for young Chinese patients with GC at baseline (all p < .05). For young U.S. patients with GC, grade, T stage, and N stage significantly affected DSS (all p < .05). These findings suggested that the independent prognostic factors at baseline were not identical between the East and West. Nevertheless, in the two groups, only T stage could continually affect DSS (p < .05) within 3 years after resection. Although the data reflected the relative stability of T stage in affecting the DSS of young patients with GC, T stage lost prognostic significance after 5 years of survival. Subgroup analysis showed that the CS3 of the two regions tended to be consistent over time in each T stage, indicating that in both the Chinese and U.S. survivors, the initial well‐known prognostic factors lost their prognostic abilities after 5 years of survival. The results and conclusions of previous comparative studies on gastric cancer in the East and the West were based on the comparison of survival at the cutoff point after surgery [52], [53]. However, prognostic factors that have a strong impact on DSS at baseline may weaken or even lose their differentiating capacities over time. Therefore, this study not only compares the clinicopathological characteristics of young patients with GC between China and the U.S. based on multicenter data but also, for the first time, evaluates the dynamic survival differences of patients between China and the U.S. Owing to the limitations of the SEER database and our retrospective data, we did not propose new independent prognostic factors that affect conditional survival but rather investigated whether well‐known prognostic factors could continuously predict survival in patients after curative‐intent resections in a variety of ways. Unlike other reported independent prognostic factors, such as tumor size, N stage, and histological grading, that affect survival at the time of surgery, our results showed that only T stage can continue to affect DSS within 3 years after surgery. In addition, after 5 years of survival, the common prognostic factors of gastric cancer at the initial time after surgery lost their capacity to differentiate DSS. These results can provide a reference for clinicians to develop long‐term follow‐up strategies. We are still looking forward to finding new prognostic factors that can persistently affect DSS in young patients with GC by further selecting a larger, wider, and more representative sample of clinical gastric cancer data, combined with the development of medical molecular biology, so as to provide a reference for clinicians to individualize long‐term follow‐up strategies for young patients with GC.
The present study not only evaluated the changes in CS in Eastern and Western populations of young patients with GC for the first time but also analyzed the dynamic changes in the prognostic factors of young survivors of GC. Although the results are more reliable based on the large amount of data and the long‐term follow‐up, several limitations of this study could not be avoided. First, because this study is a multicenter retrospective study, some potential biases are inevitable. Second, although we have included data from multiple Chinese centers and U.S. SEER database, which reflect the generalizability and applicability of the findings because of different social and economic conditions in different regions, the heterogeneity of the diagnosis level and operation level may be confounding factors when analyzing DSS and CS. More detailed and accurate information is needed to control these confounding factors in future studies. Third, as is already known, preoperative chemotherapy has a chemotherapy‐related downgrading effect. Postoperative pathological T stage (ypT) and pathological N stage (ypN) of patients with gastric cancer after preoperative therapy may have different effects on prognosis compared with the same pathological T stage (pT) and pathological N stage (pN) of patients without preoperative chemotherapy. The aim of this study was to analyze whether well‐known prognostic factors could continuously predict survival in young patients with GC after curative‐intent gastrectomy and to evaluate the CS changes in young Chinese and U.S. patients with different pT and pN. Therefore, we did not include patients with neoadjuvant therapy to avoid the impact of neoadjuvant therapy on the accuracy of the results. In addition, because SEER data do not provide detailed information on neoadjuvant therapy, we were unable to carefully study CS in young patients with GC after neoadjuvant therapy plus surgery. We look forward to further cooperation with the U.S. Gastric Cancer Treatment Center and to studying the CS of young patients with GC through more detailed clinical data in the future. Currently, in East Asian countries, including China, standard D2 lymphadenectomy plus postoperative adjuvant chemotherapy has been the standard treatment for advanced gastric cancer for many years, whereas in Western countries, including the U.S., preoperative chemotherapy plus D1 lymphadenectomy plus postoperative radiochemotherapy are the preferred treatment pattern for advanced gastric cancer [51]. Even after the same adjuvant chemotherapy, the survival benefits of gastric cancer in Eastern and Western populations are very different [54], [55]. More importantly, postoperative adjuvant treatment is not only related to tumor stage but is also affected by the side effects of chemotherapy and/or radiotherapy, the willingness of patients, and the economic situations of the patients themselves. Because of the different strategies of adjuvant treatment in the two regions during the study period (2000–2012), patients received many different treatment regimens (single or combined adjuvant chemotherapy, dosage, frequency). Therefore, we cannot accurately assess the effects of adjuvant treatment regimens on the DSS and CS of young patients with GC. We look forward to assessing the impact of adjuvant treatment on the CS of young patients with GC in the future through more stringent prospective studies.
