Sorafenib and dacarbazine have low single agent response rates in metastatic sarcomas. This article reports results of a clinical trial using a combination of sorafenib and dacarbazine in select sarcoma subtypes.
Keywords: Soft tissue sarcoma, Angiogenesis, Chemotherapy, Phase II clinical trials
Abstract
Background.
Sorafenib and dacarbazine have low single‐agent response rates in metastatic sarcomas. As angiogenesis inhibitors can enhance the efficacy of chemotherapy, we investigated the combination of sorafenib and dacarbazine in select sarcoma subtypes.
Materials and Methods.
Patients with leiomyosarcoma (LMS), synovial sarcoma (SS), or malignant peripheral nerve sheath tumors (MPNST) with up to two previous lines of therapy and adequate hepatic, renal, and marrow function received 3‐week cycles of sorafenib at 400 mg oral twice daily and dacarbazine 1,000 mg/m2 intravenously (later reduced to 850 mg/m2). Patients were evaluated for response every 6 weeks. The primary objective was to determine the disease control rate (DCR) of sorafenib plus dacarbazine in the selected sarcoma subtypes.
Results.
The study included 37 patients (19 female); median age was 55 years (range 26–87); and histologies included LMS (22), SS (11), and MPNST (4). The DCR was 46% (17/37). Median progression‐free survival was 13.4 weeks. The RECIST response rate was 14% (5/37). The Choi response rate was 51% (19/37). Median overall survival was 13.2 months. Of the first 25 patients, 15 (60%) required dacarbazine dose reductions for hematologic toxicity, with one episode of grade 5 neutropenic fever. After reducing the starting dose of dacarbazine to 850 mg/m2, only 3 of the final 12 (25%) patients required dose reduction.
Conclusion.
This phase II study met its primary endpoint with an 18‐week DCR of 46%. The clinical activity of dacarbazine plus sorafenib in patients with these diagnoses is modest.
Implications for Practice.
Metastatic soft tissue sarcomas are a heterogeneous group of relatively rare malignancies. Most patients are treated with cytotoxic chemotherapy or targeted therapy in the form of tyrosine kinase inhibitors. Response rates are relatively low, and there is a need for better therapies. This clinical trial demonstrates that combining a cytotoxic therapy (dacarbazine) with an antiangiogenic small molecule (sorafenib) is feasible and associated with favorable disease‐control rates; however, it also increases the potential for significant toxicity.
Introduction
Sarcomas comprise a group of rare malignancies that are challenging to treat. Incidence of sarcoma is ∼15,000 annually in the U.S. [1] with a high case fatality rate. Up to one fourth of patients with sarcomas present with metastatic disease, and up to one half of patients who present with locally advanced disease develop metastases despite surgical resection and radiotherapy [2], [3]. Standard chemotherapies (doxorubicin, ifosfamide; gemcitabine and docetaxel) have modest activity with single‐agent and combination response rates of 10%–20% [4], [5], [6], [7], [8] and 17%–40%, respectively [9], [10], [11], [12], [13], [14]. This study was designed and began enrollment in 2008, prior to the availability of newer drugs such as eribulin, trabectedin, pazopanib, or olaratumab [15], [16], [17], [18]. Tumor angiogenesis has been hypothesized to be necessary for transformation and progression of most malignancies [19]. Sorafenib is a multitargeted tyrosine kinase inhibitor approved for renal, thyroid, and hepatocellular carcinomas [20], [21] that targets VEGFR1, 2, and 3, CRAF, KIT, FLT3, and PDGFR [22], [23], [24]. Responses have been also been noted in neuroendocrine tumors [25], angiosarcomas [26], and gastrointestinal stromal tumors (GISTs) [27], [28]. Stabilization of disease has been observed more often than radiologic response in the majority of these tumors [29]. In many cancers, angiogenesis inhibition by itself has been ineffective, whereas it has improved efficacy of standard chemotherapy when used in some cancers, but not in uterine leiomyosarcoma [30], [31], [32], [33]. This model led us to test the combination of sorafenib and dacarbazine in selected sarcoma subtypes. Dacarbazine is a standard sarcoma agent generally reserved as a later line of therapy [8], [34]. Sorafenib and dacarbazine have been combined in the treatment of melanoma with an acceptable safety profile [35].
We previously conducted a trial of sorafenib in multiple sarcoma subtypes [26]. Only one arm, angiosarcoma, met the primary endpoint of objective RECIST response rate. Partial responses (PR), minor responses, and prolonged stable disease (SD) were observed in some patients with leiomyosarcoma (LMS), synovial sarcoma (SS), and malignant peripheral nerve sheath tumors (MPNST). Responses in LMS and SS were also documented in the sorafenib randomized discontinuation study [36]. These results led to the selection of the histologies included in the current study.
