This article reports on the association between phase angle, which is measured by bioelectric impedance analysis and reflects cellular integrity and hydration level, and survival in patients with advanced cancer.
Keywords: Electric impedance, Forecasting, Neoplasms, Palliative care, Prognosis, Survival
Abstract
Background.
Phase angle is a prognostic factor in patients with months of survival, but its accuracy has not been examined in patients with weeks/days of survival. We determined the association between phase angle and survival in patients with advanced cancer admitted to an acute palliative care unit (APCU).
Subjects, Materials, and Methods.
We prospectively assessed phase angle in consecutive patients with advanced cancer admitted to our APCU. We conducted univariate and multivariate survival analyses adjusting for established prognostic factors. Post hoc subgroup analyses examined patients with and without edema.
Results.
Among 204 patients, the median overall survival was 10 days (95% confidence interval [CI] 8–11 days). Seventy‐four (36%) did not have edema. The median phase angle was 3.7° for the entire cohort, 3.9° for the nonedematous subgroup and 3.6° for the edematous subgroup. In univariate analysis, a low phase angle was associated with decreased survival for the entire cohort (≤3° vs. >3°, median survival 7 vs. 10 days, p = .045) and the nonedematous subgroup (5 vs. 18 days, p < .001) but not the edematous subgroup (9 vs. 9 days, p = .84). In multivariate analysis, phase angle did not reach significance for the entire cohort but remained significant in the nonedematous subgroup (hazard ratio 2.46, 95% CI 1.14–5.31, p < .001). Specifically, phase angle ≤3° had an accuracy of 86% (95% CI 77%–93%) for 3‐day survival in patients without edema.
Conclusion.
Phase angle had limited prognostic utility in unselected APCU patients but was significant in the nonedematous subgroup. Further studies are required to confirm these preliminary findings.
Implications for Practice.
In this prospective study involving 204 patients with advanced cancer, phase angle as measured by bioelectric impedance analysis was a significant predictor of mortality independent of known prognostic factors in patients without edema but not patients with edema. Among patients without edema, a phase angle ≤3° had an accuracy of 86% for 3‐day survival, which may inform the diagnosis of impending death and potentially end‐of‐life decision making.
Introduction
In the last weeks of life, patients with cancer, their caregivers, and health care professionals alike are faced with many complex and difficult decisions regarding the initiation and/or discontinuation of chemotherapy, indwelling catheters, artificial nutrition, hospice care, and discharge from the hospital [1], [2]. For these issues, a patient's life expectancy (weeks vs. days) can be a major factor in the care planning process. Although clinicians often rely on their experience to make survival estimation, clinician prediction of survival (CPS) suffers from subjectivity and low accuracy [3], [4]. Prognostic models such as the Palliative Prognostic (PaP) Score have been found to be more accurate than CPS [5], but are difficult to interpret and rarely used. Thus, there is a need to develop simple, objective prognostic tools to facilitate clinical decision making.
One promising prognostic marker is phase angle, which is measured by bioelectric impedance analysis (BIA) and reflects cellular membrane integrity and hydration level. It typically ranges between 4 and 9 in healthy individuals and decreases with increasing age, female sex, lower body mass index, and various disease states [6], [7]. Phase angle has been studied in both oncology and nononcology patient populations. A lower phase angle was consistently found to be associated with a shorter survival [8], [9]. This objective, inexpensive, and noninvasive assessment can be easily conducted at bedside, making it a potentially attractive tool to facilitate clinical decision making. However, a vast majority of the studies have been conducted in patients with survival of months or longer [10], [11], [12], [13], [14], [15], [16].
To date, only one small study has examined phase angle in a small cohort of patients with days to weeks of survival [17]. If phase angle could accurately identify whether patients will die in the next few days, it may facilitate the diagnosis of impending death (defined as 3 days or less or survival) and support clinical decision making during this critical time, such as the need for investigations, appropriateness of various treatments, and discharge planning [1], [18], [19]. In this study, we determined the association between phase angle and survival in patients with advanced cancer admitted to an acute palliative care unit (APCU) who had a median survival of days to weeks.