Conclusion
Although there are differences in tumor characteristics among young patients with GC in China and the U.S., the prognosis of young patients with GC gradually improves with the prolongation of postoperative survival in any area. After a period of survival, especially after 5 years of survival, young survivors of GC in China and the U.S. have similar DSS rates, which would be similar to the general population. Insight into CS will help clinicians provide a more meaningful prognostic assessment for young survivors.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We want to dedicate this article to young patients with GC. Life is tough; please never give up your hope.
This study was supported by Scientific and Technological Innovation Joint Capital Projects of Fujian Province (2016Y9031), Construction Project of Fujian Province Minimally Invasive Medical Center ([2017]171), General Project of Miaopu Scientific Research Fund of Fujian Medical University (2015MP021), and Youth Project of Fujian Provincial Health and Family Planning Commission (2016‐1‐41).
Contributed equally.
Author Contributions
Conception/design: Qi‐Yue Chen, Qing Zhong, Ping Li, Chang‐Ming Huang
Provision of study material or patients: Qi‐Yue Chen, Qing Zhong, Wei Wang, Shi Chen, Ping Li, Jian‐Wei Xie, Jia‐Bing Wang, Jian‐Xian Lin, Jun Lu, Long‐Long Cao, Mi Lin, Ru‐Hong Tu, Ze‐Ning Huang, Ju‐Li Lin, Hua‐Long Zheng, Zhi‐Yu Liu, Chao‐Hui Zheng, Jun‐Sheng Peng, Zhi‐Wei Zhou, Chang‐Ming Huang
Collection and/or assembly of data: Qi‐Yue Chen, Qing Zhong, Wei Wang, Shi Chen, Ping Li, Jian‐Wei Xie, Jia‐Bing Wang, Jian‐Xian Lin, Jun Lu, Long‐Long Cao, Mi Lin, Ru‐Hong Tu, Ze‐Ning Huang, Ju‐Li Lin, Hua‐Long Zheng, Zhi‐Yu Liu, Chao‐Hui Zheng, Jun‐Sheng Peng, Zhi‐Wei Zhou, Chang‐Ming Huang
Data analysis and interpretation: Qi‐Yue Chen, Qing Zhong, Wei Wang, Shi Chen, Ping Li, Jian‐Wei Xie, Jia‐Bing Wang, Jian‐Xian Lin, Jun Lu, Long‐Long Cao, Mi Lin, Ru‐Hong Tu, Ze‐Ning Huang, Ju‐Li Lin, Hua‐Long Zheng, Zhi‐Yu Liu, Chao‐Hui Zheng, Jun‐Sheng Peng, Zhi‐Wei Zhou, Chang‐Ming Huang
Manuscript writing: Qi‐Yue Chen, Qing Zhong, Wei Wang, Shi Chen, Ping Li, Jun‐Sheng Peng, Zhi‐Wei Zhou, Chang‐Ming Huang
Final approval of manuscript: Qi‐Yue Chen, Qing Zhong, Wei Wang, Shi Chen, Ping Li, Jian‐Wei Xie, Jia‐Bing Wang, Jian‐Xian Lin, Jun Lu, Long‐Long Cao, Mi Lin, Ru‐Hong Tu, Ze‐Ning Huang, Ju‐Li Lin, Hua‐Long Zheng, Zhi‐Yu Liu, Chao‐Hui Zheng, Jun‐Sheng Peng, Zhi‐Wei Zhou, Chang‐Ming Huang
Disclosures
The authors indicated no financial relationships.