Because disease stabilization has been observed with sorafenib more often than objective response, we chose disease control rate (DCR; defined as RECIST complete response [CR], PR, or SD for at least 18 weeks) as the primary endpoint for the study. Data from the European Organisation for Research and Treatment of Cancer database define progression‐free survival (PFS) rates at 3 and 6 months for “active” agents as 40% PFS at 3 months and 14% PFS at 6 months and defines “inactive” agents as those associated with fewer than 20% of patients progression‐free at 3 months or fewer than 8% at 6 months, in the second‐line setting [37]. As dacarbazine achieves objective responses in some sarcomas, and because we were enrolling some patients with no prior therapy, we chose an intermediate time point of 4.5 months (18 weeks). We conducted a Simon two‐stage trial to distinguish between an 18‐week DCR rate of 20% (inactive) and a rate of 40% (active).
Materials and Methods
This was a single‐center, open label, phase II study of oral sorafenib with intravenous dacarbazine in patients with LMS, SS, and MPNST. The protocol was approved by the Memorial Sloan Kettering Cancer Center institutional review board. Each patient provided written informed consent. RECIST response assessment was made by a single musculoskeletal radiologist (R.A.L.). All pathology was reviewed by one of two dedicated sarcoma pathologists (C.R.A.). The clinicaltrials.gov study identifier was NCT00837148.
Study Design
Patients were treated with an initial dose of dacarbazine at 1,000 mg/m2 as a 1‐hour intravenous infusion every 3 weeks and sorafenib 400 mg orally twice daily. A cycle of therapy was defined as 21 days of treatment. Sorafenib dose reductions to 400 mg daily and then 200 mg daily were permitted for patients experiencing (National Cancer Institute Common Terminology Criteria for Adverse Events v3.0) events of symptomatic grade 2 or higher dermatologic reaction; grade 2 hypertension despite optimal medical therapy, or grade 3–4 hypertension; grade 3–4 drug‐related nonhematologic toxicity. Dacarbazine was held if a patient did not recover to an absolute neutrophil count of 1,500/μL or a platelet count of 75,000/μL by day 21. Growth factors were only permitted in patients who required dose reductions or delay or experienced neutropenic fever. Patients deemed intolerant of dacarbazine but still experiencing clinical benefit (PR or SD) were able to continue sorafenib at the discretion of the treating physician. Patients continued to receive therapy until unacceptable toxicity, tumor progression, or death.
Clinical examinations and laboratory testing were performed at a screening visit and at the start of each 3‐week cycle. Blood pressure was examined weekly for the first 3 weeks and then every 3 weeks thereafter. Physical examination, Eastern Cooperative Oncology Group (ECOG) performance status, complete blood count, biochemical profile, and lactate dehydrogenase (LDH) were evaluated every 3 weeks. Patients on anticoagulation had PT/INR levels monitored weekly for the first 6 weeks, and then at the discretion of the treating physician. Tumors were evaluated by computed tomography or magnetic resonance imaging every 6 weeks for the first 24 weeks, and every 9 weeks thereafter.
Sorafenib was provided by Bayer AG. Patients were treated with commercially sourced dacarbazine.
Patient Eligibility
Patients older than 18 years of age, with histologically confirmed LMS, SS, or MPNST, with zero to two prior lines of chemotherapy were eligible for enrollment. Other eligibility requirements included measurable disease (RECIST 1.0), ECOG performance status 0–2, adequate hematologic, hepatic, and renal function, use of adequate contraception, resolution of prior treatment‐related toxicities, and ability to understand and willingness to provide informed consent. Exclusion criteria included the following: prior therapy with dacarbazine, sorafenib, or other antiangiogenic agents, chemotherapy within 3 weeks or radiotherapy within 2 weeks, known brain metastasis, pregnancy or nursing, psychiatric or social situation that would limit compliance with study requirements, congestive heart failure, unstable angina or myocardial infarction within the past 6 months, cardiac ventricular arrhythmias requiring antiarrhythmic therapy, uncontrolled hypertension, human immunodeficiency virus, chronic hepatitis B or C infection, active clinically serious infection, thrombotic or embolic events within the past 6 months, deep venous thrombosis or pulmonary embolism within 2 months, pulmonary hemorrhage or other significant bleeding event within 4 weeks of first dose of study drug, or serious nonhealing wound or bone fracture.
Statistical Analysis
The primary endpoint was DCR, defined as CR, PR, or SD by RECIST 1.0 for at least 18 weeks from first date of treatment. This study employed a Simon optimum two‐stage design to distinguish between a 20% DCR rate (not promising) and a 40% DCR rate (promising). The type I error (falsely accepting a nonpromising therapy) rate was set at 0.10 and the type II error (falsely rejecting a promising therapy) rate at 0.10.
Seventeen patients would be accrued to the first stage. If there were 3 or fewer with disease control among these 17 patients, accrual would stop and the treatment would be declared ineffective. If at least 4 had disease control, then 20 additional patients would be accrued. If at least 11 had disease control among the 37 patients, the trial would be considered to have a positive result and the treatment would be considered worthy of further testing.
Secondary endpoints included PFS, response rate by RECIST, response rate by Choi criteria, evaluation of the toxicity of the combination in sarcoma patients, and overall survival (OS). Kaplan‐Meier curves along with 95% confidence intervals (CI) for PFS and OS were constructed. All analyses were univariate.