Subjects, Materials, and Methods
Eligibility Criteria
This is a single‐center, prospective, longitudinal, observational study. Adult patients (aged ≥18) with a diagnosis of advanced cancer were eligible for this study if they were within 3 days of admission to the APCU at the University of Texas MD Anderson Cancer Center in Houston, Texas, and had parenteral hydration within the past 48 hours. The requirement for parenteral hydration was because previous studies reported that dehydration could negatively affect the accuracy of BIA [20]. Patients were excluded if they had extensive local infection/wound preventing placement of BIA pads, defibrillator, or cardiac pacemaker or any implanted electrical device, or were unable to communicate in English. The APCU was selected as the study setting because many complex end‐of‐life decisions are made during this admission, and the patient population had a relatively homogenous overall survival of 3 weeks or less [21].
The Institutional Review Board at MD Anderson Cancer Center approved this study. We obtained written informed consent from patients, or written surrogate permission from the medical power of attorney or legal representative based on the understanding of the patient's values and preferences if the patient was delirious. Enrollment occurred between April 21, 2015, and August 24, 2016.
Data Collection
In this prospective study, we collected baseline patient demographics on the day of study enrollment, including age, sex, race, cancer diagnosis, disease stage, Palliative Prognostic Score (PaP), and Palliative Prognostic Index (PPI). The PaP score is a prognostic model validated for patients with advanced cancer. It consists of six variables (dyspnea at rest, anorexia, Karnofsky Performance Status (KPS), clinician prediction of survival, total leukocyte count, and lymphocyte percentage). The total score ranges between 0 and 17.5, with a higher score indicating worse survival [22], [23]. PPI represents another validated prognostic tool that includes Palliative Performance Status, oral intake, edema, dyspnea at rest, and delirium [24], [25]. PPI is scored between 0 and 15, with a score > 6 indicating poorer survival.
We assessed phase angle, Edmonton Symptom Assessment System (ESAS), and the Memorial Delirium Assessment Scale (MDAS) once daily from enrollment until discharge, except for weekends because of lack of research staff.
Phase angle was measured using the RJL Systems Quantum IV (Clinton Township, MI). With the patient in supine position, the research staff first placed an electrode over the right foot between the medial and the lateral malleoli at the ankle and another over the middle of the dorsal surface of the right hand between the distal prominence of the radius and the ulnar styloid. Subsequently, a single 50 kHz frequency alternating low voltage electrical current was applied to detect the reactance (Xc) and resistance (R) over the right hemibody, which were used to calculate the phase angle based on the following formula: Phase angle (in degrees) = Atan(Xc/R). The entire procedure took less than 5 minutes, and patients may or may not be receiving medications or fluids intravenously at the time of assessment. Phase angle has been shown to have high reliability and predictive validity in previous studies [11], [26].
ESAS is a validated 10‐item symptom battery that examines the intensity of pain, fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, appetite, feeling of well‐being, and sleep. The average intensity of each symptom over the past 24 hours is rated using a numeric rating scale that ranges from 0 (none) to 10 (worst) [27].
MDAS is a 10‐item clinician‐rated assessment scale validated for assessment of delirium in patients with cancer [28], [29]. It examines the level of consciousness, disorientation, memory, recall, attention, disorganized thinking, perceptual disturbance, delusions, psychomotor activity, and sleep, assigning a score between 0 and 3 for each item, for a total score between 0 and 30. A score of 13 or higher indicates delirium.
Survival data from study enrollment were collected from institutional databases and electronic health records.
Statistical Analysis
Based on the previous data, the median survival in the APCU population was 21 days [21]. For the purpose of sample size justification, we considered the median of phase angle as the cutoff point to divide patients equally into two groups. We estimated that 194 patients (97 patients with high phase angle and 97 with low phase angle) would provide 80% power to detect a hazard ratio (HR) of 1.5, with the overall survival rate of 43% in patients with low phase angle versus 57% in patients with high phase angle at 21 days, using the two‐sided log‐rank test with a 5% significance level. We enrolled a total of 204 patients to account for an expected attrition rate of 5%.
We summarized the baseline demographics using descriptive statistics, including mean, SD, median, interquartile range (IQR), frequency, and percentage.
We estimated overall survival using the Kaplan‐Meier method and compared among degrees of phase angle using the log‐rank test. Because the ideal cutoff has not been defined for this population, we prespecified the median value as cutoff. We also applied the Contal and O'Quigley method [30], a modified log‐rank statistical procedure that examined all available cut points of phase angle to identify the optimal cutoff based on the maximal difference in survival outcome between two groups.