References
- 1.Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Anderson WF, Camargo MC, Fraumeni JF Jr et al. Age‐specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karimi P, Islami F, Anandasabapathy S et al. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong X, Wang JL, Chen HM et al. Comparison of the clinicopathological characteristics of young and elderly patients with gastric carcinoma: A meta analysis. J Surg Oncol 2012;106:346–352. [DOI] [PubMed] [Google Scholar]
- 5.Theuer CP, Kurosaki T, Taylor TH et al. Unique features of gastric carcinoma in the young: A population‐based analysis. Cancer 2015;83:25–33. [DOI] [PubMed] [Google Scholar]
- 6.Song M, Kang D, Yang JJ et al. Age and sex interactions in gastric cancer incidence and mortality trends in Korea. Gastric Cancer 2015;18:580–589. [DOI] [PubMed] [Google Scholar]
- 7.Merchant SJ, Kim J, Choi AH et al. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer 2017;20:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maconi G, Kurihana H, Panizzo V et al. Delay of diagnosis, stage of gastric cancer (GC) and survival in young gastric cancer patients without alarm symptoms (AS). Gut 2002;51(suppl 2):97A. [DOI] [PubMed] [Google Scholar]
- 9.Santoro R, Carboni F, Lepiane P et al. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg 2007;94:737–742. [DOI] [PubMed] [Google Scholar]
- 10.Al‐Refaie WB, Hu CY, Pisters PWT et al. Gastric adenocarcinoma in young patients: A population‐based appraisal. Ann Surg Oncol 2011;18:2800–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, Margonis GA, Prescott JD et al. Curative surgical resection of adrenocortical carcinoma: Determining long‐term outcome based on conditional disease‐free probability. Ann Surg 2017;265:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, Sun Z, Wang W et al. Conditional survival of patients with gastric cancer who undergo curative resection: A multi‐institutional analysis in China. Cancer 2018;124:916–926. [DOI] [PubMed] [Google Scholar]
- 13.Zhong Q, Chen QY, Li P et al. Prediction of conditional probability of survival after surgery for gastric cancer: A study based on Eastern and Western large data sets. Surgery 2018;163:1307–1316. [DOI] [PubMed] [Google Scholar]
- 14.Skuladottir H, Olsen JH. Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol 2003;21:3035–3040. [DOI] [PubMed] [Google Scholar]
- 15.Zamboni BA, Yothers G, Choi M et al. Conditional survival and the choice of conditioning set for patients with colon cancer: An analysis of NSABP trials C‐03 through C‐07. J Clin Oncol 2010;28:2544–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi M, Becker A, Hansen J et al. Conditional survival after nephrectomy for renal cell carcinoma (RCC): Changes in future survival probability over time. BJU Int 2013;111:E283–E289. [DOI] [PubMed] [Google Scholar]
- 17.Ploussard G, Xylinas E, Lotan Y et al. Conditional survival after radical nephroureterectomy for upper tract carcinoma. Eur Urol 2015;67:803–812. [DOI] [PubMed] [Google Scholar]
- 18.Margonis GA, Buettner S, Andreatos N et al. Prognostic factors change over time after hepatectomy for colorectal liver metastases: A multi‐institutional, international analysis of 1099 patients. Ann Surg 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.American Joint Committee on Cancer . AJCC Cancer Staging Manual. 7th ed New York, NY: Springer, 2010. [Google Scholar]
- 20.American Joint Committee on Cancer . AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017. [Google Scholar]
- 21.Jin H, Pinheiro PS, Callahan KE et al. Examining the gastric cancer survival gap between Asians and whites in the United States. Gastric Cancer 2017;20:573–582. [DOI] [PubMed] [Google Scholar]
- 22.Hu C, Xing Y, Cormier JN et al. The validity of cancer specific mortality within the surveillence, epidemiology, and end results registry. J Surg Res 2011;165(2):270. [Google Scholar]
- 23.Lu J, Wang W, Zheng CH et al. Influence of total lymph node count on staging and survival after gastrectomy for gastric cancer: An analysis from a two‐institution database in China. Ann Surg Oncol 2017;24:486–493. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Huang C, Sun Y et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: A randomized controlled trial. J Clin Oncol 2016;34:1350–1357. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Sun XW, Li CF et al. Comparison of the 6th and 7th editions of the UICC TNM staging system for gastric cancer: Results of a Chinese single‐institution study of 1,503 patients. Ann Surg Oncol 2011;18:1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, Ouyang LY, Nie RC et al. Tumor size is a critical factor in adjuvant chemotherapy for T3‐4aN0M0 gastric cancer patients after D2 gastrectomy. Gastroenterol Res Pract 2017;2017:4928736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurta ML, Edwards RP, Moysich KB et al. Prognosis and conditional disease‐free survival among patients with ovarian cancer. J Clin Oncol 2014;32:4102–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cucchetti A, Piscaglia F, Cescon M et al. Conditional survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Clin Cancer Res 2012;18:4397–4405. [DOI] [PubMed] [Google Scholar]