Results
Demographics
Patient characteristics are presented in Table 1. All 37 patients received at least one dose of sorafenib and were evaluable for toxicity. Three patients did not complete 6 weeks of therapy and were not evaluable for response by RECIST; however, for the purposes of the primary DCR at 18 weeks endpoint, these 3 patients were considered failures. Median age was 55 years (range 26–87), 19 were women (53%), and median ECOG performance status was 0. Sixteen (43%) had received no previous chemotherapy. Eleven (30%) had prior surgery and sixteen (43%) had prior radiotherapy. Twenty‐two patients had LMS (eight of uterine origin), eleven SS, and four MPNSTs.
Table 1. Patient demographics.
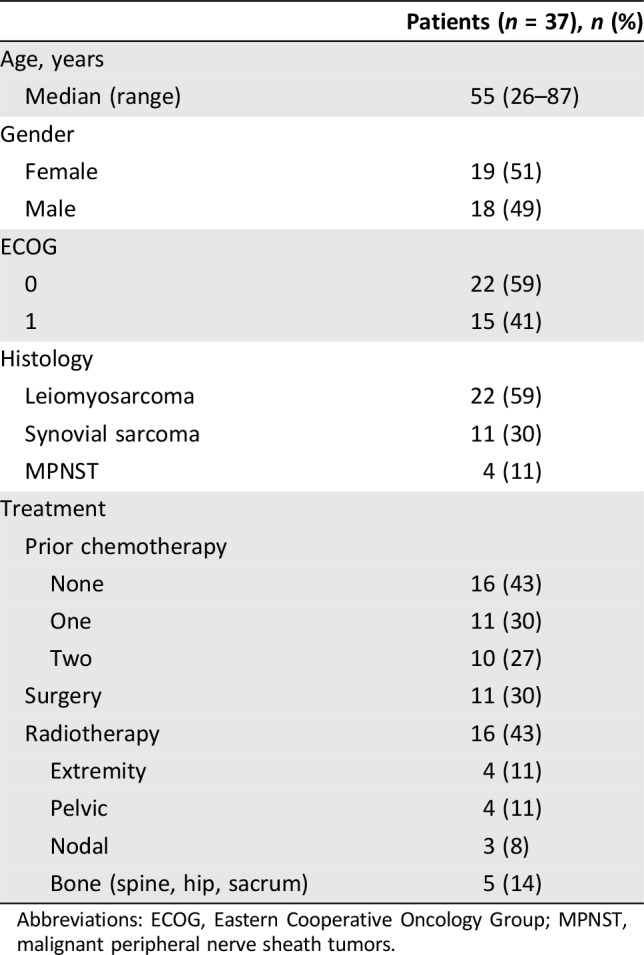
Abbreviations: ECOG, Eastern Cooperative Oncology Group; MPNST, malignant peripheral nerve sheath tumors.
Table 2. Responses.
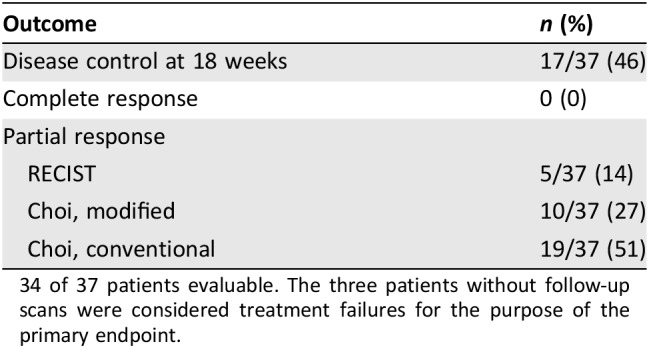
34 of 37 patients evaluable. The three patients without follow‐up scans were considered treatment failures for the purpose of the primary endpoint.