The univariate Cox Proportional Hazards model was used to assess the effect of phase angle, patient characteristics (age, sex, race, cancer type), and prognostic variables (Karnofsky performance status, PaP Score, PPI, and MDAS) on overall survival. We then included age, sex, race, cancer type, PaP Score, phase angle, and MDAS in a multivariate model with stepwise selection. PPI was not included in the model because of its high correlation with PaP Score. Because peripheral edema is common among patients with cancer in the last weeks/days of life, and edematous status may affect phase angle readings (lower resistance = high values), we conducted post hoc subgroup analyses on patients with and without clinical evidence of peripheral edema.
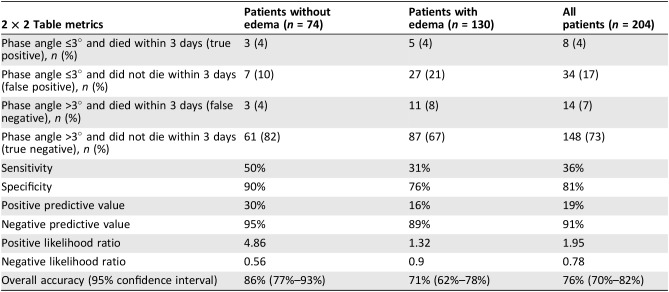
We constructed a 2 × 2 table to assess the accuracy of phase angle (≤3° based on the cutoff identified by the Contal and O'Quigley method above) to inform the diagnosis of impending death (i.e., ≤3 days of phase angle assessment) [18], [19]. We then calculated the specificity, sensitivity, positive predictive value, negative predictive value, and overall accuracy for phase angle to inform the diagnosis of impending death.
All computations were carried out in SAS 9.3 (SAS Institute, Cary, NC) and RStudio version 1.0.136 (Integrated Development for R. RStudio, Inc., Boston, MA). A p value of <.05 is considered to be statistically significant.
Results
Patient Characteristics
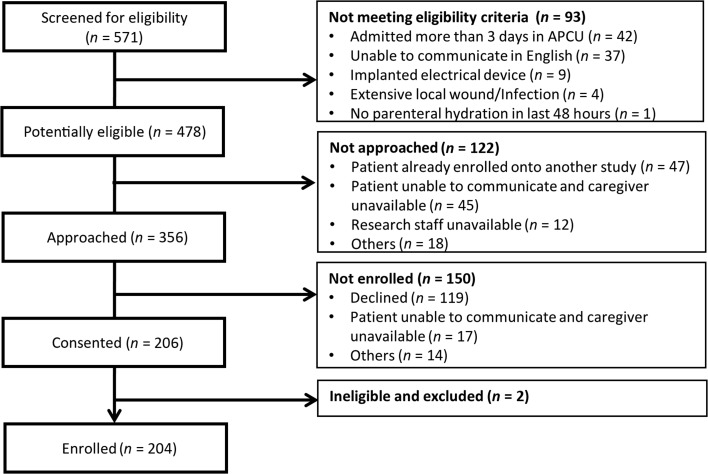
Among the 571 patients admitted to the APCU during the study period, 356 (62%) were eligible and 204 (57%) participated in this study (Fig. 1).
Figure 1.
Study flow diagram.
Abbreviation: APCU, acute palliative care unit.
