To better characterize the activity of taxanes in small bowel cancer, a single center retrospective review of patients treated with taxane‐based therapy was completed, and the results are reported in this brief communication.
Abstract
Currently, treatment of small bowel adenocarcinoma (SBA) mirrors that of colorectal cancer (CRC). Recent genomic data have demonstrated SBA to be a genetically unique entity, suggesting that therapies not traditionally utilized in CRC should be explored. In order to further characterize the activity of taxanes in this rare cancer, we completed a single‐center retrospective study. Twenty patients were found to have been treated with taxane‐based regimens (monotherapy in 3, combination therapy in 17). Median time to progression was 3.8 months (95% confidence interval [CI] 2.9–4.6), and median overall survival was 10.7 months (95% CI: 3.1–18.3). The results of this study demonstrate clinical activity from taxane‐based therapy in advanced SBA and support further clinical trial investigation.
摘要
目前,小肠腺癌 (SBA) 的治疗复制了结直肠癌 (CRC) 的治疗。当前的基因组数据证明,从遗传学角度来看,SBA 是一种独特的存在,建议我们应该探索在传统上未用于治疗 CRC 的疗法。为了进一步描绘紫杉烷在这种罕见癌症中的活性特征,我们完成了一项单中心回顾性研究。我们找到 20 名已经接受紫杉烷类方案治疗的患者(3 名患者接受单药治疗,17 名患者接受联合治疗)。中位至进展时间为 3.8 个月 [95% 置信区间(CI)2.9–4.6],中位总生存期为 10.7 个月(95% CI:3.1–18.3)。本研究结果表明紫杉烷类化疗在晚期 SBA 中具有临床活性并支持进一步开展临床试验调查。
Introduction
Approximately one third of the 10,400 new cases of small bowel cancer in 2018 will be adenocarcinomas, and an estimated 27% of these patients will present with distant metastasis [1], [2]. Phase II trials have demonstrated fluoropyrimidine plus platinum‐based chemotherapy to be the preferred initial regimen for metastatic disease, but optimal treatments in the second‐line and beyond have not been established [3], [4].
To date, the treatment approach for small bowel adenocarcinoma (SBA) closely mirrors that for colorectal cancer (CRC). However, recent genomic data demonstrates SBA to be a unique genomic entity, differing from both gastric cancer and CRC. Specifically, the rate of APC mutations in SBA was significantly different from both gastric and CRC (27% vs. 8% and 76%, respectively), and the rate of KRAS mutations was significantly different from gastric cancer (54% vs. 14%, respectively) [5]. This suggests that classes of agents not routinely utilized in CRC treatment should be explored in SBA.
One such class, taxanes, commonly used in gastric cancer have only recently been reported in the treatment of SBA. A Phase II study of nab‐paclitaxel as treatment for CpG island methylator phenotype‐high CRC included 10 patients with SBA [6]. Although the treatment demonstrated a 0% response rate in patients with CRC, there was a 20% response rate in the patients with SBA. To further characterize the activity of taxanes in SBA, we completed a single center retrospective review of patients treated with taxane‐based therapy.
Materials and Methods
Patients treated from 2010 to 2017 at The University of Texas MD Anderson Cancer Center with SBA were reviewed. Eligible patients had advanced SBA, received greater than one cycle of a taxane‐based regimen, and had a radiographic tumor response evaluation. Radiographic response was assessed retrospectively based upon the treating physician's assessment and categorized as response, stable disease, or progression. Patients with ampullary adenocarcinomas or those enrolled in a clinical trial were excluded.
Baseline characteristic data of the study population was obtained (see Table 1). Median time to progression (TTP) and median overall survival (OS) were determined using the Kaplan‐Meier method. Subgroup analysis was completed for location of disease (duodenum vs. jejunum and ileum) and type of therapy (combination vs. monotherapy). Log‐rank of p < .05 was considered statistically significant.
Table 1. Patients’ baseline characteristics.