Treatment Efficacy
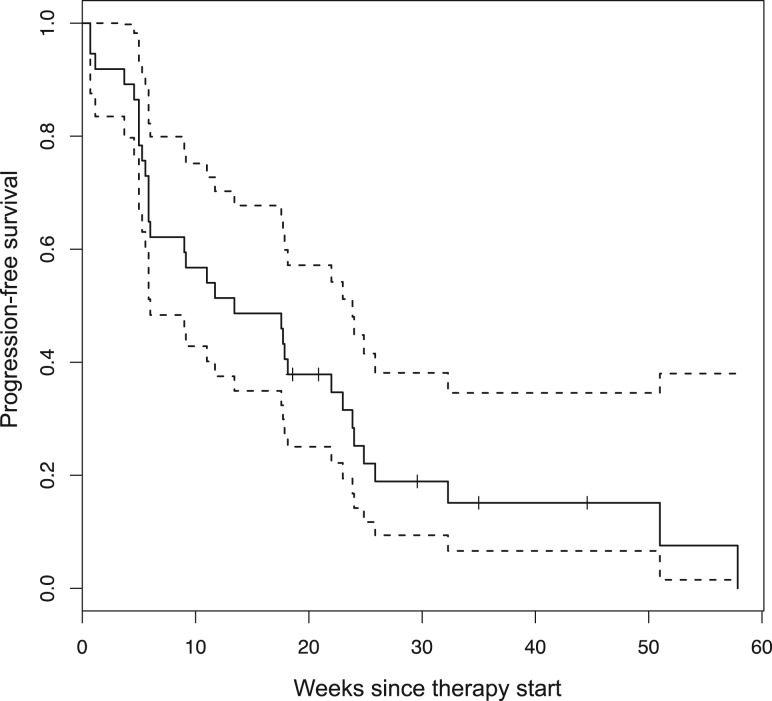
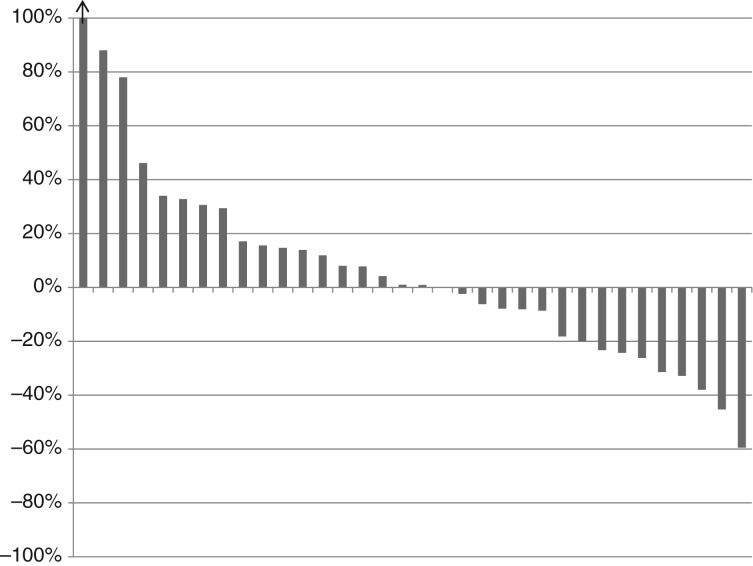
Thirty‐seven patients received 211 cycles of therapy (median 4, range 1–19). Seventeen patients (46%; lower bound of the one‐sided 90% CI: 0.35; two‐sided 95% CI: 0.30–0.63) met the primary endpoint of disease control at 18 weeks. Median PFS was 13.4 weeks (two‐sided 95% CI: 6–23.9). PFS did not differ significantly depending on number of prior therapies (0 vs. 1 vs. 2) or between LMS and SS, but was shorter for MPNST versus other (4.9 vs. 13.4 weeks, p < .01; Fig. 1). There were five partial responses by RECIST (four LMS and one SS) for an overall response rate of 14%. The modified Choi response rate (10% decrease in unidimensional disease) was 27% (10/37). The classical Choi response rate (10% decrease in unidimensional disease or 15% decrease in density by Hounsfield units [38]) was 51% (19/37). Responses are shown in Table 2 and Figure 2. Responses as assessed by the different criteria were correlated with disease control rate. There were 17 patients who achieved 18 weeks of disease control (successes) and 20 who did not (failures). Of the five patients with PR by RECIST, all met the disease control endpoint. Of the 10 patients with PR by modified Choi, 8 met the disease control endpoint. Of the 19 patients with PR by classical Choi, 12 met the disease control endpoint. Modified Choi and Choi were more sensitive at predicting disease control but less specific compared with RECIST (Table 3).
Figure 1.
Progression‐free survival (n = 37). ‐‐‐‐ = 95% confidence intervals.
Figure 2.
Waterfall plot of best response by RECIST.
Table 3. Correlation of response criteria with disease control rate.
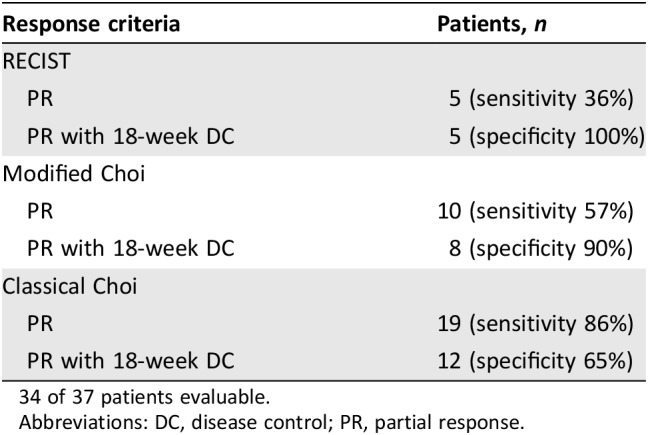
34 of 37 patients evaluable.
Abbreviations: DC, disease control; PR, partial response.