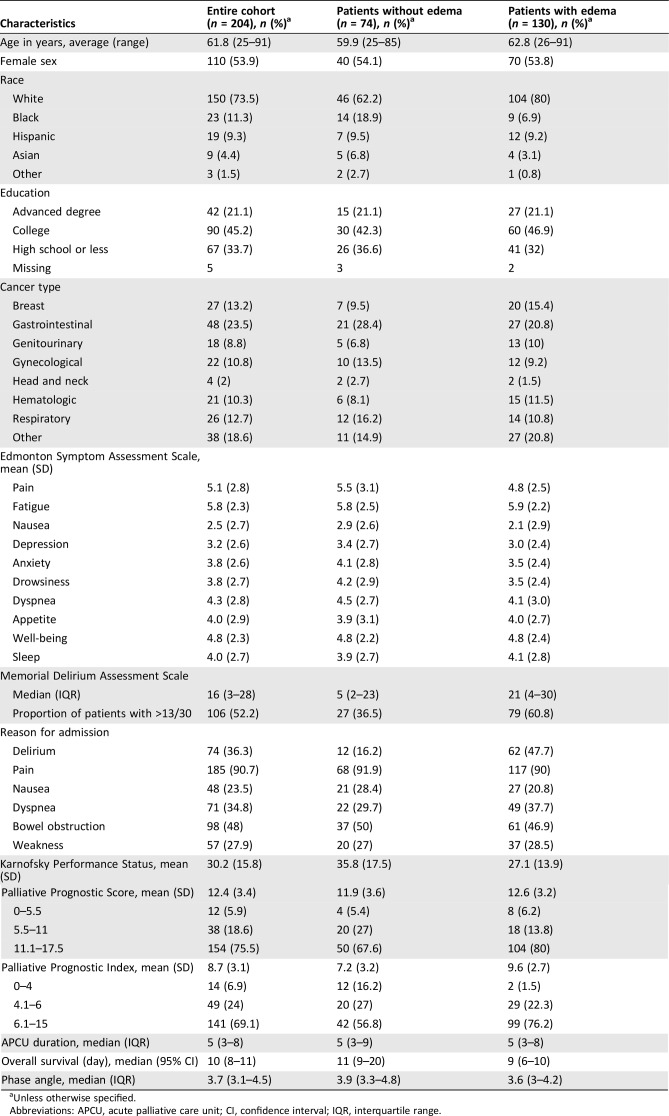
As shown in Table 1, the mean age was 62 (range 25–91), and 110 patients (54%) were female. Over half (n = 106, 52%) were delirious. The mean KPS was 30% (SD 16%). The median survival was 10 days (95% confidence interval [CI] 8–11 days) for the entire cohort and 11 days (95% CI 9–20 days) for the 74 (36%) patients without edema.
Table 1. Baseline characteristics (n = 204).
Unless otherwise specified.
Abbreviations: APCU, acute palliative care unit; CI, confidence interval; IQR, interquartile range.
Survival Analysis with Overall Cohort
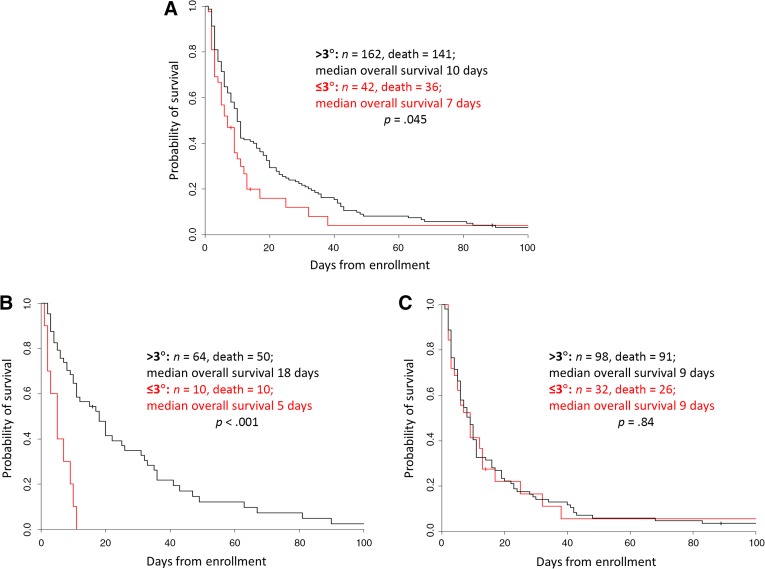
The median phase angle at the time of study enrollment was 3.7° (IQR 3.1°–4.5°). There was no significant difference in survival using 3.7° as the cutoff (log‐rank test p = .28). The Contal and O'Quigley method identified the phase angle cutoffs of 2.5° to 3° were significant for univariate overall survival analysis. Specifically, a phase angle ≤3° was associated with decreased survival compared with >3° in univariate analysis for the entire cohort (median survival 7 vs. 10 days, p = .045; Fig. 2A) and for the nonedematous cohort (median survival 5 vs. 18 days, p < .001; Fig. 2B).