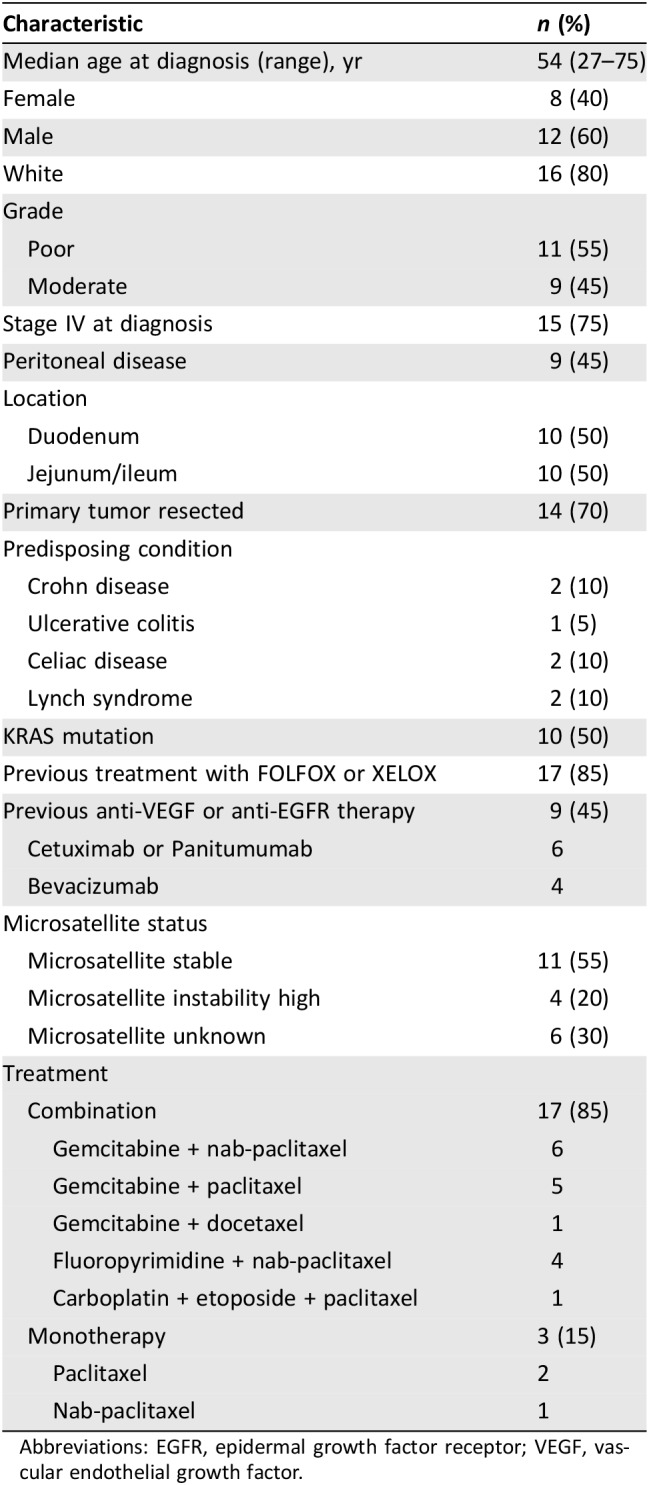
Abbreviations: EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor.
Results
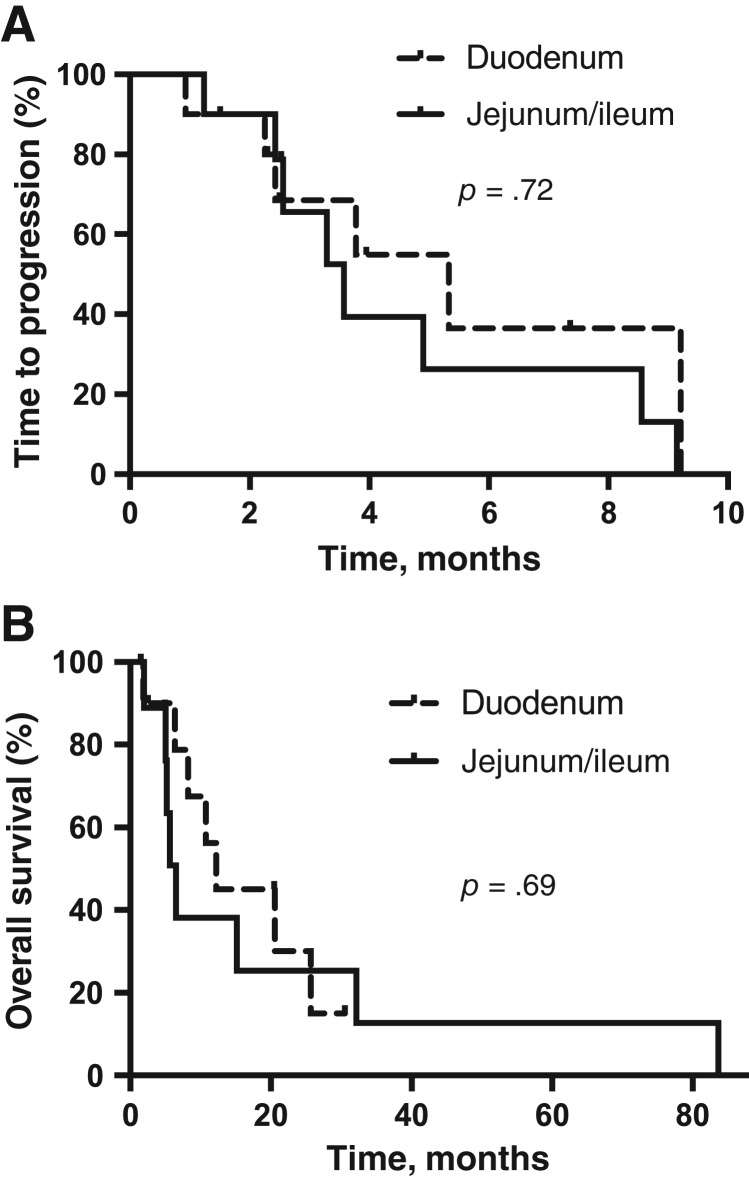
Three hundred and twelve patients with SBA were identified. Twenty patients were found to have been treated with taxane‐based therapy and met inclusion criteria. Seventeen patients were treated with combination therapy (gemcitabine combination in 12, fluoropyrimidine combination in 4, and platinum combination in 1), and three were treated with monotherapy. The taxane used was paclitaxel in two and nab‐paclitaxel in one. Tumor response was seen in 30%, stable disease in 35%, and progressive disease in 35% of patients. When stratified by tumor site, tumor response was 30% for duodenal and 30% for jejunal and ileal primaries. Median TTP was 3.8 months (95% confidence interval [CI], 2.9–4.6) and median OS was 10.7 months (95% CI, 3.1–18.3). No significant difference in TTP or OS was found between duodenum versus jejunum and ileum small bowel site (p = .72 and .69, respectively; Fig. 1). No significant differences in TTP or OS were seen between various chemotherapy combinations (supplemental online Fig. 1). Of the four patients with microsatellite instability‐high, three demonstrated a response to therapy.
Figure 1.
Kaplan‐Meier survival curves for SBA patients treated with taxane‐based therapy. (A): Time to progression. (B): Overall survival.
Discussion
Classically, taxanes have not been utilized for SBA treatment, and investigations into their efficacy is lacking. To the best of our knowledge, this is the largest cohort of patients with advanced SBA treated with taxane‐based therapy reported in the literature. The overall TTP supports the conclusion that taxanes have activity in SBA. Additionally, there was no significant difference between the TTP or OS in combination therapy versus monotherapy. Although 85% of patients had combination therapy, the majority were previously treated with fluoropyrimidine‐based regimens, and gemcitabine has not demonstrated clinical activity in intestinal cancers [7].