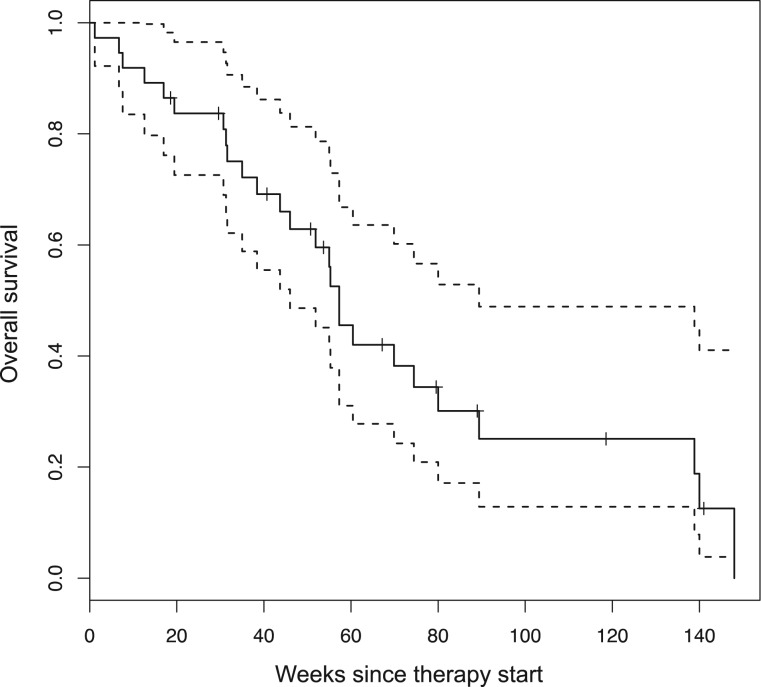
The 3‐ and 6‐month progression‐free rates were 51% and 25%, respectively. With a median follow‐up of 11 months, median OS was 13.2 months (two‐sided 95% CI: 10–17; Fig. 3).
Figure 3.
Overall survival. ‐‐‐‐ = 95% confidence intervals.
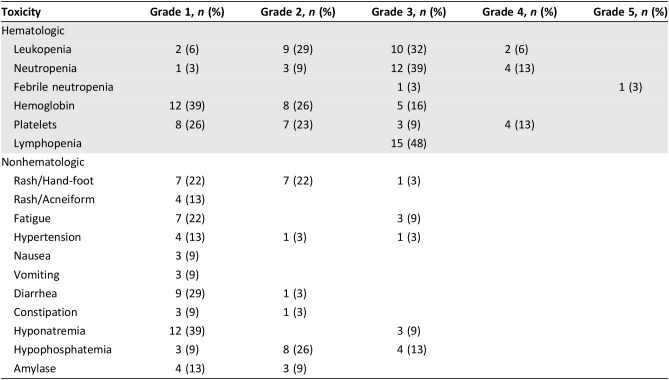
Toxicity is presented in Table 4. Protocol‐specified dose modifications to sorafenib were required for rash and hand‐foot syndrome. The primary reason for dose reduction of dacarbazine was for hematologic toxicity. Toxicity was largely as reported in single‐agent studies of sorafenib and dacarbazine with the exception of a higher frequency of grade 3 and 4 neutropenia and thrombocytopenia. There was one event of fatal febrile neutropenia (3%). This patient had synovial sarcoma and had been treated with prior chemotherapy and extremity radiotherapy. Of the first 25 patients, 15 (60%) required dacarbazine dose reductions (6 reduced one level; 9 by two levels). After the first 25 patients, the protocol was amended to reduce the starting dose of dacarbazine from 1,000 mg/m2 to 850 mg/m2. Thereafter, only 3 of the next 12 patients (25%) required a dose reduction (2 reduced one level; 1 by two levels). Of 37 patients, 18 (49%) required sorafenib dose reductions. Three patients (8%) required two dose reductions of sorafenib.
Table 4. Toxicity.
Serum LDH was measured for all patients before enrollment and at each cycle on therapy. Five (13.5%) patients had an LDH above the upper limit of normal prior to treatment. LDH increased in all patients who had at least two LDH measurements (34/37), regardless of response. Mean increase in LDH was 53%.
Discussion
The hypothesis underlying this trial was that we could improve the modest outcomes seen with sorafenib by combining it with dacarbazine. Two phase I trials explored the dosing of sorafenib and dacarbazine. Both trials noted responses in sarcoma [39]. This trial prospectively evaluated the disease control rate, RECIST, and Choi response rates of sorafenib and dacarbazine in patients with LMS, SS, and MPNST.
We observed 5 patients with disease control out of the first 17 patients, which met the first stage goal and led to second‐stage enrollment. At the completion of the trial, 17 out of 37 patients had disease control at 18 weeks, which was considered a positive result. Although the trial met its primary endpoint, it is a single‐arm study and therefore superiority to single‐agent dacarbazine or sorafenib alone is not proven. Historical literature reports PR rates of 4%–17% for sarcoma patients treated with dacarbazine.
Although RECIST has been proposed as a simpler, more reproducible disease assessment method than World Health Organization (WHO) criteria, in sarcoma RECIST may underestimate the proportion of patients with meaningful reduction in their burden of disease. Recent trials have shown a reduction in response rates compared with historical literature. For example, older trials in the 1980s and 1990s reported response rates of 20%–30% using WHO criteria for unselected sarcoma patients treated with doxorubicin. More recent trials using RECIST show response rates to doxorubicin closer to 10% [6], [40], [41]. Changes in imaging technology and techniques over time may also complicate the interpretation of historical data.