Figure 2.
Kaplan‐Meier survival curves. A phase angle value of 3.0 was identified as the ideal cutoff. A lower phase angle was associated with shorter survival. All 204 patients (A), patients without edema (B), and patients with edema (C).
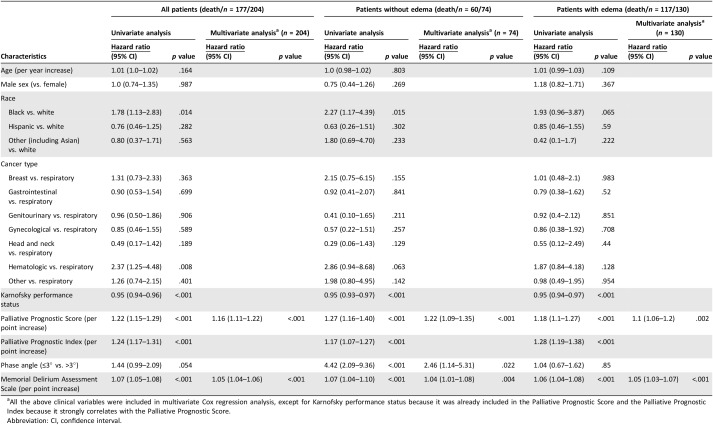
In univariate Cox regression analysis, phase angle of ≤3° was associated with a shorter survival in the nonedematous cohort (HR 4.42, 95% CI 2.09–9.36, p < .001), but not the entire cohort (HR 1.44, 95% CI 0.99–2.09, p = .054) or the edematous cohort (HR 1.04, 95% CI 0.67–1.62, p = .85). As shown in Table 2, phase angle remained an independent prognostic factor after adjusting for established prognostic factors in multivariable analysis in the nonedematous cohort (HR 2.46, 95% 1.14–5.31, p = .022).
Table 2. Univariate and multivariate Cox regression analysis.
All the above clinical variables were included in multivariate Cox regression analysis, except for Karnofsky performance status because it was already included in the Palliative Prognostic Score and the Palliative Prognostic Index because it strongly correlates with the Palliative Prognostic Score.
Abbreviation: CI, confidence interval.
Diagnosis of Impending Death
In the nonedematous cohort, phase angle ≤3° was associated with death within 3 days of phase angle assessment, with a sensitivity of 50% and specificity of 90%. The overall accuracy was 86% (95% CI 77%–93%) for 3‐day survival (Table 3).
Table 3. Association between phase angle and impending death within 3 days.
Discussion
In this study of patients admitted to an APCU and with a median survival of 10 days, the association between phase angle and overall survival was of borderline significance in the entire cohort (p = .045 in log‐rank test and p = .054 in univariate Cox regression). Post hoc subgroup analyses revealed that edematous status was a key determinant of prognostic utility. Specifically, phase angle was significantly associated with a shorter survival in patients without peripheral edema but not in patients with edema. This study highlights the importance of patient selection when applying phase angle in both clinical practice and research. Further research is needed to assess how phase angle may be used to facilitate clinical decision making in patients without edema.
Patients seen by palliative care often have advanced‐stage disease and have a short survival. The ability to differentiate if patients have months, weeks, or days of survival accurately is of paramount importance for clinical decision making. Norman et al. examined the prognostic utility of phase angle in 399 consecutive hospitalized patients with cancer (61% had advanced‐stage disease) [11]. The investigators reported that phase angle was more accurate at predicting 6‐month survival than malnutrition and disease severity in multivariate analysis.