In addition to establishing SBA as genetically unique compared with CRC and gastric cancer, the recent article by Schrock et al. also noted that genomic alterations were similar when comparing duodenal to jejunal and ileal small bowel locations [5]. This is consistent with our findings, in which we found similar efficacy irrespective of small bowel tumor site.
The study is limited by the small sample size, inability to retrospectively perform RECIST tumor assessment, and single‐institution nature. However, this is the largest collection of taxane‐treated SBA patients in the literature. Given the rarity of this cancer, small retrospective trials such as this are beneficial to the management of this cancer. Additional retrospective and prospective trials would help to further validate these results and support the use of taxane regimens for treatment of SBA.
See http://www.TheOncologist.com for supplemental material available online.
Disclosures
The authors indicated no financial relationships.
References
- 1.American Cancer Society . Key statistics for small intestine cancer. Available at: https://www.cancer.org/cancer/small‐intestine‐cancer/about/what‐is‐key‐statistics.html. Accessed June 23, 2018.
- 2.National Cancer Institute . Cancer stat facts: Small intestine cancer. Available at: https://seer.cancer.gov/statfacts/html/smint.html. Accessed June 23, 2018.
- 3.Overman M, Varadhachary G, Kopetz S et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol 2009;27(16):2598–2603. [DOI] [PubMed] [Google Scholar]
- 4.Xiang X, Liu Y, Zhang L et al. A Phase II study of modified FOLFOX as first‐line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs 2012; 23(5):551–566. [DOI] [PubMed] [Google Scholar]
- 5.Schrock A, Devoe C, McWilliams R et al. Genomic profiling of small‐bowel adenocarcinoma. JAMA Oncol 2017;3:1546–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overman M, Adam L, Raghav K et al. Phase II study of nab‐paclitaxel in refractory small bowel adenocarcinoma and CpG island methylator phenotype (CIMP)‐high colorectal cancer. Ann Oncol 2018;29(1):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiménez‐Fonseca P, Solis MP, Garrido M et al. Gemcitabine plus capecitabine (Gem‐Cape) biweekly in chemorefractory metastatic colorectal cancer. Clin Transl Oncol 2015;17:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]