Choi criteria were first developed in GIST. Response by Choi correlates to metabolic response as assessed by 18F‐FDG‐PET [38], [42]. Retrospective analysis has shown that Choi response in GIST patients correlated better with overall survival than response according to RECIST. This concept is untested in other sarcomas [43]. This trial prospectively evaluated the use of Choi criteria in a non‐GIST sarcoma population. A higher proportion of patients had Choi responses than had RECIST responses. We found decrease in tumor density did not correlate well with duration of tumor control. Several patients had decrease in density but rapid progression of disease. In contrast, a 10% decrease in unidimensional disease correlated well with disease control. Eight of ten patients with a modified Choi response had disease control for at least 18 weeks. All five patients with RECIST PR had disease control for at least 18 weeks. Additional prospective evaluation of radiologic criteria alternatives to RECIST is warranted.
A randomized trial of pazopanib, another multitargeted tyrosine kinase inhibitor, in unselected sarcoma patients has been reported [44]. This trial randomized sarcoma patients who had progressed on one to four lines of prior therapy to treatment with pazopanib or placebo. PFS for patients treated with pazopanib was significantly longer at 4.6 months compared with 1.6 months for patients treated on the placebo arm. This does appear to be longer than we observed for combination sorafenib and dacarbazine therapy. The response rate for pazopanib by itself was 4%, with most responses seen in two of the histologies we examined—synovial sarcoma and leiomyosarcoma.
Elevated LDH is classically regarded as a measure of increased tumor turnover and a poor prognostic marker. Lactic acid metabolism is generated in tissues undergoing anaerobic metabolism. It has been posited to be a measure of tumor hypoxia. There is a paucity of data in sarcoma on the prognostic utility of LDH. We hypothesized that a decline in LDH might correlate with response in sarcoma and with normalization of tumor vasculature and resolution of tumor hypoxia in response to antiangiogenic therapy. We were surprised to observe that LDH actually increased in all patients who had more than one LDH measurement (34/37) by a mean of 53%. This did not correlate with progression, response, or stable disease. It may be that sorafenib increased tumor hypoxia, anaerobic metabolism, and thereby lactic acid production. Although our data are consistent with a rise in LDH being a pharmacodynamic marker of sorafenib effect, they fail to show that change in LDH is predictive of efficacy and the results must be considered exploratory. Further research in identifying a biomarker for efficacy of antiangiogenic therapy is clearly warranted.
In our study, the hematologic toxicity we observed was greater than expected. There are a number of possible reasons. Sorafenib does not have significant hematologic toxicity by itself, but it could worsen when combined with chemotherapy. This does not seem to be the case, as multiple trials have examined sorafenib with paclitaxel and carboplatin in both lung cancer and melanoma [45], [46]. These trials did not show significantly worse toxicity on the sorafenib arm. An alternate explanation is that there may be a drug‐drug interaction between sorafenib and dacarbazine. In fact, this was seen in the phase I study, such that sorafenib decreases exposure to dacarbazine, but increases 5‐amino‐imidazole‐4‐carboxamide (AIC, a dacarbazine metabolite) effectively increasing its dose [39]. In our previous sorafenib trial, a higher proportion of patients (61%) required sorafenib dose reduction compared with 28% in the pivotal trial in renal cell carcinoma [47]. In this trial, our sorafenib dose reduction rate (49%) was lower than in the earlier study, this being likely because the dose management scheme in the new trial was more liberal, allowing drug interruptions for toxicity, and these did not automatically mandate dose reduction, which was required in the earlier trial.
Conclusion
This trial of sorafenib and dacarbazine met its primary endpoint of DCR at 18 weeks. Without randomized data, we cannot conclude that the combination treatment is superior to either single agent. The combination of dacarbazine 1,000 mg/m2 with sorafenib was associated with significant serious hematologic toxicity. However, the dose of dacarbazine 850 mg/m2 with sorafenib appeared reasonably well tolerated. The combination has modest activity in select sarcoma subtypes.
Acknowledgments
The study was funded by Bayer Pharmaceuticals, the manufacturer of sorafenib. Additional funding was provided by the NCI in the form of a Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center (P30 CA 008748).
Contributed equally.