To date, there have only been a handful of studies examining phase angle in the advanced cancer palliative care setting, all consistently validating the prognostic significance of phase angle. Davis et al. examined phase angle in 50 patients with advanced cancer from inpatient units with a median survival of approximately 2 months, and reported that a higher phase angle had a nonsignificant trend toward longer survival (univariate HR 0.81, 95% CI 0.66–1.01, p = .57; multivariate HR 0.79, 95% CI 0.61–1.01, p = .064) [15]. In another study, Perez Camargo et al. enrolled 452 patients hospitalized in a palliative care unit, and found that a phase angle of ≥4° was associated with longer survival (median 86 days vs. 163 days, p > .0001) [31]. Because the BIA device used in this study required patients to be able to stand, patients with poor Karnofsky Performance Status ≤40% or delirium were excluded. Our research team also examined the association between phase angle and survival in 222 patients with advanced cancer seen by the inpatient palliative care consultation team with a median survival of 106 days. Phase angle remained a significant prognostic marker (HR 0.86, 95% CI 0.74–0.99, p = .04) independent of the PaP Score, lean body mass, and serum albumin [10]. More recently, a separate study also confirmed the prognostic utility of phase angle among 366 patients at the outpatient palliative care clinic with a longer median overall survival of 250 days. The multivariate HR was 0.85 (95% CI 0.72–0.99, p = .048) [32]. A separate study in Mexico that included 628 palliative outpatients also identified phase angle ≤4° to be a significant prognostic marker (HR 1.9, 95% CI 1.6–2.4, p < .0001) [31].
Although the above studies have established that phase angle is a prognostic factor in patients with months of survival, it is less clear if its discriminatory power extends to the last weeks to days of life. In a small study that included 28 patients admitted to a palliative care unit in Korea, Lee et al. reported that phase angle <4.4° was associated with a shorter survival (23 days vs. 54 days, p = .013; multivariate Cox regression HR 0.64, 95% CI 0.42–0.95, p = .028) [17]. Importantly, this study excluded patients with edema, pleural effusion, pericardial effusion, and ascites. In our current study, phase angle only retained its significance in the multivariable model in the nonedematous cohort, further supporting that phase angle should be limited to patients without edema.
At the end of life, edema is particularly common because of various complications such as hypoalbuminemia, lymphatic obstruction, and renal failure. Consistent with the literature, patients with edema had poorer survival in our study (9 days vs. 11 days). These patients also had a lower phase angle (median 3.6 vs. 3.9), although phase angle was not significantly associated with survival in this subgroup. Although resistance is reduced in edematous state, cellular function may also be negatively affected, leading to decreased reactance and thus a lower phase angle [33]. Conversely, acute dehydration is associated with higher resistance, reactance, and phase angles [20]. Indeed, bioelectrical impedance vector analysis has been used to examine hydration status in patients with advanced cancer, with a shorter vector indicating more hydration and longer vector indicating less hydration [34]. Interestingly, patients with edema were often not excluded from many previous studies [11], [15]. Based on our preliminary findings, future studies may consider stricter eligibility criteria. Further research is needed to determine how the extent and location of edema may impact the prognostic accuracy of phase angle.
We found that a low phase angle of ≤3° was associated with impending death within 3 days. We were particularly interested in this time frame because it has practical implications on end‐of‐life care, such as informing family of the last chance to say goodbye, discontinuing invasive investigations or aggressive therapies, and focusing purely on comfort care. Our previous research demonstrated that the human body often undergoes various neurocognitive, neuromuscular, and cardiovascular changes in the final days of life that could be detected by various tell‐tale physical signs [18], [19], [35]. This current study supports that this deterioration also occurs at the cellular level (i.e., cellular membrane permeability affecting reactance), which could be detected by bioelectrical impedance analysis. Thus, phase angle represents a novel objective bedside assessment to facilitate diagnosis of impending death.
Similar to signs of impending death [18], [19], it has a high specificity but lower sensitivity. Thus, a phase angle of ≤3° suggests that impending death is likely, although a phase angle of >3° cannot rule out impending death. The positive likelihood ratio was 4.9, suggesting it is moderately useful to increase the post‐test probability of death within 3 days. Because phase angle is a quick measurement (<3 minutes) whereas clinical signs require more longitudinal observation, phase angle may offer additional advantage. Further studies are needed to determine how phase angle may be used in combination with the physical signs of impending death to further improve the accuracy of this challenging diagnosis.
Strengths of this study included a relatively large sample size adequately powered to detect a difference, the fact that we included patients with delirium, and inclusion of validated prognostic factors and scores in the multivariate model. This study has several limitations. First, it was conducted in a single palliative care unit in a comprehensive cancer center. We also required parenteral hydration to minimize the risk of dehydration. The patient population is unique and our findings may be less generalizable to other settings. Second, a substantial minority (n = 119, 33%) of patients or families approached by our research staff were not interested in participating in this study. Given that the median survival in this population was only 10 days and many patients were admitted to the APCU because of distress, an enrollment rate of 58% was reasonable and consistent with other studies in this setting [36]. Third, the subgroup analyses were conducted post hoc and should be considered hypothesis‐generating only. Because the nonedematous subgroup only constituted one third of patients, the small sample size and multiple comparisons may contribute to false‐negative or false‐positive findings, respectively. Future studies are needed to validate our findings.