- 29.Spolverato G, Kim Y, Ejaz A et al. Conditional probability of long‐term survival after liver resection for intrahepatic cholangiocarcinoma: A multi‐institutional analysis of 535 patients. JAMA Surg 2015;150:538–545. [DOI] [PubMed] [Google Scholar]
- 30.Takatsu Y, Hiki N, Nunobe S et al. Clinicopathological features of gastric cancer in young patients. Gastric Cancer 2016;19:472–478. [DOI] [PubMed] [Google Scholar]
- 31.Correa P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735–6740. [PubMed] [Google Scholar]
- 32.Blaser MJ, Nomura A, Lee J et al. Early‐life family structure and microbially induced cancer risk. PLoS Med 2007;4:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy G, Pfeiffer R, Camargo MC et al. Meta‐analysis shows that prevalence of Epstein‐Barr virus‐positive gastric cancer differs based on sex and anatomic location. Gastroenterology 2009;137:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr 2004;24:401–431. [DOI] [PubMed] [Google Scholar]
- 35.Derakhshan MH, Liptrot S, Paul J et al. Oesophageal and gastric intestinal‐type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut 2009;58:16–23. [DOI] [PubMed] [Google Scholar]
- 36.Lagergren JE, Bergstrom R, Lindgren A et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1998;340:2367–2368. [DOI] [PubMed] [Google Scholar]
- 37.Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015;18:23–32. [DOI] [PubMed] [Google Scholar]
- 38.Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 39.Gill S, Shah A, Le N et al. Asian ethnicity‐related differences in gastric cancer presentation and outcome among patients treated at a Canadian cancer center. J Clin Oncol 2003;21:2070–2076. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Sun CL, Mailey B et al. Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol 2010;21:152–160. [DOI] [PubMed] [Google Scholar]
- 41.Ajani JA, D'Amico TA, Almhanna K et al. Gastric cancer, version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286–1312. [DOI] [PubMed] [Google Scholar]
- 42.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 2010;97:643–649. [DOI] [PubMed] [Google Scholar]
- 44.Degiuli M, Sasako M, Ponti A et al.; Italian Gastric Cancer Study Group. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23–31. [DOI] [PubMed] [Google Scholar]
- 45.Wang SJ, Emery R, Fuller CD et al. Conditional survival in gastric cancer: A SEER database analysis. Gastric Cancer 2007;10:153–158. [DOI] [PubMed] [Google Scholar]
- 46.Kunz PL, Gubens M, Fisher GA et al. Long‐term survivors of gastric cancer: A California population‐based study. J Clin Oncol 2012;30:3507–3515. [DOI] [PubMed] [Google Scholar]
- 47.Miller KD, Siegel RL, Lin CC et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 48.McLean M, Cleland JA, Worrell M et al. “What am I going to say here?” The experiences of doctors and nurses communicating with patients in a cancer unit. Front Psychol 2011;2:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobsen PB. Clinical practice guidelines for the psychosocial care of cancer survivors: Current status and future prospects. Cancer 2010;115(S18):4419–4429. [DOI] [PubMed] [Google Scholar]
- 50.Zebrack B, Isaacson S. Psychosocial care of adolescent and young adult patients with cancer and survivors. J Clin Oncol 2012;30:1221–1226. [DOI] [PubMed] [Google Scholar]
- 51.Ajani JA, D'Amico TA, Baggstrom M et al. NCCN Clinical Practice Guidelines in Oncology: Gastric cancer, version 2.2018. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2018. [Google Scholar]
- 52.Strong VE, Song KY, Park CH et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010;251:640–646. [DOI] [PubMed] [Google Scholar]
- 53.Shim JH, Song KY, Jeon HM et al. Is gastric cancer different in Korea and the United States? Impact of tumor location on prognosis. Ann Surg Oncol 2014;21:2332–2339. [DOI] [PubMed] [Google Scholar]
- 54.Macdonald JS. Gastric cancer: Nagoya is not New York. J Clin Oncol 2011;29:4348–4350. [DOI] [PubMed] [Google Scholar]
- 55.Ajani JA, Blum MA, Estrella JS et al. Gastric cancer: Apples will always be apples. J Clin Oncol 2012;30:1019–1020. [DOI] [PubMed] [Google Scholar]