Author Contributions
Conception/design: David R. D'Adamo, Mary L. Keohan, Richard D. Carvajal, Martee L. Hensley, Catherine M. Hirst, Linda Ahn, Li‐Xuan Qin, Cristina R. Antonescu, Robert A. Lefkowitz, Robert G. Maki, Gary K. Schwartz
Provision of study material or patients: David R. D'Adamo, Mark A. Dickson, Mary L. Keohan, Richard D. Carvajal, Martee L. Hensley, Catherine M. Hirst, Marietta O. Ezeoke, Linda Ahn, Li‐Xuan Qin, Cristina R. Antonescu, Robert A. Lefkowitz, Robert G. Maki, Gary K. Schwartz, William D. Tap
Collection and/or assembly of data: David R. D'Adamo, Mark A. Dickson, Mary L. Keohan, Richard D. Carvajal, Martee L. Hensley, Catherine M. Hirst, Marietta O. Ezeoke, Linda Ahn, Li‐Xuan Qin, Cristina R. Antonescu, Robert A. Lefkowitz, Robert G. Maki, Gary K. Schwartz, William D. Tap
Data analysis and interpretation: David R. D'Adamo, Mark A. Dickson, Mary L. Keohan, Richard D. Carvajal, Martee L. Hensley, Catherine M. Hirst, Marietta O. Ezeoke, Linda Ahn, Li‐Xuan Qin, Cristina R. Antonescu, Robert A. Lefkowitz, Robert G. Maki, Gary K. Schwartz, William D. Tap
Manuscript writing: David R. D'Adamo, Mark A. Dickson, Mary L. Keohan, Richard D. Carvajal, Martee L. Hensley, Li‐Xuan Qin, Robert G. Maki, Gary K. Schwartz, William D. Tap
Final approval of manuscript: David R. D'Adamo, Mark A. Dickson, Mary L. Keohan, Richard D. Carvajal, Martee L. Hensley, Catherine M. Hirst, Marietta O. Ezeoke, Linda Ahn, Li‐Xuan Qin, Cristina R. Antonescu, Robert A. Lefkowitz, Robert G. Maki, Gary K. Schwartz, William D. Tap
Disclosures
David R. D'Adamo: Eisai Inc. (E), Bayer (RF [institution]); Robert G. Maki: Bayer (C/A, SAB); Martee L. Hensley: Sanofi (E [spouse]), EMD Serono, Janssen, Tesaro, Eli Lilly and Company (C/A), Janssen, Bristol‐Myers Squibb (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Singer S, Demetri GD, Baldini EH et al. Management of soft‐tissue sarcomas: An overview and update. Lancet Oncol 2000;1:75–85. [DOI] [PubMed] [Google Scholar]
- 3.Skubitz KM, D'Adamo DR. Sarcoma. Mayo Clin Proc 2007;82:1409–1432. [DOI] [PubMed] [Google Scholar]
- 4.Spath‐Schwalbe E, Genvresse I, Koschuth A et al. Phase II trial of gemcitabine in patients with pretreated advanced soft tissue sarcomas. Anticancer Drugs 2000;11:325–329. [DOI] [PubMed] [Google Scholar]
- 5.Mouridsen HT, Bastholt L, Somers R et al. Adriamycin versus epirubicin in advanced soft tissue sarcomas. A randomized phase II/phase III study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer Clin Oncol 1987;23:1477–1483. [DOI] [PubMed] [Google Scholar]
- 6.Lorigan P, Verweij J, Papai Z et al. Phase III trial of two investigational schedules of ifosfamide compared with standard‐dose doxorubicin in advanced or metastatic soft tissue sarcoma: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 2007;25:3144–3150. [DOI] [PubMed] [Google Scholar]
- 7.Verweij J, Lee SM, Ruka W et al. Randomized phase II study of docetaxel versus doxorubicin in first‐ and second‐line chemotherapy for locally advanced or metastatic soft tissue sarcomas in adults: A study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 2000;18:2081–2086. [DOI] [PubMed] [Google Scholar]
- 8.Borden EC, Amato DA, Rosenbaum C et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–850. [DOI] [PubMed] [Google Scholar]
- 9.Antman K, Crowley J, Balcerzak SP et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–1285. [DOI] [PubMed] [Google Scholar]
- 10.Bay JO, Ray‐Coquard I, Fayette J et al. Docetaxel and gemcitabine combination in 133 advanced soft‐tissue sarcomas: A retrospective analysis. Int J Cancer 2006;119:706–711. [DOI] [PubMed] [Google Scholar]
- 11.Edmonson JH, Ryan LM, Blum RH et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–1275. [DOI] [PubMed] [Google Scholar]
- 12.Hensley ML, Maki R, Venkatraman E et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J Clin Oncol 2002;20:2824–2831. [DOI] [PubMed] [Google Scholar]
- 13.Hensley ML, Blessing JA, Mannel R et al. Fixed‐dose rate gemcitabine plus docetaxel as first‐line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol Oncol 2008;109:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensley ML, Blessing JA, Degeest K et al. Fixed‐dose rate gemcitabine plus docetaxel as second‐line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecol Oncol 2008;109:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoffski P, Maki RG, Italiano A et al. Randomized, open‐label, multicenter, phase III study of eribulin versus dacarbazine in patients (pts) with leiomyosarcoma (LMS) and adipocytic sarcoma (ADI). J Clin Oncol 2015;33(suppl 18):LBA10502a. [Google Scholar]
- 16.Demetri GD, von Mehren M, Jones RL et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Graaf WT, Blay JY, Chawla SP et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 18.Tap WD, Jones RL, Van Tine BA et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft‐tissue sarcoma: An open‐label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med 1971;285:1182–1186. [DOI] [PubMed] [Google Scholar]