Conclusion
Phase angle ≤3° was independently associated with a shorter survival in patients without edema after accounting for established prognostic factors and was predictive of impending death within 3 days. Phase angle had limited prognostic value in patients with edema. Upon further validation, phase angle may be used to facilitate clinical decision making at the end of life.
Acknowledgments
We thank all the patients and families who participated in this study. We are also grateful to all the clinicians at the acute palliative care unit at MD Anderson Cancer Center for their support of this project. Part of this manuscript will be presented in a Poster Discussion session at ASCO 2018 Annual Meeting, Chicago, Illinois, USA. This work was supported in part by a National Institutes of Health grants (R21CA186000‐01A1; 1R01CA214960‐01A1; R21NR016736‐01 to D.H.), an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (MRSG‐14‐1418‐01‐CCE to D.H.), and the Andrew Sabin Family Fellowship (to D.H.). This work was also supported by the National Institutes of Health Cancer Center Support Grant (CA016672 to M.P. and D.L.).
Author Contributions
Conception/design: David Hui, Jessica Moore, Minjeong Park, Diane Liu, Eduardo Bruera
Provision of study material or patients: David Hui, Jessica Moore
Collection and/or assembly of data: David Hui, Jessica Moore
Data analysis and interpretation: David Hui, Jessica Moore, Minjeong Park, Diane Liu, Eduardo Bruera
Manuscript writing: David Hui
Final approval of manuscript: David Hui, Jessica Moore, Minjeong Park, Diane Liu, Eduardo Bruera
Disclosures
The authors indicated no financial relationships.
References
- 1.Hui D. Prognostication of survival in patients with advanced cancer: Predicting the unpredictable? Cancer Control 2015;22:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weeks JC, Catalano PJ, Cronin A et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012;367:1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui D, Kilgore K, Nguyen L et al. The accuracy of probabilistic versus temporal clinician prediction of survival for patients with advanced cancer: A preliminary report. The Oncologist 2011;16:1642–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez‐Cruz PE, Dos Santos R, Silva TB et al. Longitudinal temporal and probabilistic prediction of survival in a cohort of patients with advanced cancer. J Pain Symptom Manage 2014;48:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui D, Park M, Liu D et al. Clinician prediction of survival versus the Palliative Prognostic Score: Which approach is more accurate? Eur J Cancer 2016;64:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbosa‐Silva MC, Barros AJ, Wang J et al. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am J Clin Nutr 2005;82:49–52. [DOI] [PubMed] [Google Scholar]
- 7.Norman K, Stobaus N, Pirlich M et al. Bioelectrical phase angle and impedance vector analysis‐‐Clinical relevance and applicability of impedance parameters. Clin Nutr 2012;31:854–861. [DOI] [PubMed] [Google Scholar]
- 8.Barbosa‐Silva MC, Barros AJ. Bioelectrical impedance analysis in clinical practice: A new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care 2005;8:311–317. [DOI] [PubMed] [Google Scholar]
- 9.Abad S, Sotomayor G, Vega A et al. The phase angle of the electrical impedance is a predictor of long‐term survival in dialysis patients. Nefrologia 2011;31:670–676. [DOI] [PubMed] [Google Scholar]
- 10.Hui D, Bansal S, Morgado M et al. Phase angle for prognostication of survival in patients with advanced cancer: Preliminary findings. Cancer 2014;120:2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman K, Stobaus N, Zocher D et al. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr 2010;92:612–619. [DOI] [PubMed] [Google Scholar]
- 12.Gupta D, Lammersfeld CA, Burrows JL et al. Bioelectrical impedance phase angle in clinical practice: Implications for prognosis in advanced colorectal cancer. Am J Clin Nutr 2004;80:1634–1638. [DOI] [PubMed] [Google Scholar]
- 13.Gupta D, Lis CG, Dahlk SL et al. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr 2004;92:957–962. [DOI] [PubMed] [Google Scholar]
- 14.Gupta D, Lammersfeld CA, Vashi PG et al. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer 2008;8:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MP, Yavuzsen T, Khoshknabi D et al. Bioelectrical impedance phase angle changes during hydration and prognosis in advanced cancer. Am J Hosp Palliat Care 2009;26:180–187. [DOI] [PubMed] [Google Scholar]