- 20.Escudier B, Eisen T, Stadler WM et al. Sorafenib in advanced clear‐cell renal‐cell carcinoma. N Engl J Med 2007;356:125–134. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 22.Awada A, Hendlisz A, Gil T et al. Phase I safety and pharmacokinetics of BAY 43‐9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer 2005;92:1855–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strumberg D, Voliotis D, Moeller JG et al. Results of phase I pharmacokinetic and pharmacodynamic studies of the Raf kinase inhibitor BAY 43‐9006 in patients with solid tumors. Int J Clin Pharmacol Ther 2002;40:580–581. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm SM, Carter C, Tang L et al. BAY 43‐9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–7109. [DOI] [PubMed] [Google Scholar]
- 25.Hobday TJ, Rubin J, Holen K et al. MC044h, a phase II trial of sorafenib in patients (pts) with metastatic neuroendocrine tumors (NET): A Phase II Consortium (P2C) study. J Clin Oncol 2007;25(suppl 18):4504a. [Google Scholar]
- 26.Maki RG, D'Adamo DR, Keohan ML et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 2009;27:3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiebe L, Kasza KE, Maki RG et al. Activity of Sorafenib (SOR) in patients (pts) with Imatinib (IM) and Sunitinib (SU)‐resistant (RES) gastrointestinal stromal tumors (GIST): A phase II trial of the University of Chicago Phase II Consortium. J Clin Oncol 2008;26(suppl 15):10502a. [Google Scholar]
- 28.Reichardt P, Montemurro M, Gelderblom H et al. Sorafenib fourth‐line treatment in imatinib‐, sunitinib‐, and nilotinib‐resistant metastatic GIST: A retrospective analysis. J Clin Oncol 2009;27(suppl 15):10564a. [Google Scholar]
- 29.Ratain MJ, Eisen T, Stadler WM et al. Phase II placebo‐controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24:2505–2512. [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 31.Sandler A, Gray R, Perry MC et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006;355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 32.Giantonio BJ, Catalano PJ, Meropol NJ et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539–1544. [DOI] [PubMed] [Google Scholar]
- 33.Hensley ML, Miller A, O'Malley DM et al. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first‐line treatment for metastatic uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol 2015;33:1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buesa JM, Mouridsen HT, van Oosterom AT et al. High‐dose DTIC in advanced soft‐tissue sarcomas in the adult. A phase II study of the E.O.R.T.C. Soft Tissue and Bone Sarcoma Group. Ann Oncol 1991;2:307–309. [DOI] [PubMed] [Google Scholar]
- 35.McDermott DF, Sosman JA, Gonzalez R et al. Double‐blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: A report from the 11715 Study Group. J Clin Oncol 2008;26:2178–2185. [DOI] [PubMed] [Google Scholar]
- 36.Pacey S, Ratain MJ, Flaherty KT et al. Efficacy and safety of sorafenib in a subset of patients with advanced soft tissue sarcoma from a Phase II randomized discontinuation trial. Invest New Drugs 2011;29:481–488. [DOI] [PubMed] [Google Scholar]
- 37.Van Glabbeke M, Verweij J, Judson I et al. Progression‐free rate as the principal end‐point for phase II trials in soft‐tissue sarcomas. Eur J Cancer 2002;38:543–549. [DOI] [PubMed] [Google Scholar]
- 38.Choi H, Charnsangavej C, Faria SC et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753–1759. [DOI] [PubMed] [Google Scholar]
- 39.Brendel E, Ludwig M, Lathia C et al. Pharmacokinetic results of a phase I trial of sorafenib in combination with dacarbazine in patients with advanced solid tumors. Cancer Chemother Pharmacol 2011;68:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Judson I, Radford JA, Harris M et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: A study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2001;37:870–877. [DOI] [PubMed] [Google Scholar]
- 41.D'Adamo DR, Anderson SE, Albritton K et al. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft‐tissue sarcomas. J Clin Oncol 2005;23:7135–7142. [DOI] [PubMed] [Google Scholar]
- 42.Choi H. Critical issues in response evaluation on computed tomography: Lessons from the gastrointestinal stromal tumor model. Curr Oncol Rep 2005;7:307–311. [DOI] [PubMed] [Google Scholar]
- 43.Benjamin RS, Choi H, Macapinlac HA et al. We should desist using RECIST, at least in GIST. J Clin Oncol 2007;25:1760–1764. [DOI] [PubMed] [Google Scholar]
- 44.Van Der Graaf WT, Blay J, Chawla SP et al., PALETTE: A randomized, double‐blind, phase III trial of pazopanib versus placebo in patients (pts) with soft‐tissue sarcoma (STS) whose disease has progressed during or following prior chemotherapy—An EORTC STBSG Global Network Study (EORTC 62072). J Clin Oncol 2011;29(suppl 18):LBA10002a. [DOI] [PubMed] [Google Scholar]
- 45.Scagliotti G, Novello S, von Pawel J et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non‐small‐cell lung cancer. J Clin Oncol 2010;28:1835–1842. [DOI] [PubMed] [Google Scholar]
- 46.Hauschild A, Agarwala SS, Trefzer U et al. Results of a phase III, randomized, placebo‐controlled study of sorafenib in combination with carboplatin and paclitaxel as second‐line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 2009;27:2823–2830. [DOI] [PubMed] [Google Scholar]
- 47.Escudier B, Szczylik C, Eisen T et al. Randomized phase III trial of the Raf kinase and VEGFR inhibitor sorafenib (BAY43‐9006) in patients with advanced renal cell carcinoma (RCC). J Clin Oncol 2005;23(suppl 16):LBA4510a. [Google Scholar]