- 16.Gupta D, Lammersfeld CA, Vashi PG, et al. Bioelectrical impedance phase angle in clinical practice: Implications for prognosis in stage IIIB and IV non‐small cell lung cancer. BMC Cancer 2009;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SY, Lee YJ, Yang JH et al. The association between phase angle of bioelectrical impedance analysis and survival time in advanced cancer patients: Preliminary study. Korean J Fam Med 2014;35:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui D, Dos Santos R, Chisholm G et al. Bedside clinical signs associated with impending death in patients with advanced cancer: Preliminary findings. Cancer 2015;121:960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui D, dos Santos R, Chisholm G et al. Clinical signs of impending death in cancer patients. The Oncologist 2014;19:681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dal Cin S, Braga M, Molinari M et al. Role of bioelectrical impedance analysis in acutely dehydrated subjects. Clin Nutr 1992;11:128–133. [DOI] [PubMed] [Google Scholar]
- 21.Hui D, Elsayem A, Palla S et al. Discharge outcomes and survival of patients with advanced cancer admitted to an acute palliative care unit at a comprehensive cancer center. J Palliat Med 2010;13:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glare P, Virik K. Independent prospective validation of the PaP score in terminally ill patients referred to a hospital‐based Palliative Medicine consultation service. J Pain Symptom Manage 2001;22:891–898. [DOI] [PubMed] [Google Scholar]
- 23.Maltoni M, Nanni O, Pirovano M et al. Successful validation of the palliative prognostic score in terminally ill cancer patients. Italian Multicenter Study Group on Palliative Care. J Pain Symptom Manage 1999;17:240–247. [DOI] [PubMed] [Google Scholar]
- 24.Morita T, Tsunoda J, Inoue S et al. The Palliative Prognostic Index: A scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer 1999;7:128–133. [DOI] [PubMed] [Google Scholar]
- 25.Stone CA, Tiernan E, Dooley BA. Prospective validation of the palliative prognostic index in patients with cancer. J Pain Symptom Manage 2008;35:617–622. [DOI] [PubMed] [Google Scholar]
- 26.Dittmar M. Reliability and variability of bioimpedance measures in normal adults: Effects of age, gender, and body mass. Am J Phys Anthropol 2003;122:361–370. [DOI] [PubMed] [Google Scholar]
- 27.Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: Past, present and future developments. J Pain Symp Manage 2017;53:630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breitbart W, Rosenfeld B, Roth A et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage 1997;13:128–137. [DOI] [PubMed] [Google Scholar]
- 29.Fadul N, Kaur G, Zhang T et al. Evaluation of the memorial delirium assessment scale (MDAS) for the screening of delirium by means of simulated cases by palliative care health professionals. Support Care Cancer 2007;15:1271–1276. [DOI] [PubMed] [Google Scholar]
- 30.Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal 1999;30:253–270. [Google Scholar]
- 31.Perez Camargo DA, Allende Perez SR, Rivera Franco MM et al. Phase angle of bioelectrical impedance analysis as prognostic factor in palliative care patients at the National Cancer Institute in Mexico. Nutr Cancer 2017;69:601–606. [DOI] [PubMed] [Google Scholar]
- 32.Hui D, Dev R, Pimental L et al. Association between multi‐frequency phase angle and survival in patients with advanced cancer. J Pain Symptom Manage 2017;53:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J 2008;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nwosu AC, Mayland CR, Mason S et al. The Association of hydration status with physical signs, symptoms and survival in advanced cancer‐The use of bioelectrical impedance vector analysis (BIVA) technology to evaluate fluid volume in palliative care: An observational study. PLoS One 2016;11:e0163114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hui D, Hess K, dos Santos R et al. A diagnostic model for impending death in cancer patients: Preliminary report. Cancer 2015;121:3914–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hui D, Frisbee‐Hume S, Wilson A et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: A randomized clinical trial. JAMA 2017;318:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]